Abstract
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are created in normal hepatocytes and are critical for normal physiological processes including oxidative respiration, growth, regeneration, apoptosis, and microsomal defense. When the levels of oxidation products exceed the capacity of normal antioxidant systems, oxidative stress occurs. This type of stress, in the form of ROS and RNS, can be damaging to all liver cells, including hepatocytes, Kupffer cells, stellate cells, and endothelial cells, through induction of inflammation, ischemia, fibrosis, necrosis, apoptosis, or through malignant transformation by damaging lipids, proteins, and/or DNA. In part I of this review, we will discuss basic redox biology in the liver, including a review of ROS, RNS, and antioxidants, with a focus on nitric oxide as a common source of RNS. We will then review the evidence for oxidative stress as a mechanism of liver injury in hepatitis (alcoholic, viral, non-alcoholic). In part II of this review, we will review oxidative stress in common pathophysiological conditions including ischemia/reperfusion injury, fibrosis, hepatocellular carcinoma, iron overload, Wilson's disease, sepsis and acetaminophen overdose. Finally, biomarkers, proteomic, and antioxidant therapies will be discussed as areas for future therapeutic interventions.
Keywords: nitric oxide, hepatocytes, oxidative stress, reactive oxygen species, hepatitis, ethanol induced hepatitis, fibrosis, ischemia/reperfusion, antioxidants, proteomics
Outline for Part I and II
-
Redox regulation in Healthy Hepatocytes
Reactive oxygen and nitrogen species
Antioxidants
Nitric Oxide
Redox in apoptosis
-
Redox in Pathologic Hepatocytes
-
Hepatitis
Alcohol-induced Liver Disease
Viral Hepatitis
Non-alcoholic fatty Liver Disease
Ischemia/ Reperfusion Injury
Hemorrhagic Shock
Sepsis
Fibrosis
Iron overload
Wilson's Disease
Acetaminophen-Induced liver injury
-
-
Implication for future interventions
Biomarkers/ Redox proteomics
Antioxidants
Redox Regulation in Healthy Hepatocytes
Reactive Oxygen and Reactive Nitrogen Species
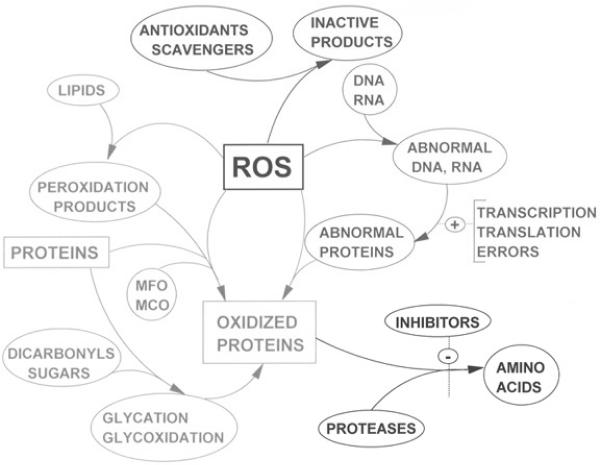
Reactive oxygen species (ROS), reactive nitrogen species (RNS), and other free radicals are critical intermediates in the normal physiology and pathophysiology of hepatocytes. ROS, including, H2O2, OH., and , are important in the creation of oxidative stimuli required for normal physiological homeostasis of hepatocytes. When the equilibrium between ROS generation and the antioxidant defense of the cell is disrupted, a net oxidative stress results [143]. In the liver, free radicals triggered by ROS and RNS are created by neutrophils, Kupffer cells, mitochondria, and cytochromes P450 [70]. The damage created by oxidative stress affects hepatocytes, endothelial, Kupffer, and stellate cells by inducing inflammation, ischemia, apoptosis, necrosis, and regeneration. ROS are know to affect lipids, protein, and DNA and have been implicated in the pathophysiology of atherosclerosis, adult respiratory distress syndrome, cystic fibrosis, cataracts, macular degeneration, cancer, liver disease, diabetes, neurological conditions, and ischemia/reperfusion injuries [100] (Figure 1). Currently, it is believed that ROS also affect signal transduction pathways that, when unbalanced, may lead to hepatic inflammation, necrosis, fibrosis and/or apoptosis [64].
Figure 1.
Reactive oxygen species (ROS) are involved in the oxidation of proteins, lipids, and nucleic acids. Reprinted by permission from dir.nhlbi.nih.gov/labs/lb/es/index.asp. [147]
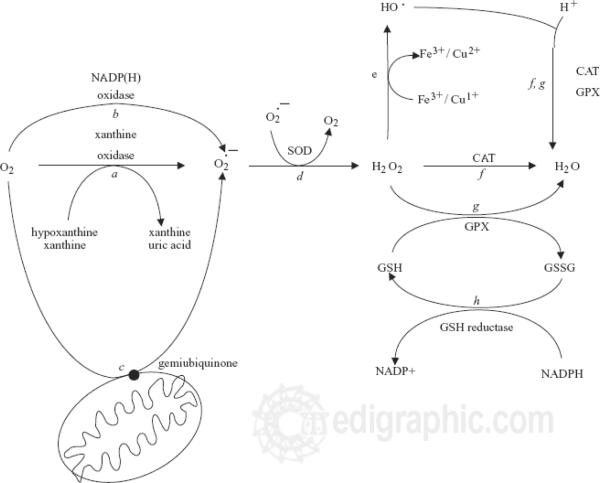
ROS include superoxide (O2.-), hydrogen peroxide (H2O2) and hydroxyl radical (OH.), while RNS includes nitric oxide (NO.) and peroxynitrite (ONOO-). (Table 1) Of these, those with an unpaired electron are known as “radicals” [59]. Superoxide (O2.-) is a common radical that can serve as a precursor for several other ROS and RNS, although O2.- itself is also a potent oxidant. Superoxide is constitutively produced by mitochondria as a byproduct of oxidative phosphorylation, as the mitochondria will reduce approximately 1–3% of respiratory oxygen molecules to superoxide anions [124]. Inherent in these cells are enzymes and compounds that consume ROS, including superoxide dismutase that converts superoxide anion (O2−) to hydrogen peroxide (H2O2), catalases (H2O2 dismutases), and peroxidases that reduce H2O2 to water, frequently by employing the reducing power of NAD(P)H (Table 2; Figure 2). Metals including iron and copper can further react with hydrogen peroxide to produce hydroxyl radicals via the Fenton reaction [156]. (Table 2, Figure 2) The Fenton reaction recycles iron from Fe (II) to Fe (III) by oxidiziong superoxide anion to oxygen. Hydroxyl radicals are then created. Increases in superoxide can increase the levels of bioactive iron. This can occur in Kupffer cells in the liver in pathologic condition such as cirrhosis. The ability of superoxide to activate this reaction makes it an important factor in oxidative stress.
Table 1.
List of Common Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
| Free Radicals |
Non-radicals |
|---|---|
| Reactive oxygen species (ROS) |
Reactive oxygen species (ROS) |
| Superoxide (O2•-) | Hydrogen peroxide (H2O2) |
| Hydroxyl radical (HO•) | Hypochlorous acid (HOCl) |
| Peroxyl radical (RO2•) | Hypobromous acid (HOBr) |
| Alkoxyl radical (RO•) | Ozone (O3) |
| Hydroperoxyl radical (HO2•) | Organic peroxides (ROOH) |
| Singlet oxygen (1O2) | Perioxynitrous acid (ONOOHc) |
| Peroxynitrate (O2NOO-) | |
| Peroxynitrite (ONOO-c) |
| Reactive nitrogen species (RNS) |
Reactive nitrogen species (RNS) |
|---|---|
| Nitric oxide (•NO) | Nitryl chloride (NO2Cl) |
| Nitrogen dioxide (•NO2) | Nitrous acid (HNO2) |
| Nitrite (•NO3) | Nitrosyl cation (NO+) |
| Nitrosyl anion (NO.) | |
| Dinitrogen tetroxide (N22O4) | |
| Dinitrogen trioxide (N2O3) | |
| Peroxynitrite (ONOO-) | |
| Peroxynitrous acid (ONOOH) | |
| Alkyl peroxynitrites (ROONO) | |
| Nitronium cation (NO2+) |
Table 2.
Chemical equations relevant to reactive oxygen and reactive nitrogen species generation.
| Reactive oxygen species generation |
| O2 + e- → O2-• (superoxide anion) |
| O2-• + H2O → HO2• (hydroperoxyl radical) |
| HO2• + e- + H → H2O2 (hydrogen peroxide) |
| H2O2 + e- → OH- + •OH (hydroxyl radical) |
| Reactive nitrogen species generation |
| L-arginine + O2 → •NO (nitric oxide) + L-citrulline |
| O2-• + •NO → ONOO- (peroxynitrite) |
| Fenton reaction (catalyzed by transition metals) |
| H2O2 + Fe2+ → OH- + •OH + Fe3+ |
| Haber-Weiss Reaction (catalyzed by transition metals) |
| H2O2 + O2-• → O2 + •OH + OH- |
Reprinted with permission from Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 44:239–267,2004. [78]
Figure 2.
Pathway for formation of reactive oxygen species. (ROS) Reprinted by permission from Cesaratto L, Vascotto C, Calligaris S, Tell G. The importance of redox state in liver damage. Ann Hepatol. 3:86–92,2004. [23]
Reactive oxygen species are necessary for many normal physiological functions. Nitric oxide specifically is critically important in microbial defense, neuronal signaling, vascular tone, platelet aggregation, and cardiac contractility. Reactive oxygen species are also implicated in cell signaling and are considered by some to be second messengers which can trigger cytokines, hormones, and growth factors [89]. In this way, ROS can also affect gene expression. ROS are implicated in the normal induction of apoptosis though the exact mechanisms are unclear. Specifically, nitric oxide has been implicated in both the induction and suppression of apoptosis [28, 96]. Since ROS and RNS are ubiquitous in the normal physiology of so many processes, it is not surprising that when excess ROS and RNS are produced, many functions of the cell are disrupted.
Antioxidants
To consume excess ROS and RNS, the body utilizes antioxidants. Endogenous antioxidants are usually small molecular weight molecules that are able to prevent or limit oxidative damage by detoxifying ROS and RNS [53]. Common antioxidants in hepatocytes include glutathione (GSH), glutathione peroxidase and reductase enzymes, superoxide dismutase (SOD), catalase, dismutase, thioredoxin, heme oxygenase (HO), peroxidases, and metal binding proteins. (Table 3) Low molecular weight compounds, such as bilirubin, melatonin, lipoic acid, coenzyme Q, and uric acid, also have antioxidant properties.
Table 3.
Common antioxidant reactions and the enzymes that catalyze them.
| Superoxide Dismutase (SOD) |
| 202- + 2H+ → H2O2 + O2 |
| Catalase: |
| 2H2O2 → 2H2O + O2 |
| Glutathione peroxidase |
| 2GSH +H2O2 → GSSG (glutathione disulfide) + 2H2O |
| Glutathione disulfide reductase |
| GSSG + NADPH + H+ → 2GSH + NADP+ |
| Glutathione S-transferase (GST) |
| RX + GSH → RSG + HX |
Glutathione is a ubiquitous tripeptide whose main function is to react with hydrogen peroxide and, via glutathione peroxidase, to create glutathione disulfide (GSSG) (Table 3). GSH also scavenges other ROS and RNS, chelates copper, and prevents oxidation of protein sulfhydryl groups. In addition to glutathione, catalase and peroxidases are able to break down hydrogen peroxide to less reactive metabolites. Catalase converts hydrogen peroxide to water and oxygen, while peroxidase is also able to reduce it to water with reducing equivalents, usually thiol-containing molecules such as thioredoxin. Thioredoxin contains two sulfhydryl groups, which are oxidized to a disulfide.
An antioxidant enzyme is superoxide dismutase (SOD), which catalyzes the reaction of two molecules of superoxide radical anions with each other, leading to the formation of one molecule of molecular oxygen and one molecule of hydrogen peroxide. Peroxide is relatively stable but, in the presence of a transition metal, may form hydroxyl radicals. Because transition metals may create ROS, metal-binding proteins may also serve antioxidant roles. Iron may be bound by transferrin and lactoferrin, while ceruloplasmin and albumin bind copper. Hemoglobin and myoglobin both contain iron and heme. When exposed to excessive oxidative stress, the heme group and the iron may disassociate, thus promoting lipid peroxidation. When this occurs, hemoglobin binding proteins such as haptoglobin and heme binding proteins, like hemopexin, bind these proteins to decrease the level of lipid peroxidation [57].
Exogenous antioxidants can be consumed through the diet; common dietary forms include vitamin C (ascorbate), vitamin E, carotenoids, and plant phenols. Lipophilic α-tocopherol, the active form of vitamin E, scavenges peroxyl radicals and prevents lipid peroxidation [162]. Ascorbate is critical for the biosynthesis of collagen and is able to scavenge superoxide anions, hydroxyl, peroxyl, thiyl, and oxysulfur radicals, and peroxynitrite [12, 58]. Carotenoids are a source of vitamin A and also scavenge free radicals [103]. Plant phenols are able to scavenge ROS and RNS and to chelate metals. Many therapeutic interventions, discussed later in this review, have been aimed at increasing dietary antioxidants in order to prevent and/or treat diseases associated with increased ROS, although the results, as of yet, are inconclusive.
Nitric Oxide
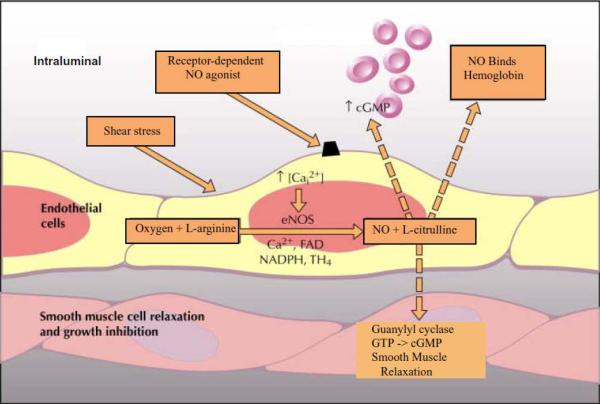
Nitric oxide (NO) is a reactive nitrogen species critical in the redox biology of hepatocytes. It is a hydrophobic, freely diffusible, small molecule with a half-life of seconds (or less). It is created by nitric oxide synthase (NOS) which is present in three forms: neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). nNOS was first discovered in neuronal tissue and is thought to be constitutively active. It has since been identified as having importance in the regulation of skeletal muscle contractions as well. eNOS was first discovered in the endothelium and is constitutively active as well. It is vitally important in the regulation of blood flow and pressure. While initially thought to only be constitutively active, it is now understood that eNOS production may be also be induced by shear stress. iNOS is an inducible NOS found in a variety of cells, although it was first described in macrophages and hepatocytes after treatment with endotoxins and cytokines. Further research has shown that iNOS can also be constitutively expressed as well, particularly in epithelial cells of the respiratory tract and nasal sinuses. Both eNOS and nNOS are calcium dependent, while iNOS binds calmodulin but is calcium independent.
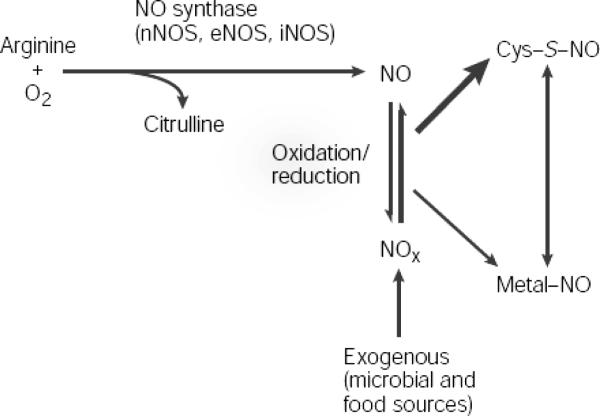
NOS utilizes L-arginine and oxygen, in combination with electrons from NADPH and cofactors FAD, FMN, BH4, CaM, and heme, to create L-citrulline and NO. (Figure 3) When constitutively expressed, NOS releases small amounts of NO based on calcium. This NO then serves as an intra- and extracellular signaling molecule. In iNOS however, transcriptional activation leads to increased protein levels and increased expression of the enzyme. iNOS is then able to produce NO, independent of calcium and in large amounts (up to micromolar concentrations). This induction of iNOS may be protective or destructive to the cell, depending on the type of stimulus and the amount and duration of iNOS expression.
Figure 3.
The formation of nitric oxide. Reprinted by permission from Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 6:150–166,2005. [60]
NO itself interacts with multiple molecular targets including thiols, transitional metals (such as iron), oxygen, and other free radicals [149]. It readily binds to heme, thus affecting the activation or inhibition of various proteins. Specifically, NO activates guanylate cyclase, which results in increased cGMP synthesis. This increase then affects the activation or inhibition of other molecules. NO can also inhibit cytochrome p450, which affects the metabolism of many compounds [72, 146, 168]. NO can inhibit cytochrome oxidase by binding to the oxygen binding site, thus reducing ATP (adenosine triphosphate) production, [52, 141] and can bind and inhibit NOS and catalase [14, 56].
The interaction of NO and oxygen results in other reactive nitrogen species (RNS) such as dinitrogen trioxide (N2O3), which can nitrosate amines producing N-nitrosamines or may nitrosate cysteines on various proteins (RSH) to form S-nitrosothiols (RSNO) [14, 17, 149] RSNO can transport and store NO as well as affect protein interaction and activity [41, 42, 152]. NO also reacts with a peroxidate at a diffusion limiting rate creating peroxynitrite (ONOO-) which depending on the amount and duration, may have beneficial (antimicrobial) or detrimental (cell damage/death) effects on the cell [80, 125]. Peroxynitrite affects many molecules and can result in alterations in DNA, in lipid and protein oxidation and in protein nitration. (Figure 1; Table 2)
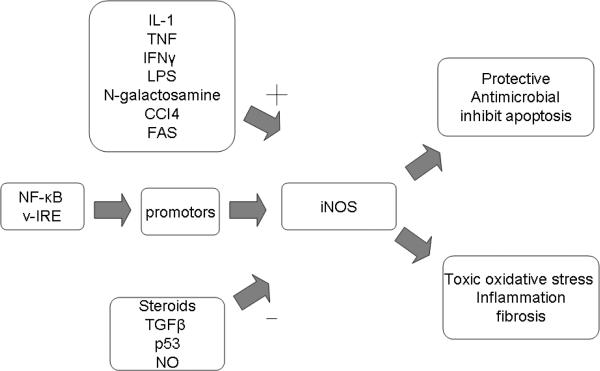
Hepatocytes was one of the first human cell types where iNOS was described. It the liver iNOS was found to be critical in the development and propagation of inflammation [25, 158]. (Figure 4) In the liver, iNOS is known to be expressed in all cells (hepatocytes, Kupffer cells, vascular endothelial cells, and stellate/Ito cells) and its expression is induced by IL-1β or IL-1β in combination with TNF, IFNγ, and/or LPS [11, 34, 35, 51, 140, 145]. (Table 4) NF-κB and γ-interferon response elements (γ-IRE) have both been found to affect the promoter of iNOS [138, 158, 159]. iNOS expression is also known to be down-regulated by steroids, TGF-β, the heat shock response, p53, and NO itself [158]. (Figure 4) Cytokine activation of iNOS leads to peroxynitrite (ONOO-) formation and thus oxidative stress, which can be beneficial in the setting of microbial infection or deleterious in the setting hepatitis or acetaminophen-induced liver injury. (Table 4)
Figure 4.
Important promoter, inhibitor, and effects of iNOS in the liver. iNOS is expressed hepatocytes, Kupffer cells, vascular endothelial cells, and stellate/Ito cells in the liver. The level of iNOS activity is determined by a variety of stimuli. Based on the amount and duration of activity iNOS can be either protective or toxic.
Table 4.
Roles of iNOS production in liver damage.
| Condition/Inducers |
NO Effect |
Mechanism |
|---|---|---|
| In vivo | ||
| Endotoxemia | Protective | Inhibits apoptosis |
| Toxic | Oxidative stress, circulatory failure | |
| TNFα + N-galactos-amine | Protective | Inhibits apoptosis |
| CCl4 | Protective | Decreases oxidative stress |
| Liver regeneration | Protective | Inhibits apoptosis |
| Ischemia-reperfusion | Toxic | Oxidative damage |
| Hemorrhagic shock | Toxic | Direct toxicity, activates inflammation |
| Alcoholic liver injury |
Protective |
unclear |
| In vitro (hepatocytes) | ||
| TNFα, Fas antibody | Protective | Inhibits caspase/apoptosis HSP70 upregulation |
| H2O2 | Protective | Heme oxygenase-1 upregulation |
| Acetaminophen | Protective | Modulates GSH levels |
Reprinted with permission from Li J, Billiar TR. Nitric Oxide. IV. Determinants of nitric oxide protection and toxicity in liver. Am J Physiol. 276:G1069–1073,1999. [94]
The role of iNOS in liver injury is complex. The amount and duration of iNOS expression determines the amount of NO and thus, the level of reactive nitrogen species created. The effects of iNOS are also dependent on the other proinflammatory cascades active in the cell at the time of NO production. For example, iNOS activation is protective in both preventing sepsis and by inhibiting apoptosis but it is also associated with deleterious effects in both ischemia-reperfusion injury and hemorrhagic shock due to oxidative damage and activation of inflammatory cascades. [94] When iNOS is activated by TNF-α and N-galactosamine or by CCl4, it plays a protective role by inhibiting apoptosis and decreasing oxidative stress. In cultured hepatocytes, iNOS is activated by TNF-α and Fas antibody, and NO is protective in these cells as it inhibits caspases and thus inhibits apoptosis. Similarly, hepatocytes stimulated by hydrogen peroxide are protected by NO induced heme oxygenase-1 upregulation. [94] These examples of iNOS activation and subsequent downstream regulation of protein/gene expression is just a sample of the complexity inherent in redox signaling in hepatocytes.
In hepatocytes, eNOS is found primarily in the endothelial cells of the sinusoids (Figure 5). eNOS is critical in the maintenance and regulation of vascular tone by the basal and inducible release of NO. Release of NO by the endothelial cells results in vascular smooth muscle relaxation while NO scavenging, by cell free hemoglobin for example, results in vasoconstriction due to relative NO depletion. eNOS production is critical for healthy hepatocyte blood flow. Loss of eNOS induced NO has been implicated in the pathology of early ischemia/reperfusion injury, as described below.
Figure 5.
Critical components in the formation of nitric oxide by eNOS and its role in hepatocytes.
Redox in Apoptosis and Regeneration
Reactive oxygen species have been implicated in regulation of apoptosis and in the regeneration of hepatocytes. Apoptosis can be induced by direct chemical interactions or indirectly by activation of various ligands, including TGF-β, Fas, TNF-α/D-Gal, or TNF-α/actinomycin D [16, 47, 74]. Apoptosis may be induced by superoxide or H2O2 from exogenous sources, such as occurs in monocytes and neutrophils in the setting of liver injury,[64] or from intracellularly generated ROS/RNS as occurs from bile acids, ischemia, or hepatotoxins including alcohol, acetaminophen, and chemotherapeutic drugs [62]. Many of these drugs are metabolized by the cytochrome P450 isoform CYP2E1, which results in increased oxidative stress and apoptosis.
When appropriately induced, Kupffer cells can express the death ligand TNF-α, TNF related apoptosis inducing ligand (TRAIL) and Fas ligand [19]. TNF-α plays a critical role in physiological apoptosis and in pathophysiological liver inflammation, ischemia, and necrosis. TNF-α induced cell death may involve the binding of type I TNF receptor, activation of caspases, and recruitment of intracellular proteins, although caspase independent pathways have also been described [66]. Normally, hepatocytes are resistant to TNF-α but many become sensitized to it after oxidative stress-induced gene alterations [66]. The Fas/CD95 death receptor pathway, as well as cytoplasmic c-Abl, has also been implicated in ROS induced apoptosis [62, 121, 155].
NO appears to play a role in apoptosis but the exact mechanisms are unclear. Hepatic cellular proliferation may be suppressed by NO [90, 91] and this effect is prevented by the addition of NOS inhibitors [90, 91]. NO induced apoptosis has been suggested by the demonstration of apoptosis in various cells cocultured with NO producing cells [44, 87, 166]. On the other hand, other studies have demonstrated that NO can protect the liver from TNF-α induced apoptosis [74, 165], so the amount of NO and the previous redox conditions of the cell seem to be important in determining the role of NO as an inducer or inhibitor of apoptosis.
Regeneration is associated with increased iNOS expression, which in turn is associated with increased cell proliferation [114]. These protective effects are both cGMP dependent and independent [76]. cGMP analogs decrease caspase activity, while ODQ decreases the inhibition of caspase-3-like activity. NO also results in S-nitrosation of cysteines, causing in inhibition of caspase activity [73, 75, 76, 95, 112, 113]. These inhibitory effects can be partially reversed by dithiothreitol [76, 95]. NO also decreases recombinant caspase, inhibits Bid and Bcl-2 cleavage, and reduces cytochrome c release; all of these are apoptotic stimuli [75, 95]. NO induced heat shock protein 70 (HSP70) also protects the liver from apoptosis [74].
Following partial hepatectomy, iNOS expression increases within 4–6 hours [61]. iNOS appears to be critical in the regeneration of hepatocytes after resection, particularly in iNOS knockout mice that show impaired liver regeneration and increased cell death [136]. The NOS inhibitors, aminoguanidine and L-NMMA, demonstrate the need for NO in DNA synthesis [21], while other factors, including cytokine IL-6, TNF-α, NF-κB, and STAT3, also appear to be important in liver regeneration [2, 29, 33, 36, 97, 169].
In summary, the production of ROS is critical for hepatic homeostasis, but when production overwhelms consumption, oxidative stress develops. Oxidative stress, in turn, can damage all of the cells present in the liver by induction of disruptive processes ranging from inflammation to apoptosis to malignant transformation [105]. The primary sources of this oxidative stress tend to be neutrophils, Kupffer cells, the P450 system of hepatocytes ER, and the hepatocyte mitochondria with nitric oxide playing a critical role. Markers for this oxidative stress include not only increased levels of ROS/RNS, lipid peroxidation, transcription or translational errors, and upregulation of known associated genes/protein expression, but can also include decreased levels of antioxidants including glutathione, vitamin E, ascorbate, and selenium [20, 105].
Hepatitis
Alcohol-induced Liver Injury
Oxidative stress is a well-documented cause of alcoholic liver damage. Ethanol is metabolized via alcohol dehydrogenase (ADH), the microsomal ethanol-oxidizing system (MEOS), and catalase in the peroxisomes [98, 99, 102], although the alcohol dehydrogenase pathway is responsible for a majority of ethanol metabolism. In this pathway, nicotinamide adenine dinucleotide (NAD) is reduced by a transfer of hydrogen to NADH with concomitant production of acetaldehyde. NADP can also be reduced to NADPH and this increase in reducing equivalents in the cytosol (NADH and NADPH) changes the redox potential of the cell. Hydrogen equivalents from ethanol, but not NADH, are transferred from the cytosol to the mitochondria via a shuttle mechanism such as the malate cycle, the fatty acid elongation cycle, and/or the α-glycerophosphate cycle. This replaces the citric acid cycle as the source of hydrogen and the mitochondria become more reduced.
Metabolism of ethanol occurs in the CYP2E1 of the endoplasmic reticulum within hepatocytes. This oxidative metabolism uses NADPH oxidase to generate superoxide anion, hydrogen peroxide, and hydroxyethyl radicals, which can lead to lipid peroxidation [22, 37, 118, 148, 163]. (Figure 6) Acetaldehyde is also created as a product of the ADH reaction and is then oxidized to acetate via mitochondrial aldehyde dehydrogenase. Acetate has been associated with up-regulation of transcription factors such as AP-1 (activator protein-1) and NF-κB, which can upregulate chemokines, inflammatory cytokines (IL-1β, IL-6, IL-18, and others), adhesion molecules, and Fas ligands [137]. This can further lead to apoptosis via caspase cascades as well as necrosis from ATP depletion [133].
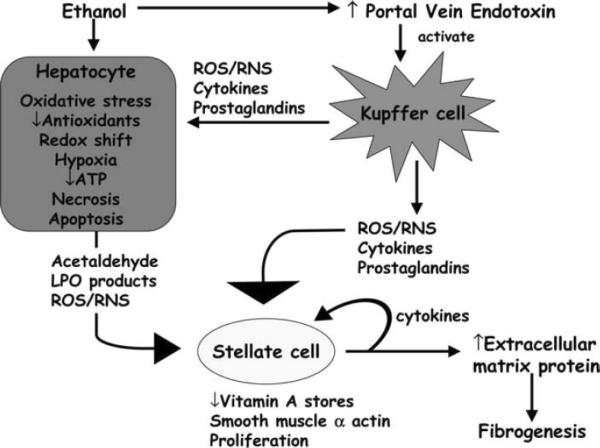
Figure 6.
Mechanism of alcohol-induced liver injury. Alcohol metabolism causes acetaldehyde and reactive oxygen species to be generated, both of which can activate stellate cells. ROS/RNS from Kupffer cells can also active stellate cells causing increasing collagen deposition and eventual fibrosis. Reprinted by permission from Halliwell B, JMC G. Free Radicals in Biology and Medicine. 4th ed. oxford: Oxford University Press; 2007. [59]
One of the mechanisms of alcohol-related liver damage is through oxidative stress and cytokine production leading to hepatic necrosis and fibrosis [131]. Animal models of alcoholic liver disease have demonstrated that increasing oxidative stress increases the severity of liver injury,[71, 106, 122] while reducing oxidative stress may prevent liver injury [101, 167]. Chronic alcohol exposure can lead to mitochondrial abnormalities including megamitochondria and can result in increased CYP2E1 activity. Ethanol induces an isoform of the cytochrome P450 family, CYP2E1, resulting in oxidative stress in hepatocytes and Kupffer cells [13, 82–84], while polymorphisms of CYP2E1 may correlate with susceptibility of alcoholic liver disease [164]. This isoform generates more ROS than do other P450 isoforms via reduction of molecular oxygen through superoxide anion to hydrogen peroxide [6, 39]. Animal models of mice that overexpress CYP2E1 showed enhanced alcohol-induced liver injury, although CYP2E1 knockout mice can still develop alcohol-induced liver injury [117]. This increased CYP2E1 activity is noted in animals and humans exposed to chronic intoxication as measured by increased amounts of 1-hydroxyethyl free radicals [6, 79]. These react with macromolecules, thiols, and epitopes that affect both structure and function. In models of intoxicated mice, inhibition of CYP2E1 is associated with decreased lipid peroxidation and hydroxyethyl radical production [4, 119].
Since mitochondria lack catalase, glutathione (GSH) becomes very important in protection against oxidative stress. GSH is imported into the mitochondria from the cytosol and chronic alcohol exposure results in an impairment of this transport, possibly a result of the accumulation of acetylaldehyde. Mitochondria then become glutathione depleted [104]. Decreased mitochondrial GSH from decreased GSH synthase sensitizes hepatocytes to TNF-α, resulting in increased hepatic damage [31, 107]. Glutathione is synthesized from S-adenosylmethionine (SAMe) via methionine synthase (MS) and methionine adenosyltransferase (MAT). In patients with alcoholic liver disease, decreased hepatic SAMe and GSH were observed, and the administration of SAM can reverse the effects of ethanol exposure by increasing liver GSH availability [129]. Patients who drink ethanol heavily are also noted to have decreased levels of a number of antioxidants including selenium,[38] vitamin A,[92] vitamin E,[161] and Coenzyme Q [10] perhaps from decrease intake, decrease absorption, or both.
In the setting of oxidative stress, NO production is also increased, which leads to further damage [171]. Alcohol and lipopolysaccharides (LPS) together create increased hepatic damage, but the addition of aminoguanidine decreases this damage by decreasing NO synthesis [24]. GSH depletion and increase in transaminase activity could be prevented by the inhibition of iNOS [3]. The NO induced vasodilation counters the alcohol-induced vasoconstriction so it is not only the ROS generated by NO, but also the vasoactive properties, that play a role in liver biology [127].
The role of NADPH oxidase and iNOS in Kupffer cells is a compelling source of oxidative stress in alcohol-induced liver injury. NADPH oxidase (p47phox) knockout mice are resistant to alcohol-induced liver damage [81]. Mice given diphenyleneiodonium sulfate (an NADPH oxidase blocker) and alcohol were protected against alcohol-induced liver damage. Ethanol treated mice were noted to have severe liver injury involving gut-derived endotoxin, CD14 receptor, free radicals (detected by ESR), activation of NF-κB, and release of TNF-α from activated Kupffer cells. In contrast, NADPH oxidase-deficient mice had no notable liver pathology, no increase in free radicals, no activation of NF-κB, and no increase in TNF-α. This suggests that NADPH oxidase is important in the development of alcohol-induced liver injury as it relates to NF-κB and TNFα expression [81].
iNOS appears to be required for the formation of alcohol-induced liver injury by McKim et al.[116] Studies on mice reveal that after weeks of ethanol exposure, serum alanine aminotransferase (ALT) levels were significantly increased in wild-type mice but blunted in iNOS knockout mice. Similarly, if wild-type mice were treated with N-(3-aminomethyl) benzyl-acetamindine (1400W), an iNOS inhibitor, their levels of hepatic dysfunction were also attenuated. The administration of ethanol in turn induced inflammation, fatty accumulation, and necrosis in the wild-type mice but not in iNOS knockout mice. The iNOS knockout mice also did not have accumulation of lipid peroxidase proteins (4-hydroxynonenal) or reactive nitrogen species (3-nitrotyrosine) [116]. These results suggest that iNOS is critical in the development of alcohol-induced liver injury.
Measures of lipid peroxidation, 4-hydroxy-2,3-nonenal (HNE) and malonaldehyde (MDA) protein adducts, are noted in the plasma of alcoholics who have with no signs of hepatic dysfunction. MDA is noted in both protein and lipid free solution of alcoholic patients [30], while HNE is noted by immunohistochemistry of the liver of alcoholics[128], and the magnitude of lipid peroxidation correlates with the degree of liver injury [144], being most prevalent in the perivenular region where liver injury is usually most significant [128]. Oxidation of n-6-polyunsaturated fatty acids produces hydroxyalkenal HNE; male rats chronically intoxicated have increased HNE levels in mitochondria and microsomes [68]. HNE and other hydroxyalkenals can also induce the up-regulation of procollagen type I gene in human stellate cell cultures, thus resulting in increased transcription and synthesis of collagen type 1 [130, 133]. HNE induces expression and synthesis of the fibrogenic cytokine transforming growth factor β1 (TGFβ1) in rat liver and macrophage-derived cell lines [93]. HNE also activates the transcription of the heat shock protein (hsp70) in HepG2 cells, a human hepatoma cell line [18]. All of these processes have been implicated in the development of alcoholic liver fibrosis. Kupffer cells, in particular, are involved in the generation of protein adducts with both acetaldehyde and ethanol-induced lipid peroxidation products in alcoholic liver disease [123].
Animal models have also used electron spin resonance to detect free radicals seen in alcohol-induced liver injury [6, 7, 81]. When hepatocytes were incubated with NADPH, ethanol and the spin trapping agent 4-pyridyl-1-oxide-t-butyl nitrone (4-POBN) produced an electron spin resonance (ESR) signal. The free radical formation was dependent upon the activity of the microsomal monoxygenase system and increased with ethanol and oxide, while catalase and P-450 inhibitors decreased it [6]. This free radical formation in part appeared to be initiated by the hepatic Kupffer cells and was associated with activation of the transcription factor NF-κB, and release of cytotoxic TNF-α from activated Kupffer cells [81].
Impairment of proteasome function, appearing as the loss of the ability to break down oxidized proteins, is also associated with alcohol-induced liver damage. Proteasome inhibition has been associated with increased TNF-mediated hepatocyte death and inflammation [67] and TNF can cause oxidative stress and impair mitochondrial function. Monocytes of alcoholic hepatitis patients produce TNF and have an increased TNF response to endotoxins or lipopolysaccharides (LPS) [115]. Animal models have demonstrated increased LPS stimulated serum TNF levels, while increased Kupffer cell TNF production has been observed in alcohol-fed rats. Inhibition of TNF secretion by antisense oligonucleotides prevented liver injury in ethanol-fed rats [134].
The development of hepatocellular carcinoma has also been associated with alcohol-related liver injury. As noted previously, oxidative stress appears to be important in the development of alcohol-induced liver injury and alcohol-induced liver injury is associated with an increased incidence of hepatocellular carcinoma, although data correlating these two conditions is lacking. Rodents chronically exposed to alcohol produce an ethanol-derived α-hydroxyethyl radical (CH3C.HOH) which may form neoantigens [150], although the role of these neoantigens in the development of carcinoma is unclear. While many studies have documented an association of oxidative stress with alcohol-induced liver injury, and there is clinical evidence of the association between alcohol-induced liver injury and hepatocellular carcinoma, there is little evidence for a mechanism by which alcohol-induced liver injury could lead to hepatocellular carcinoma.
In addition to injury, chronic alcohol exposure is also related to apoptosis of hepatocytes [9, 54], which can be inhibited by the administration of antioxidants. Specifically, mice with chronic ethanol exposure were noted to have an increase in the number of apoptotic bodies, which was depend on the duration of ethanol exposure. After a period of abstinence, this increase in apoptosis was reversed [54]. The increased apoptotic bodies seen involved the parenchymal cells and were often observed with adjacent mononuclear infiltration. This histology was also significant for structural alterations of hepatocytes, mitochondrial pleomorphisms, increases in smooth endoplasmic reticulum and increased lipid deposition.
Viral Hepatitis
Hepatitis B and Hepatitis C virus (HBV/HCV) are common causes of viral hepatitis that result in hepatic inflammation, steatosis, fibrosis, and malignant degeneration. This damage is caused by the virus itself and the subsequent increase in inflammation and oxidative stress. Patients with HCV may experience increased oxidative stress due to activation of NADPH oxidase, increased production of mitochondrial ROS/RNS, decreased antioxidants, iron overload, increased cytokines, and increased Cox-2 and CYP2E1 [15, 27, 55, 126].
Clinically, the serum of HCV patients may show increased ROS [26]. Levels of lipid peroxidation products are increased in serum, white blood cells, and liver specimens in HCV patients. Increased levels of 8-OH-dG in leukocytes are a reliable marker of oxidative stress in patients with chronic HCV infection and this marker was correlated with clinical diagnosis, ferritin levels, and amount of liver steatosis [43]. Mahmood et al. also noted increased levels of 4-hydroxynonenal (HNE) and 8-hydroxyguanosine levels, both of which are measures of oxidative stress, in HCV patients [110]. Serum of HCV patients also contained elevated serum thioredoxin levels that correlate with the severity of disease. Patients who became HCV-RNA negative after 14 days of interferon therapy had lower pretreatment thioredoxin levels that did those who remained positive [154]. These patients were also noted to have increase 8-hydroxydeoxyguanosine, a measure of DNA damage [77].
In HCV, increases in NADPH oxidase generate increased ROS and RNS which may lead to the chronic inflammation seen in these patients [27]. The virus itself, specifically the NS3 protein, can activate NADPH oxidase on membranes and phagosomes leading to increased ROS and thus increased apoptosis and T cell dysfunction [48, 160]. The core protein of hepatitis C itself may also inhibit the electron transport chain, increase ROS, increase mitochondrial permeability, increase intracellular calcium levels, and deplete mitochondrial glutathione stores [1, 85, 86, 120, 126]. The NS5A protein of HCV can also elicit oxidative stress as demonstrated by increased heme oxygenase-1 (HO-1), increased catalase, increased GSH, activation of AP1, and induction of MnSOD [1, 157].
HCV is also strongly associated with the development of hepatocellular carcinoma (HCC). The development and transformation of infection to carcinoma is likely related to oxidative DNA damage from ROS/RNS, specifically from iNOS [111]. Viral hepatitis is associated with an increased iNOS expression [109, 111], with a questionable association with iNOS expression and severity of disease [50, 69, 88]. HBV is able to induce iNOS expression, while iNOS levels are increased in patients infected with HBV compared with levels in patients with other types of hepatic disease [111]. HCV infection can also stimulate the production of NO through activation of the gene for iNOS by the viral core protein and the NS3 protein [109]. HCV patients have increased levels of iNOS, which correlates with high levels of HCV proteins and/or level of disease, as measured by histology [50, 69, 142].
This increased RNS from increased iNOS expression is associated with DNA damage and, since DNA damage is associated with the development of cancer, NO is implicated in the development of hepatocellular carcinoma. In mice, core protein from HCV can increase ROS, increase lipid peroxidation products, and induce antioxidant gene expression. By blocking mitochondrial electron transport, the formation of ROS is inhibited. HCV transgenic mice have an increased sensitivity to oxidative stress, in which increased intrahepatic lipid peroxidation products occurs in response to carbon tetrachloride [126]. In patients with HCC, the combined negative expression of iNOS and COX-2 on histology has a significant impact on patient survival [135]. Again, these studies are suggestive but not conclusive as to the relationship between oxidative stress, viral infection, and the development of hepatocellular carcinoma. Further studies are needed to clarify this correlation.
Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease (NAFLD), also known as non-alcoholic steatohepatitis (NASH), is syndrome of liver injury similar to that found in alcoholics, but occurs in patients who deny alcohol use. Risk factors include obesity, gender (female), diabetes, and hyperlipidemia. Although it was originally considered a benign disorder, the pathological histology of patients with NAFLD reveals the presence of macrovescicular steatosis, lobular inflammation, fibrosis, and occasionally cirrhosis, suggestive of a pathologic process [65, 108].
It is postulated that NAFLS develops because of increased liver fat accumulation, which makes the liver vulnerable to hepatocyte injury. Although the mechanism of NAFLD is not known, oxidative stress appears to play a role via lipid peroxidation, cytokine induction, and Fas ligand induction (Figure 7). Lipid peroxidation causes the release of malondialdehyde (MDA) and 4-hydroxynonenal (HNE), which bind proteins and create neoantigens that can then cause immune reactions, stellate cell activation, and neutrophil chemotaxis [5, 170]. Ikura et al. noted a correlation between oxidized phophatidylcholine, a lipid peroxide that serves as a ligand for scavenger receptors, and disease progression [63].
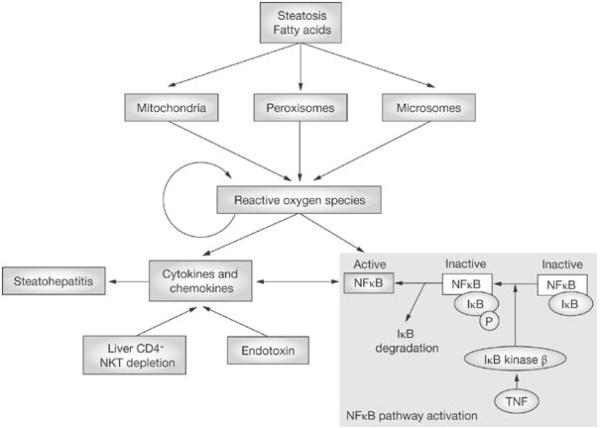
Figure 7.
In non-alcoholic fatty liver disease, the oxidation of free fatty acids increases the production of reactive oxygen species, which, in turn, release cytokines and chemokines, thus causing more inflammation. There is also activation of NF-κB and TNF-α, which again leads to further inflammation. Reprinted by permission from Perlemuter G, Bigorgne A, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 3:458–469,2007. [132]
Exposure of free fatty acids to hepatocytes resulted in the translocation of Bax to lysosomes and subsequent lysosomal destabilization, with release of cathepsin B (ctsb), a lysosomal cysteine protease, into the cytosol. The accumulation of free fatty acids was also associated with NF-κB induced cytokine expression, specifically IL-6 and IL-1β [46] (Figure 7). Patients with NAFLD were also noted to have release of ctsb into the cytoplasm, which correlated with disease severity. In a mouse model of NAFLD, either genetic or pharmacological inactivation of ctsb was protective against the development of hepatic steatosis and liver injury [46].
In addition to lipid peroxidation, oxidative stress may be induced in NAFLD by activation or expression of TNF-α or Fas. Crespo et al. noted that obese patients with NAFLD had increased TNF-α expression and increased TNF-α adipose expression, and that this increased expression correlated with degree of fibrosis [32]. TNFα is a potent proinflammatory cytokine that is capable of inducing ROS/RNS and affecting downstream signaling, resulting in more inflammation. Unfortunately, the data suggest only an association, not a causation, so further investigation is necessary. These patients are also noted to have increased Fas-ligand expression as well as active caspases 3 and 7, suggesting increased apoptosis [45].
Animal studies on obese mice indicated an increased production of ROS, increased expression of NADPH oxidase, and decreased expression of antioxidative enzymes. When these mice were treated with a NADPH oxidase inhibitor, ROS production was reduced[49]. Human patients with NAFLD also have higher levels of ROS, including xanthine oxidase, and lower levels of antioxidants [8]. The resulting increase in oxidative stress may lead to increased lipid peroxidation, increased H2O2 production, and CYP2E1 induction. Patients with NAFLD and increased cytochrome CYP2E1 were noted to have improved levels after gastric bypass surgery and weight loss [40]. NAFLD patients were also noted to have elevated levels of thioredoxin, a thiol-containing antioxidant, and that thioredoxin levels correlated with severity of disease[153], although in general in NAFLD, ROS could deplete antioxidant enzymes including glutathione, vitamin E, and ascorbate, thus increasing hepatic susceptibility to oxidative stress [139, 151].
Conclusion
Part I of this review discussed the role of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in normal physiological function of hepatocytes including oxidative respiration, cell signaling, and protein modification required for normal cellular growth, regeneration, apoptosis, and microsomal defense. However, ROS and RNS can damage any cells in the liver causing inflammation, ischemia, fibrosis, necrosis, apoptosis, or malignant transformation. Here we discussed the pathology of hepatitis as it relates to redox biology in the liver. In Part II of this review, we will discuss the pathology of ischemia/reperfusion injury, fibrosis, iron overload, Wilson's disease, sepsis, and acetaminophen overdose as it relates to redox biology. We will also discuss redox proteomics and the potential of antioxidant therapy in the attenuation of disease progression.
Abbreviations
- AP
apurinic/apyrimidinic
- AP-1
activator protein-1
- APE/Ref-1
apurinic/apyrimidinic endonuclease/redox factor 1
- ATP
adenosine triphosphate
- ALT
aminotransferase
- Bcl-2
B-cell lymphoma-2
- BER
base excision repair
- BH4
tetrahydrobiopterin
- Bid
A BH3 domain-only death agonist protein
- CaM
calmodulin
- CAT
catalase
- cGMP
cyclic guanosine monophosphate
- COX-2
cyclooxygenase-2
- CSF
colony stimulating factor
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- EPO
erythropoietin
- eNOS
endothelial nitric oxide synthase
- ESR
electron spin resonance
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- γ-IRE
γ-interferon response element
- G-CSF
granulocyte colony stimulating factor
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIF
hypoxia-inducible factor
- HO-1
heme oxygenase
- HNE
4-hydroxynonenal
- HSP70
heat shock protein 70
- IL
interleukin
- INFγ
interferon gamma
- im
intramuscular
- iNOS
inducible nitric oxide synthase
- iv
intravenous
- JNK
c-Jun NH2-terminal kinase
- KLF6
a zinc finger molecule
- L-NIL
N-iminoethyl-L-lysine
- L-NMMA
L-N(G)-monomethyl arginine citrate
- L-NNA
L-Nω-nitro-L-arginine
- LPS
lipopolysaccharides
- MAP kinase
mitogen-activated protein kinase
- MAT
methionine adenosyltransferase
- MDA
malondialdehyde
- MDS
myelodysplastic syndrome
- MEOS
microsomal ethanol-oxidizing system
- MMP
matrix metalloproteinases
- MnSOD
manganese-containing superoxide dismutase
- MS
methionine synthase
- NAC
N-acetylcysteine
- NADQI
N-acetyl-p-benzoquinone imine
- NAFLD
non-alcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NOX
nitric oxide scavenger
- nNOS
neuronal nitric oxide synthase
- NADH
reduced nicotinamide adenine dinucleotide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide; reactive halogen species
- NS5A
non-structural 5A protein
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- OLT
orthotopic liver transplant
- PEG-poly SNO-BSA
polyethylene glycol-conjugated bovine serum albumin
- PMN
polymorphonuclear leukocytes
- po
oral
- PT
prothrombin time
- RHS
reactive hydrogen species
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SAMe
S-adenosylmethionine
- sGC
soluble guanylate cyclase
- SMAD
mothers against decapentaplegic
- SOD
superoxide dismutase
- TGFα/β
transforming growth factor α/β
- TIMP1
tissue inhibitor metalloproteinase-1
- TNFα/β
tumor necrosis factor α/β
- TRAIL
TNF related apoptosis inducing ligand
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
- 1400W
N-(3-aminomethyl)benzyl-acetamindine
- 8-OH-dG
8-hydroxydeoxyguanosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005;76:489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- 2.Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- 3.Alam K, Nagi MN, Al-Shabanah OA, Al-Bekairi AM. Beneficial effect of nitric oxide synthase inhibitor on hepatotoxicity induced by allyl alcohol. J Biochem Mol Toxicol. 2001;15:317–321. doi: 10.1002/jbt.10008. [DOI] [PubMed] [Google Scholar]
- 4.Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- 5.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–993. doi: 10.1136/gut.2004.057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albano E, Tomasi A, Goria-Gatti L, Dianzani MU. Spin trapping of free radical species produced during the microsomal metabolism of ethanol. Chem Biol Interact. 1988;65:223–234. doi: 10.1016/0009-2797(88)90108-1. [DOI] [PubMed] [Google Scholar]
- 7.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- 8.Baskol G, Baskol M, Kocer D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin Biochem. 2007;40:776–780. doi: 10.1016/j.clinbiochem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Benedetti A, Brunelli E, Risicato R, Cilluffo T, Jezequel AM, Orlandi F. Subcellular changes and apoptosis induced by ethanol in rat liver. J Hepatol. 1988;6:137–143. doi: 10.1016/s0168-8278(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi GP, Fiorella PL, Bargossi AM, Grossi G, Marchesini G. Reduced ubiquinone plasma levels in patients with liver cirrhosis and in chronic alcoholics. Liver. 1994;14:138–140. doi: 10.1111/j.1600-0676.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 11.Billiar TR, Curran RD, Stuehr DJ, West MA, Bentz BG, Simmons RL. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989;169:1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birlouez-Aragon I, Tessier FJ. Antioxidant vitamins and degenerative pathologies. A review of vitamin C. J Nutr Health Aging. 2003;7:103–109. [PubMed] [Google Scholar]
- 13.Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–241. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- 14.Brown GC. Reversible binding and inhibition of catalase by nitric oxide. Eur J Biochem. 1995;232:188–191. doi: 10.1111/j.1432-1033.1995.tb20798.x. [DOI] [PubMed] [Google Scholar]
- 15.Bureau C, Bernad J, Chaouche N, Orfila C, Beraud M, Gonindard C, Alric L, Vinel JP, Pipy B. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J Biol Chem. 2001;276:23077–23083. doi: 10.1074/jbc.M100698200. [DOI] [PubMed] [Google Scholar]
- 16.Bursch W, Oberhammer F, Jirtle RL, Askari M, Sedivy R, Grasl-Kraupp B, Purchio AF, Schulte-Hermann R. Transforming growth factor-beta 1 as a signal for induction of cell death by apoptosis. Br J Cancer. 1993;67:531–536. doi: 10.1038/bjc.1993.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler AR, Rhodes P. Chemistry, analysis, and biological roles of S-nitrosothiols. Anal Biochem. 1997;249:1–9. doi: 10.1006/abio.1997.2129. [DOI] [PubMed] [Google Scholar]
- 18.Cajone F, Bernelli-Zazzera A. The action of 4-hydroxynonenal on heat shock gene expression in cultured hepatoma cells. Free Radic Res Commun. 1989;7:189–194. doi: 10.3109/10715768909087941. [DOI] [PubMed] [Google Scholar]
- 19.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 20.Cardin R, Saccoccio G, Masutti F, Bellentani S, Farinati F, Tiribelli C. DNA oxidative damage in leukocytes correlates with the severity of HCV-related liver disease: validation in an open population study. J Hepatol. 2001;34:587–592. doi: 10.1016/s0168-8278(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 21.Carnovale CE, Scapini C, Alvarez ML, Favre C, Monti J, Carrillo MC. Nitric oxide release and enhancement of lipid peroxidation in regenerating rat liver. J Hepatol. 2000;32:798–804. doi: 10.1016/s0168-8278(00)80249-4. [DOI] [PubMed] [Google Scholar]
- 22.Cederbaum AI. Ethanol-related cytotoxicity catalyzed by CYP2E1-dependent generation of reactive oxygen intermediates in transduced HepG2 cells. Biofactors. 1998;8:93–96. doi: 10.1002/biof.5520080116. [DOI] [PubMed] [Google Scholar]
- 23.Cesaratto L, Vascotto C, Calligaris S, Tell G. The importance of redox state in liver damage. Ann Hepatol. 2004;3:86–92. [PubMed] [Google Scholar]
- 24.Chamulitrat W, Spitzer JJ. Nitric oxide and liver injury in alcohol-fed rats after lipopolysaccharide administration. Alcohol Clin Exp Res. 1996;20:1065–1070. doi: 10.1111/j.1530-0277.1996.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 25.Chartrain NA, Geller DA, Koty PP, Sitrin NF, Nussler AK, Hoffman EP, Billiar TR, Hutchinson NI, Mudgett JS. Molecular cloning, structure, and chromosomal localization of the human inducible nitric oxide synthase gene. J Biol Chem. 1994;269:6765–6772. [PubMed] [Google Scholar]
- 26.Choi J, Lee KJ, Zheng Y, Yamaga AK, Lai MM, Ou JH. Reactive oxygen species suppress hepatitis C virus RNA replication in human hepatoma cells. Hepatology. 2004;39:81–89. doi: 10.1002/hep.20001. [DOI] [PubMed] [Google Scholar]
- 27.Choi J, Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol. 2006;290:G847–851. doi: 10.1152/ajpgi.00522.2005. [DOI] [PubMed] [Google Scholar]
- 28.Chung KC, Park JH, Kim CH, Ahn YS. Tumor necrosis factor-alpha and phorbol 12-myristate 13-acetate differentially modulate cytotoxic effect of nitric oxide generated by serum deprivation in neuronal PC12 cells. J Neurochem. 1999;72:1482–1488. doi: 10.1046/j.1471-4159.1999.721482.x. [DOI] [PubMed] [Google Scholar]
- 29.Clavien PA. IL-6, a key cytokine in liver regeneration. Hepatology. 1997;25:1294–1296. doi: 10.1002/hep.510250544. [DOI] [PubMed] [Google Scholar]
- 30.Clot P, Tabone M, Arico S, Albano E. Monitoring oxidative damage in patients with liver cirrhosis and different daily alcohol intake. Gut. 1994;35:1637–1643. doi: 10.1136/gut.35.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 32.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, Fernandez-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 33.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 34.Curran RD, Billiar TR, Stuehr DJ, Hofmann K, Simmons RL. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989;170:1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curran RD, Billiar TR, Stuehr DJ, Ochoa JB, Harbrecht BG, Flint SG, Simmons RL. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990;212:462–469. doi: 10.1097/00000658-199010000-00009. discussion 470-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz-Guerra MJ, Velasco M, Martin-Sanz P, Bosca L. Nuclear factor kappaB is required for the transcriptional control of type II NO synthase in regenerating liver. Biochem J. 1997;326(Pt 3):791–797. doi: 10.1042/bj3260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dryden GW, Jr., Deaciuc I, Arteel G, McClain CJ. Clinical implications of oxidative stress and antioxidant therapy. Curr Gastroenterol Rep. 2005;7:308–316. doi: 10.1007/s11894-005-0024-y. [DOI] [PubMed] [Google Scholar]
- 38.Dworkin B, Rosenthal WS, Jankowski RH, Gordon GG, Haldea D. Low blood selenium levels in alcoholics with and without advanced liver disease. Correlations with clinical and nutritional status. Dig Dis Sci. 1985;30:838–844. doi: 10.1007/BF01309514. [DOI] [PubMed] [Google Scholar]
- 39.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 40.Emery MG, Fisher JM, Chien JY, Kharasch ED, Dellinger EP, Kowdley KV, Thummel KE. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology. 2003;38:428–435. doi: 10.1053/jhep.2003.50342. [DOI] [PubMed] [Google Scholar]
- 41.Espey MG, Miranda KM, Thomas DD, Wink DA. Distinction between nitrosating mechanisms within human cells and aqueous solution. J Biol Chem. 2001;276:30085–30091. doi: 10.1074/jbc.M101723200. [DOI] [PubMed] [Google Scholar]
- 42.Espey MG, Thomas DD, Miranda KM, Wink DA. Focusing of nitric oxide mediated nitrosation and oxidative nitrosylation as a consequence of reaction with superoxide. Proc Natl Acad Sci U S A. 2002;99:11127–11132. doi: 10.1073/pnas.152157599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farinati F, Cardin R, Degan P, De Maria N, Floyd RA, Van Thiel DH, Naccarato R. Oxidative DNA damage in circulating leukocytes occurs as an early event in chronic HCV infection. Free Radic Biol Med. 1999;27:1284–1291. doi: 10.1016/s0891-5849(99)00161-6. [DOI] [PubMed] [Google Scholar]
- 44.Fehsel K, Kroncke KD, Meyer KL, Huber H, Wahn V, Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–2865. [PubMed] [Google Scholar]
- 45.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 46.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 47.Fiorucci S, Mencarelli A, Palazzetti B, Del Soldato P, Morelli A, Ignarro LJ. An NO derivative of ursodeoxycholic acid protects against Fas-mediated liver injury by inhibiting caspase activity. Proc Natl Acad Sci U S A. 2001;98:2652–2657. doi: 10.1073/pnas.041603898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forman HJTM, Fukuto J. Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles. Kluwer Academic; Boston: 2003. [Google Scholar]
- 49.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Monzon C, Majano PL, Zubia I, Sanz P, Apolinario A, Moreno-Otero R. Intrahepatic accumulation of nitrotyrosine in chronic viral hepatitis is associated with histological severity of liver disease. J Hepatol. 2000;32:331–338. doi: 10.1016/s0168-8278(00)80080-x. [DOI] [PubMed] [Google Scholar]
- 51.Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC, Simmons RL, Billiar TR. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993;90:522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giuffre A, Sarti P, D'Itri E, Buse G, Soulimane T, Brunori M. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J Biol Chem. 1996;271:33404–33408. doi: 10.1074/jbc.271.52.33404. [DOI] [PubMed] [Google Scholar]
- 53.Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11:1031–1047. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- 54.Goldin RD, Hunt NC, Clark J, Wickramasinghe SN. Apoptotic bodies in a murine model of alcoholic liver disease: reversibility of ethanol-induced changes. J Pathol. 1993;171:73–76. doi: 10.1002/path.1711710115. [DOI] [PubMed] [Google Scholar]
- 55.Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griscavage JM, Hobbs AJ, Ignarro LJ. Negative modulation of nitric oxide synthase by nitric oxide and nitroso compounds. Adv Pharmacol. 1995;34:215–234. doi: 10.1016/s1054-3589(08)61088-1. [DOI] [PubMed] [Google Scholar]
- 57.Gutteridge JM. Iron and oxygen: a biologically damaging mixture. Acta Paediatr Scand Suppl. 1989;361:78–85. doi: 10.1111/apa.1989.78.s361.78. [DOI] [PubMed] [Google Scholar]
- 58.Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 59.Halliwell B, JMC G. Free Radicals in Biology and Medicine. Oxford University Press; oxford: 2007. [Google Scholar]
- 60.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 61.Hortelano S, Dewez B, Genaro AM, Diaz-Guerra MJ, Bosca L. Nitric oxide is released in regenerating liver after partial hepatectomy. Hepatology. 1995;21:776–786. [PubMed] [Google Scholar]
- 62.Hug H, Strand S, Grambihler A, Galle J, Hack V, Stremmel W, Krammer PH, Galle PR. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–28193. doi: 10.1074/jbc.272.45.28191. [DOI] [PubMed] [Google Scholar]
- 63.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S, Kawada N, Arakawa T, Ueda M. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 64.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol. 2000;15:718–724. doi: 10.1046/j.1440-1746.2000.02207.x. [DOI] [PubMed] [Google Scholar]
- 65.James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495–501. doi: 10.1016/s0168-8278(98)80073-1. [DOI] [PubMed] [Google Scholar]
- 66.Jones BE, Lo CR, Liu H, Srinivasan A, Streetz K, Valentino KL, Czaja MJ. Hepatocytes sensitized to tumor necrosis factor-alpha cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. J Biol Chem. 2000;275:705–712. doi: 10.1074/jbc.275.1.705. [DOI] [PubMed] [Google Scholar]
- 67.Joshi-Barve S, Barve SS, Butt W, Klein J, McClain CJ. Inhibition of proteasome function leads to NF-kappaB-independent IL-8 expression in human hepatocytes. Hepatology. 2003;38:1178–1187. doi: 10.1053/jhep.2003.50470. [DOI] [PubMed] [Google Scholar]
- 68.Kamimura S, Gaal K, Britton RS, Bacon BR, Triadafilopoulos G, Tsukamoto H. Increased 4-hydroxynonenal levels in experimental alcoholic liver disease: association of lipid peroxidation with liver fibrogenesis. Hepatology. 1992;16:448–453. doi: 10.1002/hep.1840160225. [DOI] [PubMed] [Google Scholar]
- 69.Kandemir O, Polat A, Kaya A. Inducible nitric oxide synthase expression in chronic viral hepatitis and its relation with histological severity of disease. J Viral Hepat. 2002;9:419–423. doi: 10.1046/j.1365-2893.2002.00382.x. [DOI] [PubMed] [Google Scholar]
- 70.Kang KJ. Mechanism of hepatic ischemia/reperfusion injury and protection against reperfusion injury. Transplant Proc. 2002;34:2659–2661. doi: 10.1016/s0041-1345(02)03465-6. [DOI] [PubMed] [Google Scholar]
- 71.Kessova IG, Ho YS, Thung S, Cederbaum AI. Alcohol-induced liver injury in mice lacking Cu, Zn-superoxide dismutase. Hepatology. 2003;38:1136–1145. doi: 10.1053/jhep.2003.50450. [DOI] [PubMed] [Google Scholar]
- 72.Khatsenko OG, Gross SS, Rifkind AB, Vane JR. Nitric oxide is a mediator of the decrease in cytochrome P450-dependent metabolism caused by immunostimulants. Proc Natl Acad Sci U S A. 1993;90:11147–11151. doi: 10.1073/pnas.90.23.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim YM, Chung HT, Simmons RL, Billiar TR. Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J Biol Chem. 2000;275:10954–10961. doi: 10.1074/jbc.275.15.10954. [DOI] [PubMed] [Google Scholar]
- 74.Kim YM, de Vera ME, Watkins SC, Billiar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- 75.Kim YM, Kim TH, Chung HT, Talanian RV, Yin XM, Billiar TR. Nitric oxide prevents tumor necrosis factor alpha-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology. 2000;32:770–778. doi: 10.1053/jhep.2000.18291. [DOI] [PubMed] [Google Scholar]
- 76.Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 77.Kitada T, Seki S, Iwai S, Yamada T, Sakaguchi H, Wakasa K. In situ detection of oxidative DNA damage, 8-hydroxydeoxyguanosine, in chronic human liver disease. J Hepatol. 2001;35:613–618. doi: 10.1016/s0168-8278(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 78.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 79.Knecht KT, Bradford BU, Mason RP, Thurman RG. In vivo formation of a free radical metabolite of ethanol. Mol Pharmacol. 1990;38:26–30. [PubMed] [Google Scholar]
- 80.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 81.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6:724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- 83.Koop DR, Chernosky A, Brass EP. Identification and induction of cytochrome P450 2E1 in rat Kupffer cells. J Pharmacol Exp Ther. 1991;258:1072–1076. [PubMed] [Google Scholar]
- 84.Koop DR, Tierney DJ. Multiple mechanisms in the regulation of ethanol-inducible cytochrome P450IIE1. Bioessays. 1990;12:429–435. doi: 10.1002/bies.950120906. [DOI] [PubMed] [Google Scholar]
- 85.Korenaga M, Okuda M, Otani K, Wang T, Li Y, Weinman SA. Mitochondrial dysfunction in hepatitis C. J Clin Gastroenterol. 2005;39:S162–166. doi: 10.1097/01.mcg.0000155517.02468.46. [DOI] [PubMed] [Google Scholar]
- 86.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 87.Kurose I, Higuchi H, Yonei Y, Ebinuma H, Watanabe N, Hokari R, Fukumura D, Miura S, Takaishi M, Saito H, Nakatsumi RC, Ishii H. Rat Kupffer cell-derived nitric oxide suppresses proliferation and induces apoptosis of syngeneic hepatoma cells. Gastroenterology. 1996;111:1058–1070. doi: 10.1016/s0016-5085(96)70075-6. [DOI] [PubMed] [Google Scholar]
- 88.Lake-Bakaar G, Sorbi D, Mazzoccoli V. Nitric oxide and chronic HCV and HIV infections. Dig Dis Sci. 2001;46:1072–1076. doi: 10.1023/a:1010770230422. [DOI] [PubMed] [Google Scholar]
- 89.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 90.Laskin DL, Heck DE, Gardner CR, Feder LS, Laskin JD. Distinct patterns of nitric oxide production in hepatic macrophages and endothelial cells following acute exposure of rats to endotoxin. J Leukoc Biol. 1994;56:751–758. doi: 10.1002/jlb.56.6.751. [DOI] [PubMed] [Google Scholar]
- 91.Laskin DL, Rodriguez del Valle M, Heck DE, Hwang SM, Ohnishi ST, Durham SK, Goller NL, Laskin JD. Hepatic nitric oxide production following acute endotoxemia in rats is mediated by increased inducible nitric oxide synthase gene expression. Hepatology. 1995;22:223–234. [PubMed] [Google Scholar]
- 92.Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med. 1982;307:597–601. doi: 10.1056/NEJM198209023071006. [DOI] [PubMed] [Google Scholar]
- 93.Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851–857. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Billiar TR. Nitric Oxide. IV. Determinants of nitric oxide protection and toxicity in liver. Am J Physiol. 1999;276:G1069–1073. doi: 10.1152/ajpgi.1999.276.5.G1069. [DOI] [PubMed] [Google Scholar]
- 95.Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 96.Li J, Bombeck CA, Yang S, Kim YM, Billiar TR. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J Biol Chem. 1999;274:17325–17333. doi: 10.1074/jbc.274.24.17325. [DOI] [PubMed] [Google Scholar]
- 97.Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- 98.Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology. 1994;106:1085–1105. doi: 10.1016/0016-5085(94)90772-2. [DOI] [PubMed] [Google Scholar]
- 99.Lieber CS. Hepatic, metabolic and toxic effects of ethanol: 1991 update. Alcohol Clin Exp Res. 1991;15:573–592. doi: 10.1111/j.1530-0277.1991.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 100.Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997;38:601–628. doi: 10.1016/s1054-3589(08)61001-7. [DOI] [PubMed] [Google Scholar]
- 101.Lieber CS. S-Adenosyl-L-methionine and alcoholic liver disease in animal models: implications for early intervention in human beings. Alcohol. 2002;27:173–177. doi: 10.1016/s0741-8329(02)00230-6. [DOI] [PubMed] [Google Scholar]
- 102.Lieber CS, DeCarli LM. Ethanol oxidation by hepatic microsomes: adaptive increase after ethanol feeding. Science. 1968;162:917–918. doi: 10.1126/science.162.3856.917. [DOI] [PubMed] [Google Scholar]
- 103.Liebler DC, McClure TD. Antioxidant reactions of beta-carotene: identification of carotenoid-radical adducts. Chem Res Toxicol. 1996;9:8–11. doi: 10.1021/tx950151t. [DOI] [PubMed] [Google Scholar]
- 104.Lluis JM, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124:708–724. doi: 10.1053/gast.2003.50089. [DOI] [PubMed] [Google Scholar]
- 105.Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 106.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu SC, Tsukamoto H, Mato JM. Role of abnormal methionine metabolism in alcoholic liver injury. Alcohol. 2002;27:155–162. doi: 10.1016/s0741-8329(02)00226-4. [DOI] [PubMed] [Google Scholar]
- 108.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 109.Machida K, Cheng KT, Sung VM, Lee KJ, Levine AM, Lai MM. Hepatitis C virus infection activates the immunologic (type II) isoform of nitric oxide synthase and thereby enhances DNA damage and mutations of cellular genes. J Virol. 2004;78:8835–8843. doi: 10.1128/JVI.78.16.8835-8843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahmood S, Kawanaka M, Kamei A, Izumi A, Nakata K, Niiyama G, Ikeda H, Hanano S, Suehiro M, Togawa K, Yamada G. Immunohistochemical evaluation of oxidative stress markers in chronic hepatitis C. Antioxid Redox Signal. 2004;6:19–24. doi: 10.1089/152308604771978318. [DOI] [PubMed] [Google Scholar]
- 111.Majano PL, Garcia-Monzon C, Lopez-Cabrera M, Lara-Pezzi E, Fernandez-Ruiz E, Garcia-Iglesias C, Borque MJ, Moreno-Otero R. Inducible nitric oxide synthase expression in chronic viral hepatitis. Evidence for a virus-induced gene upregulation. J Clin Invest. 1998;101:1343–1352. doi: 10.1172/JCI774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 113.Marshall HE, Stamler JS. Nitrosative stress-induced apoptosis through inhibition of NF-kappa B. J Biol Chem. 2002;277:34223–34228. doi: 10.1074/jbc.M201638200. [DOI] [PubMed] [Google Scholar]
- 114.Martin-Sanz P, Hortelano S, Callejas NA, Goren N, Casado M, Zeini M, Bosca L. Nitric oxide in liver inflammation and regeneration. Metab Brain Dis. 2002;17:325–334. doi: 10.1023/a:1021909902310. [DOI] [PubMed] [Google Scholar]
- 115.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 116.McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Arteel GE. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 117.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 118.Morimoto M, Hagbjork AL, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, Albano E, French SW. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol. 1993;10:459–464. doi: 10.1016/0741-8329(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 119.Morimoto M, Hagbjork AL, Wan YJ, Fu PC, Clot P, Albano E, Ingelman-Sundberg M, French SW. Modulation of experimental alcohol-induced liver disease by cytochrome P450 2E1 inhibitors. Hepatology. 1995;21:1610–1617. [PubMed] [Google Scholar]
- 120.Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T, Imai K, Todoroki T, Kimura S, Koike K. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365–4370. [PubMed] [Google Scholar]
- 121.Muller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, Galle PR. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest. 1997;99:403–413. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nanji AA, Mendenhall CL, French SW. Beef fat prevents alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1989;13:15–19. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 123.Niemela O, Parkkila S, Bradford B, Iimuro Y, Pasanen M, Thurman RG. Effect of Kupffer cell inactivation on ethanol-induced protein adducts in the liver. Free Radic Biol Med. 2002;33:350–355. doi: 10.1016/s0891-5849(02)00894-8. [DOI] [PubMed] [Google Scholar]
- 124.Nohl H, Gille L, Kozlov A, Staniek K. Are mitochondria a spontaneous and permanent source of reactive oxygen species? Redox Rep. 2003;8:135–141. doi: 10.1179/135100003225001502. [DOI] [PubMed] [Google Scholar]
- 125.O'Reilly P, Hickman-Davis JM, McArdle P, Young KR, Matalon S. The role of nitric oxide in lung innate immunity: modulation by surfactant protein-A. Mol Cell Biochem. 2002;234–235:39–48. [PubMed] [Google Scholar]
- 126.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 127.Oshita M, Takei Y, Kawano S, Hijioka T, Masuda E, Goto M, Nishimura Y, Nagai H, Iio S, Tsuji S, et al. Endogenous nitric oxide attenuates ethanol-induced perturbation of hepatic circulation in the isolated perfused rat liver. Hepatology. 1994;20:961–965. doi: 10.1002/hep.1840200427. [DOI] [PubMed] [Google Scholar]
- 128.Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135–142. doi: 10.1053/jhep.1997.v26.pm0009214462. [DOI] [PubMed] [Google Scholar]
- 129.Paredes SR, Kozicki PA, Fukuda H, Rossetti MV, Batlle AM. S-adenosyl-L-methionine: its effect on aminolevulinate dehydratase and glutathione in acute ethanol intoxication. Alcohol. 1987;4:81–85. doi: 10.1016/0741-8329(87)90003-6. [DOI] [PubMed] [Google Scholar]
- 130.Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993;194:1044–1050. doi: 10.1006/bbrc.1993.1927. [DOI] [PubMed] [Google Scholar]
- 131.Pennington HL, Hall PM, Wilce PA, Worrall S. Ethanol feeding enhances inflammatory cytokine expression in lipopolysaccharide-induced hepatitis. J Gastroenterol Hepatol. 1997;12:305–313. doi: 10.1111/j.1440-1746.1997.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 132.Perlemuter G, Bigorgne A, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–469. doi: 10.1038/ncpendmet0505. [DOI] [PubMed] [Google Scholar]
- 133.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 134.Ponnappa BC, Israel Y, Aini M, Zhou F, Russ R, Cao QN, Hu Y, Rubin R. Inhibition of tumor necrosis factor alpha secretion and prevention of liver injury in ethanol-fed rats by antisense oligonucleotides. Biochem Pharmacol. 2005;69:569–577. doi: 10.1016/j.bcp.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 135.Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H, Nagasue N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325–1332. [PubMed] [Google Scholar]
- 136.Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci U S A. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roman J, Colell A, Blasco C, Caballeria J, Pares A, Rodes J, Fernandez-Checa JC. Differential role of ethanol and acetaldehyde in the induction of oxidative stress in HEP G2 cells: effect on transcription factors AP-1 and NF-kappaB. Hepatology. 1999;30:1473–1480. doi: 10.1002/hep.510300623. [DOI] [PubMed] [Google Scholar]
- 138.Sakitani K, Nishizawa M, Inoue K, Masu Y, Okumura T, Ito S. Synergistic regulation of inducible nitric oxide synthase gene by CCAAT/enhancer-binding protein beta and nuclear factor-kappaB in hepatocytes. Genes Cells. 1998;3:321–330. doi: 10.1046/j.1365-2443.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 139.Sastre J, Pallardo FV, Llopis J, Furukawa T, Vina JR, Vina J. Glutathione depletion by hyperphagia-induced obesity. Life Sci. 1989;45:183–187. doi: 10.1016/0024-3205(89)90293-2. [DOI] [PubMed] [Google Scholar]
- 140.Schmidt HH, Pollock JS, Nakane M, Gorsky LD, Forstermann U, Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991;88:365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994;204:169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- 142.Schweyer S, Mihm S, Radzun HJ, Hartmann H, Fayyazi A. Liver infiltrating T lymphocytes express interferon gamma and inducible nitric oxide synthase in chronic hepatitis C virus infection. Gut. 2000;46:255–259. doi: 10.1136/gut.46.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sies H. Oxidative stress:introductory remarks. Academic Press; London: 1985. [Google Scholar]
- 144.Situnayake RD, Crump BJ, Thurnham DI, Davies JA, Gearty J, Davis M. Lipid peroxidation and hepatic antioxidants in alcoholic liver disease. Gut. 1990;31:1311–1317. doi: 10.1136/gut.31.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spitzer JA. Cytokine stimulation of nitric oxide formation and differential regulation in hepatocytes and nonparenchymal cells of endotoxemic rats. Hepatology. 1994;19:217–228. [PubMed] [Google Scholar]
- 146.Stadler J, Trockfeld J, Schmalix WA, Brill T, Siewert JR, Greim H, Doehmer J. Inhibition of cytochromes P4501A by nitric oxide. Proc Natl Acad Sci U S A. 1994;91:3559–3563. doi: 10.1073/pnas.91.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stadtman ER. NHLBI Research at the Bethesda Campus: Enzyme Section. [Google Scholar]
- 148.Stal P, Johansson I, Ingelman-Sundberg M, Hagen K, Hultcrantz R. Hepatotoxicity induced by iron overload and alcohol. Studies on the role of chelatable iron, cytochrome P450 2E1 and lipid peroxidation. J Hepatol. 1996;25:538–546. doi: 10.1016/s0168-8278(96)80214-5. [DOI] [PubMed] [Google Scholar]
- 149.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 150.Stewart SF, Vidali M, Day CP, Albano E, Jones DE. Oxidative stress as a trigger for cellular immune responses in patients with alcoholic liver disease. Hepatology. 2004;39:197–203. doi: 10.1002/hep.20021. [DOI] [PubMed] [Google Scholar]
- 151.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733. [PubMed] [Google Scholar]
- 152.Sumbayev VV, Budde A, Zhou J, Brune B. HIF-1 alpha protein as a target for S-nitrosation. FEBS Lett. 2003;535:106–112. doi: 10.1016/s0014-5793(02)03887-5. [DOI] [PubMed] [Google Scholar]
- 153.Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, Nakajima Y, Ishikawa H, Mitsuyoshi H, Okanoue T, Kashima K, Nakamura H, Yodoi J. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32–38. doi: 10.1016/s0168-8278(02)00331-8. [DOI] [PubMed] [Google Scholar]
- 154.Sumida Y, Nakashima T, Yoh T, Nakajima Y, Ishikawa H, Mitsuyoshi H, Sakamoto Y, Okanoue T, Kashima K, Nakamura H, Yodoi J. Serum thioredoxin levels as an indicator of oxidative stress in patients with hepatitis C virus infection. J Hepatol. 2000;33:616–622. doi: 10.1034/j.1600-0641.2000.033004616.x. [DOI] [PubMed] [Google Scholar]
- 155.Sun X, Majumder P, Shioya H, Wu F, Kumar S, Weichselbaum R, Kharbanda S, Kufe D. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem. 2000;275:17237–17240. doi: 10.1074/jbc.C000099200. [DOI] [PubMed] [Google Scholar]
- 156.Sutton HC, Winterbourn CC. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic Biol Med. 1989;6:53–60. doi: 10.1016/0891-5849(89)90160-3. [DOI] [PubMed] [Google Scholar]
- 157.Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005;13:159–163. doi: 10.1016/j.tim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 158.Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc) 1998;63:766–781. [PubMed] [Google Scholar]
- 159.Taylor BS, Geller DA. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock. 2000;13:413–424. doi: 10.1097/00024382-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 160.Thoren F, Romero A, Lindh M, Dahlgren C, Hellstrand K. A hepatitis C virus-encoded, nonstructural protein (NS3) triggers dysfunction and apoptosis in lymphocytes: role of NADPH oxidase-derived oxygen radicals. J Leukoc Biol. 2004;76:1180–1186. doi: 10.1189/jlb.0704387. [DOI] [PubMed] [Google Scholar]
- 161.Thurnham DI, Davies JA, Crump BJ, Situnayake RD, Davis M. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Ann Clin Biochem. 1986;23(Pt 5):514–520. doi: 10.1177/000456328602300505. [DOI] [PubMed] [Google Scholar]
- 162.Traber MG. Determinants of plasma vitamin E concentrations. Free Radic Biol Med. 1994;16:229–239. doi: 10.1016/0891-5849(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 163.Tsutsumi M, Lasker JM, Takahashi T, Lieber CS. In vivo induction of hepatic P4502E1 by ethanol: role of increased enzyme synthesis. Arch Biochem Biophys. 1993;304:209–218. doi: 10.1006/abbi.1993.1341. [DOI] [PubMed] [Google Scholar]
- 164.Tsutsumi M, Takada A, Wang JS. Genetic polymorphisms of cytochrome P4502E1 related to the development of alcoholic liver disease. Gastroenterology. 1994;107:1430–1435. doi: 10.1016/0016-5085(94)90546-0. [DOI] [PubMed] [Google Scholar]
- 165.Tzeng E, Billiar TR, Williams DL, Li J, Lizonova A, Kovesdi I, Kim YM. Adenovirus-mediated inducible nitric oxide synthase gene transfer inhibits hepatocyte apoptosis. Surgery. 1998;124:278–283. [PubMed] [Google Scholar]
- 166.Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 2000;60:5862–5869. [PubMed] [Google Scholar]
- 167.Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, Dikalova A, Kadiiska M, Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 168.Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993;300:115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 169.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O, Autelli R, Colombatto S, Dianzani MU, Pinzani M, Parola M. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 171.Zima T, Fialova L, Mestek O, Janebova M, Crkovska J, Malbohan I, Stipek S, Mikulikova L, Popov P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J Biomed Sci. 2001;8:59–70. doi: 10.1007/BF02255972. [DOI] [PubMed] [Google Scholar]