Abstract
Zebrafish embryos demonstrate robust swimming behavior, which consists of smooth, alternating body bends. In contrast, several motility mutants have been identified that perform sustained, bilateral trunk muscle contractions which result in abnormal body shortening. Unlike most of these mutants, accordion (acc)dta5 demonstrates a semidominant effect: Heterozygotes exhibit a distinct but less severe phenotype than homozygotes. Using molecular-genetic mapping and candidate gene analysis, we determined that accdta5 mutants harbor a novel mutation in atp2a1, which encodes SERCA1, a calcium pump important for muscle relaxation. Previous studies have shown that eight other acc alleles compromise SERCA1 function, but these alleles were all reported to be recessive. Quantitative behavioral assays, complementation testing, and analysis of molecular models all indicate that the accdta5 mutation diminishes SERCA1 function to a greater degree than other acc alleles through either haploinsufficient or dominant-negative molecular mechanisms. Since mutation of human ATP2A1 results in Brody disease, an exercise induced impairment of muscle relaxation, accdta5 mutants may provide a particularly sensitive model of this disorder.
Keywords: Danio rerio, zebrafish, mutant, behavior, muscle, Brody disease
Zebrafish embryos perform an initial sequence of three motor behaviors as they develop. Initially, at about 17 hours postfertilization (hpf), embryos demonstrate spontaneous, alternating tail coils. Around 21 hpf, embryos begin to respond to touch by performing more rapid tail coils. Finally, embryos begin to swim at approximately 27 hpf, and react to touch by moving at least one body length away from the touch stimulus (Downes and Granato, 2006; Saint-Amant and Drapeau, 1998). Granato et al. utilized this series of robust behaviors as the basis for extensive mutagenesis screens to identify mutants with specific deficits in embryonic motility (Granato et al., 1996). Many mutants demonstrated similar behavioral deficits, which was used as criteria to group them into several different phenotypic classes.
All members of the accordion class of motility mutants exhibit similar, characteristic behavior. Beginning at the onset of the touch response, accordion class mutants perform bilateral trunk muscle contractions, which cause the embryo to contract along the rostral-caudal axis reminiscent of an accordion. The genes have been identified for several accordion class mutants and, despite the similar behavior of the mutants, they encode proteins essential for diverse aspects of spinal cord, neuromuscular synapse or muscle function (Downes and Granato, 2004; Gleason et al., 2004; Hirata et al., 2005; Hirata et al., 2004; Lefebvre et al., 2004; Wang et al., 2008).
A single allele of dta5 was isolated in a previous genetic screen for dominant mutants that exhibit abnormal locomotor behavior (van Eeden et al., 1999). As a first step to determine the molecular and cellular defects in dta5 mutants, we analyzed their locomotive behavior over the course of development using a high-speed video camera. Homozygous mutant embryos perform abnormal behavior that can first be distinguished from wild-type embryos around 24 hours postfertilization (hpf). At this time point, wild-type embryos respond to touch with smooth, alternating tail coils (Supporting information: Movie1). In contrast, dta5 homozygotes appear to contract their trunk muscles bilaterally, which results in prolonged compression along the rostral-caudal axis and an aberrant dorsal bend (Supporting information: Movie2). dta5 heterozygotes demonstrate a intermediate phenotype consisting of slower or incomplete tail coils. The heterozygous phenotype is variable and challenging to quantify at 24hpf, but analysis of a population of embryos obtained from crossing dta5 heterozygotes revealed that 61.3% exhibit abnormal behavior at this stage of development (n=186; data not shown). According to Mendelian ratios, about 25% of the embryos would be predicted to show abnormal behavior if dta5 were a recessive allele. Therefore, this observation shows that dta5 homozygotes and some heterozygotes can be identified at 24hpf and it demonstrates the dominant mode of inheritance of dta5.
At 48hpf, wild-type larvae exhibit robust swimming behavior, but both dta5 heterozygotes and homozygotes continue to display abnormal motor behavior. Kinematic analysis illustrates that wild-type larvae respond to touch with a rapid burst of swimming, consisting of fast, alternating body bends (Figure 1a, d; Supporting information: Movie3). dta5 homozygotes do not swim in response to touch stimuli. They typically perform a small amplitude body bend towards one side and appear stiff, likely due to bilateral trunk muscle contractions (Figure 1c, f; Supporting information: Movie4). The rostral-caudal compression and dorsal bend observed at early ages are less pronounced at 48hpf, presumably because of the mechanical resistance provided by the increased stiffness of the notochord. dta5 heterozygotes display an intermediate phenotype (Figure 1b, e). They are able to swim; however, their swimming behavior consists of slow, small-amplitude body bends. As a result, dta5 heterozyotes swim much more slowly than wild-type embryos (compare Figure 1a and 1b; Supporting information: Movie5).
Figure 1. dta5 exhibits a semi-dominant mode of inheritance.
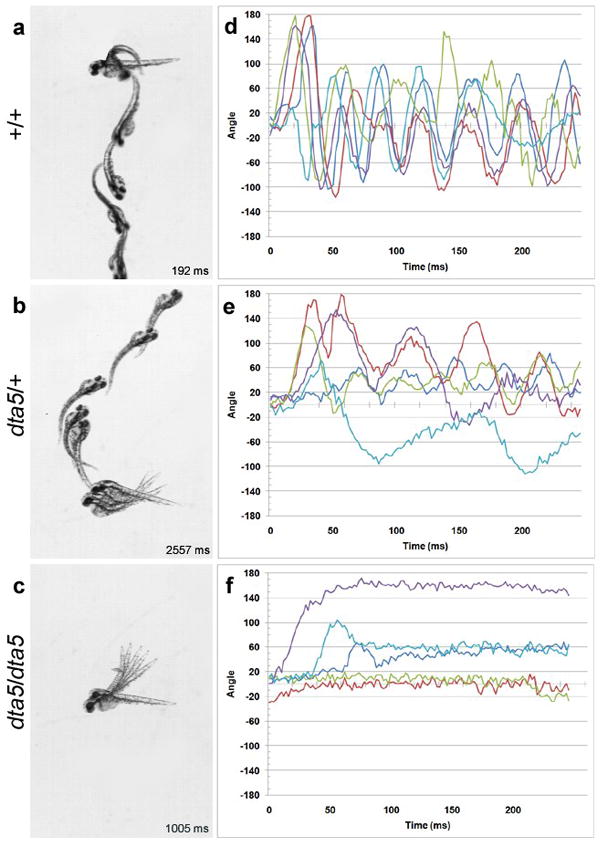
Selected, superimposed frames from a high-speed video camera illustrate escape behaviors of (a) wild-type, (b) heterozygous, and (c) homozygous mutant larvae at 48hpf. Gentle touch with a probe was used to stimulate escape behavior, and it has been removed from each frame for clarity. The duration of each response is indicated in milliseconds (ms). (d–f) Kinematic traces are shown for 48hpf larvae. For each condition, five representative traces are shown from the first 250ms of a response. Each response is from a different larva. The body angles are shown in degrees with 0 indicating a straight body, and positive and negative values indicating body bends in opposite directions. Note that mutants carrying one dta5 allele display a reduced number of body bends and ability to swim. Swimming behavior is further reduced in mutants with two dta5 alleles.
Since we observed a clear correlation between swimming speed and genotype, we developed a behavioral assay to measure the amount of time it takes larval zebrafish to swim a 4mm distance. Using this time-trial assay, we found that a population of wild-type larvae swim with an average time of 137ms at 48hpf (Figure 2a). Wild-type larvae swim faster over the course of development, with average times of 76ms at 96hpf and 43ms at 144hpf (Figure 2b, c). A population of larvae obtained from crossing dta5 heterozygotes showed a range of swimming times at 48hpf. We expected to observe a Mendelian distribution of 25% wild-type with normal swimming times, 50% heterozygotes with abnormally slow swimming times, and 25% homozygotes that are unable to swim. Instead, we noted that about 36% exhibit wild-type like swimming times, 34% demonstrate abnormally slow swimming times, defined as more than 500ms, and 30% are unable to swim (Figure 2d). This suggests that dta5 heterozygotes exhibit a wide range of swimming phenotypes at 48hpf, with some indistinguishable from wild-type or dta5 homozygotes. Regardless of genotype, nearly all larvae obtained from crossing dta5 heterozygotes acquire the ability to swim (Figure 2e, f). While most dta5 homozygotes can swim by 144hpf, their swimming is not normal. Abnormalities in tail-flip frequency and left-right alternation were observed (data not shown). These larvae fail to inflate the swim bladder and soon die, likely due to an inability to feed. We did not observe any differences in swimming behavior between dta5 heterozygotes and wild-type larvae at 144hpf, and dta5 heterozygotes are viable to adulthood.
Figure 2. Wild-type and dta5 mutant larvae increase swimming speeds over the course of development.
The amount of time for each larva to swim 4mm was measured. The total number (n) of larvae tested, the average speed in mm/s (a.s.), and the average time (a.t.) for all of the larvae that can swim are indicated. Larvae that do not swim are not included in the average speed or time. The percentages of larvae from the total number of tested are plotted in time bins, with numbers indicating the upper limit of a time bin. 50ms bins are shown to the left of the dashed line and 250ms bins are show to the right of the dashed line. Embryos that do not swim are plotted in the time bin furthest to the right. (a) All wild-type larvae swim the 4mm distance within 500ms at 48hpf. Wild-type embryos swim the 4mm distance increasingly faster at (b) 96hpf and (c) 144hpf. (d) Crosses between dta5 heterozygotes yield a population of larvae in which some swim with wild-type times, defined as faster than 500ms, some heterozygotes swim slowly, and a group of mostly homozygotes do not swim at all. Regardless of genotype, most larvae have acquired the ability to swim at (e) 96hpf and (f) 144hpf.
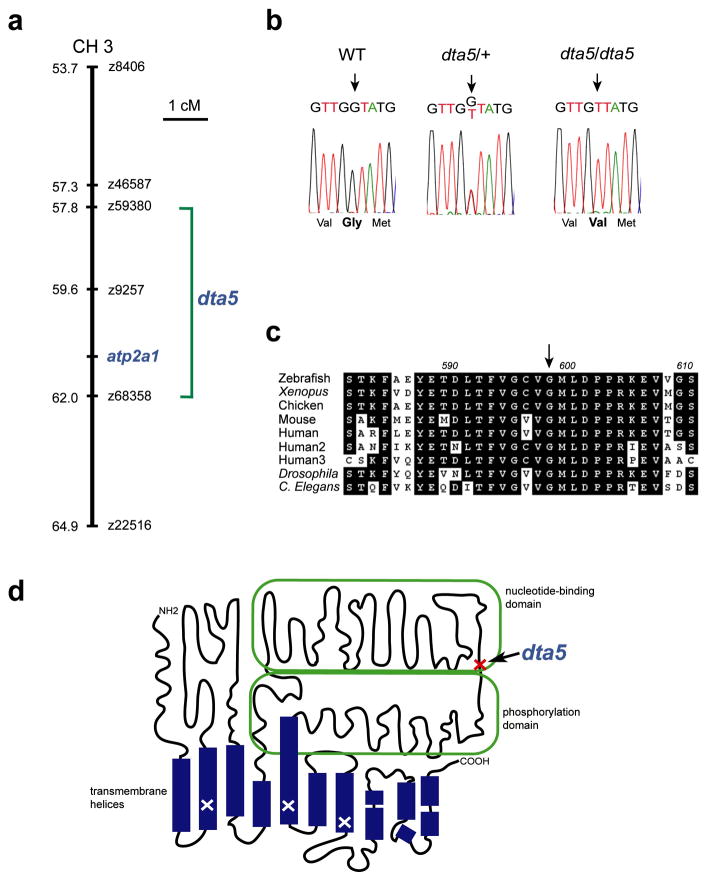
To determine the molecular identity of dta5, we mapped its genomic location using a three-generation map cross panel. Genomic DNA from pooled and individual wild-type and homozygous mutant embryos was screened with a panel of simple sequence length polymorphism (SSLP) markers. We found that dta5 maps to chromosome 3 near markers z59380 and z68358 (both 0 recombinants in 184 meioses, Figure 3a). Genome database analysis indicated that atp2a1 lies within this vicinity. atp2a1 encodes Sarco(endo)plasmic Reticulum Ca2+ ATPase 1 (SERCA1), a calcium ATPase that plays an important role in muscle relaxation. SERCA1 transports calcium from the cytosol into the sarco(endo)plasmic reticulm of muscle, which allows actin and myosin to disassociate, resulting in muscle relaxation. Previous studies described the zebrafish accordion (acc) mutants, in the accordion class, which contain mutations in atp2a1 and exhibit a behavioral phenotype similar to dta5 (Gleason et al., 2004; Hirata et al., 2004).
Figure 3. The dta5 mutation disrupts atp2a1/SERCA1, a calcium ATPase important for muscle relaxation.
(a) A portion of chromosome 3 is shown with map positions, CA-repeat markers, and the location of the Atp2a1 gene indicated. dta5 maps to a large 0 recombinant interval (from 92 mutants) from marker z59380 to z68358. (b) Chromatogram sequence traces, with nucleotide sequence above and translated sequence below, from wild-type, dta5 heterozygous, and dta5 homozygous embryos. A single mutation was observed, which segregates along with the phenotype, and results in the substitution of Gly598 to Val. (c) Alignment of the Gly598 region of SERCA1 from different species demonstrates its precise conservation. Shading indicates amino acid identity and the arrow indicates Gly598. Gly598 is also conserved among the other two SERCA-encoding genes in humans and single orthologs in Drosophila and C. Elegans. (d) A schematic of the SERCA1 crystal structure illustrates the locations of SERCA1 functional domains, the accordion mutations previously identified in zebrafish (white X), and the dta5 mutation (red X with arrow). The schematic is adapted from (Toyoshima and Inesi, 2004).
Since atp2a1 was a promising candidate gene for dta5, we cloned and sequenced atp2a1 transcripts from heterozygous and homozygous larvae. We found a single point mutation that segregates along with the phenotype and results in the substitution of Gly598 for a Val (Figure 3b). This residue is precisely conserved among each of the three SERCA-encoding genes in vertebrates and single orthologs in C. Elegans and Drosophila, which strongly argues that Gly598 is crucial for SERCA function (Figure 3c).
Structural studies of mammalian SERCA molecules also suggest that Gly598 is important for SERCA function. Mammalian SERCA1 has been examined extensively through X-ray crystallography and site-directed mutagenesis (Toyoshima and Inesi, 2004). Based upon these studies, Gly598 is located in the ATP binding domain near where it connects to the phosphorylation domain (Figure 3d). Although this residue has not been the target of site-directed mutagenesis, mutation of nearby Asp601 and Pro603 has been shown to severely compromise Ca2+ transport activity (Clarke et al., 1990). These residues are thought to be important in guiding the transition of SERCA from the E1P to the E2P state, which is important in translocating Ca2+ across the membrane. The substitution of Gly598 with the slightly larger Val likely disrupts the function of this critical region and greatly diminishes or abolishes SERCA1 activity. Based upon our mapping data, the nature of the dta5 mutation, and similarity to the acc mutant phenotype, we propose that dta5 is an allele of acc, and hereafter refer to dta5 as accdta5.
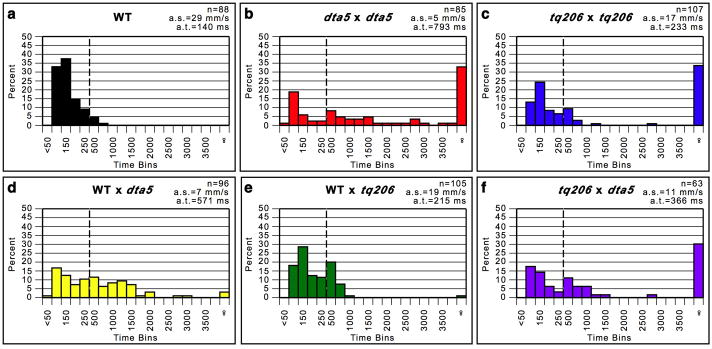
Although eight other acc mutant alleles have been identified, each is believed to demonstrate a recessive mode of inheritance and no heterozygous phenotype has been reported (Gleason et al., 2004; Granato et al., 1996; Hirata et al., 2004). To confirm these observations and compare one of these alleles to accdta5, acctq206, the strongest previously reported allele, was used for time-trial assays (Figure 4). A population of larvae obtained from crossing accdta5 heterozygotes again show a range of swimming times and demonstrate the consistent results obtained using this assay (Figure 4b). 39% exhibit wild-type like swimming, 28% perform slower swimming, and 33% do not swim at all. The difference in average swim times between the populations of accdta5 larvae shown in Figure 2d and 4b is not statistically significant (t(92) = 0.02, p=.49). Since acctq206 were reported as recessive, we expected to observe a roughly Mendelian distribution of 75% with a wild-type phenotype and 25% that do not swim. Instead we observe that 62% perform wild-type like swimming, 5% demonstrate slower swimming, and 34% do not swim, which suggests that acctq206 may not be fully recessive. Since accdta5 demonstrates a semi-dominant mode of inheritance, we expected that crosses between between accdta5 heterozygotes and wild-type fish would yield a population of larvae in which some swim like wild-type and others swim slowly. We observe that 59% swim with wild-type swim speeds, 38% swim slowly, and 3% do not swim at all, which provides additional evidence that accdta5 is semi-dominant and that accdta5 heterozygotes demonstrate a range of swimming phenotypes (Figure 4d). If acctq206 is completely recessive, we would predict that crosses between acctq206 heterozygotes and wild-type would yield a population of larvae in which all perform wild-type swimming. We find that 90% perform wild-type swimming, but 9% swim slowly and 1% do not swim. Moreover, the average swim time is significantly slower than wild-type (t(158) = 10.08, p<.01) (Figure 4e). These results further suggest that acctq206 is not completely recessive. Lastly, we performed complementation testing between accdta5 and acctq206 to confirm that they are allelic (Figure 4f). We observe the expected failure to complement. 52% exhibit wild-type swimming speeds, 17% perform abnormally slow swimming, and 30% do not swim. Altogether, these data indicate that accdta5 is semi-dominant, acctq206 has a subtle semi-dominant effect, accdta5 is a stronger allele than acctq206, and both alleles disrupt SERCA1 function.
Figure 4. accdta5 is a semi-dominant allele of acc.
Time-trial assays of various crosses are shown. Crosses involving mutants consist of mating heterozygous adults since homozygotes do not survive to adulthood. All larvae were assayed at 48hpf. The total number of larvae and average swimming times are indicated. The calculation of swimming times and depiction of time bins are identical to Figure 2. (a) Wild-type (WT) larvae swim quickly, with nearly all (99%) swimming 4mm in less than 500ms. (b) Crosses between accdta5 heterozygous adults yield larvae that swim with wild-type swim times, those that swim slowly, and those that do not swim at all. The WT and accdta5 crosses shown here are different populations than those in Figure 2 and illustrate the consistent results obtained using this assay. The differences in the average times between this and Figure 2 are not statistically significant. (c) Crosses between acctq206 heterozygotes produce larvae that swim quickly, few that swim slowly, and larvae that do not swim. (d) Crosses between wild-type and accdta5 heterozygotes yield populations that swim with wild-type times and those that swim slowly, illustrating the semi-dominant effect. (e) Crosses between wild-type and acctq206 heterozygotes reveal larvae that swim like wild-type and those that swim slightly slower, indicating a subtle semi-dominant effect. (f) Crosses between acctq206 and accdta5 show a failure to complement, which demonstrates that they are allelic to each other.
In this study, we used novel quantification of swimming behavior, molecular-genetic mapping and candidate gene analysis to characterize the accdta5 mutant over the course of development and reveal that it harbors a semi-dominant mutation in atp2a1. atp2a1 is expressed in muscle (see Supporting Information: Figure 5) and it is important for muscle relaxation, so accdta5 mutants develop abnormal swimming due to a reduced ability to relax axial muscle. Intriguingly, previous studies have indicated that eight acc alleles disrupt atp2a1 and demonstrate a recessive mode of inheritance (Gleason et al., 2004; Granato et al., 1996; Hirata et al., 2004). The sensitivity of our time-trial assay allowed us to uncover that the acctq206 allele has a weak semi-dominant effect, with some heterozyotes having a subtle motility phenotype. This assay also shows that the acctq206 allele is not as strong as accdta5, which is the strongest allele reported to date.
It is not entirely clear why the accdta5 allele is stronger than the other acc alleles, but one factor is that a different portion of the SERCA1 protein is disrupted in accdta5 mutants. The precise mutation has been determined for three acc alleles: accmi25i Ile97 is mutated to Asn, acctq206 Ser766 is mutated to Phe, and accmi289a Thr848 is mutated to Ile (see Figure 3). Although these mutations are distributed across the SERCA1 protein, each is in a transmembrane domain and results in altered hydrophobicity. accdta5, which mutates Gly598 to Val, is the only acc allele identified that disrupts the SERCA1 ATP binding domain.
accdta5 could result in a dominant mode of inheritance through either haploinsufficient or dominant-negative molecular mechanisms. According to a haploinsufficient model, two functional copies of atp2a1 are required in the zebrafish embryo. accdta5 would be a semi-dominant mutation because it is diminishes SERCA1 function to a greater degree than other acc mutations, which all have reduced, but not abolished, SERCA1 function. According to a dominant-negative model, accdta5 SERCA1 protein interferes with wild-type SERCA1 function. This model implies that SERCA1 requires assembly of macromolecular complexes to function. SERCA1 is generally thought to operate as a monomer but this view is being increasingly challenged by reports that SERCA1 forms oligomers (Maguire and Ohlendieck, 1996; Mahaney et al., 2008). Additional analysis of the accdta5 mutation could yield new insight into the molecular mechanisms of SERCA1 function.
We observed that both accdta5 homozygotes and heterozygotes demonstrated a marked improvement in swimming performance over the course of development (see Figure 2). The cellular and molecular basis of this improvement is not known. It is possible that functional SERCA1 molecules accumulate over the course of development or that SERCA2, a homolog of SERCA1 that is also expressed in muscle, can partially compensate for loss of SERCA1 (Ebert et al., 2005). It is also possible that non-SERCA mechanisms develop to regulate cytoplasmic calcium levels. Examining calcium regulation and/or SERCA2 in accdta5 mutants may enhance our understanding of muscle function.
Mutation of atp2a1 has been shown to disrupt muscle function in a variety of species. In humans, both missense and presumptive null mutations in ATP2A1 result in Brody disease, a condition in which individuals exhibit a normal life span but slowed relaxation of skeletal muscle (Periasamy and Kalyanasundaram, 2007). Deletion of Atp2a1 in mice results in a more severe impairment of skeletal muscle function than in humans. Knockout mice die soon after birth due to diaphragm muscle hypercontractility (Pan et al., 2003). The ATP2A1 gene has also been identified as mutated in two muscle-contraction disorders in two breeds of cattle, Chianina and Belgian Blue. The Chianina disorder results in a more mild, Brody disease-like muscle relaxation defect (Sacchetto et al., 2009). The Belgian Blue disorder results in death a few weeks after birth due to respiratory complications, similar to the Atp2a1 knockout mice (Charlier et al., 2008). In human, mice, and cattle, the disorders that disrupt SERCA1 function seem to be recessive and no phenotype has reported in any of the heterozygotes, thus the semi-dominant accdta5 mutant could provide a particularly sensitive model for SERCA1 disorders.
METHODS
Zebrafish Maintenance and Breeding
Zebrafish were raised and maintained in our fish facility according to established protocols. Embryos were staged as described in Kimmel et al. (Kimmel et al., 1995). The accdta5 and acctq206 alleles were maintained in a Tuebingen/TL genetic background.
Behavioral Analysis
To examine embryos at 24 hpf, touch stimuli was applied using a probe and the responses were examined using a dissecting microscope. The percentage of embryos that perform accordion-like behavior was determined by examining populations of wild-type, accdta5 and acctq206 without knowledge of genotype.
To examine swimming behavior at 48 hpf, touch stimuli was applied using a probe and the responses were recorded using a high-speed video camera recording at 1000 frames/second (TroubleShooter 1000 Fastec Imaging). Kinematic analysis was performed by measuring the head-to-tail angle during each frame using a custom designed computer program. Angles were calculated using three landmark points along the body: the head, the caudal end of the yolk, and the tip of the tail. The angles were graphed over time using Microsoft Excel.
To perform time-trial assays, single 48 hpf zebrafish larvae were placed at the center of two concentric circles. The distance between the inner and outer circle was 4mm. The larvae were gently touched on the head and the escape response was recorded using the high-speed video camera recording at 1000 frames/second. The amount of time was measured from when the larval head crossed over the boundary of the inner circle and touched the outer circle. Individual larvae were tested once, entire clutches were assayed, and each genetic cross was performed multiple times. The results were compiled into graphs using DeltaGraph. Statistical analysis was performed to compare the average swim times between all genetic crosses using the student’s t-test.
Chromosomal Mapping and cDNA Analysis
Crosses between Tuebingen/TL strain fish carrying the accdta5 allele and polymorphic WIK fish were used to generate a three-generation map cross panel using standard techniques. F2 accdta5 homozygous mutant and wild-type sibling embryos were collected, sorted based on the motility phenotype, and stored in MeOH at −20°C. Bulked-segregant analysis was performed at the Zebrafish Mutant Mapping Facility at the University of Louisville. DNA was extracted from pools of 20 mutant and 20 wild-type embryos and analyzed using a variety of CA-repeat markers. To determine the accdta5 linkage interval, several nearby markers on chromosome 3 were used with DNA from 96 individual F2 mutant embryos.
To clone atp2a1 cDNA, RNA was extracted from 48hpf wild-type, accdta5 heterozygous and accdta5 homozygous embryos and used for RT-PCR. The Accusript RT-PCR system (Stratagene) was used with oligo-dT and gene-specific primers to reverse transcribe and amplify atp2a1. The PCR products were gel purified, sequenced, and the nucleic acid sequences were analyzed using MacVector 9.0. RT-PCR was performed and analyzed multiple times with each genotype to decrease the possibility of amplification artifacts.
In Situ Hybridization
In situ hybridization was performed using established protocols (Thisse and Thisse, 2008). The atp2a1 probe was generated by using RT-PCR to amplify a 572bp region at the 3′ end of atp2a1. This fragment was subcloned, linearized, and used for in vitro transcription using the digoxigenin RNA labeling kit (Roche). A compound microscope attached to a digital cameral (Zeiss) was used to acquire images, and the pictures were processed using Autostitch, Abode Photoshop and Illustrator.
Supplementary Material
In situ hybridization was performed using a probe directed against atp2a1. 48hpf larvae are shown and the in situ signal is purple. Lateral views show that atp2a1 is expressed in the heart and somites in (a) wild-type, (b) heterozygous, and (c) homozygous mutant larvae. The scale bar = 0.5mm and applies to figures A–C. The line dorsal to the larva in A shows the approximate location of the cross-sections. accdta5 homozygous larvae are compressed along the rostral-caudal axis, which obscures the somite boundaries (d–f) Cross-sectional views illustrate that atp2a1 is expressed in the somites but is not detected in the spinal cord. S.C. indicates the location of spinal cord, while N.C. indicates the location of the notochord. The scale bar = 0.1mm and applies to figures D–F.
Wild-type embryos respond to touch by coiling their tails in one direction and then in the opposite direction. Two full tail coils, like that shown here, are common. Note the smooth motion of the tail compared to dta5 homozygotes. This and all subsequent movies are recorded at 1000 frames/s. This movie was recorded over a 1173ms time period.
dta5 homozygous mutants perform slow, incomplete tail coils. They exhibit seemingly bilateral axial muscle contractions, which cause the notochord to become distorted and induce an abnormal dorsal tail bend. This movie was recorded over a 1257ms time period.
A wild-type escape response consists of an initial large-amplitude body bend away from the touch stimulus, followed by rhythmic swimming. This movie was recorded over a 192ms time period.
dta5 homozygotes exhibit bilateral axial muscle contractions, which cause them to appear stiff, and they frequently bend to one side as shown. This movie was recorded over a 1005ms time period.
Larvae with one dta5 allele perform bilateral axial muscle contractions and swim slowly. Note the abnormal dorsal body bend, low amplitude tail flips, and repeated tail bends towards one side of the body. This movie was recorded over a 2556ms time period.
Acknowledgments
We thank Michael Granato for generously sharing the accdta5 mutant, Paul Brehm for providing the acctq206 mutant, and Saunders Whittlesley, Kelly Anne McKeown, Syeda Sanam Al Rafia and Maya Bialik for help with kinematic analysis and time-trial assays. We also thank Ron Gregg and Greg Willer for initial mapping and members of the Downes laboratory for numerous discussions and comments on this manuscript.
Grant Sponsor: National Institute of Health K01NS057409 to G.B.D. The zebrafish mapping facility is supported by R01RR020357 to Ronald Gregg.
References
- Charlier C, Coppieters W, Rollin F, Desmecht D, Agerholm JS, Cambisano N, Carta E, Dardano S, Dive M, Fasquelle C, Frennet JC, Hanset R, Hubin X, Jorgensen C, Karim L, Kent M, Harvey K, Pearce BR, Simon P, Tama N, Nie H, Vandeputte S, Lien S, Longeri M, Fredholm M, Harvey RJ, Georges M. Highly effective SNP-based association mapping and management of recessive defects in livestock. Nat Genet. 2008;40:449–454. doi: 10.1038/ng.96. [DOI] [PubMed] [Google Scholar]
- Clarke DM, Loo TW, MacLennan DH. Functional consequences of alterations to amino acids located in the nucleotide binding domain of the Ca2(+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1990;265:22223–22227. [PubMed] [Google Scholar]
- Downes GB, Granato M. Acetylcholinesterase function is dispensable for sensory neurite growth but is critical for neuromuscular synapse stability. Dev Biol. 2004;270:232–245. doi: 10.1016/j.ydbio.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Downes GB, Granato M. Supraspinal input is dispensable to generate glycine-mediated locomotive behaviors in the zebrafish embryo. J Neurobiol. 2006;66:437–451. doi: 10.1002/neu.20226. [DOI] [PubMed] [Google Scholar]
- Ebert AM, Hume GL, Warren KS, Cook NP, Burns CG, Mohideen MA, Siegal G, Yelon D, Fishman MC, Garrity DM. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc Natl Acad Sci U S A. 2005;102:17705–17710. doi: 10.1073/pnas.0502683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MR, Armisen R, Verdecia MA, Sirotkin H, Brehm P, Mandel G. A mutation in serca underlies motility dysfunction in accordion zebrafish. Dev Biol. 2004;276:441–451. doi: 10.1016/j.ydbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Downes GB, Cui WW, Zhou W, Granato M, Kuwada JY. Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor {beta}-subunit. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0500862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Waterbury J, Cui W, Zhou W, Li Q, Goldman D, Granato M, Kuwada JY. accordion, a zebrafish behavioral mutant, has a muscle relaxation defect due to a mutation in the ATPase Ca2+ pump SERCA1. Development. 2004;131:5457–5468. doi: 10.1242/dev.01410. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Ono F, Puglielli C, Seidner G, Franzini-Armstrong C, Brehm P, Granato M. Increased neuromuscular activity causes axonal defects and muscular degeneration. Development. 2004;131:2605–2618. doi: 10.1242/dev.01123. [DOI] [PubMed] [Google Scholar]
- Maguire PB, Ohlendieck K. Oligomerization of sarcoplasmic reticulum Ca2+-ATPase from rabbit skeletal muscle. FEBS Lett. 1996;396:115–118. doi: 10.1016/0014-5793(96)01106-4. [DOI] [PubMed] [Google Scholar]
- Mahaney JE, Thomas DD, Farrance IK, Froehlich JP. Intermolecular interactions in the mechanism of skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase (SERCA1): evidence for a triprotomer. Biochemistry. 2008;47:13711–13725. doi: 10.1021/bi801024a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zvaritch E, Tupling AR, Rice WJ, de Leon S, Rudnicki M, McKerlie C, Banwell BL, MacLennan DH. Targeted disruption of the ATP2A1 gene encoding the sarco(endo)plasmic reticulum Ca2+ ATPase isoform 1 (SERCA1) impairs diaphragm function and is lethal in neonatal mice. J Biol Chem. 2003;278:13367–13375. doi: 10.1074/jbc.M213228200. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Testoni S, Gentile A, Damiani E, Rossi M, Liguori R, Drogemuller C, Mascarello F. A defective SERCA1 protein is responsible for congenital pseudomyotonia in Chianina cattle. Am J Pathol. 2009;174:565–573. doi: 10.2353/ajpath.2009.080659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Inesi G. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 2004;73:269–292. doi: 10.1146/annurev.biochem.73.011303.073700. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Odenthal J, Haffter P. Developmental mutant screens in the zebrafish. Methods Cell Biol. 1999;60:21–41. doi: 10.1016/s0091-679x(08)61892-0. [DOI] [PubMed] [Google Scholar]
- Wang M, Wen H, Brehm P. Function of neuromuscular synapses in the zebrafish choline-acetyltransferase mutant bajan. J Neurophysiol. 2008;100:1995–2004. doi: 10.1152/jn.90517.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In situ hybridization was performed using a probe directed against atp2a1. 48hpf larvae are shown and the in situ signal is purple. Lateral views show that atp2a1 is expressed in the heart and somites in (a) wild-type, (b) heterozygous, and (c) homozygous mutant larvae. The scale bar = 0.5mm and applies to figures A–C. The line dorsal to the larva in A shows the approximate location of the cross-sections. accdta5 homozygous larvae are compressed along the rostral-caudal axis, which obscures the somite boundaries (d–f) Cross-sectional views illustrate that atp2a1 is expressed in the somites but is not detected in the spinal cord. S.C. indicates the location of spinal cord, while N.C. indicates the location of the notochord. The scale bar = 0.1mm and applies to figures D–F.
Wild-type embryos respond to touch by coiling their tails in one direction and then in the opposite direction. Two full tail coils, like that shown here, are common. Note the smooth motion of the tail compared to dta5 homozygotes. This and all subsequent movies are recorded at 1000 frames/s. This movie was recorded over a 1173ms time period.
dta5 homozygous mutants perform slow, incomplete tail coils. They exhibit seemingly bilateral axial muscle contractions, which cause the notochord to become distorted and induce an abnormal dorsal tail bend. This movie was recorded over a 1257ms time period.
A wild-type escape response consists of an initial large-amplitude body bend away from the touch stimulus, followed by rhythmic swimming. This movie was recorded over a 192ms time period.
dta5 homozygotes exhibit bilateral axial muscle contractions, which cause them to appear stiff, and they frequently bend to one side as shown. This movie was recorded over a 1005ms time period.
Larvae with one dta5 allele perform bilateral axial muscle contractions and swim slowly. Note the abnormal dorsal body bend, low amplitude tail flips, and repeated tail bends towards one side of the body. This movie was recorded over a 2556ms time period.