Abstract
Acetaminophen (APAP) overdose, which causes liver injury in animals and humans, activates c-jun N-terminal kinase (JNK). Although it was shown that the JNK inhibitor SP600125 effectively reduced APAP hepatotoxicity, the mechanisms of protection remain unclear. C57Bl/6 mice were treated with 10 mg/kg SP600125 or vehicle (8% dimethylsulfoxide) 1h before 600 mg/kg APAP administration. APAP time-dependently induced JNK activation (detected by JNK phosphorylation). SP600125, but not the vehicle, reduced JNK activation, attenuated mitochondrial Bax translocation and prevented the mitochondrial release of apoptosis-inducing factor at 4–12 h. Nuclear DNA fragmentation, nitrotyrosine staining, tissue GSSG levels and liver injury (plasma ALT release and necrosis) were partially attenuated by the vehicle (−65%) and completely eliminated by SP600125 (−98%) at 6 and 12h. Furthermore, SP600125 attenuated the increase of inducible nitric oxide synthase (iNOS) mRNA and protein. However, APAP did not enhance plasma nitrite+nitrate levels (NO formation); SP600125 had no effect on this parameter. The iNOS inhibitor L-NIL did not reduce NO formation or injury after APAP but prevented NO formation caused by endotoxin. Since SP600125 completely eliminated the increase in hepatic GSSG levels, an indicator of mitochondrial oxidant stress, it is concluded that the inhibition of peroxynitrite was mainly caused by reduced superoxide formation. Our data suggest that the JNK inhibitor SP600125 protects against APAP-induced liver injury in part by attenuation of mitochondrial Bax translocation but mainly by preventing mitochondrial oxidant stress and peroxynitrite formation and thereby preventing the mitochondrial permeability transition pore opening, a key event in APAP-induced cell necrosis.
Keywords: Acetaminophen, Hepatotoxicity, Mitochondria, JNK, SP600125, Bax, Oxidant Stress
INTRODUCTION
An overdose of acetaminophen (APAP), a commonly used analgesic drug, can cause hepatic necrosis and even liver failure in humans and animals. APAP overdose is currently the most frequent cause of drug-induced liver failure in the US (Larson et al. 2005). APAP-induced liver cell injury is initiated by the formation of a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which can be generated by several cytochrome P-450 isoenzymes, especially CYP2E1 (Nelson, 1990). NAPQI is detoxified by glutathione (GSH), resulting in the depletion of this sulfhydryl reagent (Mitchell et al., 1973). Once cellular GSH is consumed, NAPQI covalently binds to cellular proteins (Cohen et al., 1997). However, cell injury correlates less with total protein binding but more with the capacity to bind to mitochondrial proteins (Tirmenstein and Nelson 1989; Qiu et al., 2001). These findings support the hypothesis that covalent binding to mitochondrial proteins may be responsible for mitochondrial dysfunction (Jaeschke et al., 2003; Jaeschke and Bajt, 2006). The well established effects of an APAP overdose on mitochondria include inhibition of mitochondrial respiration, enhanced formation of reactive oxygen species and peroxynitrite, mitochondrial DNA damage, release of mitochondrial intermembrane proteins, which translocate to the nucleus and cause nuclear DNA degradation, and ultimately opening of the mitochondrial membrane permeability transition pore with collapse of the membrane potential (Jaeschke, et al., 2003; Jaeschke and Bajt, 2006).
c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinase (MAPK) superfamily (Hibi et al., 1993). JNK can activate a variety of signal cascades through its phosphorylation of not only transcription factors such as c-jun (Li et al., 2004), p53 (Cheng et al., 2003), and ATF-2 (Hayakawa et al., 2003), but also members of the Bcl-2 family (Latchoumycandane et al. 2007). JNK has 2 ubiquitously expressed isoforms (JNK1, JNK2) and a tissue-specific isoform (JNK3), which is predominately located in neurons of the central nervous system (Resnick and Fennell, 2004). JNK1 mediates the majority of c-jun phosphorylation and JNK2 mainly regulates c-jun stability (Sabapathy et al., 2004).
Studies with APAP overdose clearly demonstrated prolonged activation of JNK before cell death. Furthermore, pharmacological inhibition of JNK or the silencing of JNK gene expression resulted in reduced liver injury after APAP overdose (Gunawan et al., 2006; Hanawa et al., 2008; Henderson et al., 2007). However, the use of JNK-deficient mice yielded mixed results. One study showed a partial protection with JNK2- but not JNK1-deficient mice (Gunawan et al., 2006). Other studies did not find a protection with either JNK knockout mice (Henderson et al., 2007; Bourdi et al., 2008). In contrast, one report suggested a beneficial role of JNK2 in tissue repair after APAP (Bourdi et al., 2008). Although apoptosis signal-regulating kinase 1 (ASK1), a member of the mitogen-activated protein kinase kinase kinase family, has been identified as one of the upstream activators of JNK (Nakagawa et al., 2008), the downstream mechanisms by which JNK affects APAP hepatotoxicity are less clear. It was suggested that JNK may act by triggering Bax activation and translocation to the mitochondria (Gunawan et al., 2006). However, Bax-deficient mice are only temporarily protected against APAP-induced hepatotoxicity (Bajt et al., 2008a) suggesting that additional mechanisms may be operative. More recently it was proposed that JNK is activated by reactive oxygen species generated by GSH-depleted mitochondria, which triggers the translocation of activated JNK to mitochondria resulting in the induction of the mitochondrial permeability transition (Hanawa et al. 2008). A caveat of this hypothesis is that reactive oxygen species have not been detected outside the mitochondria (Jaeschke, 1990) and that the more aggressive oxidant peroxynitrite, generated in mitochondria, was identified as a critical mediator of the injury process (Cover et al., 2005; Knight et al., 2002). A third mechanism, JNK-mediated activation of inducible nitric oxide synthase (iNOS), was also suggested (Latchoumycandane et al., 2007). However, inhibition of iNOS was not consistently beneficial in APAP hepatotoxicity (Hinson et al., 2002; Gardner et al., 2002). Therefore, our goal was to assess all three mechanisms simultaneously and evaluate by which mechanism the pharmacological and genetic inhibition of JNK protects in an in vivo murine model of APAP hepatotoxicity.
MATERIALS AND METHODS
Animals
Male C57Bl/6J mice (8-10 weeks old), JNK2-deficient mice (B6.129S2-Mapk9tm1Flv/J) or age-matched wild type (C57Bl/6J) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals”. The experimental protocols were approved by the institutional animal care and use committee of Kansas University Medical Center.
Experimental Protocols
All animals were fasted overnight and some animals received JNK inhibitor, 10 mg/kg SP600125 (LC Laboratories, Woburn, MA) dissolved in 8.3 % DMSO in phosphate-buffered saline (PBS) (1 mg in 125 μl of DMSO diluted with 1375 μl of PBS) or the vehicle alone (15ml/kg) (Hanawa et al., 2008). JNK inhibitor and vehicle were injected 1 h prior to 300 or 600 mg/kg APAP (Sigma-Aldrich Chemical Co., St. Louis, MO). APAP was dissolved in warm saline (15 mg/ml) and injected i.p. To study the effect of glutathione depletion and oxidant stress on JNK activation, some animals were treated i.p. with 1 mmol/kg tert-butylhydroperoxide (Sigma), 100 mg/kg phorone (Sigma) (dissolved in corn oil) or both (Jaeschke, 1991). Additional animals received 2 mg/kg endotoxin (Salmonella abortus equi, Sigma) by i.p. injection with or without 3.3 mg/kg of the iNOS inhibitor L-N-(1-iminoethyl)lysine (L-NIL,; Cayman, Ann Arbor, MI) or vehicle (PBS) at 0 and 3 h (Zhang et al., 2000). Groups of animals were killed by cervical dislocation under isoflurane anesthesia at various times after APAP or endotoxin injection. Blood was drawn from the vena cava into heparinized syringes and centrifuged. The plasma was used for determination of alanine aminotransferase (ALT) activities. Immediately after collecting the blood, the livers were excised and rinsed in saline. A small section from each liver was placed in 10% phosphate buffered formalin to be used for H&E staining and immunohistochemical analysis. The remaining liver was frozen in liquid nitrogen and stored at −80°C.
Methods
Plasma ALT activities were determined with the kinetic test kit 68-B (Biotron Diagnostics, Inc., Hernet, CA) and expressed as IU/liter. In addition, the plasma concentrations of nitrite/nitrate were determined with a Nitrate/Nitrite Colorimetric Assay Kit (Cayman) based on the Griess reaction. Total soluble GSH and GSSG were measured in the liver homogenate with a modified method of Tietze as described in detail (Jaeschke and Mitchell 1990; Knight et al., 2002). Briefly, the frozen tissue was homogenized at 0°C in 3% sulfosalicylic acid containing 0.1 mM EDTA. For GSSG measurement, GSH was trapped with 10 mM N-ethylmaleimide. After dilution with 0.01 N HCl, the sample was centrifuged and the supernatant was diluted with 100 mM potassium phosphate buffer (KPP), pH 7.4. The samples were assayed using dithionitrobenzoic acid. All data are expressed in GSH-equivalents.
Quantitative Real-time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Expression of selected genes was quantified using qRT-PCR analysis as previously described (Bajt et al., 2008b). Briefly, total RNA was reverse transcribed with MuLV reverse transcriptase and aligo-dT primers. The forward and reverse primers for all genes were designed using Primer Express software (Applied Biosystems, Foster City, CA). The SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA) was used for real-time PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values. Ct values for the various genes were first normalized with that of β-actin in the same sample, and then relative differences between groups were expressed as relative increases setting control as 1.0. Assuming that the Ct value is reflective of the initial starting copy and that there is 100 % efficiency, a difference of one cycle is equivalent to a two-fold difference in starting copy using the 2⋀ (−ddCt) formula.
Histology and immunohistochemistry
Formalin-fixed tissue samples were embedded in paraffin and 4 μm sections were cut. Replicate sections were stained with hematoxylin and eosin (H&E) for evaluation of necrosis (Gujral et al., 2002). Sections were also stained for nitrotyrosine (NT) protein adducts with the DAKO LSAB peroxidase Kit (K684) (DAKO Corp., Carpinteria, CA) and an anti-nitrotyrosine antibody (Molecular Probes, Eugene, OR) (Knight et al., 2002). For the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, sections of liver were stained with the In Situ Cell Death Detection Kit, AP (Roche Diagnostics, Indianapolis, IN) as described in the manufacturer’s instructions (Gujral et al., 2002).
Isolation of subcellular fractions and western blotting
Mitochondria and cytosolic fractions were isolated as described (Cover et al., 2005). Briefly, the liver was homogenized in ice cold isolation buffer (pH7.4) containing 220 mM mannitol, 70 mM sucrose, 2.5 mM HEPES, 10 mM EDTA, 1 mM EGTA, and 0.1% bovine serum albumin. Mitochondria were isolated by differential centrifugation (10,000g) and washed with 2 ml of isolation buffer. The supernatant of the 10,000 g spin was centrifuged at 100,000 g and the supernatant represented the cytosolic fraction. Following the isolation, mitochondrial and cytosolic content of Bax, apoptosis-inducing factor (AIF), iNOS, JNK2, P-JNK and β-actin were analyzed by Western blotting as described in detail (Bajt et al., 2000). The following antibodies were used: a rabbit anti-Bax polyclonal antibody (Cell Signaling Technology, Danvers, MA), a rabbit anti-AIF monoclonal antibody (Epitomics, Burlingame, CA), a rabbit anti-iNOS polyclonal antibody (BD Biosciences Pharmingen, San Jose, CA), a rabbit anti-phospho-JNK polyclonal antibody (Cell signaling Technology, Danvers, MA), a rabbit anti-JNK antibody (recognizes only JNK2) (Santa Cruz Biotechnology Inc., Santa Cruz, CA), and a rabbit anti-β-actin monoclonal antibody. A horseradish peroxidase-coupled anti-rabbit IgG (Santa Cruz)) was used as secondary antibody. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech. Inc., Piscataway, NJ).
Statistics
Data are expressed as means ± S.E. Comparison between two groups were performed with Student’s t-test or one-way ANOVA followed by Bonferroni t-test for multiple groups. If the data were not normally distributed, the Mann-Whitney test was applied for comparison of two groups and the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test for multiple groups. P<0.05 was considered significant.
RESULTS
Functional significance of JNK activation after APAP overdose: JNK2 KO Mice
For studying mechanisms of APAP-induced liver injury, the most frequently used dose by us and others is 300 mg/kg in overnight fasted animals (Knight et al., 2001, 2002; Cover et al., 2005; Hinson et al., 1998; Gardner et al., 2002). This dose consistently causes severe liver injury in almost all mouse strains. Administration of 300 mg/kg APAP resulted in the activation of JNK in the livers of C57Bl/6 mice as indicated by the appearance of the phosphorylated form (P-JNK) with an early peak at 2 h and second peak at 6 h (Figure 1A). At 24 h, no P-JNK was detectable. The phosphorylation of both JNK isoforms (JNK1, 46 kDa; JNK2, 54 kDa) observed after APAP is consistent with previous reports (Hanawa et al., 2008; Henderson et al. 2007; Latchoumycandane et al. 2007). Delayed treatment with GSH, which effectively scavenged peroxynitrite and hydrogen peroxide (Knight et al., 2002) or treatment with ZnCl2, which induced metallothionein and partially prevented the mitochondrial oxidant stress (Saito et al., 2010a), also prevented JNK activation at 6 h after APAP (Figure 1B). These data suggest that JNK activation is linked to the early formation of oxidant stress in this model. Both, the scavenging of reactive oxygen by GSH administration and the partial prevention of mitochondrial oxidant stress by Zn pretreatment together with the inhibition of JNK activation resulted in significantly reduced liver injury (Figure 1C). Consistent with previous reports (Hanawa et al., 2008; Henderson et al. 2007), there was no change in total JNK2. To evaluate if GSH depletion, an oxidant stress or a combination of both might be responsible for JNK activation, hepatic GSH levels were depleted with phorone and an oxidant stress was induced by treatment with 1 mmol/kg t-BHP without GSH depletion (Figure 2). Neither GSH depletion nor the tBHP-induced oxidant stress alone was able to activate JNK. In contrast, the combined effect of GSH depletion and oxidant stress caused strong JNK activation (Figure 2). However, there was neither liver injury (increase in plasma ALT activities) nor evidence for nitrotyrosine staining in the phorone/tBHP group (data not shown). These data suggest that APAP-mediated JNK activation was most likely also caused by a combined effect of GSH depletion and oxidant stress. However, JNK activation alone was not able to induce liver cell injury.
Figure 1.
JNK activation in response to treatment with 300 mg/kg acetaminophen (APAP). A. Time course of P-JNK formation as indicator of JNK activation in response to APAP treatment. β-actin protein expression is shown as loading control. B. Total JNK2 and P-JNK protein expression were measured in untreated controls (C) and in animals treated with 300 mg/kg APAP alone (6 h) or together with either 0.65 mmol/kg GSH (i.v.) administered at 1.5 h after APAP or 100 μmol/kg ZnCl2 administered for 3 days as described in details in the methods section. C. Plasma alanine aminotransferase (ALT) activities as an indicator for APAP-induced hepatotoxicity were measured in C57BL/6 mice 6 h after 300 mg/kg APAP administration. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to untreated controls). #P<0.05 (compared to APAP/PBS).
Figure 2.
JNK activation in response to GSH depletion with phorone (100 mg/kg; PH), oxidant stress (1 mmol/kg tBHP ) or a combination of both was determined 2 h after phorone or 1 h after tBHP. Total JNK2 protein expression is shown as loading control. B. Total glutathione levels were measured 2 h after phorone or 1 h after tBHP. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to untreated controls).
A previous report suggested that JNK2 is mainly responsible for the effect of JNK in this model (Gunawan et al., 2006) To evaluate the pathophysiological role of JNK2, wild type and JNK2 KO mice were treated with 300 mg/kg APAP. At 6 h, plasma ALT levels increased to a similar degree in both WT and JNK2 KO mice (Figure 3A) reflecting severe centrilobular necrosis (Figure 3B). To confirm the findings with JNK2 KO mice, C57Bl/6 mice were treated with the general JNK inhibitor SP600125 or the vehicle DMSO/PBS. Although 300 mg/kg APAP alone caused severe liver injury at 6 h (plasma ALT: 5220 ± 1085 U/L), treatment with the vehicle alone or with SP600125 completely prevented any liver injury (data not shown). The reason for the complete protection by the vehicle alone is that the dose of DMSO (1.2 ml/kg) necessary to dissolve the inhibitor effectively blocks the metabolic activation of APAP (Jaeschke et al., 2006). Thus, a higher dose of APAP (600 mg/kg) had to be used to overcome this block (Hanawa et al., 2008).
Figure 3.
A. Plasma alanine aminotransferase (ALT) activities as an indicator for APAP-induced hepatotoxicity were measured in C57BL/6 wild type and JNK2-deficient mice 6 h after 300 mg/kg APAP administration. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to untreated controls). B. Representative liver sections of animals treated for 6 h with 300 mg/kg APAP were stained with H&E. (×50 for all panels).
Functional significance of JNK activation after APAP overdose: SP600125
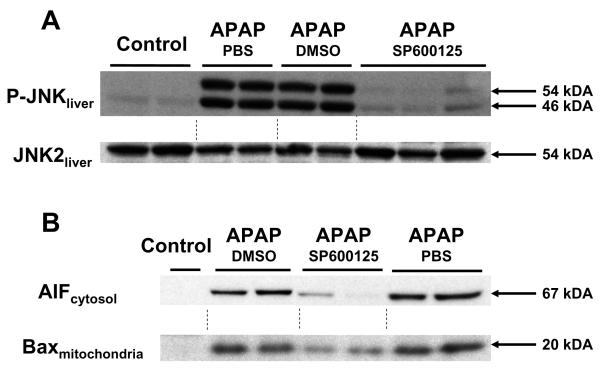
Treatment of C57Bl/6 mice with 600 mg/kg APAP resulted in a rapid loss of hepatic GSH content due to NAPQI formation (Figure 4). Within 20 min GSH levels decreased by 62% and within 2 h 92% was lost. Due to the high dose of APAP, there was only very limited recovery at 6 h (levels were still 78% below baseline) (Figure 4). However, pretreatment with SP600125 or its vehicle substantially delayed the initial decline but caused also an 81-88% loss of hepatic GSH levels by 2 h (Figure 4). At 6 h, PBS and DMSO treated animals had the same low GSH levels but the SP600125-treated animals showed some minor recovery compared to the vehicle-treated groups (Figure 4). These data indicate that the high doses of APAP overcame some of the early inhibition of the metabolic activation in the presence of DMSO. To evaluate the functional significance of JNK, the effect of SP600125 on liver injury was evaluated at 6 and 12 h after APAP (Figure 5A,B). Compared to PBS-treated animals, the DMSO-containing vehicle substantially attenuated liver injury at both time points as reflected by the approximately 65% reduction in ALT release at both time points (Figure 5A) and the reduced area of necrosis at 12 h (Figure 5B). However, liver injury was essentially completely prevented by pretreatment with SP600125 (Figure 5A,B). These findings were confirmed by using the TUNEL assay to evaluate nuclear DNA damage (Figure 5C). Previous reports from our laboratory provided evidence for the translocation of mitochondrial intermembrane proteins such as apoptosis-inducing factor (AIF) and endonuclease G to the nucleus as the main cause of the nuclear DNA damage after APAP overdose (Bajt et al., 2006). This effect was caused initially by formation of Bax pores in the outer membrane and subsequently by mitochondrial swelling and rupture of the outer membrane due to the MPT (Bajt et al., 2008a). In support of these events, JNK activation, release of AIF into the cytosol and translocation of Bax to the mitochondria was observed 12 h after APAP (Figure 6A,B). Although the vehicle was without effect on these parameters, SP600125 effectively reduced JNK activation, mitochondrial AIF release and mitochondrial Bax translocation (Figure 6A,B). To confirm that these events are not just consequences of the protection observed at 12 h, the same parameters were also measured at an earlier time point (4 h) where only minimal injury occurred (Figure 6C). Again, APAP caused JNK activation, mitochondrial Bax translocation and release of AIF, all of which was effectively attenuated by SP600125 (Figure 6C). The vehicle DMSO also prevented mitochondrial AIF release at this early time point but had no relevant effect on JNK activation and mitochondrial Bax translocation (Figure 6C). Together these data support the conclusion that JNK activation causes, at least in part, mitochondrial Bax translocation, which is responsible for the initial AIF and endonuclease G release from the mitochondria and early nuclear DNA damage. However, studies with Bax-deficient mice showed no effect of Bax on mitochondrial oxidant stress and peroxynitrite formation, which ultimately was responsible for the later release of AIF and endonuclease G and the resulting DNA degradation and cell death (Bajt et al., 2008a). Since SP600125 effectively prevented cell death even at later time points, these data suggest that JNK must have additional, more critical effects than just causing Bax activation.
Figure 4.
The liver content of total glutathione (GSH + GSSG) was determined in untreated controls (C) or 20 min, 2 h or 6 h after injection of APAP (600 mg/kg, ip). Some of the animals were pretreated with either 15 ml/kg PBS, 15 ml/kg DMSO (8.3% in PBS) or SP600125 (10 mg/kg in DMSO/PBS). Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to C) #P<0.05 (compared to APAP- PBS).
Figure 5.

A. Plasma ALT activities were measured in untreated controls (C) and at 6 or 12 h after administration of 600 mg/kg APAP in mice with either 15 ml/kg PBS, 15 ml/kg DMSO in PBS or 10 mg/kg of the JNK inhibitor SP600125 at 1 h before APAP. Data represent means ± SE of n= 4-6 animals per group. *P<0.05 (compared to C), #P<0.05 (compared to APAP-PBS) and $P<0.05 (compared to DMSO). B. Histological assessment of liver injury in representative H&E-stained tissue sections obtained 12 h after APAP administration. C. DNA fragmentation was evaluated by the TUNEL assay in animals treated with 600 mg/kg APAP for 12 h. (×50 for all panels).
Figure 6.
P-JNK and JNK2 protein expression were determined in whole liver homogenates by Western blot analysis in untreated controls and at 12 h (A/B) and 4 h (C) after APAP (600 mg/kg). The APAP-treated animals received either 15 ml/kg PBS, 15 ml/kg DMSO/PBS or 10 mg/kg of the JNK inhibitor SP600125 in DMSO/PBS. B. Apoptosis-inducing factor (AIF) and Bax protein levels were determined by Western blot analysis in the cytosol and mitochondria, respectively, from animals treated with APAP and PBS, DMSO or SP600125 as described in panel A. The same parameters as in panel A/B were again measured at 4 h after APAP (C).
Effect of SP600125 on iNOS expression and peroxynitrite formation
It is well established that APAP overdose causes peroxynitrite formation in cells undergoing necrotic cell death (Hinson et al., 1998; Knight et al., 2001). To evaluate if JNK activation is associated with peroxynitrite formation, liver tissue was stained for nitrotyrosine protein adducts (Figure 7). APAP overdose caused substantial peroxynitrite formation in centrilobular hepatocytes at 6 h (Figure 7) and at 12 h (data not shown). Pretreatment with the vehicle DMSO partially reduced and treatment with SP600125 completely eliminated nitrotyrosine staining at both 6 and 12 h (Figure 7). These data suggest that JNK activation is involved in peroxynitrite formation. Since it was previously hypothesized that JNK activation may enhance liver injury by promoting peroxynitrite formation through iNOS induction (Latchoumycandane et al., 2007), the effect of APAP and JNK on iNOS was investigated. APAP overdose caused a 3.5-fold and a 7-fold increase of iNOS mRNA at 6 and 12 h, respectively (Figure 8A). This translated into a minor increase in iNOS protein expression at 6 h (Figure 8B) and at 12 h (data not shown). However, plasma nitrite + nitrate levels as indicators of NO formation did not change significantly (Figure 8C). Although SP600125 attenuated iNOS mRNA and protein levels, there was no significant effect on plasma nitrite + nitrate concentrations (Figure 8). In addition, the iNOS inhibitor L-Nil, affected neither plasma nitrite + nitrate levels (Figure 8C) nor APAP-induced liver injury (Figure 8D) or nitrotyrosine staining (Figure 7). As a positive control, endotoxin treatment substantially enhanced plasma nitrite + nitrate concentrations, which where reduced to baseline by L-Nil (Figure 8C). Although these data confirm that JNK was involved in a minor transcriptional induction of iNOS after APAP overdose, the beneficial effect of inhibiting JNK activation on peroxynitrite formation and liver injury was independent of iNOS.
Figure 7.
Nitrotyrosine staining as indicator of peroxynitrite formation in mice treated with 600 mg/kg APAP for 6 h or 12 h. Some of the animals received PBS, DMSO or the JNK inhibitor SP600125 at 1 h before APAP administration. One group of animals was treated with the iNOS inhibitor L-N-(1-iminoethyl)lysine (L-NIL; 3.3 mg/kg) at 0 and 3 h after APAP.
Figure 8.
A. Hepatic iNOS mRNA expression was measured by real time PCR in mice at 6 h and 12 h after 600 mg/kg APAP treatment. Data are expressed as the iNOS-to-actin mRNA ratio; the value of untreated control animals was set as 1. Some of the animals received 15 ml/kg PBS, 15 ml/kg DMSO/PBS or 10 mg/kg SP600125 in DMSO/PBS at 1 h before APAP administration. Data represent means ± SE of n = 3 animals per group. *P<0.05 (compared to C), #P<0.05 (compared to APAP/PBS). B. Western blot analysis of iNOS expression in livers of controls or animals treated with 600 mg/kg APAP for 6 h. One to three representative samples are shown from each group. The numbers under the blot show the results of the densitometric analysis. Data of control animals were set as 1. C. Plasma nitrate plus nitrite, which were used as a marker of NO production, and D. plasma ALT activities were measured at 6 h after 600 mg/kg APAP or 2 mg/kg endotoxin (ET). Some of the animals received 15 ml/kg PBS (P), 15 ml/kg DMSO (8.3% in PBS) (D) or 10 mg/kg SP600125 in DMSO/PBS (SP) at 1 h before APAP administration. An additional group was treated with 3.3 mg/kg of the iNOS inhibitor L-N-(1-iminoethyl)lysine (NIL) at 0 and 3 h after APAP or LPS administration. Data represent means ± SE of n= 3-4 animals per group. *P<0.05 (compared to C), #P<0.05 (compared to P).
Role of JNK in APAP-induced mitochondrial oxidant stress
Since APAP-induced peroxynitrite formation did not appear to be caused by JNK-mediated enhanced NO generation, especially from iNOS, formation of reactive oxygen was assessed. It was previously demonstrated that increased tissue levels of GSSG after APAP reflect mainly mitochondrial superoxide/hydrogen peroxide but not peroxynitrite formation (Jaeschke, 1990; Knight et al., 2001, 2002). Thus, GSH and GSSG levels were measured at 12 h after APAP (Figure 9). The total hepatic glutathione content (GSH+GSSG) was still partially depleted after treatment with APAP alone but GSSG levels were significantly elevated above controls (Figure 9A,B). This resulted in an increased GSSG-to-GSH ratio from less than 0.5 to more than 2.5 (Figure 9C). The vehicle-treated group, which was partially protected against APAP-induced liver injury (Figure 5), showed higher total glutathione and GSSG levels resulting in a significantly elevated GSSG-to-GSH ratio (Figure 9). These data indicate that the vehicle DMSO accelerated the recovery of liver GSH levels and improved the detoxification of reactive oxygen species but did not prevent the APAP-induced oxidant stress. In contrast, the JNK inhibitor SP600125 did not only promote the faster recovery of the hepatic GSH content, it completely prevented the increase in GSSG levels and the change in the GSSG-to-GSH ratio (Figure 9). These data are consistent with the conclusion that the JNK inhibitor completely prevented the APAP-induced oxidant stress.
Figure 9.
Hepatic content of total GSH (GSH + GSSG) (Panel A) and GSSG (Panel B) were measured in untreated controls (C) and in animals treated with 600 mg/kg APAP for 12 h. In addition, the GSSG-to-GSH ratio (Panel C) was calculated from each animal. Some of the animals received 15 ml/kg PBS (P), 15 ml/kg DMSO (8.3% in PBS) (D) or 10 mg/kg SP600125 in DMSO/PBS (SP) at 1 h before APAP administration. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to C), #P<0.05 (compared to P).
DISCUSSION
Protection against APAP hepatotoxicity by JNK inhibition
The main goal of this study was to evaluate the relative importance of several potential mechanisms of the involvement of JNK signaling in APAP-induced liver injury. JNK activation was monitored by formation of P-JNK due to the autophosphorylation of JNK during activation, and the fact that JNK can phosphorylate a variety of downstream proteins not always to the same degree (Wicovsky et al., 2007). Overall, our data showing JNK activation after APAP overdose and a protective effect with the specific JNK inhibitor SP600125 (Bennett et al., 2001) are in agreement with several previous studies (Gunawan et al., 2006; Hanawa et al., 2008; Henderson et al., 2007; Latchoumycandane et al., 2007). Furthermore, the importance of JNK in APAP-induced liver injury was demonstrated by the elimination of ASK, an upstream activator of JNK (Nakagawa et al., 2008). Our studies also support the conclusion that inhibition of JNK2 alone is not effective in reducing APAP hepatotoxicity (Bourdi et al., 2008; Hanawa et al., 2008; Henderson et al., 2007). Although these findings appear to be different from an earlier report (Gunawan et al., 2006), these initial studies that showed a beneficial effect in JNK2-deficient mice were done with DMSO as solvent for APAP and a dose of 800 mg/kg APAP. Because all studies including our own that did not find a protection by eliminating only JNK2 were done in the absence of DMSO, this organic solvent may unmask a preferential role of JNK2 in the pathophysiology of APAP hepatotoxicity. In addition, selective elimination of JNK1 was also ineffective (Bourdi et al., 2008; Gunawan et al., 2006; Henderson et al., 2007). However, we did not observe an increase in APAP-induced liver injury at the 6 h time point using JNK2-/- mice as was reported by Bourdi et al. (2008) for 12 -32 h after APAP. Another important observation in our study is the fact that the solvent used to administer SP600125 (DMSO in PBS) clearly inhibits the early metabolic activation of APAP and accounts for a substantial protection in this model. Although the inhibitor provides a significant additional effect, it cannot be excluded that the high efficacy of SP600125 may involve some solvent effect. In addition, the use of a very high overdose of APAP (600 mg/kg) in the presence of DMSO also bears the risk that additional mechanisms of injury, not present at the lower overdose, may be involved. However, since a peptide inhibitor of JNK in the absence of DMSO showed similar protection after 350 mg/kg APAP as SP600125 after 600 mg/kg APAP (Henderson et al., 2007), it can be concluded that the beneficial effect is predominantly caused by inhibition of JNK rather than the initial inhibition of the metabolism.
JNK activation and Bax translocation
Our data indicate that inhibition of JNK reduces, at least in part, the mitochondrial translocation of Bax and the release of AIF from the mitochondria at early and late time points after APAP administration. At 4 h, DMSO treatment strongly attenuated AIF release without affecting mitochondrial Bax translocation (Figure 6C). This could indicate a direct effect of the vehicle on AIF release, which is also consistent with the reduced early nuclear DNA fragmentation. However, these preliminary findings need to be studied in more detail in the future. Nevertheless, the effect of JNK on Bax is in agreement with previous reports (Gunawana et al., 2006; Hanawa et al., 2008). We have shown that mitochondrial Bax translocation is an early event (beginning at 1 h) after APAP overdose, which is responsible for the early release of intermembrane proteins (Jaeschke and Bajt, 2006; Bajt et al., 2008a). Although the release of cytochrome c and the second mitochondria-derived activator of caspases (Smac) does not result in activation of caspases (Knight and Jaeschke, 2002), the nuclear translocation of AIF and endonuclease G appears to be mainly responsible for the characteristic nuclear DNA fragmentation observed after APAP (Bajt et al., 2006). Since Bax-deficient mice show a strong reduction of intermembrane protein release, nuclear DNA damage and cell injury, it can be concluded that these Bax-mediated events contribute to cell death at these early time points (Bajt et al., 2008a). However, mitochondrial Bax translocation does not affect mitochondrial oxidant stress and peroxynitrite formation, which subsequently will trigger the MPT resulting in mitochondrial swelling and rupture of the outer membrane (Bajt et al., 2008a). At this later time point, intermembrane proteins are released independent of Bax. Thus, even complete elimination of Bax results only in a temporary protection against APAP overdose. This means that the protective effect of JNK inhibition, which lasts during the first 12-24 h, can at best be mediated in part by inhibiting mitochondrial Bax translocation.
The possibility that JNK activation leads to inactivation of the protective Bcl-2 family members Bcl-2 and Bcl-xL by phosphorylation could also contribute to the mitochondrial dysfunction (Latchoumycandane et al., 2007). However, the protective role of Bcl-2 in APAP hepatotoxicity has been questioned as Bcl-2 overexpressing mice show higher injury compared to wild type animals (Adams et al., 2001). Other mechanisms suggested by which inhibition of JNK activation could be protective include the inhibition of Bid processing by caspase-8 inactivation (Wang et al., 2006) and downregulation of Bad expression (Takamura et al., 2007). However, this appears to be a mechanism mainly relevant for TNF-mediated apoptosis. Although not all of the individual Bcl-2 family members have been assessed in the APAP model, Bid-deficient mice are not protected (H. Jaeschke and X.M. Yin, unpublished) and pancaspase inhibitors do not prevent mitochondrial dysfunction and liver injury after APAP (Lawson et al., 1999; Jaeschke et al., 2006) suggesting that potential effects of JNK on caspase-8 activation and Bid may not be relevant for the injury process.
JNK activation and iNOS induction
Peroxynitrite, which is formed by the combination of the two radical species superoxide and nitric oxide (NO), is generated in cells undergoing necrosis during APAP hepatotoxicity (Hinson et al., 1998). We identified mitochondria as the primary site of peroxynitrite formation within the cells (Cover et al., 2005). Scavenging of this aggressive oxidant and nitrating agent by GSH resulted in a profound protection and improved regeneration (Knight et al., 2002; Bajt et al., 2003; Saito et al., 2010b). However, the source of NO is still controversial. An induction of iNOS during APAP-induced liver injury has been reported (Gardner et al., 2002; Latchoumycandane et al., 2007) but peroxynitrite formation in the absence of iNOS induction was also observed (Knight et al., 2001). In the current study we found a relevant increase of iNOS mRNA but only a moderate increase in iNOS protein expression. Furthermore, no significant elevation of plasma nitrite+nitrate levels as indicators of actual NO formation was found. The potent iNOS inhibitor L-Nil (Zhang et al., 2000), which completely prevented the endotoxin-induced increase in plasma nitrite+nitrate levels, had no effect on the formation of NO or peroxynitrite during APAP hepatotoxicity and did not affect liver injury. These data do not support the hypothesis that iNOS-derived NO is critical for APAP-induced peroxynitrite formation and liver injury under our present conditions. A potential reason for the limited importance of iNOS during APAP-induced liver injury may be the formation of IL-10, which can suppress pro-inflammatory gene expression, including iNOS, thereby reducing a potential contribution of iNOS to the pathophysiology (Bourdi et al., 2002). Although SP600125 attenuated iNOS mRNA and also the minor increase in protein expression, there was no effect on plasma nitrite+nitrate levels. Together these data suggest that JNK activation may contribute to a limited iNOS induction during APAP hepatotoxicity. However, consistent with previous data reported by us and others (Knight et al., 2001; Hinson et al., 2002), iNOS did not play a relevant role and therefore it is unlikely that the profound protective effect of JNK inhibition was mediated by effects on iNOS. These conclusions are different than those of Latchoumycandane et al. (2006, 2007). However, these investigators used leflonamide, which may have had additional effects beyond just inhibiting JNK.
JNK activation and oxidant stress
Our previous studies with APAP hepatotoxicity documented the presence of a mitochondrial oxidant stress (Jaeschke, 1990; Knight et al., 2001) and mitochondrial peroxynitrite formation (Cover et al., 2005), which preceded cell death and occurred as soon as GSH was depleted (Bajt et al., 2004). Furthermore, when peroxynitrite was being scavenged and more ROS was detoxified by accelerated recovery of mitochondrial GSH levels initiated by treatment with GSH (Knight et al., 2002; Saito et al., 2010b), JNK activation was prevented (Figure 1). Likewise, when the mitochondrial oxidant stress is inhibited by scavenging of NAPQI by metallothionein induction (Saito et al., 2010a), JNK activation was prevented (Figure 1). In addition, the combined effect of GSH depletion and oxidant stress induced by tBHP caused JNK activation (Figure 2). These findings suggest that in agreement with previous findings (Hanawa et al., 2008), an early oxidant stress in the presence of low GSH levels was the main trigger of JNK activation after APAP overdose. However, our findings also indicate that GSH depletion alone was not able to induce JNK activation. There is no evidence that massive GSH depletion alone causes relevant oxidant stress leading to cell injury in the liver in vivo. These findings are in agreement with previous reports showing in isolated hepatocytes and in vivo that a massive chemical-induced oxidant stress leads to JNK activation (Czaja et al., 2003; Conde de la Rosa et al., 2006; Hong et al., 2009). However, the oxidant stress most likely does not activate JNK directly, but targets upstream events such promoting either the dissociation of thioredoxin and apoptosis signal-regulating kinase 1 (ASK1) (Nakagawa et al., 2008) or the Ras pathway (Saha and Nandi, 2009). Alternatively, JNK can be released from a complex with glutathione-S-transferase Pi (GST-Pi) by binding of NAPQI to GST (Elsby et al., 2003). This would be in agreement with AMAP treatment not activating JNK (Hanawa et al., 2008) and the fact that JNK activation occurs in the cytosol and oxidant stress occurs mainly in mitochondria. In addition, the fact that JNK was activated by GSH depletion and oxidant stress without causing injury suggested that additional effects involving protein binding of NAPQI and not just JNK activation are required for APAP hepatotoxicity.
Although JNK appears to be activated by the initial oxidant stress, given the fact that nitrotyrosine staining of the tissue was eliminated by the JNK inhibitor at 6 and 12 h after APAP and that there was no increase of tissue GSSG or the GSSG-to-GSH ratio, it can be concluded that SP600125 effectively prevented the formation of reactive oxygen species. Since ROS and peroxynitrite are mainly formed in mitochondria, this suggested that JNK activation promotes the formation of ROS in this cell organelle. Interestingly, the solvent of the JNK inhibitor (DMSO in PBS) did not prevent the oxidant stress (judged by GSSG formation) but appears to allow a faster recovery of hepatic GSH levels, which seem to scavenge some of the ROS and peroxynitrite, and thereby reduces tissue injury. The effect of DMSO is attributed to its inhibitory effect on APAP activation, which limits the injury and promotes recovery. Nevertheless, the JNK inhibitor has clearly additional effects that prevent the mitochondrial oxidant stress. Hanawa et al (2008) proposed that translocation of activated JNK may induce the MPT. Given the time sequence of rapid GSH depletion and mitochondrial dysfunction followed by oxidant stress, eventually the MPT and then cell necrosis (Bajt et al., 2004; Kon et al., 2004), it appears that JNK activation enhances the oxidant stress and peroxynitrite formation, which subsequently induces the MPT. Mitochondrial oxidant stress is a potent inducer of the MPT (Nieminen et al., 1997). This does not exclude that JNK can synergistically promote the MPT through acting directly on proteins involved in the MPT (Hanawa et al., 2008). However, it appears highly unlikely that JNK inhibition can prevent the MPT in the presence of a substantial mitochondrial oxidant stress and peroxynitrite formation. Further studies are necessary to identify the various targets of JNK in the mitochondria.
In summary, our data support the hypothesis that prolonged JNK activation, which is a critical factor in cell injury, occurs after APAP overdose (Figure 10). Both GSH depletion and an oxidant stress are required for JNK activation, which seems to control at least in part the early release of intermembrane proteins and DNA fragmentation through mitochondrial Bax translocation. However, the most important effect of JNK inhibition was a profound suppression of peroxynitrite formation, which was not caused by inhibition of iNOS induction. In contrast, there was clearly a complete elimination of the mitochondrial oxidant stress suggesting an effect of JNK activation on mitochondrial ROS formation. Although the exact target of JNK in the mitochondria has to be identified, it appears to be upstream of the MPT. Because of the critical role of oxidant stress and peroxynitrite for the propagation of cell injury, especially mitochondrial viability, these data demonstrate that JNK could be an important target to limit cell injury and liver failure, at least during the first 24 h after APAP overdose.
Figure 10.
Proposed intervention points of JNK activation during acetaminophen (APAP)-induced liver injury.
Abbreviations: AIF, apoptosis-inducing factor; ASK, apoptosis signal-regulating kinase 1; GSH, glutathione; GSSG, glutathione disulfide; GST-Pi, glutathione S-transferase Pi; JNK, c-jun-N-terminal kinase; MOMP, mitochondrial outer membrane permeabilization; MPT, mitochondrial membrane permeability transition pore; NAPQI, N-acetyl-p-benzoquinone imine; NT adducts, nitrotyrosine adducts;
ACKNOWLEDGMENT
This investigation was supported in part by National Institutes of Health Grants R01 DK070195 and R01 AA12916, and by grants P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol. Pharmacol. 2001;60:907–915. doi: 10.1124/mol.60.5.907. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–25. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2008a;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Yan HM, Farhood A, Jaeschke H. Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol. Sci. 2008b;104:419–427. doi: 10.1093/toxsci/kfn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Arthur PG. Inhibitors of c-Jun N-terminal kinases: JuNK no more? Biochim. Biophys. Acta. 2008;1784:76–93. doi: 10.1016/j.bbapap.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Korrapati MC, Chakraborty M, Yee SB, Pohl LR. Protective role of c-Jun N-terminal kinase 2 in acetaminophen-induced liver injury. Biochem. Biophys. Res. Commun. 2008;374:6–10. doi: 10.1016/j.bbrc.2008.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289–298. doi: 10.1053/jhep.2002.30956. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Zheng X, Quimby FR, Roneker CA, Lei XG. Low levels of glutathione peroxidase 1 activity in selenium-deficient mouse liver affect c-Jun N-terminal kinase activation and p53 phosphorylation on Ser-15 in pro-oxidant-induced aponecrosis. Biochem. J. 2003;370:927–934. doi: 10.1042/BJ20021870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- de la Rosa L. Conde, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J. Hepatol. 2006;44:918–929. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Czaja MJ, Liu H, Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology. 2003;37:1405–1413. doi: 10.1053/jhep.2003.50233. [DOI] [PubMed] [Google Scholar]
- Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR, Park BK. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J. Biol. Chem. 2003;278:22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang XJ, DeGeorge GL, Laskin JD, Laskin DL. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology. 1998;27:748–754. doi: 10.1002/hep.510270316. [DOI] [PubMed] [Google Scholar]
- Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordon MK, Gerecke DR, Zhou P, Laskin DL. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol. Appl. Pharmacol. 2002;184:27–36. [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Bucci TJ, Irwin LK, Michael SL, Mayeux PR. Effect of inhibitors of nitric oxide synthase on acetaminophen-induced hepatotoxicity in mice. Nitric Oxide. 2002;6:160–167. doi: 10.1006/niox.2001.0404. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem. Res. Toxicol. 1998;11:604–607. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- Hong JY, Lebofsky M, Farhood A, Jaeschke H. Oxidant stress-induced liver injury in vivo: role of apoptosis, oncotic necrosis, and c-Jun NH2-terminal kinase activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G572–G581. doi: 10.1152/ajpgi.90435.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H. Vascular oxidant stress and hepatic ischemia/reperfusion injury. Free Radic. Res. Commun. 1991;12-13:737–743. doi: 10.3109/10715769109145853. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol. Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol. Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol. Appl. Pharmacol. 2002;181:133–141. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Seah QM, Tan RC, Sattabongkot J, Beerheide W, Boelsterli UA. Leflunomide or A77 1726 protect from acetaminophen-induced cell injury through inhibition of JNK-mediated mitochondrial permeability transition in immortalized human hepatocytes. Toxicol. Appl. Pharmacol. 2006;217:125–133. doi: 10.1016/j.taap.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Lahti A, Sareila O, Kankaanranta H, Moilanen E. Inhibition of p38 mitogen-activated protein kinase enhances c-Jun N-terminal kinase activity: implication in inducible nitric oxide synthase expression. BMC Pharmacol. 2006;6:5. doi: 10.1186/1471-2210-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol. Appl. Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Li L, Feng Z, Porter AG. JNK-dependent phosphorylation of c-Jun on serine 63 mediates nitric oxide-induced apoptosis of neuroblastoma cells. J. Biol. Chem. 2004;279:4058–4065. doi: 10.1074/jbc.M310415200. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am. J. Physiol. 1997;272:C1286–C1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3′-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv. Exp. Med. Biol. 2001;500:663–673. doi: 10.1007/978-1-4615-0667-6_99. [DOI] [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 1990;106:346–351. doi: 10.1016/0041-008x(90)90254-r. [DOI] [PubMed] [Google Scholar]
- Resnick L, Fennell M. Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discov. Today. 2004;9:932–939. doi: 10.1016/S1359-6446(04)03251-9. [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell. 2004;15:713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Saha B, Nandi D. Farnesyltransferase inhibitors reduce Ras activation and ameliorate acetaminophen-induced liver injury in mice. Hepatology. 2009;50:1547–1557. doi: 10.1002/hep.23180. [DOI] [PubMed] [Google Scholar]
- Saito C, Yan HM, Artigues A, Villar MT, Farhood A, Jaeschke H. Mechanism of protection by metallothionein against acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010a;242:182–190. doi: 10.1016/j.taap.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010b;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura M, Matsuda Y, Yamagiwa S, Tamura Y, Honda Y, Suzuki K, Ichida T, Aoyagi Y. An inhibitor of c-Jun NH2-terminal kinase, SP600125, protects mice from D-galactosamine/lipopolysaccharide-induced hepatic failure by modulating BH3-only proteins. Life Sci. 2007;80:1335–1344. doi: 10.1016/j.lfs.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. Embo J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J. Biol. Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicovsky A, Müller N, Daryab N, Marienfeld R, Kneitz C, Kavuri S, Leverkus M, Baumann B, Wajant H. Sustained JNK activation in response to tumor necrosis factor is mediated by caspases in a cell type-specific manner. J. Biol. Chem. 2007;282:2174–2183. doi: 10.1074/jbc.M606167200. [DOI] [PubMed] [Google Scholar]
- Zhang C, Walker LM, Hinson JA, Mayeux PR. Oxidant stress in rat liver after lipopolysaccharide administration: effect of inducible nitric-oxide synthase inhibition. J. Pharmacol. Exp. Ther. 2000;293:968–972. [PubMed] [Google Scholar]