Abstract
Objective
To test the hypothesis that mu-opioid receptor signaling in the nucleus accumbens contributes to hedonic (over)eating and obesity. To investigate the effects of chronic mu-opioid antagonism in the nucleus accumbens core or shell on intake of a palatable diet, and the development of diet-induced obesity in rats.
Methods and Design
Chronic blockade of mu-opioid receptor-signaling in the nucleus accumbens core or shell was achieved by means of repeated injections (every 4–5 days) of the irreversible receptor antagonist β-Funaltrexamine (BFNA) over 3–5 weeks. The diet consisted of either a choice of high-fat chow, chocolate-flavored Ensure, and regular chow (each nutritionally complete), or regular chow only. Intake of each food item, body weight, and body fat mass were monitored throughout the study.
Results
BFNA injections aimed at either the core or shell of the nucleus accumbens resulted in significantly attenuated intake of palatable diet, body weight gain, and fat accretion, compared with vehicle control injections. BFNA in the core did not significantly change these parameters in chow-fed control rats. BFNA in the core and shell differentially affected intake of the two palatable food items: in the core BFNA significantly reduced intake of high-fat, but not of Ensure, whereas in the shell, it significantly reduced intake of Ensure, but not of high-fat, compared with vehicle-treatment.
Conclusions
Endogenous mu-opioid receptor-signaling in the nucleus accumbens core and shell is necessary for palatable diet-induced hyperphagia and obesity to fully develop in rats. Sweet and non-sweet fatty foods may be differentially processed in subcomponents of the ventral striatum.
Keywords: High-fat chow, chocolate Ensure, nucleus accumbens core and shell, fat mass, body weight, hedonics, reward
Introduction
A combination of increased availability of tasty, energy dense foods and sedentary behavior is thought to be the major drive in the rapidly increasing prevalence of overweight and obesity in children and adults (1, 2). In genetically prone individuals, this “toxic” environment can lead to overeating, obesity, diabetes, and a number of other diseases of the metabolic syndrome (3). Regulation of appetite appears to be central, as most of the known genes associated with human obesity are involved in the control of food intake (4, 5). Thus, understanding the neural mechanisms of ingestive behavior is crucial for symptomatic treatments of this modern disease.
Eating is normally driven by metabolic need, but reinforcement by its acute pleasurable experience and the subsequent state of satisfaction makes it prone to abuse. The rewarding experience associated with eating can lead to eating in the absence of metabolic need, and has been suggested to be a form of addiction (6–11).
The neural pathways and processes that allow reward-driven eating to override homeostatic controls is not well understood, but likely involve a subconscious component making it partially resistant to voluntary control. Given the key roles of cortico-limbic structures in reward-driven eating and the hypothalamus in homeostatic eating (12, 13), interactions between these two systems are likely candidates. Chemical stimulation of the nucleus accumbens has been used as a model to elicit robust, satiation-resistant eating in rats (14–17). In particular, stimulation with the mu-opioid receptor agonist DAMGO induces voracious intake of palatable high-fat diet or sucrose, even in pre-satiated rodents (15–18). Although a number of brain areas are activated by opioid-induced accumbens stimulation (16, 19), we have previously shown that activation of orexin neurons in the lateral hypothalamus and subsequent orexin-signaling in the ventral tegmental area is crucial for the expression of the behavioral effects(20). Anatomical studies have also clearly demonstrated direct and indirect projections from the nucleus accumbens to the lateral hypothalamus (21–24). Although these findings demonstrate that a mu-opioid receptor-dependent, accumbens-hypothalamic pathway can acutely overpower satiation signals, its potential role in contributing to chronically reward-driven overeating and the development of diet-induced obesity is not known.
We hypothesized that chronic suppression of mu-opioid receptor signaling in the nucleus accumbens should lead to an attenuation of palatable food intake and development of obesity. β-funaltrexamine (BFNA) is a mixed short-term κ-opioid receptor agonist and irreversible mu-opioid receptor antagonist (25, 26), which leads to long-lasting uncoupling of the mu-opioid receptor from its G-protein (25, 27, 28). A number of previous studies demonstrated effects of this antagonist injected either into the cerebral ventricles (29–31) or directly into the nucleus accumbens (15, 27, 32–34) on food intake. While most of these studies examined acute food intake, chronic lateral ventricular administration of BFNA for eleven days reduced food intake and body weight in rats on a palatable diet (30) and in lean and obese Zucker rats for seven days (31). Furthermore, administration of BFNA directly into the nucleus accumbens selectively decreased sucrose but not chow consumption in rabbits for 4 days (27). Therefore, in the absence of anatomically-selective mu-opioid receptor deletion techniques, repeated local microinjection of BFNA may be a reasonably selective tool to chronically suppress mu-opioid signaling in the nucleus accumbens for more thoroughly testing its role in the development of diet-induced obesity.
The aim of our study was to assess the effects of repeated BFNA administration over several weeks to the nucleus accumbens core or shell on food intake, body weight gain, and changes in body composition. To mimic the Western diet, we offered Sprague-Dawley rats a three-choice diet consisting of the nutritionally complete and medium-sweet liquid food Ensure, high-fat chow (60% of energy from fat), and regular chow.
Research Design and Methods
Animals
Male adult Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually in hanging stainless steel mesh cages at 22±2°C with lights on at 0700h and lights off at 1900h. Food and water were available ad libitum except where noted in Experimental protocol and measurement of food intake below. All experimental protocols were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee and in compliance with NIH and USDA regulations.
Brain cannulation and DAMGO injections
The animals weighed ~300 to 370 g at the time of surgery and after pretreatment with atropine (1 mg/kg, i.p.), were anesthetized with ketamine-acepromazine-xylazine cocktail (80/1.6/5.4 mg/kg, s.c.). Bilateral cannulas (24 gauge) were aimed such that injections would be made into the nucleus accumbens core (from Bregma: AP ± 1.4, LM ± 1.8, DV −5.3; n = 28) or shell (AP ± 1.4, LM ± 1.0, DV −5.3; n = 16) using a stereotaxic apparatus and flat skull techniques and secured to the skull using dental cement and three screws. Postoperative care included fluid replacement (saline, 5–10 ml, i.p.) and analgesic (carprofen, 5 mg/kg, i.p.).
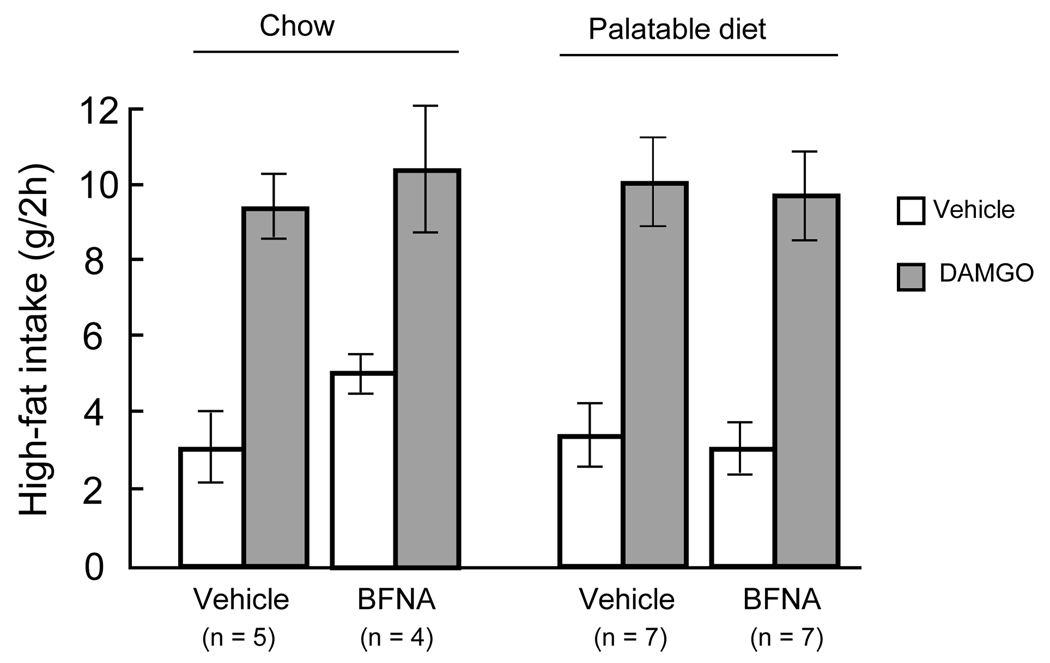
Rats were allowed to recover from the surgery for ten days, during which they were maintained on chow, but familiarized to the taste of chocolate Ensure (Abott Nutrition, Columbus, OH) and high-fat diet (D12492; Research Diets, New Brunswick, NJ), and screened for their response to DAMGO. Rats were adapted to the injection procedure by 2 mock injections. After a 1-h pre-exposure to high-fat diet, DAMGO (D-Ala2, N-Me-Phe4, Gly-ol5-enkephalin; 250 ng/0.5 ul), or sterile saline was injected unilaterally on separate days using 31-gauge injectors extending 2.5 mm past the cannula tip, and further intake of high-fat diet was measured for 2-h after injections. Twenty-three of twenty-eight rats showed increased high-fat intake of at least 3 g after DAMGO injection compared with vehicle injection and were assigned to the 4 groups as described below. Mean DAMGO-induced high-fat intake was significantly higher than after vehicle and was similar for the 4 treatment groups (Fig. 6).
Fig. 6.
Functional verification of accumbens core injection sites. Two-hour high-fat intake of rats with bilateral cannulas aimed at either the core or shell of the nucleus accumbens and assigned to either chronic vehicle or BFNA-treatment. Intake for left and right cannula injections was tested separately and the mean intake from both sides was used for each rat. BFNA stimulated high-fat intake to a similar extent in all 4 groups.
Experiment 1: BFNA injections into the nucleus accumbens core
After the recovery and adaptation period, rats exhibiting a significant ingestive response to DAMGO were randomly assigned to 4 groups. Two groups were continuously fed chow diet, one receiving injections of the mu opioid receptor antagonist β-funaltrexamine (BFNA, 10 nmol/0.5 ul on each side; Tocris, Ellisville, MO; n = 4), and one receiving vehicle (50% DMSO in saline; n = 5), every 4–5 days, for 36 days (8 injections plus 2 behavioral screening injections). The interval of 4–5 days between BFNA injections was based on in vitro (26) and in vivo studies (27) demonstrating that the mu opioid receptor antagonist activity lasts for 3–5 days.
The two other groups were switched to a three-choice diet consisting of standard chow (58% of energy from carbohydrates, 13.5% fat, 28.5% protein; # 5001 LabDiet, Purina, Richmond, IN) high-fat diet (20% carb, 60% fat, 20% prot; D12492, research Diets, New Brunswick, NJ), provided in separate food hoppers, and chocolate-flavored Ensure (64% carb, 21.6% fat, 14.4% prot; Abbott Ross, Columbus, OH) provided in a bottle, and received either BFNA (n = 7) or its vehicle (n = 7), as above. All injections were performed over 1 min per side using a 1-µl Hamilton syringe connected to PE-20 tubing and 30- to 31-gauge injectors; the injectors were left in place for 1 min to minimize backflow. This dose of BFNA has been previously shown to completely block DAMGO-induced chow intake in the nucleus accumbens (33).
Body weight as well as intake of chow, high-fat diet, and Ensure were measured daily and corrected for spillage. Fresh Ensure was provided daily in clean bottles and spouts to prevent bacterial contamination and thickening.
Body composition (fat, lean, and fluid mass) was measured weekly using nuclear magnetic resonance (NMR) analysis (Bruker Minispec LF90 Time Domain NMR Analyzer; Bruker Optics, Billerica, MA). Unanesthetized rats were placed into a restraining tube and measurements were made in triplicate over a period of 3 minutes. In addition, we also harvested and weighed the inguinal, epididymal, retroperitoneal, and visceral fat pads after euthanasia.
Experiment 2: BFNA injections into the nucleus accumbens shell
To avoid unnecessary tissue damage at the injection site before BFNA administration, the behavioral screening injections with saline and DAMGO were omitted. Furthermore, no chow-fed control groups were run, and rats with cannulas aimed at the nucleus accumbens shell were randomly assigned to 2 groups only, both exposed to the 3-choice diet after the start of either BFNA (n = 6) or vehicle (n = 7) injections. The experiment was terminated after 22 days (5 injections), when it was clear that no further separation of the body weight curves occurred. All other procedures and measures were the same as in Experiment 1.
Verification of injection sites
At the end of experiments, blue dye (0.5µl of 1% Chicago Blue) was injected through each cannula before euthanasia and perfusion. Brains were extracted and 30 µm-thick frontal sections were examined under the microscope. Injection sites were mapped on the nearest plates from the Paxinos and Watson rat stereotaxic atlas (35).
Statistical analysis
Body weight, fat mass, and food intake were analyzed using one-way or two-way ANOVA with repeated measures with adjusted Bonferroni or Newman-Keuls posthoc tests. Differences in fat pad weights between the 4 groups in Experiment 1 were analyzed using two-way ANOVA followed by Newman-Keuls posthoc tests and for the 2 groups in Experiment 2 with 2-tailed Student's t-tests. Significance was set at p<0.05. To explicitly compare the effects of BFNA-treatment in the core vs. shell on intake of high-fat diet and Ensure, percent suppression of intake of either high-fat diet or Ensure based on the mean intake of the same diet with vehicle-treatment was calculated and subjected to ANOVA.
Results
BFNA reduces body weight and fat mass gain more on the palatable than on the regular chow diet
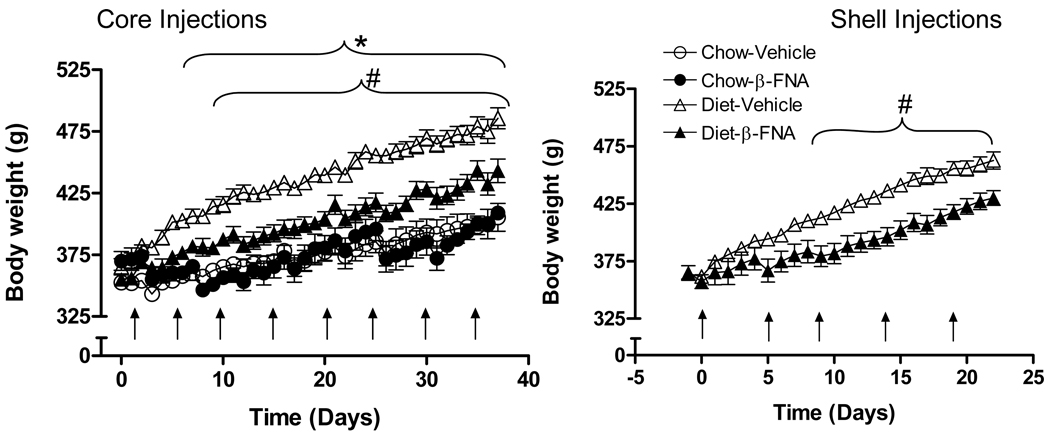
As expected, rats exposed to the palatable diet gained significantly more weight and fat mass compared to chow control. In Experiment 1 (core injections), saline-injected control rats gained 114 g body weight during 37 days on the palatable diet, compared with a gain of 45 g body weight on chow (p<0.01). Body weight was significantly higher starting on day 5 in vehicle-treated palatable-diet fed rats compared to chow-fed rats (p < 0.05, Fig.1). Although no chow group was run in the slightly shorter Experiment 2 (shell injections), similar diet-induced weight (+ 97 g) gain was observed over the 23 day experimental period (Fig. 1).
Fig. 1.
Nucleus accumbens administration of β-funaltrexamine (BFNA) attenuates palatable diet-induced body weight gain. Separate groups of rats (n = 4–7/group) received multiple injections of BFNA (10 nmol/side) or vehicle (arrows) into the nucleus accumbens core (left panel) or shell (right panel) and were fed either a three-choice palatable diet consisting of liquid Ensure, high-fat chow, and regular chow (diet), or just regular chow (core injections only). Compared to vehicle control injections, BFNA significantly attenuated palatable diet-induced body weight gain starting at day 10 with core injections and at day 9 with shell injections (# p<0.05, based on ANOVA and Bonferroni-adjusted multiple posthoc comparisons). Core injections of BFNA did not change body weight gain on regular chow diet. Body weight gain was significantly increased by the palatable diet compared to chow diet (* p<0.05).
Palatable diet-induced weight gain was significantly attenuated but not abolished by repeated injections of the mu-opioid receptor antagonist BFNA into the core (Fig. 1, Fig. 2AB) or shell Fig. 1, Fig. 2CD) of the nucleus accumbens. In Experiment 1 (core injections), BFNA-treated rats on the palatable diet gained 88 g body weight compared to 114 g with vehicle (−26 g). On chow, BFNA rats gained 30 g compared with 45 g with vehicle (−15 g). Two-way ANOVA revealed a significant treatment effect (p = 0.014) and a significant interaction between diet and treatment (p = 0.016), suggesting that BFNA reduced body weight on the palatable diet more than on the standard chow diet. Furthermore, Bonferroni-adjusted multiple comparisons revealed significant differences in body weight between BFNA and vehicle-treated rats on the palatable diet beginning on day 10 (p < 0.05), but not on the chow diet (Fig. 1).
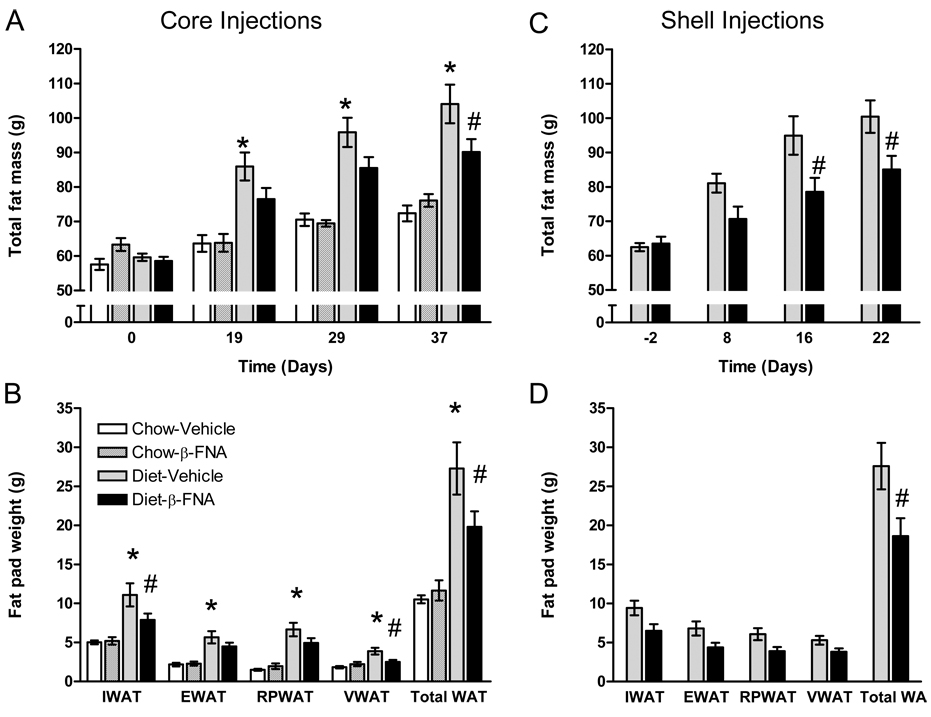
Fig. 2.
Nucleus accumbens administration of BFNA attenuates palatable diet-induced fat mass gain. Compared to vehicle control injections, BFNA (for details of injections see legend to Fig. 1) attenuated palatable food-induced gain in total fat mass (as measured with NMR; A, C) and reduced total fat pad weights (B, D) with both core and shell injections (# p<0.05). Core injections of BFNA did not change fat mass gain and fat pad weights on regular chow diet. However, fat mass and fat pad weights were significantly increased by the palatable diet compared to chow diet (* p<0.05). IWAT, inguinal; EWAT, epididymal; RPWAT, retroperitoneal; VWAT, visceral abdominal; Total WAT=total white adipose tissue.
In Experiment 2 (shell injections), one-way repeated measures ANOVA for weight gain revealed significant treatment (p < 0.01) and time (p < 0.01) effects and follow-up multiple comparisons showed significant differences in body weight between BFNA and vehicle-treated rats starting on day 9 (Fig. 1).
Fat mass measured in vivo by whole body NMR relaxometry showed that in Experiment 1 (core injections), saline-injected control rats gained 45 g fat mass during 37 days on the palatable diet, compared with a gain of 14 g on chow (p < 0.05; Fig. 2). Fat mass was significantly higher starting on day 19 in vehicle-treated palatable diet-fed rats compared to chow-fed rats (p < 0.05, Fig. 2). A similar diet-induced fat mass gain (+ 38 g) was observed over the 23 day experimental period in Experiment 2 (shell injections). In addition, measurements of individual fat pads at the end of the experiments confirm and extend whole body NMR results. Inguinal, epididymal, retroperitoneal and total fat pad weights were doubled in vehicle-treated palatable diet-fed rats compared to chow-fed rats (Fig. 2C).
Similarly to body weight, palatable diet-induced fat mass gain was significantly attenuated but not abolished by repeated injections of the mu-opioid receptor antagonist BFNA into the core or shell of the nucleus accumbens (Fig. 2). In Experiment 1 (core injections), BFNA-treated rats on the palatable diet gained 32 g fat mass compared to 45 g with vehicle (−13 g). On chow, BFNA rats gained 13 g compared with 14 g with vehicle (−1 g). Although the treatment effect revealed by ANOVA was not significant (p = 0.2), posthoc comparisons revealed that total fat mass and total fat pad weight in rats on the palatable diet injected with BFNA in the nucleus accumbens core was significantly lower than those of animals injected with vehicle (p < 0.05) (Figure 2A,B). BFNA effects were significant in inguinal and visceral fat pads but did not reach significance in the other fat pads.
In Experiment 2 (shell injections), BFNA-treated rats on the palatable diet gained 22 g fat mass compared to 38 g with vehicle (−17 g). Repeated measures ANOVA revealed significant effects of treatment (p < 0.01) and time (p < 0.01), and direct posthoc comparisons showed that total fat mass was significantly reduced by BFNA after 16 days, and both total fat mass and total fat pad weight were significantly reduced (p<0.05) at the termination of the experiment (Figure 2C,D).
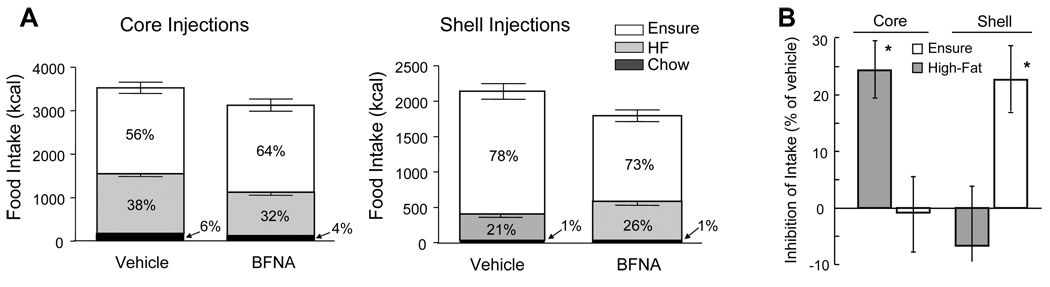
β-FNA reduces total energy intake on high-fat but not regular chow diet
All rats consumed more energy from Ensure than from high-fat diet and less than 6% of energy from chow, regardless of treatment (Fig. 3, Fig. 4). Preference for Ensure was somewhat higher in Experiment 2, regardless of treatment.
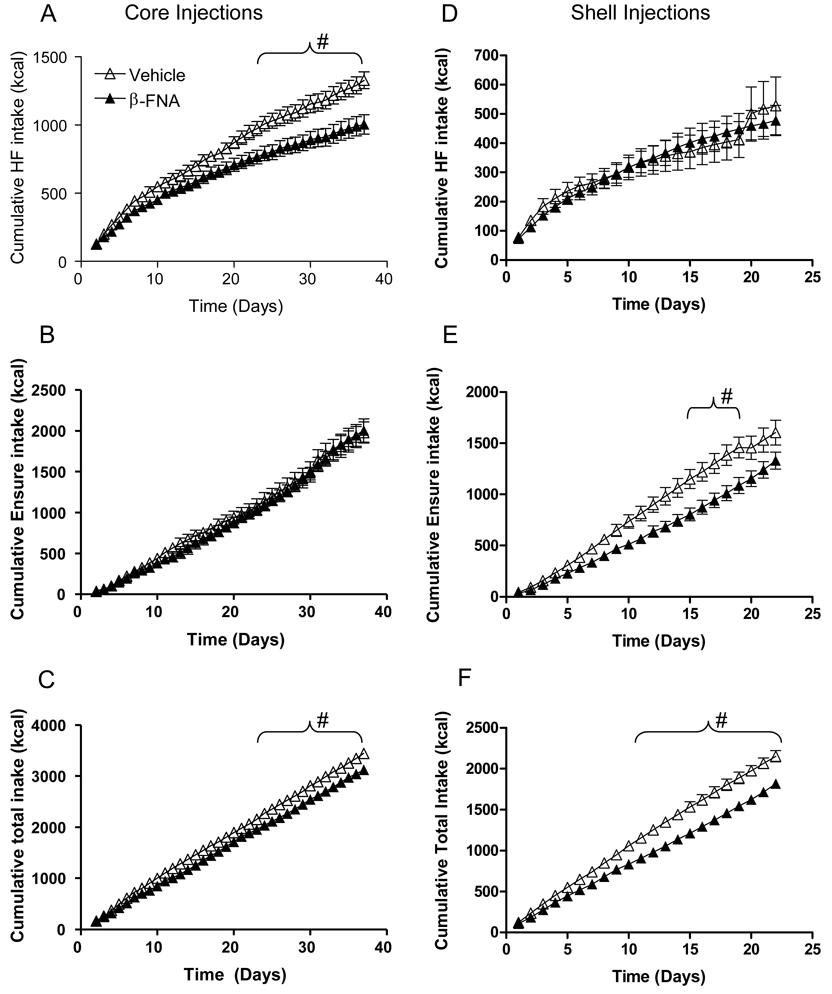
Fig. 3.
Nucleus accumbens administration of BFNA attenuates palatable food intake. Multiple core injections of BFNA (for details of injections see legend to Fig. 1) significantly reduce intake of high-fat diet but not Ensure (A, B), while shell injections reduce intake of Ensure but not high-fat diet (D, E) (# p<0.05, based on repeated measures ANOVA followed by Bonferroni-adjusted multiple comparisons). Total energy intake from all dietary components was also significantly reduced with BFNA injections into either core (C) or shell (F), compared with vehicle control injections.
Fig. 4.
Nucleus accumbens core and shell administration of BFNA differentially affects high-fat and Ensure intake. A: Percentage of total calorie intake from Ensure (white bars), high-fat chow (gray bars), and regular chow (black bars) for vehicle and BFNA treated rats with accumbens core and shell injections. B: Percent inhibition of Ensure and high-fat diet intake by BFNA in the core and shell. * p < 0.05 between suppression of ensure and high-fat intake.
With core injections, total cumulative energy intake on the 3-choice palatable diet was significantly lower in BFNA-treated rats compared with saline-treated rats (−10%, p < 0.05), while cumulative energy intake of the chow-fed groups was unchanged by BFNA (−2%, n.s.; Fig. 3, Fig. 4). Direct posthoc comparisons showed significant BFNA-induced decreases in total palatable diet intake starting on day 22 (Fig. 3).
With shell injections, BFNA also significantly reduced total cumulative palatable diet intake (−16%, p < 0.05) (Fig. 3, Fig. 4). Direct posthoc comparisons showed significant BFNA-induced decreases in total palatable diet intake starting on day 10 (Fig. 3F).
BFNA in the shell and core differentially affect intake of Ensure and high-fat diet
When the individual components of the three-choice palatable diet are examined, we observed differential effects of BFNA in the nucleus accumbens core versus the shell. BFNA injected into the core significantly reduced intake of high-fat diet, but not of Ensure, whereas BFNA injected into the shell significantly reduced intake of Ensure, but not of high-fat diet when compared to the respective vehicle control groups. Using separate repeated measure ANOVAs showed that cumulative intake of high-fat diet was significantly lower (P < 0.05) with BFNA-treatment of the core compared with vehicle-treatment for days 23–37, and that cumulative intake of Ensure was significantly lower (P < 0.05) with BFNA-treatment of the shell for days 15–19 (Fig. 3). Furthermore, explicit comparison of core and shell using percent suppression of intake of either high-fat or Ensure based on the mean intake of the same diet with vehicle-treatment yielded a significant interaction between diet and injection site (F1,25 = 12.85; P < 0.002), and significant effects of diet within core (t = 2.45; p = 0.023) and diet within shell (t = 2.61; p = 0.016; Fig. 4).
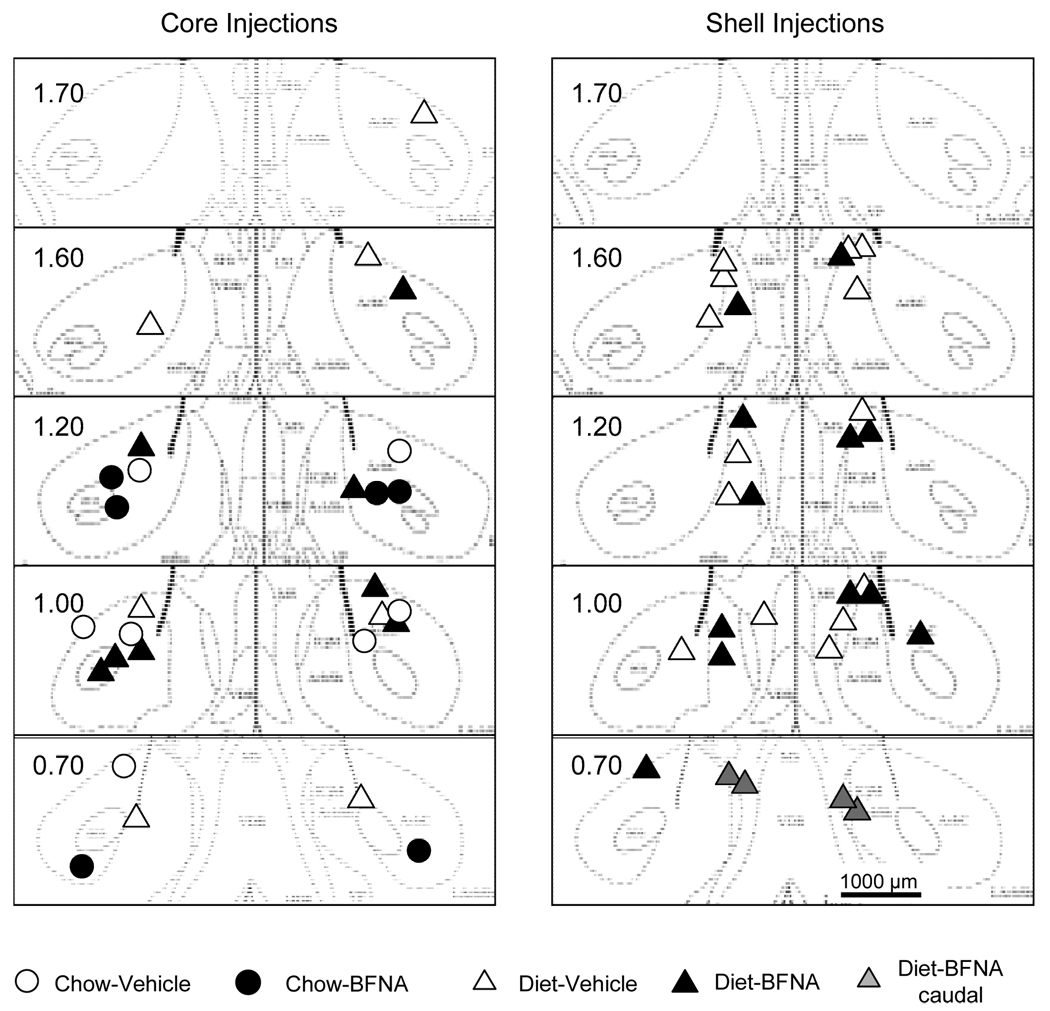
Nucleus accumbens core and shell injection sites
Because of problems with tissue processing, not all core injection sites were histologically verified. The sites successfully verified showed the expected scatter around the intended location either 1000 µm (shell) or 1800 µm (core) lateral to the midline (Fig. 5). Although some sites were up to 400 µm off the intended location, the 500 nl volume of drug injected [occupying a pore space of > 1,200 µm diameter (36)] was more than enough to affect the intended location. This is also supported by the functional verification with DAMGO injections before the chronic BFNA/vehicle treatments. Rats were only assigned to the treatment groups if their average intake with left and right DAMGO-administration was at least 3 g higher than after vehicle administration (Fig. 6).
Fig. 5.
Histological verification of nucleus accumbens injection sites. Location of injection sites aimed at the nucleus accumbens core (left panel) and shell (right panel) superimposed on images from a stereotaxic atlas (35). Note that rats with shell injections were only tested with palatable food. Two rats with bilateral injection cannulas located in the caudal shell (gray triangles) and injected with BFNA did not show reduced body weight and food intake and were not included in the analysis.
To assure that the effects of BFNA injected into the core were similar for the rats with histologically verified and non-verified cannula placements, we analyzed all dependent variables for only the rats with histologically verified cannula placements. This analysis showed very similar effects for body weight gain, fat mass gain, total food intake, and differential Ensure vs. high-fat intake. Furthermore, in the functional verification test, the average DAMGO-induced high-fat intake was not different for the histologically verified and non-verified rats.
Two rats with injections in the most caudal portion of the nucleus accumbens (Figure 5) were excluded from analysis. They both received BFNA treatment, but behaved like saline-treated rats in all measures.
Discussion
The nucleus accumbens is recognized as a key brain area involved in reward processing, and here we show that chronic suppression of endogenous mu-opioid receptor signaling in the nucleus accumbens significantly attenuates development of diet-induced obesity by reducing intake of palatable preferred foods in Sprague-Dawley rats. Because BFNA has some short-term κ-agonist activity (25, 26, 29), we cannot rule out co-involvement of κ-opioid receptor signaling in the observed effects. However, the continuous mu-opioid receptor antagonism is likely to play the dominant role, with only minor effects of the brief κ-agonist stimulation every 4–5 days.
The findings suggest that mu-opioid receptor signaling in the nucleus accumbens significantly contributes to the hyperphagia, body weight gain, and body fat accretion observed when rodents are exposed to a palatable cafeteria diet. We chose two food items that are both very palatable to rats but also very different. High-fat chow is a creamy, non-sweet solid food, while Ensure is a medium sweet (8% sugar) creamy liquid. On average, Ensure was slightly preferred over the high-fat chow, and both were highly preferred over regular laboratory chow (Fig. 4A). Thus, the 3-choice diet is more closely mimicking human food intake than a single item diet.
However, the relatively mild hypophagia and only partial prevention of the obesogenic effect of the palatable diet clearly show that this is only one mechanism contributing to diet-induced obesity. Contributions of mu-opioid receptor-mediated mechanisms in other brain areas as well as non-mu-opioid receptor-mediated mechanisms in the nucleus accumbens and other brain areas are likely also involved. There is an extensive literature on opioids and food intake, but most studies address acute rather than chronic effects. We will first discuss some of these relevant earlier observations before focusing on the differential effects with core and shell injections, and the effects on body weight and fat mass.
Opioids, food intake, and obesity
Systemic administration of opioid agonists and antagonists increases and decreases food intake respectively, suggesting that the opioid system plays a role in the regulation of ingestive behavior. For example, repeated systemic injections of the prototypical mu opioid receptor agonist, morphine, increases food intake (37), whereas systemic injection of the mu-opioid receptor antagonist BFNA (38) and the nonselective opioid receptor antagonist naltrexone (39) reduce food intake [for a recent review, see (40)]. In addition, daily oral treatment with a non-selective opioid receptor antagonist [LY255582], reduced food intake throughout the 16-week treatment period, and pair-feeding recapitulated much of the decreased body weight and adiposity seen in drug-treated rats (41).
Opioid receptors expressed in several brain regions [nucleus of the solitary tract (42), parabrachial nucleus (43), arcuate nucleus (44, 45), paraventricular nucleus of the hypothalamus (46), and nucleus accumbens (47, 48)] likely mediate the effects of systemically administered opioids.
Surprisingly, the mu-opioid receptor deficient mouse shows only a subtle, and sexually dimorphic, phenotype regarding food intake and body weight/adiposity. Hypophagia is not or only variably observed, but when challenged with palatable high-fat diets, knockout mice do resist development of obesity compared to wildtype controls (49, 50). These findings suggest that mu-opioid receptor signaling, perhaps in peripheral organs, might also have suppressive effects on food intake and/or stimulatory effects on fat oxidation and energy expenditure, and demonstrate the need for more localized manipulations of mu-opioid receptor signaling.
Chronic BFNA-treatment of the nucleus accumbens delays development of diet-induced obesity
BFNA-treatment centered around the core or shell of the nucleus accumbens significantly attenuated body weight gain and body fat accretion on the palatable diet. In contrast, BFNA-treatment of the core had a smaller and non-significant effect on body weight gain and no effect on fat mass gain in chow-fed control groups, consistent with the many reports demonstrating more or less selective modulation of “hedonic” eating by opioids [e.g. (18, 38, 51), and see (52) for a review]. We based our study on an earlier observation in rabbits, showing that a single injection of BFNA into the nucleus accumbens shell uncoupled the mu-opioid receptor from its G-protein and selectively decreased daily 4-h sucrose but not 20-h chow intake for up to 4 days (27). Because sucrose was only offered for a short period every day, no decrease in body weight was observed. Here, we show that chronic [3–5 weeks] suppression of mu-opioid receptor signaling in the nucleus accumbens with BFNA reduces development of obesity when rats have continuous access to a variety of palatable foods.
Two studies have examined the effects of chronic intraventricular administration of BFNA on the development of diet-induced obesity (30) and body weight in obese Zucker rats (31), but the observation periods were limited to 11 and 7 days and body composition was not measured. In both models, BFNA significantly reduced body weight compared with vehicle-treatment. Our results suggest that at least one of the sites necessary for these chronic weight-lowering effects of BFNA is the area of the nucleus accumbens, and that endogenous mu opioid receptor-signaling in this nucleus is required for the full extent of diet-induced obesity to develop.
Although it is clear that decreased energy intake caused some of the observed changes in body weight and composition (see below), changes in energy expenditure could have contributed to the loss of body weight and fat mass. We did not notice any major changes in physical activity levels during the treatment periods. Locomotor activity after BFNA injections into the nucleus accumbens has not been reported, but injecting BFNA into the lateral ventricle reduced locomotor activity for 2 days after injection (53). If BFNA injections into the nucleus accumbens would similarly decrease locomotor activity, the reduced weight gain observed in our experiments would be the more impressive, as reduced locomotor activity would lead to increased weight gain. However, measurement of energy expenditure, substrate oxidation, and locomotor activity will be necessary to determine the relative contribution of energy intake and expenditure on body weight and adiposity.
BFNA reduces total intake and differentially affects intake of high-fat and Ensure in the core and shell
We selected a three-choice diet composed of high-fat (60% energy), chocolate-flavored Ensure, and standard chow, all nutritionally complete, as a palatable diet to induce obesity. Normal Sprague-Dawley rats can be divided into two populations, one resistant, and one susceptible, to diet-induced obesity on a high-energy diet. However, the addition of Ensure to the high-fat diet makes most Sprague-Dawley rats obese (54). It has been shown that intermittent access to sucrose but not high-fat diet can produce addictive-type behavior in rats (8). Therefore, our three-choice diet is more likely to mimic all aspects of hedonic appetite mechanisms also found in humans, than single high-fat diet.
Whereas core injections of BFNA selectively reduced intake of high-fat diet, shell injections selectively reduced intake of Ensure compared with vehicle injections. Unfortunately, we screened for DAMGO effects only with core injections, but the effect on high-fat intake is in agreement with a study demonstrating that core injections of DAMGO more robustly increased high-fat intake compared with shell injections (55). Similarly, the selective effect of shell injections on Ensure (a sweet tasting liquid) intake is in agreement with studies examining the effect of DAMGO on sucrose solutions - DAMGO robustly increased sucrose ingestion when injected into the ventral shell of the nucleus accumbens, although no a priori attempt to restrict injections to either the core or the shell of the accumbens was made (15). Furthermore, DAMGO, when administered into a 1 mm3 “hotspot” of the nucleus accumbens, robustly increases the affective responses to sucrose ingestion, an effect that is regarded as increased ‘liking’ of sucrose (56). Although our injection sites do not exactly overlap this “hotspot” for sucrose affective responses, they are close to sites that increase sucrose ingestion (56).
A limitation of our study is the lack of histological verification of a subset of injection cannulas aimed at the core. However, all cannulas were functionally verified by demonstrating a robust high-fat feeding response to the injection of DAMGO. Furthermore, analysis of the major outcome measures in only those animals with histologically verified cannulas showed very similar effects of BFNA observed in the complete set of animals.
Conclusions
By chronically blocking endogenous signaling, the present studies suggest that mu opioid receptors in the nucleus accumbens shell and core at least partially mediate the hyperphagia and development of obesity when exposed to a palatable diet. The findings corroborate some studies demonstrating reduced susceptibility to diet-induced obesity in Oprm-1 knockout mice and with chronic systemic administration of opioid receptor antagonists in rats, and point to the nucleus accumbens as an important site of action. The results show that reduction of palatable food intake is at least one mechanism leading to reduced weight gain, but changes in energy expenditure and nutrient partitioning cannot be ruled out.
Given the wide distribution of mu-opioid receptors in the brain and periphery, it will be necessary to use tissue-selective loss-of-function approaches to further dissect the relative contribution of various brain areas and peripheral mechanisms. Understanding the role of mu-opioid receptor signaling in stimulation of palatable food intake may lead to new targets for drug development.
Acknowledgements
This research was supported by National Institutes of Diabetes and Digestive and Kidney Disease Grant DK071082.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare
References
- 1.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Weinsier RL, Hunter GR, Heini AF, Goran MI, Sell SM. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med. 1998;105:145–150. doi: 10.1016/s0002-9343(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 4.O'Rahilly S, Farooqi IS. Genetics of obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1095–1105. doi: 10.1098/rstb.2006.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 6.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers PJ, Smit HJ. Food craving and food "addiction": a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav. 2000;66:3–14. doi: 10.1016/s0091-3057(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 12.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 13.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 14.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 15.Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–1507. [PubMed] [Google Scholar]
- 16.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- 19.Zheng H, Patterson LM, Berthoud H-R. Orexin-signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007 doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that Regulate Food Intake: Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- 21.Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- 22.Otake K, Nakamura Y. Possible pathways through which neurons of the shell of the nucleus accumbens influence the outflow of the core of the nucleus accumbens. Brain Dev. 2000;22(Suppl 1):S17–S26. doi: 10.1016/s0387-7604(00)00142-x. [DOI] [PubMed] [Google Scholar]
- 23.Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27:6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 25.Portoghese PS, Larson DL, Sayre LM, Fries DS, Takemori AE. A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem. 1980;23:233–234. doi: 10.1021/jm00177a002. [DOI] [PubMed] [Google Scholar]
- 26.Ward SJ, Portoghese PS, Takemori AE. Improved assays for the assessment of kappa- and delta-properties of opioid ligands. Eur J Pharmacol. 1982;85:163–170. doi: 10.1016/0014-2999(82)90461-7. [DOI] [PubMed] [Google Scholar]
- 27.Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci. 2006;23:1605–1613. doi: 10.1111/j.1460-9568.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- 28.Ward HG, Simansky KJ. Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 2006;187:435–446. doi: 10.1007/s00213-006-0463-7. [DOI] [PubMed] [Google Scholar]
- 29.Arjune D, Standifer KM, Pasternak GW, Bodnar RJ. Reduction by central beta-funaltrexamine of food intake in rats under freely-feeding, deprivation and glucoprivic conditions. Brain Res. 1990;535:101–109. doi: 10.1016/0006-8993(90)91828-5. [DOI] [PubMed] [Google Scholar]
- 30.Cole JL, Leventhal L, Pasternak GW, Bowen WD, Bodnar RJ. Reductions in body weight following chronic central opioid receptor subtype antagonists during development of dietary obesity in rats. Brain Res. 1995;678:168–176. doi: 10.1016/0006-8993(95)00181-o. [DOI] [PubMed] [Google Scholar]
- 31.Cole JL, Berman N, Bodnar RJ. Evaluation of chronic opioid receptor antagonist effects upon weight and intake measures in lean and obese Zucker rats. Peptides. 1997;18:1201–1207. doi: 10.1016/s0196-9781(97)00074-0. [DOI] [PubMed] [Google Scholar]
- 32.Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- 33.Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 2000;876:76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- 34.Znamensky V, Echo JA, Lamonte N, Christian G, Ragnauth A, Bodnar RJ. gamma-Aminobutyric acid receptor subtype antagonists differentially alter opioid-induced feeding in the shell region of the nucleus accumbens in rats. Brain Res. 2001;906:84–91. doi: 10.1016/s0006-8993(01)02558-6. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Second Edition edn. San Diego: Academic Press; 1986. [Google Scholar]
- 36.Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- 37.Martin WR, Wikler A, Eades CG, Pescor FT. Tolerance to and Physical Dependence on Morphine in Rats. Psychopharmacologia. 1963;4:247–260. doi: 10.1007/BF00408180. [DOI] [PubMed] [Google Scholar]
- 38.South T, Deng C, Huang XF. AM 251 and beta-Funaltrexamine reduce fat intake in a fat-preferring strain of mouse. Behav Brain Res. 2007;181:153–157. doi: 10.1016/j.bbr.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Holtzman SG. Behavioral effects of separate and combined administration of naloxone and d-amphetamine. J Pharmacol Exp Ther. 1974;189:51–60. [PubMed] [Google Scholar]
- 40.Bodnar RJ. Endogenous opiates and behavior: 2006. Peptides. 2007;28:2435–2513. doi: 10.1016/j.peptides.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Statnick MA, Tinsley FC, Eastwood BJ, Suter TM, Mitch CH, Heiman ML. Peptides that regulate food intake: antagonism of opioid receptors reduces body fat in obese rats by decreasing food intake and stimulating lipid utilization. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1399–R1408. doi: 10.1152/ajpregu.00632.2002. [DOI] [PubMed] [Google Scholar]
- 42.Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol. 1997;272:R1028–R1032. doi: 10.1152/ajpregu.1997.272.4.R1028. [DOI] [PubMed] [Google Scholar]
- 43.Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–R1065. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- 44.Vaccarino FJ, Taube MR. Intra-arcuate opiate actions stimulate GRF-dependent and protein-selective feeding. Peptides. 1997;18:197–205. doi: 10.1016/s0196-9781(96)00283-5. [DOI] [PubMed] [Google Scholar]
- 45.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R388–R394. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mc Lean S, Hoebel BG. Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides. 1983;4:287–292. doi: 10.1016/0196-9781(83)90134-1. [DOI] [PubMed] [Google Scholar]
- 47.Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–224. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- 48.Mac Donald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- 49.Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ, et al. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a "thrifty gene". Diabetes. 2005;54:3510–3516. doi: 10.2337/diabetes.54.12.3510. [DOI] [PubMed] [Google Scholar]
- 50.Zuberi AR, Townsend L, Patterson L, Zheng H, Berthoud HR. Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2008;585:14–23. doi: 10.1016/j.ejphar.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- 52.Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav. 2004;82:57–61. doi: 10.1016/j.physbeh.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 53.Leventhal L, Cole JL, Bodnar RJ. Reductions in locomotor activity following central opioid receptor subtype antagonists in rats. Physiol Behav. 1996;60:833–836. doi: 10.1016/0031-9384(96)00103-5. [DOI] [PubMed] [Google Scholar]
- 54.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol. 1998;274:R412–R419. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 56.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]