Abstract
PGC-1α is a transcriptional coactivator that controls energy homeostasis through regulation of glucose and oxidative metabolism. Both PGC-1α expression and oxidative capacity are decreased in skeletal muscle of patients and animals undergoing atrophy, suggesting that PGC-1α participates in the regulation of muscle mass. PGC-1α gene expression is controlled by calcium- and cAMP-sensitive pathways. However, the mechanism regulating PGC-1α in skeletal muscle during atrophy remains unclear. Therefore, we examined the mechanism responsible for decreased PGC-1α expression using a rodent streptozotocin (STZ) model of chronic diabetes and atrophy. After 21d, the levels of PGC-1α protein and mRNA were decreased. We examined the activation state of CREB, a potent activator of PGC-1α transcription, and found that phospho-CREB was paradoxically high in muscle of STZ-rats, suggesting that the cAMP pathway was not involved in PGC-1α regulation. In contrast, expression of calcineurin (Cn), a calcium-dependent phosphatase, was suppressed in the same muscles. PGC-1α expression is regulated by two Cn substrates, MEF2 and NFATc. Therefore, we examined MEF2 and NFATc activity in muscles from STZ-rats. Target genes MRF4 and MCIP1.4 were both significantly reduced, consistent with reduced Cn signaling. Moreover, levels of MRF4, MCIP1.4, and PGC-1α were also decreased in muscles of CnAα-/- and CnAβ-/- mice without diabetes indicating that decreased Cn signaling, rather than changes in other calcium- or cAMP-sensitive pathways, were responsible for decreased PGC-1α expression. These findings demonstrate that Cn activity is a major determinant of PGC-1α expression in skeletal muscle during diabetes and possibly other conditions associated with loss of muscle mass.
Keywords: skeletal muscle, atrophy, calcineurin, PGC-1α, diabetes, streptozotocin
1. INTRODUCTION
Diabetes mellitus (DM) is an epidemic public health issue that threatens the health and quality of life of people globally. DM can result from insulin deficiency or resistance and is characterized by a variety of metabolic disturbances including reduced cellular glucose uptake, decreased fatty acid oxidation, and dysfunctional mitochondria [1]. Skeletal muscle is a major site of insulin action and loss of insulin signaling induces muscle atrophy, which leads to reduced functional capacity and muscle weakness [1].
PGC-1α is a transcriptional coactivator that participates in the regulation of skeletal muscle metabolism, particularly energy homeostasis. It controls glucose transport and is necessary for mitochondrial biogenesis and the maintenance of the oxidative phenotype of muscle fibers [2]. Skeletal muscles of mice transgenically overexpressing PGC-1α have an increased proportion of oxidative fibers [3]. Recently, PGC-1α has been implicated in the regulation of muscle mass and protein turnover. Glycolytic fibers express a lower level of PGC-1α and they exhibit a greater degree of atrophy than oxidative fibers in disease or systemic models of muscle atrophy [4-6]. In general, PGC-1α expression is lower in skeletal muscles undergoing atrophy, including those of Type II DM patients [7-9]. The mechanism of the atrophy-related reduction of PGC-1α in skeletal muscle is unknown.
Given the multi-faceted role of PGC-1α in energy metabolism, it is not surprising that the control of its transcription is complex. Several signaling pathways (e.g., MAP kinase, cAMP/PKA) induce PGC-1α transcription by phosphorylating Ser133 in the cAMP response element binding protein (CREB). Activated CREB forms a complex with other proteins (e.g., p300, CREB Binding Protein or CBP) which then bind to a cAMP response element located in the promoter region of the PGC-1α gene [10,11]. Thus, a reduction in CREB activity could be responsible for the reduction in PGC-1α during muscle atrophy. Another pathway known to regulate PGC-1α involves the calcium-dependent phosphatase calcineurin (Cn). Two downstream substrates of calcineurin, NFATc and MEF2 have been proposed to work in concert to increase the transcription of prototypical type I oxidative muscle fiber genes, including PGC-1α [12]. Importantly, transgenic expression of Cn in skeletal muscle recapitulated the effects of PGC-1α overexpression by inducing a switching from glycolytic to oxidative fibers [3,13,14]. Thus, a second possible mechanism that could lead to down-regulation of PGC-1α expression is a reduction in Cn signaling.
In the present study, we found that chronic DM induced by a low-dose of streptozotocin increased skeletal muscle protein degradation and decreased PGC-1α expression. This led us to examine the possible mechanisms that could be responsible for reducing PGC-1α transcription. Our results demonstrate that insulin deficiency leads to suppression of Cn signaling and a resulting decrease in PGC-1α transcription.
2. METHODS
2.1 Materials
Streptozotocin (STZ) was purchased from Sigma-Aldrich (St. Louis, MO). The following commercially available antibodies were used: anti-actin (Sigma-Aldrich), anti-Akt and anti-phospho(S473) Akt (Cell Signaling: Danvers, MA), anti-calcineurin pan A (Chemicon: Billerica, MA), anti-CREB and anti-phospho(S133) CREB (Cell Signaling), anti-glycogen synthase and anti-phospho(S641) glycogen synthase (Cell Signaling), anti-GSK-3β and anti-phospho(S9) GSK-3β (Cell Signaling), anti-NFATc1 (Abcam: Cambridge, MA), anti-MEF2 (Santa Cruz: Santa Cruz, CA), anti-PGC-1 (Calbiochem: San Diego, CA), HRP-anti-rabbit IgG (Amersham: Piscataway, NJ). The following primers were purchased from Invitrogen (Carlsbad, CA) for real-time RT-PCR: DHPRα1s primers FWD 5′ – TTTCCCACAGGCCGTGCTGCTGCTCTTCA – 3′ and REV 5′ – CATGTAGAAGCTGATGAA – 3′[15,16], MCIP1.4 primers previously published [18], MRF4 primers previously published [19], and PGC-1α primers previously published [20], rUbC primers previously published [15].
2.2 Animals
2.2.1 STZ-DM Rats
All studies were approved by the Emory University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats weighing ~150 g were anesthetized using isoflourane and given a single tail-vein injection of 60mg/kg body weight of streptozotocin (STZ) prepared fresh in 0.1M sodium citrate buffer (pH4.0); control rats were injected with the vehicle alone. Chronically diabetic animals were fed the standard diet ad libitum for 21days. At the time of sacrifice, animals were anesthetized and the gastrocnemius muscles were immediately frozen in liquid nitrogen and then stored at −80°C. Arterial blood was collected for glucose measurements.
2.2.2 STZ-DM Transgenic Mice
Transgenic mice expressing a NFAT-luciferase (NFAT-luc) reporter gene in all tissues were created by Dr. J. Molkentin (Cincinnati Children’s Hospital, Cincinnati, OH) [17,18]. Mice weighing 25-30 g were given an interperitoneal injection of either 55 mg/kg body weight STZ in 0.1M sodium citrate buffer (pH. 4.0) or sodium citrate buffer alone daily for four days. Blood glucose levels were monitored for one week following the last injection of STZ. Mice with blood glucose levels more than 200 mg/dL were considered diabetic. Diabetic mice were fed standard chow and water ad libitum for 21 days and blood glucose levels were monitored weekly. At the time of sacrifice, blood was obtained to measure glucose prior to harvesting the gastrocnemius muscle. Blood glucose levels were similar to those for STZ-treated rats (data not shown). Dissected muscles were immediately frozen in liquid nitrogen and stored at −80°C.
2.2.3 Calcineurin knock-out mice
Mice lacking the gene for the α isoform of the CnA catalytic subunit were created by Dr. J. Seidman (Howard Hughes Medical Institute, Harvard Medical School, Boston, MA) [19]. Mice lacking the gene for the β isoform of the CnA catalytic subunit were created by Dr. J. Molkentin (Cincinnati Children’s Hospital, Cincinnati, OH) [17,18].
2.3 Western Blot Analysis
Muscle samples were homogenized in 20 μl/mg of lysis buffer. Two different lysis buffers were used. A hypotonic buffer consisting of 50mM Tris (pH7.5), 1mM EDTA, 1mM EGTA, 0.5mM DTT, 0.1% NP-40, and complete mini protease inhibitor cocktail tablets (Roche: Indianapolis, IN) was used for analysis of Cn, NFAT, MEF2, and PGC-1α. The samples were subjected to three freeze/thaw cycles using liquid nitrogen and a 37°C water bath. The homogenates were centrifuged and the supernatant was used for analysis. A buffer consisting of 50mM Hepes (pH 7.4), 137mM NaCl, 1mM MgCl2, 1mM CaCl2, 10mM Na pyrophosphate, 10mM Na fluoride, 2mM EDTA, 10% glycerol, 1% NP-40, 2mM Na3VO4, 2mM PMSF, 10μg/mL aprotinin, 10μg/mL leupeptin, and 10mM benzamidine, was used for analysis of AKT, CREB, GSK-3β, and glycogen synthase (GS). Supernatant protein concentrations were measured using a BioRad DC Protein Assay Kit (BioRad: Hercules, CA). Protein samples (50 μg) were separated by SDS-PAGE, transferred to nitrocellulose membranes and blocked in Tris-buffered saline (pH7.5) containing 5% non-fat milk and 0.1% Tween-20(Sigma: St. Louis, MO). Blots were incubated with primary antibodies (see Materials, 2.1) and detected using chemiluminescence technology. Equal protein loading and transfer were confirmed by Ponceau S Red staining of the membranes.
2.4 Real-Time RT-PCR
Rat or mouse gastrocnemius muscle RNA was isolated using Trizol Reagent (Invitrogen: Carlsbad, CA) according to the manufacturer’s instructions, treated with DNase and reverse transcribed using M-MLV reverse transcriptase and random hexamer primers. Real-Time PCR was performed using a BioRad iCycler with target-specific primers and iQ SYBR Green (BioRad: Hercules, CA); the 18S rRNA was used as a normalization control. The data were analyzed for fold change (ΔΔCt) using the iCycler software.
2.5 Endogenous NFATc Activity Assay
Luciferase activity in the gastrocnemius muscles of control and STZ-treated NFATc-luc mice was measured using a commercial kit (Promega: Madison, WI). Tissue samples were homogenized in 20μl/□g passive lysis buffer and particulate matter removed by centrifugation. Total protein content of the supernatant was measured using a BioRad DC Protein Assay Kit (Hercules, CA) and all lysates were diluted to a concentration of 1μg/μL with passive lysis buffer. Luciferase assay reagent (50μL) was added to 10μl of supernatant and luminescence was measured for 10 seconds using a luminometer (Turner Biosystems, Sunnyvale, CA).
2.6 Measurement of Muscle Protein Degradation
The rate of total protein degradation was measured in isolated, mixed-fiber epitrochlearis muscles as described [20]. Briefly, freshly isolated muscles were incubated for 2h at 37°C in Krebs Ringer Bicarbonate buffer containing cyclohexamide to inhibit protein synthesis. Free tyrosine released into the media by proteolysis was measured fluorometrically [21]. The rate of tyrosine release was calculated as the nmol tyrosine released/ mg wet muscle weight/ hour.
3. RESULTS
3.1 Rats treated with streptozotocin experience skeletal muscle atrophy due to increased protein degradation
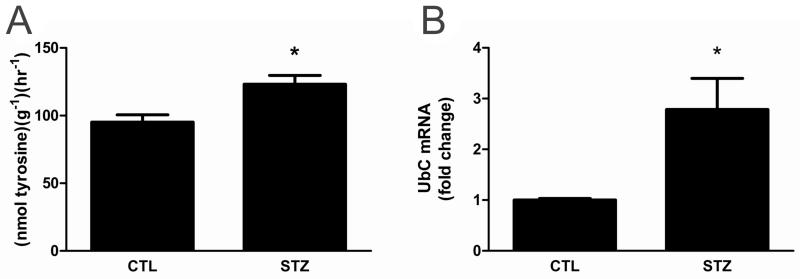
Streptozotocin (STZ) was administered to rats to induce Type I DM (i.e., insulin deficiency and hyperglycemia) and cachexia. After 21 days, STZ-rats were hyperglycemic with significantly smaller body and wet muscle weights than controls (Table 1). To confirm that increased muscle protein turnover contributed to the lower muscle mass, total protein degradation was measured in isolated epitrochlearis muscles. The epitrochlearis is a small, flat, mixed-fiber type muscle located in the fore-limb. The size of the muscle allows for extended incubation times after dissection without induction of necrosis. Additionally, protein degradation rates measured in the epitrochlearis are comparable to those of the perfused hind-limb[22]. Therefore, the rate measured in the epitrochlearis is representative of whole body protein breakdown. The proteolytic rate was increased in epitrochlearis muscles of STZ-treated rats (Fig. 1A). A second independent measure of protein degradation, expression of rat ubiquitin (UbC) mRNA, was also increased in gastrocnemius muscle from STZ-treated rats versus controls (Fig. 1B).
Table 1.
Overall body weight and muscle weights are reduced in 21day STZ-treated rats.
| CTL | STZ | |
|---|---|---|
| Glucose (mg/dL) | 153 ± 14.7 | 427 ± 29.8* |
| Initial Body Wt. (g) | 157 ± 5.3 | 154 ± 5.9 |
| Final Body Wt. (g) | 346 ± 11.7 | 254 ± 8.4* |
| Gastrocnemius. Wt. (g) | 2.2 ± 0.08 | 1.3 ± .011* |
| Epitrochlearis. Wt. (mg) | 57.1 ± 3.4 | 39.1 ± 4.7* |
21d DM rats presented with hyperglycemia and significantly smaller body and wet skeletal muscle (gastrocnemius and epitrochlearis) weights compared to controls. Mean ± SEM, n=8
P<0.05.
Figure 1. The rate of protein degradation and ubiquitin expression are increased in 21day STZ-treated rat muscle.
(A) Protein degradation was measured in isolated mixed-fiber epitrochlearis skeletal muscles. Muscles from control and 21d STZ-treated rats were incubated in Krebs-Ringer Bicarbonate Buffer with cycloheximide to inhibit protein synthesis. Free tyrosine released into the media was measured fluorometrically after being converted to a nitrosonapthol derivative. (B) Ubiquitin C (rUbC) mRNA in the gastrocnemius muscles was measured by real-time RT-PCR. Data were normalized to 18S RNA and expressed as mean fold increase over control. Data are expressed as the mean ± SEM; n=7/group, P<0.05
3.2 PGC-1α expression is decreased in muscle from STZ-treated rats
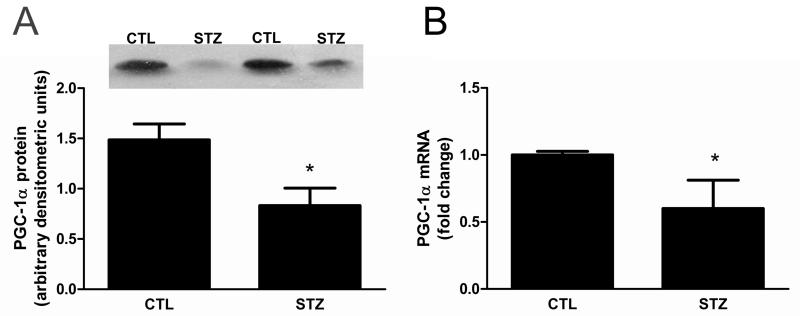
Expression of the PGC-1α transcriptional co-activator has been reported to be downregulated in skeletal muscle of humans with insulin resistance or Type II DM [8]. To determine whether PGC-1α is similarly regulated in skeletal muscle of rats with chronic diabetes mellitus, we evaluated PGC-1α protein and found it was decreased in the gastrocnemius muscle of STZ-treated rats (Fig. 2A). The decrement in PGC-1α protein appears to result from a pre-translational mechanism because PGC-1α mRNA was also significantly decreased in STZ-treated rat muscle (Fig. 2B).
Figure 2. PGC-1α expression is decreased in 21day STZ-treated rat muscle.
(A) PGC-1α protein was evaluated by Western blot analysis. Equal protein loading and transfer was confirmed by Ponceau S staining. n=9/group, *P<0.05. (B) PGC-1α mRNA in gastrocnemius muscle was measured by real time RT-PCR, normalized to 18S RNA, and expressed as mean fold change relative to control ± SEM; n=7/group, *P<0.05.
3.3 Decreased PGC-1α transcription is not due to decreased CREB activity
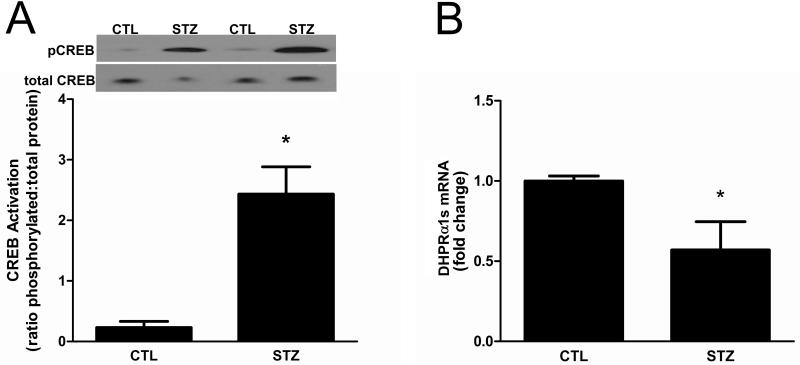
One of the major signaling pathways that regulates PGC-1α transcription involves the cAMP regulatory element binding protein (CREB) via a CREB binding element in the cofactor’s promoter [11]. CREB is activated by phosphorylation of Ser 133 by various Ca2+-sensing and stress pathways including CaMKII, PKA, and ERK, thus making it relevant in the context of reduced insulin-signaling [23]. To determine if the reduction in PGC-1α transcription could be attributed to attenuated CREB activity, we evaluated the amounts of CREB phospho-Ser133 relative to total CREB protein. In contrast to the reduction of PGC-1α, the amount of activated CREB was markedly increased ~8 fold in STZ-treated rat muscle compared to controls (Fig. 3A). This finding suggests that CREB function may be abnormal in the muscle of STZ-treated rats and led us to evaluate the expression of a known CREB target. The dihydropyridine receptor (DHPR) α1s subunit is expressed exclusively in skeletal muscle, and its transcription is regulated by CREB [24]. The level of DHPRα1s mRNA was decreased in muscles from STZ-treated rats (Fig. 3B).
Figure 3. CREB signaling is abnormal in 21day STZ-treated rat muscle.
(A) The phosphorylation (i.e., activation) status of CREB in gastrocnemius muscles of control and 21d STZ-treated rats was examined by Western blot analysis using antibodies that detect phospho-Ser133 and total CREB. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM. Equal protein loading and transfer were confirmed by Ponceau S staining. n=6/group, *P<0.05. (B) To evaluate CREB function, the amounts of DHPRα1s, a gene target of CREB, were measured in gastrocnemius muscles by real time RT-PCR. Data are expressed as the mean ± SEM; n=8/group, *P<0.05.
3.4 Calcineurin signaling is down-regulated in muscle from STZ-treated rat
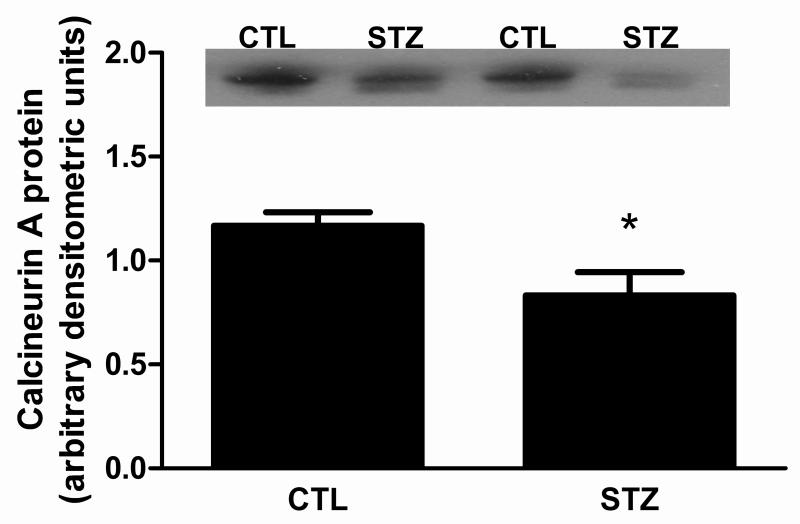
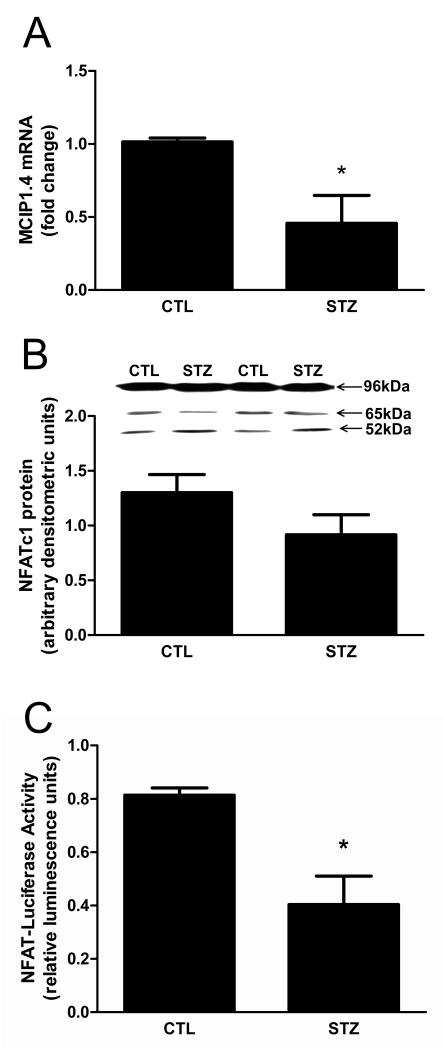
The calcium-dependent calcineurin (Cn) signaling pathway has also been implicated in regulating PGC-1α transcription [25], prompting us to investigate its role in regulating PGC-1α in our 21d STZ-treated rats. The amount of Cn catalytic A subunit (CnA) was decreased in muscles from STZ-treated rats (Fig. 4). One of the best characterized substrates of Cn is the NFATc transcription factor family. To determine if NFATc activity was reduced, we measured the amount of mRNA encoding the modulatory calcineurin interacting protein MCIP1.4. MCIP1.4 is an NFATc responsive gene that has been used previously as a surrogate reporter for Cn activity in skeletal muscle [26,27]. MCIP1.4 expression was decreased in gastrocnemius of STZ-rats (Fig. 5A). To determine whether the reduction in NFATc activity was a result of a change in NFAT protein, the amount of NFATc1 protein was measured since it is the major subclass of NFATc in muscle [28]. NFATc1 protein expression was not significantly changed in STZ-rat muscles (Fig. 5B). As a way of confirming that STZ-induced, chronic diabetes mellitus attenuated NFATc activity in muscle, we compared luciferase activity in the gastrocnemius muscle of control and STZ-treated mice expressing a NFATc responsive luciferase transgene in all tissues. Luciferase activity was decreased in STZ-treated mice (Fig. 5C).
Figure 4. Cn catalytic A subunit protein is decreased in 21day STZ-treated rat muscle.
The levels of Cn A subunit (CnA) protein in gastrocnemius muscles of control and 21d STZ-treated rats were measured by Western blot analysis using a pan antibody that recognized all CnA isoforms. Equal protein loading and transfer were confirmed by Ponceau S staining. Data are expressed as the mean ± SEM; n=12/group, *P<0.05.
Figure 5. NFATc activity is decreased in 21 day STZ-treated rat muscle.
Changes in Cn activity are reflected in the activity of the transcription factor NFATc. (A) The amount of mRNA encoding the NFATc target MCIP1.4 in gastrocnemius muscles was measured using real time RT-PCR. Values were normalized to 18S RNA and expressed as mean fold change relative to control ± SEM; n=8/group, *P<0.05. (B) The levels of NFATc1 proteins were evaluated by Western blot analysis. n=10/group, P=0.14. (C) Transgenic mice expressing a luciferase reporter gene under the control of a NFAT-responsive promoter were injected with STZ (an intraperitoneal injection of STZ (55 mg/kg) daily for four days). After 21d muscle NFATc activity was evaluated by measuring luciferase activity in lysates prepared from gastrocnemius muscles. Data are expressed as mean luminesence units ± SEM; n=3/group, *P<0.05.
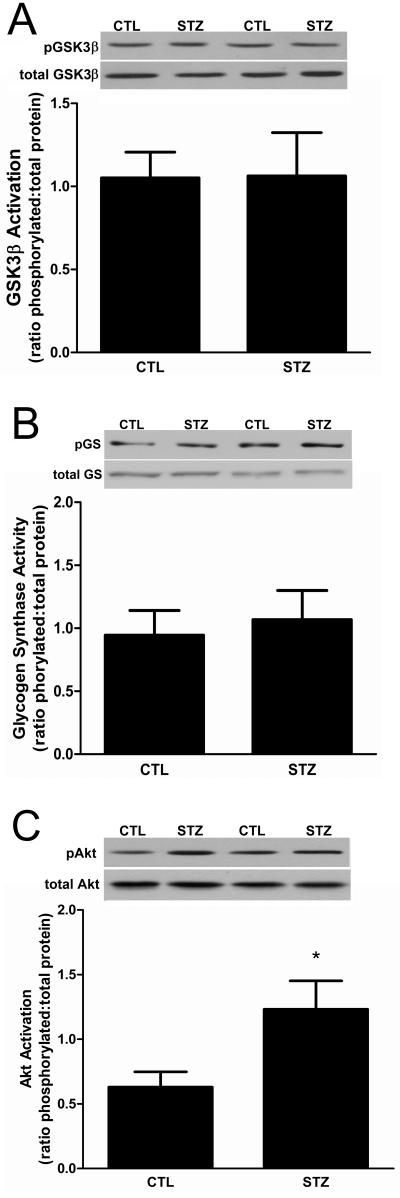
The activities of NFATc proteins can be regulated by either Cn or glycogen synthase kinase −3β (GSK-3β) which phosphorylates and inactivates the transcription factors [29]. In response to insulin, GSK-3β is inactivated by AKT-mediated phosphorylation [30]. To exclude the possibility that DM activated GSK-3β, we examined the phosphorylation status of GSK-3β Ser-9. There was no difference in the level of phosphorylated enzyme or enzyme expression in STZ-treated and control rat muscles (Fig. 6A). To confirm that GSK-3β activity was unchanged, we measured the levels of glycogen synthase (GS) phosphorylation at Ser-641, the canonical target of GSK-3β. There was no difference in either the phosphorylation status or total protein expression between STZ-treated versus control rat muscles (Fig. 6B). We also analyzed the phosphorylation status of Ser-473 in Akt and found that it was increased slightly but significantly in STZ-treated rat muscle (Fig. 6C). Together, these results indicate that GSK-3β signaling was unchanged in STZ-treated animals.
Figure 6. GSK-3β signaling is unchanged in 21day STZ-treated rat muscle.
(A) The phosphorylation (i.e., inactivation) of GSK-3β on Ser-9 in gastrocnemius muscles was examined by Western blot analysis using antibodies that detect phospho-S9 and total GSK-3β. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM. n= 8/group; P=0.31 (B) The phosphorylation of glycogen synthase on Ser-641 also was examined as a downstream target of GSK-3β activity by Western blot analysis using antibodies that detect total and phospho-Ser-641 glycogen synthase. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM. n= 8/group; P=0.69 (C) Western blot analysis of Akt phosphorylation was performed using antibodies that detect total and phospho-Ser-473 Akt. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM: P<0.05. For each experiment equal protein loading and transfer were confirmed by Ponceau S staining. n=7/group.
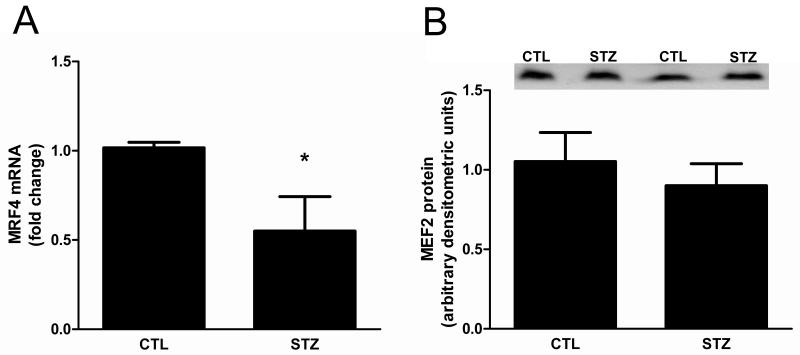
MEF2 is another transcription factor that can regulate PGC-1α expression and Cn has been shown to affect its activity. To evaluate MEF2 activity, the amount of myogenic regulatory factor 4 (MRF4) mRNA was measured because MEF2 is its primary regulator in differentiated muscle cells [31,32]. In STZ-treated rat muscle, MRF4 mRNA was decreased relative to control rat muscle (Fig. 7A). This response was not due to a change in the amount of MEF2 protein (Fig. 7B).
Figure 7. MEF2 activity is decreased in 21day STZ-treated rat muscle.
(A) The amount of mRNA encoding the MEF2 target MRF4 in gastrocnemius muscles was measured using real time RT-PCR, normalized to 18S RNA and expressed as mean fold change relative to control ± SEM. n=6/group, *P<0.05. (B) MEF2A protein in gastrocnemius muscles of control and 21d STZ-treated rats was evaluated by Western blot analysis. Equal protein loading and transfer were confirmed by Ponceau S staining. n=8/group, P=0.74
3.5 Loss of calcineurin signaling results in decreased PGC-1α transcription in skeletal muscle
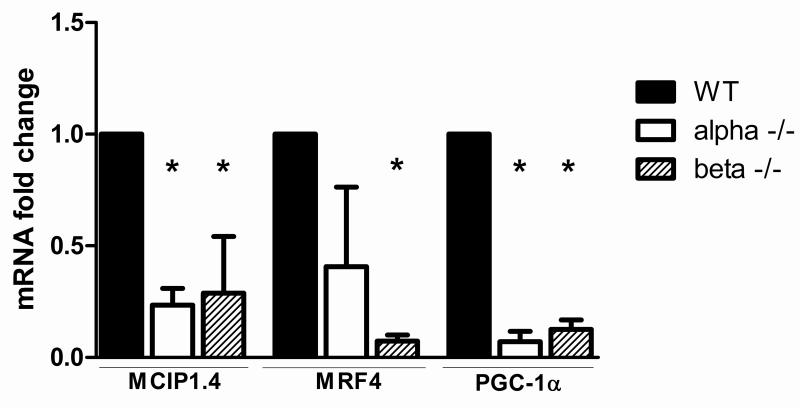
To directly examine the relationship between Cn signaling and PGC-1α expression, the level of PGC-1α mRNA was measured in gastrocnemius muscles of CnAα-/- and CnAβ-/- mice. PGC-1α expression was drastically reduced in both CnA-/- strains. Moreover, NFAT activity, as measured by MCIP1.4 expression, was significantly decreased when either CnA subunit was absent (Fig. 8). MRF4 expression, a measure of MEF2 activity, was also significantly decreased in CnAβ-/- mice and tended to be lower in CnAα-/- mice (Fig. 8).
Figure 8. MEF2 and NFATc signaling and PGC-1α mRNA are decreased in muscles of CnAα-/- and CnAβ-/- mice.
MRF4, MCIP1.4 and PGC-1α mRNAs in the gastrocnemius muscle of CnAα-/- and CnAβ-/- mice were measured by real-time RT-PCR, normalized to 18S RNA, and expressed as mean fold change relative to control ± SEM; n≥3/group, *P<0.05.
4. DISCUSSION
PGC-1α is a coactivator protein that participates in the transcriptional regulation of a variety of genes involved in energy metabolism, including the glucose transporter 4 (GLUT4) and mitochondrial biogenesis [2,33-36]. Hyperglycemia in Type II DM patients has been attributed, in part, to decreased expression of PGC-1α [8,37]. Other complications of Type I and Type II DM in experimental animals include skeletal muscle atrophy and PGC-1α has been linked to the regulation of skeletal muscle protein turnover (i.e., muscle mass) and fiber type [5,20,38]. Furthermore, atrophy occurs preferentially in muscles comprised predominantly of type II glycolytic fibers during DM and other systemic disease states are also associated with loss of muscle oxidative capacity [5,39]. This preferential atrophy could be linked to the reduction in PGC-1α expression since the cofactor helps to maintain the type I oxidative fiber phenotype in muscle [3].
In the present studies, the levels of PGC-1α protein and mRNA in skeletal muscle were decreased in a model of chronic (21d) STZ-induced DM. To elucidate the mechanism regulating PGC-1α expression in this system, we first tested whether CREB-mediated signaling was altered because CREB is a potent regulator of PGC-1α expression [40]. Paradoxically, the activation state of CREB was increased in skeletal muscle of 21d STZ-treated rats. This led us to examine a relevant downstream target of CREB, DHPRα1s. DHPRα1s forms the L-type calcium channel in the T-tubules and is a voltage sensor of excitation-contraction (EC) coupling [41]. A recent report demonstrated that knock-down of DHPRα1s results in the induction of muscle atrophy [41]. In our study, DHPRα1s mRNA was decreased in muscles from STZ-treated rats, a finding that is suggests that CREB-mediated transcription is abnormal. Several proteins are known to interact with CREB to mediate its activity, including p300 or CBP [10]. One or more of these interacting proteins could be altered in skeletal muscle of STZ-treated rats, which would result in dysfunctional CREB signaling and decreased expression of downstream targets like DHPRa1s and PGC-1a.
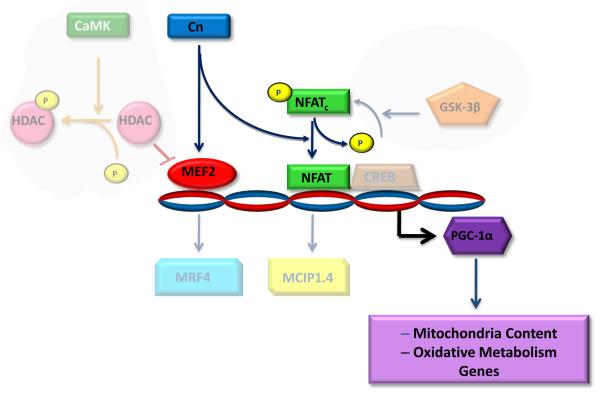
The Cn/NFATc/MEF2 signaling pathway has been linked to the maintenance of overall muscle mass. Cn activity, which is induced by exercise or other stimuli that raise intracellular calcium, activates two relevant downstream substrates, MEF2 and NFATc [42,43]. These transcription factors regulate the expression of genes involved in maintenance of overall muscle mass and the oxidative fiber phenotype, including PGC-1α (Fig.9) [42].
Figure 9. Decreased Cn signaling downregulates PGC-1α during chronic diabetes mellitus.
Functional capacity and muscle mass are regulated by contractile and activity and the associated calcium signaling. Two parallel calcium-dependent pathways involving calcium-calmodulin kinase (CaMK) and calcineurin (Cn) can regulate MEF2 whereas Cn is the primary pathway that regulates NFATc. Since both NFATc and MEF2 activities are attenuated during chronic diabetes mellitus as well as in both CnAα and CnAβ knockout mice, we propose that reduced Cn signaling is predominantly responsible for the decreased level of PGC-1α expression. Consequently, mitochondrial biogenesis, and the expression of proteins associated with oxidative metabolism in skeletal muscle are likely to be affected. Suppression of Cn/NFATc/MEF2 signaling, and subsequent downregulation of PGC-1α may perpetuate the decrease in muscle functional capacity and mass that accompanies diabetes mellitus.
Since, NFATc can be inactivated by phosphorylation by the kinase GSK-3β, we examined Akt/GSK-3β signaling and found no evidence that the pathway was activated in STZ-treated rats. Although phosphorylation of Akt on Ser-473 was slightly elevated, the increase did not seem to be physiologically relevant with regard to GSK-3β signaling or suppression of protein degradation. Tong et. al. reported similar paradoxical findings involving increased atrophy-related responses despite augmented levels of phosphorylated Akt [44].
In muscles of STZ-treated rats, extensive evidence was found to indicate that the decrease in CnA protein was the primarycause of the reduction in overall pathway signaling. The role of Cn in the regulation of PGC-1α gene expression was confirmed when PGC-1α mRNA was found to be lower in the muscles of CnAα-/- and CnAβ-/- mice. Together, these results suggest three possible interpretations. First, CnAα and CnAβ may have overlapping actions with regard to activating NFATc and MEF2. Second, CnAα and CnAβ may exhibit selectivity for either MEF2 or NFATc and inactivation of either transcription factor is sufficient to reduce PGC-1α expression regardless of the activation status of the other protein. Lastly, MEF2 and NFAT could act cooperatively to regulate PGC-1α and inactivation of either protein is sufficient to affect cofactor expression. Further studies will be necessary to delineate the intricacies of this mechanism in the context of diabetes. The reduction in PGC-1α expression may contribute to the chronic muscle atrophy induced by DM. This cofactor has been proposed to protect against atrophy through several different mechanisms. First, PGC-1α can antagonize FoxO-dependent transcription of AT-1 [5]. Decreased PGC-1α protein could perpetuate atrophy through the loss of FoxO inhibition and subsequent upregulation of AT-1. In light of the fact that AT-1 mRNA was not increased in 21 day STZ-treated rat muscle, it would seem other mechanisms are responsible for sustaining muscle wasting (data not shown). Second, the loss of PGC-1α could affect oxidative metabolism as PGC-1α is necessary to maintain the oxidative muscle fiber phenotype and helps to protect muscle cells against increased oxidative stress [3,45]. In other studies, we found that chronic DM reduced the proportion of oxidative MHC Type I fibers relative to glycolytic MHC II fibers in soleus muscles (TKR et al., manuscript submitted). Therefore, the loss of PGC-1α may contribute to the progression of muscle atrophy by increasing the number of glycolytic fibers, which are more prone to undergo atrophy induced by systemic pathologic conditions [6,46]. This could account for the perpetuation of atrophy over long periods of time.
In summary, our studies have shown that DM suppresses the Cn/NFATc/MEF2 signaling pathway and that this may perpetuate muscle wasting by downregulating PGC-1α expression. Recent reports indicate that increasing muscle contractile activity through resistance exercise may help to attenuate the muscle atrophy process [47,48]. Our findings suggest that contractile activity may attenuate chronic wasting by increasing the activation state of Cn or by activating alternative calcium signaling pathways that bypass Cn, such as calmodulin-dependent kinase (CaMK) (Fig. 9). Lastly, our results provide a possible explanation for the myopathy that frequently develops in transplant recipients who receive Cn inhibitors as part of their immunosuppressive regimen and suggests that these drugs may make the post-surgical rehabilitation of these patients more difficult [49]. A better understanding of the role of Cn in muscle atrophy will be important for improving rehabilitation of patients suffering from systemic disease or receiving immunosuppressive therapies.
ACKNOWLEDGMENTS
We thank Dr. S. K. Donaldson, Dr. Christine A. Griffin, and Brian Roberts for technical assistance and comments on the manuscript. This work was supported by National Institutes of Health Grants DK61521 (SRP), DK50740 (SRP) and DK066422 (JLG) and the Department of Veterans Affairs (JLG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sun Z, Liu L, Liu N, Liu Y. Muscular response and adaptation to diabetes mellitus. Front Biosci. 2008;13:4765–94. doi: 10.2741/3038. [DOI] [PubMed] [Google Scholar]
- 2.Michael L, Wu Z, Cheatham R, Puigserver P, Adelmant G, Lehman J, Kelly D, Spiegelman B. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001 Mar;98(7):3820–5. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J, Wu H, Tarr P, Zhang C, Wu Z, Boss O, Michael L, Puigserver P, Isotani E, Olson E, Lowell B, Bassel-Duby R, Spiegelman B. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002 Aug;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 4.Sacheck J, Hyatt J, Raffaello A, Jagoe R, Roy R, Edgerton V, Lecker S, Goldberg A. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007 Jan;21(1):140–55. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 5.Sandri M, Lin J, Handschin C, Yang W, Arany Z, Lecker S, Goldberg A, Spiegelman B. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006 Oct;103(44):16260–5. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiao G, Lieberman M, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol. 1997 Mar;272(3 Pt 2):R849–56. doi: 10.1152/ajpregu.1997.272.3.R849. [DOI] [PubMed] [Google Scholar]
- 7.Mootha V, Lindgren C, Eriksson K, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly M, Patterson N, Mesirov J, Golub T, Tamayo P, Spiegelman B, Lander E, Hirschhorn J, Altshuler D, Groop L. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003 Jul;34(3):267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 8.Patti M, Butte A, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker E, Goldfine A, Mun E, DeFronzo R, Finlayson J, Kahn C, Mandarino L. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003 Jul;100(14):8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008 Oct;18(5):426–34. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–80. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 11.Herzig S, Long F, Jhala U, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001 Sep;413(6852):179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 12.Chin E, Olson E, Richardson J, Yang Q, Humphries C, Shelton J, Wu H, Zhu W, Bassel-Duby R, Williams R. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998 Aug;12(16):2499–509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naya F, Mercer B, Shelton J, Richardson J, Williams R, Olson E. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000 Feb;275(7):4545–8. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- 14.Olson E, Williams R. Calcineurin signaling and muscle remodeling. Cell. 2000 Jun;101(7):689–92. doi: 10.1016/s0092-8674(00)80880-6. [DOI] [PubMed] [Google Scholar]
- 15.Savignac M, Gomes B, Gallard A, Narbonnet S, Moreau M, Leclerc C, Paulet P, Mariame B, Druet P, Saoudi A, Fournie GJ, Guery JC, Pelletier L. Dihydropyridine receptors are selective markers of Th2 cells and can be targeted to prevent Th2-dependent immunopathological disorders. J Immunol. 2004 May 1;172(9):5206–12. doi: 10.4049/jimmunol.172.9.5206. [DOI] [PubMed] [Google Scholar]
- 16.Sheridan DC, Carbonneau L, Ahern CA, Nataraj P, Coronado R. Ca2+-dependent excitation-contraction coupling triggered by the heterologous cardiac/brain DHPR beta2a-subunit in skeletal myotubes. Biophys J. 2003 Dec;85(6):3739–57. doi: 10.1016/S0006-3495(03)74790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4586–91. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkins B, Dai Y, Bueno O, Parsons S, Xu J, Plank D, Jones F, Kimball T, Molkentin J. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004 Jan;94(1):110–8. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 19.Zhang BW, Zimmer G, Chen J, Ladd D, Li E, Alt FW, Wiederrecht G, Cryan J, O’Neill EA, Seidman CE, Abbas AK, Seidman JG. T cell responses in calcineurin A alpha-deficient mice. J Exp Med. 1996 Feb 1;183(2):413–20. doi: 10.1084/jem.183.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price S, Bailey J, Wang X, Jurkovitz C, England B, Ding X, Phillips L, Mitch W. Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin-proteasome proteolytic pathway by a mechanism including gene transcription. J Clin Invest. 1996 Oct;98(8):1703–8. doi: 10.1172/JCI118968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waalkes T, Udenfriend S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–6. [PubMed] [Google Scholar]
- 22.Clark AS, Mitch WE. Comparison of protein synthesis and degradation in incubated and perfused muscle. Biochem J. 1983 Jun 15;212(3):649–53. doi: 10.1042/bj2120649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001 Aug;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z, Wang ZM, Delbono O. Insulin-like growth factor-1 increases skeletal muscle dihydropyridine receptor alpha 1S transcriptional activity by acting on the cAMP-response element-binding protein element of the promoter region. J Biol Chem. 2002 Dec 27;277(52):50535–42. doi: 10.1074/jbc.M210526200. [DOI] [PubMed] [Google Scholar]
- 25.Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002 Dec;23(12):569–75. doi: 10.1016/s0165-6147(02)02111-9. [DOI] [PubMed] [Google Scholar]
- 26.Fenyvesi R, Racz G, Wuytack F, Zador E. The calcineurin activity and MCIP1.4 mRNA levels are increased by innervation in regenerating soleus muscle. Biochem Biophys Res Commun. 2004 Jul 23;320(2):599–605. doi: 10.1016/j.bbrc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Rothermel B, Vega R, Frey N, McKinsey T, Olson E, Bassel-Duby R, Williams R. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000 Dec;87(12):E61–8. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 28.Olson EN, Williams RS. Calcineurin signaling and muscle remodeling. Cell. 2000 Jun 23;101(7):689–92. doi: 10.1016/s0092-8674(00)80880-6. [DOI] [PubMed] [Google Scholar]
- 29.Beals C, Sheridan C, Turck C, Gardner P, Crabtree G. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997 Mar;275(5308):1930–4. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 30.Cross D, Alessi D, Vandenheede J, McDowell H, Hundal H, Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994 Oct;303(Pt 1):21–6. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes S, Konieczny S. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–61. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- 32.Black B, Martin J, Olson E. The mouse MRF4 promoter is trans-activated directly and indirectly by muscle-specific transcription factors. J Biol Chem. 1995 Feb;270(7):2889–92. doi: 10.1074/jbc.270.7.2889. [DOI] [PubMed] [Google Scholar]
- 33.Finck B, Kelly D. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006 Mar;116(3):615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J, Tarr P, Yang R, Rhee J, Puigserver P, Newgard C, Spiegelman B. PGC-1beta in the regulation of hepatic glucose and energy metabolism. J Biol Chem. 2003 Aug;278(33):30843–8. doi: 10.1074/jbc.M303643200. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers J, Lerin C, Haas W, Gygi S, Spiegelman B, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005 Mar;434(7029):113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 36.Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006 May;3(5):333–41. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Soyal S, Krempler F, Oberkofler H, Patsch W. PGC-1alpha: a potent transcriptional cofactor involved in the pathogenesis of type 2 diabetes. Diabetologia. 2006 Jul;49(7):1477–88. doi: 10.1007/s00125-006-0268-6. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006 Sep;147(9):4160–8. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 39.Phielix E, Mensink M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav. 2008 May;94(2):252–8. doi: 10.1016/j.physbeh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003 Feb;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 41.Pietri-Rouxel F, Gentil C, Vassilopoulos S, Baas D, Mouisel E, Ferry A, Vignaud A, Hourde C, Marty I, Schaeffer L, Voit T, Garcia L. DHPR alpha1S subunit controls skeletal muscle mass and morphogenesis. EMBO J. Feb 3;29(3):643–54. doi: 10.1038/emboj.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassel-Duby R, Olson E. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 43.Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007 Aug;22:269–78. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- 44.Tong JF, Yan X, Zhu MJ, Du M. AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes. J Cell Biochem. 2009 Oct 1;108(2):458–68. doi: 10.1002/jcb.22272. [DOI] [PubMed] [Google Scholar]
- 45.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006 Oct 20;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 46.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004 Aug;114(3):370–8. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alkner BA, Tesch PA. Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiol Scand. 2004 Jul;181(3):345–57. doi: 10.1111/j.1365-201X.2004.01293.x. [DOI] [PubMed] [Google Scholar]
- 48.Fluckey JD, Dupont-Versteegden EE, Montague DC, Knox M, Tesch P, Peterson CA, Gaddy-Kurten D. A rat resistance exercise regimen attenuates losses of musculoskeletal mass during hindlimb suspension. Acta Physiol Scand. 2002 Dec;176(4):293–300. doi: 10.1046/j.1365-201X.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 49.Rennie MJ, Wilkes EA. Maintenance of the musculoskeletal mass by control of protein turnover: the concept of anabolic resistance and its relevance to the transplant recipient. Ann Transplant. 2005;10(4):31–4. [PubMed] [Google Scholar]