Abstract
Evidence has accumulated from previous studies that vagal fibers in the lungs are involved in the genesis of dyspnea. In a series of human studies, based on our previous animal data (J Physiol. 1998;508:109-18;J Appl Physiol 1998;84:417-24; J Appl Physiol. 2003;95:1315-24) we established that intravenous adenosine has a dyspnogenic effect (J Appl Physiol 2005; 98:180-5; Respir Res 2006;7:139; Pulm Pharmacol Ther 2008; 21:208-13), strongly implicating a role for vagal C fibers in the genesis of dyspnea. We have now analysed the relative effects of blockade of vagal C fibers by two methods and routes of delivery: by inhibition of the sodium channel and interruption of action potential conduction in the nerve by inhaled local anesthetic (lidocaine), and by blockade by systemic theophylline, a known, nonselective adenosine receptor antagonist. Both techniques significantly (p<0.05) attenuated the dyspneic response to intravenous adenosine. However, the attenuation was significantly (p<0.05) greater with pretreatment with systemic theophylline (mean change in response, ΔAUC - 44%) versus pretreatment with inhaled lidocaine (mean change in response, ΔAUC -11.8%). These differences in the results of airway sensory nerve blockade probably reflect different populations of C fiber receptors and may explain conflicting results of previous studies of dyspnea and airway anesthesia.
Keywords: Dyspnea, Vagal C Fibres, Adenosine, Theophylline, Lidocaine
INTRODUCTION
Dyspnea is perhaps the commonest symptom associated with cardiopulmonary diseases; however, the neural mechanisms of dyspnea are not well understood, although central, peripheral, and chemoreceptor mechanism are clearly involved. Peripheral mechanisms include lung receptors, and these sensory receptors are innervated by the vagus nerve, specifically the unmyelinated C fibers. Previous studies in humans have shown that the dyspneic sensation is decreased or abolished by vagal blockade or section (1,2), and we have presented the first evidence that activation of vagal C fiber receptors by adenosine is dyspnogenic (3,4,5). On the other hand, studies utilizing other chemical stimuli to the C fibers have given conflicting results, with no significant dyspnea reported with capsaicin, phenyldiguanide or lobeline (6, 7, 8, 9, 10). A possible explanation for these differences in dyspnogenic responses to C fiber activation may be provided by recent animal data showing that there are differences in C fiber responsiveness depending on their ganglionic origin (11, 12).
In order to elucidate the dyspnogenic mechanisms of vagal C fiber activation in the light of these animal data, we have compared the effects of vagal nerve blockade by two different methods: by inhibition of the sodium channel and interruption of action potential conduction in the nerve by inhaled local anesthetic (lidocaine), and by blockade by systemic theophylline, a known, nonselective adenosine receptor antagonist.
METHODS
Studies were performed in two groups of normal, nonsmoking, healthy subjects; the subjects had no history of asthma or any pulmonary or general systemic disease or abnormality. The effects of airway anesthesia with inhaled lidocaine were studied in one group and the effects of oral theophylline were studied in the other group. Written informed consent, approved by the Institutional Review Board was obtained from each subject prior to the experiment.
The adenosine injection protocol consisted of cannulation of a forearm vein in the seated subject; the catheter was connected to a normal saline drip. A curtain between the subject and the cannulated forearm prevented the subject from being able to see when an injection was given (see below). The subject breathed via a mouthpiece attached to a two-way valve (Hans-Rudolph, Kansas City, MO); expiratory flow and electronically integrated volume were recorded via a differential pressure transducer (Hans-Rudolph, Kansas City, MO) and recorded (theophylline studies) on a multi-channel recorder (Grass Medical Instruments, Astro-Med Inc, West Warwick, RI) or direct to computer hard disk (Lidocaine studies). The system was calibrated before each experiment, using a calibrated syringe (Spirometrix, Inc).
Airways resistance (Rint) was measured by the interrupter technique (13, 14): Inspiratory flow was interrupted with an electronically operated shutter at a flow rate of approximately 0.5 L.sec−1, and the corresponding change in mouth pressure was measured at the mouthpiece by a pressure transducer. These signals were then used to calculate airways resistance as we have previously reported (13).
Each subject indicated the presence and intensity of dyspneic sensation by hand grip dynamometry (3, 15, 16); the subject was instructed to respond immediately any sensation was felt, with a magnitude of handgrip force proportional to the intensity of the sensation. Subjects were asked to focus on respiratory symptoms such as chest tightness, shortness of breath, increased urge to breathe, burning sensation in the chest and throat; preliminary studies had indicated that the commonest symptoms expressed were shortness of breath and chest tightness. Subjects were cued to repeat the handgrip responses every 20 seconds following the initial response and the maximum deflection for each response was recorded.
End-tidal CO2 was sampled at the mouthpiece and analyzed by a CO2 meter (Ohmeda, Englewood, CO), the output from which was continuously recorded.
Arterial O2 saturation (SaO2) was recorded continuously using a pulse oximeter (Criticare Systems, Inc. Waukesha, WI). Electrocardiogram lead II was recorded continuously and heart rate was measured from the R-R interval.
Baseline measurements of Rint were made and when the subject had a stable breathing pattern (as judged by <5% variation in the end-tidal CO2), minute ventilation (Ve), ventilatory pattern, end-tidal CO2, heart rate (H.R), and SaO2 were recorded over three minutes, and a baseline handgrip dynamometry score was recorded.
The subject then received an injection of 10 mg adenosine, care being taken to avoid awareness of the injection by the subject. The time latency between the bolus injection and the initial change in ventilation, hand grip and heart rate were measured and further measurements of ventilation, hand grip dynamometry, end-tidal CO2, SaO2 and HR were made continuously over the next 3 minutes; our previous studies (3, 4, 5) had shown that all measured parameters returned to baseline 90 – 120 seconds after adenosine injection. Rint was measured at baseline and 60 to 90 seconds after the injection.
In the lidocaine group, twelve healthy, nonsmoking subjects (mean age 32.4 ± 10.2 yrs, range 18–56 yrs, 7 females) were studied on two separate days. Each subject inhaled either normal saline or 4% lidocaine solution in a randomized sequence, through a fine particle nebulizer (MicroMist; Hudson RCI, Temecula, CA; 75% of the particles produced by this nebulizer are <5 mm in diameter); details of the technique of inhalation have been previously published (5). Immediately after the saline or lidocaine inhalation, the subjects inhaled hypertonic saline (10% sodium chloride solution) aerosol to assess the presence or absence of the cough reflex. Following this the experiments with adenosine injection were performed as noted above.
In the theophylline group (mean age: 24.8 ± 9.4 yrs, range 18 – 48 yrs, 5 females) studies were also performed on two separate days: subjects received tablets of placebo on one day and theophylline (6 mg/Kg body weight) on the other day, in an individualized random sequence, blinded to the subject and observers. On each day, two hours after tablet ingestion, a venous blood sample was drawn for analysis of serum theophylline levels, and the subject then underwent the adenosine injection protocol as described above.
Differences in the time latency of the initial hand grip, ventilatory, and heart rate response to intravenous adenosine between placebo and theophylline and placebo and lidocaine treatment were analysed by paired t test within each group (17).
Within each group, the significance of changes in these responses from baseline after adenosine injection were analysed by one-way repeated measures ANOVA, with post hoc Tukey’s analysis (17). Statistical comparisons of the differences in the responses between placebo and theophylline or lidocaine pretreatment were performed by two-way ANOVA.
Finally, the effects of theophylline and of lidocaine treatment on these responses, in terms of percent change in the area under the curve (AUC) from the placebo state were compared by unpaired t test.
RESULTS
There were no serious adverse effects from the theophylline ingestion; two subjects complained of mild anxiety and tachycardia. Blood levels of serum theophylline were mean 11.61 ± 5.8mg% standard deviation (sd) after theophylline and 0.98±1.81 mg% on the placebo day. Similarly, there were no adverse effects of the lidocaine inhalation.
The time latencies for hand grip and minute ventilation responses after adenosine injection increased significantly (p<0.05) after theophylline pretreatment compared to the control state (Table 1), but not after lidocaine. The time latencies for heart rate did not change significantly (p>0.1) in either condition.
Table 1.
Time latencies in seconds after adenosine injection
| (mean±sd) | |||
|---|---|---|---|
| Isotonic saline | Theophylline | P value (paired t-test) | |
| Hand Grip | 17.8±4.0 | 22.2±5.4 | <0.04 |
| Ventilation | 19.1±3.3 | 23.7±7.0 | <0.05 |
| Heart rate | 19.6±4.1 | 20.6±6.1 | ns |
| Isotonic saline | Lidocaine | ||
| Hand Grip | 20.6±3.3 | 19.3±3.8 | ns |
| Ventilation | 22.5±4.1 | 21.1±4.0 | ns |
| Heart rate | 20.5±4.5 | 19.0±5.8 | ns |
ns = not significant, P>0.05
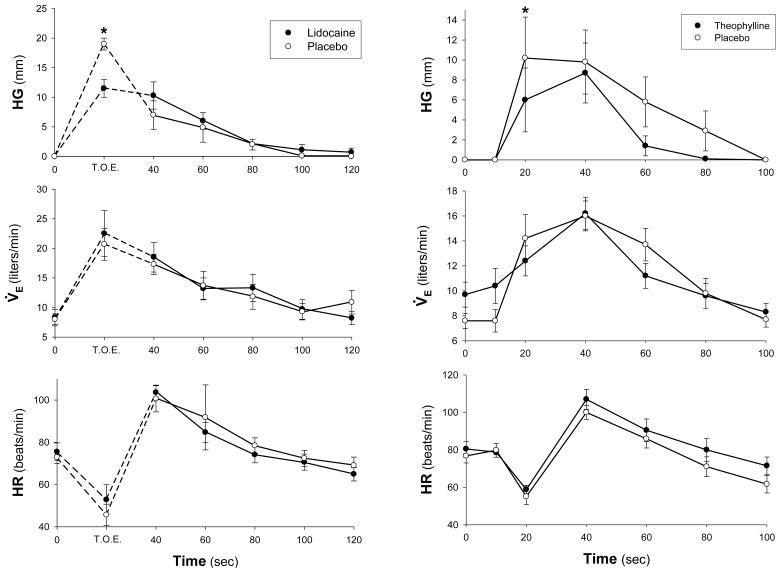
In both the control and theophylline or lidocaine treated states, intravenous adenosine resulted in significant dyspnea, increase in ventilation, and tachycardia (Fig. 1). The increase in Ve was primarily due to a significant increase (p<0.05) in tidal volume. The ventilatory and heart rate effects of intravenous adenosine were not significantly altered by theophylline or lidocaine pretreatment. On the other hand, there was a significant decrease (p<0.05) in the dyspneic sensation response to adenosine injection after both theophylline and lidocaine compared to placebo (Figure 1).
Figure 1.
Comparison of changes in hand grip signal (HG), minute ventilation (V̇E), and heart Rate (HR) in response to intravenous adenosine after placebo (open circles) and theophylline or lidocaine (closed circles). Mean ± SEM; *, significant difference (p<0.05) between the responses to adenosine after theophylline or lidocaine and placebo.
Intravenous adenosine did not produce a cough in any subject. Subjects described the adenosine-induced sensation variously as “difficulty in breathing” or “hard to breathe” or “shortness of breath” (n= 6, theophylline group, n=10 lidocaine group) or “pressure or tightness in the chest” (n=4, theophylline group, n=2, lidocaine group), and the verbal description was similar within each subject between the control and theophylline or lidocaine states. There was a significant (p<0.002) decrease in the verbalized intensity of the sensation with theophylline and lidocaine.
As shown previously (3, 4), Rint did not change significantly following adenosine injection in the placebo state (Baseline Rint: 2.13±0.23 cmH20/L/s, and 2.13±0.21 cmH20/L/s after adenosine, p>0.1, n=10); similarly, there was no significant change in Rint after theophylline or lidocaine.
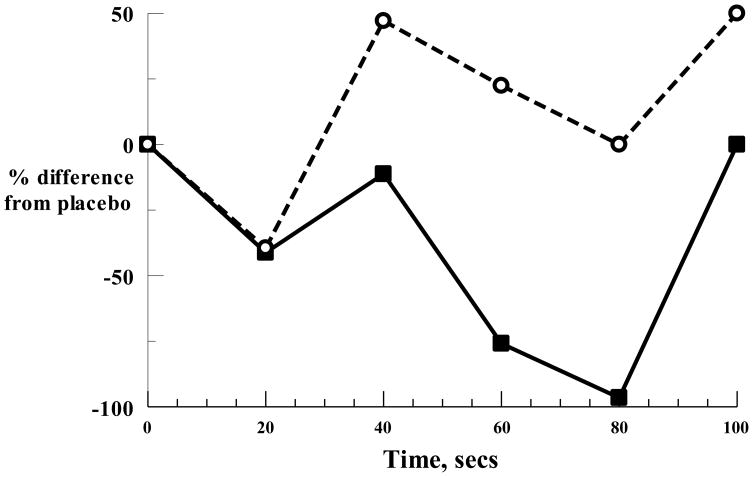
A comparison of the percent change from placebo in the hand grip response to adenosine injection after lidocaine, versus the percent change from placebo after theophylline treatment (Fig. 2) revealed a significantly (p<0.04) greater decrease after theophylline compared to lidocaine (ΔAUC - 44.2% for theophylline, ΔAUC -11.8% for lidocaine); in contrast there were no significant differences in the heart rate or ventilatory responses between placebo and lidocaine or theophylline.
Figure 2.
Percent difference from placebo in hand grip response to adenosine with lidocaine (open circles, broken line) and theophylline (continuous line).
DISCUSSION
We have shown in rats that adenosine directly stimulates pulmonary vagal C fibers through activation of A1 receptors (18). We translated these studies to human subjects and showed that intravenous adenosine results in a dyspnogenic response, most likely mediated by the pulmonary C fibers (3, 4, 5). These studies have shown that intravenous adenosine results in a dyspnogenic response, with an increase in ventilation and initial bradycardia followed by tachycardia and that intravenous adenosine is not associated with bronchospasm. Adenosine is known to activate carotid chemoreceptors in cats; however, the effects of hyperoxia as well as the time latency (3) argue against chemoreceptor activation as the basis for the dyspnogenic effect of adenosine (3, 4, 5). Analysis of our data has strongly implicated the vagal C fibers in the lungs as the dyspnogenic locus of the effects of adenosine (3, 4, 5).
The results of the present study are in conformity with these findings, and also indicate that systemic theophylline was more effective in attenuating the dyspnogenic response than inhaled lidocaine.
Lidocaine is believed to act by blocking the Na channel and interruption of action potential conduction (19). Seventy five percent of the lidocaine particles generated for inhalation in the present study were <5 μm mass median aerodynamic diameter (MMAD); particles of this size are known to be deposited in the proximal airways (20); this was confirmed by blockade of the cough reflex. A smaller proportion of the generated aerosol particles <2 μm MMAD are likely to have been deposited in the very distal airways (20), however, the extent of the blockade to more distal afferent neuroreceptors is uncertain. In contrast, theophylline acts as an adenosine receptor antagonist and is likely to have reached most of the airway/pulmonary adenosine receptors through its systemic circulatory distribution.
However, previous studies in man aimed at elucidating the role of vagal C fibers have given conflicting results. Seventy five percent of the afferent neural traffic in the vagus nerve is supplied by non-myelinated C fibers (21); these have been considered to be involved in respiratory sensations in response to noxious stimuli. Studies in man by Guz and colleagues (1, 2) have shown that the dyspneic sensation is decreased or abolished by vagal blockade or section, indicating a role of the vagal nerves in this sensation. On the other hand, studies utilizing chemical stimuli to the C fibers have given conflicting results: capsaicin is known to stimulate TRPV-1 receptors on C fibers (7, 22). Winning et al (6) noted a sensation of ‘raw, burning’ feeling after capsaicin injection into the superior vena cava and an arm vein; no subject reported feeling breathless, and one subject developed a cough with a higher dose of capsaicin. The chest sensations were blocked by prior inhalation of 5% bupivacine anesthetic aerosol. Inhaled capsaicin induces cough (7) but has not been documented to have any dyspnogenic effect. Phenyldiguanide is a selective 5-HT3 receptor agonist which can lead to stimulation of pulmonary C-fibers (11). However, studies with phenyldiguanide (8) have not noted any breathlessness. Finally, lobeline has been thought to stimulate vagal C fibers (8); however, intravenous injection of lobeline in humans produces cough and sensations of respiratory discomfort referred to the throat and upper chest (9, 10), but sensations of breathlessness are not believed to be associated with lobeline stimulation of C fibers (23).
Airway anesthesia with lidocaine has no effect on resting ventilatory pattern (24), but does appear to alter the ventilatory pattern response to CO2 and exercise (24, 25). It is unclear whether it has any specific effect on dyspnea.
The reason for these apparent differences in the effects of various substances believed to act on airway nerves, and the fact that only adenosine appears to be specifically dyspnogenic, may be explained by recent animal studies of vagal sensory fibers (11, 12, 22). It has been shown in guinea pigs, that C-fibers arising from the jugular ganglion (neural crest) are associated with the large airways, i.e. extrapulmonary airways and large intrapulmonary bronchi, whereas the C-fibers deeper in the lungs have cell bodies more often situated in the nodose ganglia (26). The activation profile of jugular C-fibers is distinct from the nodose (placodal) C-fibers. Thus, in guinea pigs, selectively activating vagal C-fibers arising from the nodose ganglia with either adenosine or 2-methyl-5-HT evokes only tachypnea, whereas selectively activating vagal afferents arising from the jugular ganglia induces respiratory slowing and apnea (22).
In addition, it has been shown that the placodal C-fibers are effectively stimulated by adenosine (via A1 receptors and, in some studies, also A2a receptors), and purinergic agonists such as ATP (via P2X2,3 heteromeric receptors), as well as 5-hydroxytryptamine (via 5-HT3 receptors) (18, 26, 27), whereas the jugular C-fibers are unaffected by these stimuli.
In the present study a significantly greater ameliorating effect was noted with oral theophylline compared to inhaled lidocaine on adenosine induced dyspnea. Inhaled lidocaine, because of the pattern of deposition, is likely to preferentially block jugular C fibers since they are primarily located in the larger airways, with a lesser effect on the placodal C fibers which are located deeper in the lung. On the other hand, systemic theophylline is more likely to access and block adenosine receptors in the placodal C fiber endings, and this probably explains the results.
These differences between the activation profiles of jugular and placodal C fibers also probably explain the differences between the effects of capsaicin and intravenous adenosine; first, inhaled capsaicin is probably preferentially deposited in the distribution of the jugular C fibers; in addition, since capsaicin acts on the TRPV-1 receptor but not on the adenosine receptors, it is possible that the dyspneic sensation is specifically related to the adenosine receptors on C fibers. While lobeline and phenyldiguanide have not been studied in terms of their effects on these two different C fiber phenotypes, it is probable that their primary effects are on the jugular C fibers. Finally, increasing evidence indicates that the afferent nerves regulating cough in anesthetized guinea pigs are distinct from either C-fibers or intrapulmonary rapidly adapting receptors (22) and it is possible that both capsaicin and phenyldiguanide also act on these cough receptors.
In summary, the present study documents a difference in the effects of vagal sensory nerve blockade by inhaled lidocaine compared to systemic theophylline and this is very likely due to different C fiber phenotypes accessible by the inhaled and systemic routes. These data suggest that the finding of different phenotypes of vagal C fibers in guinea pigs is probably also applicable to man and may explain the conflicting results seen with different C fiber activators.
Acknowledgments
Supported by USPHS NIH grant HL-65486.
Footnotes
The data with lidocaine have been partially published previously (Pulm Pharmacol & Therap 2008; 21(1), 208-13
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guz A. Respiratory sensations in man. Br Med Bull. 1977;33(2):175–7. doi: 10.1093/oxfordjournals.bmb.a071419. [DOI] [PubMed] [Google Scholar]
- 2.Davies SF, McQuaid KR, Iber C, McArthur CD, Path MJ, Beebe DS, Helseth HK. Extreme dyspnea from unilateral pulmonary venous obstruction. Demonstration of a vagal mechanism and relief by right vagotomy. Am Rev Respir Dis. 1987;136:184–8. doi: 10.1164/ajrccm/136.1.184. [DOI] [PubMed] [Google Scholar]
- 3.Burki NK, Dale WJ, Lee L-Y. Intravenous adenosine and dyspnea in man. Journal of Applied Physiology. 2005;98:180–185. doi: 10.1152/japplphysiol.00913.2004. [DOI] [PubMed] [Google Scholar]
- 4.Burki NK, Alam M, Lee LY. The pulmonary effects of intravenous adenosine in asthmatic subjects. Respiratory Research. 2006;7:139. doi: 10.1186/1465-9921-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burki NK, Sheatt M, Lee L-Y. Effects of Airway Anesthesia on Dyspnea and Ventilatory Response to Intravenous Injection of Adenosine in Healthy Human Subjects. Pulm Pharmacol & Therap. 2008;21(1):208–13. doi: 10.1016/j.pupt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Winning AJ Hamilton RD, Shea SA, Guz A. Respiratory and cardiovascular effects of central and peripheral intravenous injections of capsaicin in man: evidence for pulmonary chemosensitivity. Clin Sci. 1986;71:519–526. doi: 10.1042/cs0710519. [DOI] [PubMed] [Google Scholar]
- 7.Dicpinigaitis PV. Experimentally induced cough. Pulm Pharmacol Ther. 2007;20:319–324. doi: 10.1016/j.pupt.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Jain SK, Subramanian S, Julka DB, Guz A. Search for evidence of lung chemoreflexes in man: study of respiratory and circulatory effects of phenyldiguanide and lobeline. Clinical Science. 1972;42:163–177. doi: 10.1042/cs0420163. [DOI] [PubMed] [Google Scholar]
- 9.Raj H, Bakshi GS, Tiwari RR, Anand A, Paintal AS. How does lobeline injected intravenously produce a cough? Respir Physiol Neurobiol. 2005;145:79–90. doi: 10.1016/j.resp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Gandevia SC, Butler JE, Taylor JL, Crawford MR. Absence of viscerosomatic inhibition with injections of lobeline designed to activate human pulmonary C fibres. J Physiol. 1998;511:289–300. doi: 10.1111/j.1469-7793.1998.289bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Resp Physiol Neurobiol. 2009;167:36–44. doi: 10.1016/j.resp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol. 2009;167(1):26–35. doi: 10.1016/j.resp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burki N. Effects of bronchodilation on magnitude estimation of added resistive loads in asthmatic subjects. Am Rev Respir Dis. 1984;129:225–229. [PubMed] [Google Scholar]
- 14.Liistro G, Stanescu D, Rodenstein D, Veriter C. Reassessment of the interruption technique for measuring airflow resistance in humans. J Appl Physiol. 1989;67:933–937. doi: 10.1152/jappl.1989.67.3.933. [DOI] [PubMed] [Google Scholar]
- 15.Zechman FW, Wiley RL, Davenport PW. Ability of healthy men to discriminate between added inspiratory resisitive and elastic loads. Respir Physiol. 1981;45:111–120. doi: 10.1016/0034-5687(81)90053-0. [DOI] [PubMed] [Google Scholar]
- 16.Muza SR, Zechman FW. Scaling of added loads to breathing: magnitude estimation vs handgrip matching. J Appl Physiol. 1984;57:888–891. doi: 10.1152/jappl.1984.57.3.888. [DOI] [PubMed] [Google Scholar]
- 17.Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. 4. Blackwell Science Ltd, Iowa State University Press; Ames, Iowa: 2002. pp. 225–227.pp. 432–435. [Google Scholar]
- 18.Hong JL, Ho CY, Kwong K, Lee LY. Activation of pulmonary C fibers by adenosine in anaesthetized rats: role of adenosine A1 receptors. J Physiol (Lond) 1998;508:109–118. doi: 10.1111/j.1469-7793.1998.109br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhary BA, Burki NK. The effects of airway anesthesia on detection of added inspiratory elastic loads. Am Rev Respir Dis. 1980;122:635–40. doi: 10.1164/arrd.1980.122.4.635. [DOI] [PubMed] [Google Scholar]
- 20.Dolovich MB. 18F-fluorodeoxyglucose positron emission tomographic imaging of pulmonary functions, pathology, and drug delivery. Proc Am Thorac Soc. 2009;6(5):477–85. doi: 10.1513/pats.200904-023AW. [DOI] [PubMed] [Google Scholar]
- 21.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst. 1982;5:165–176. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 22.Chou YL, Scarupa MD, Mori N, Canning BJ. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1572–84. doi: 10.1152/ajpregu.90382.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler JE, Anand A, Crawford MR, Glanville AR, McKenzie DK, Paintal AS, Taylor JL, Gandevia SC. Changes in respiratory sensations induced by lobeline after human bilateral lung transplantation. J Physiol. 2001;534(Pt 2):583–593. doi: 10.1111/j.1469-7793.2001.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winning AJ, Hamilton RD, Shea SA, Knott C, Guz A. The effect of airway anaesthesia on the control of breathing and the sensation of breathlessness in man. Clin Sci (Lond) 1985;68(2):215–25. doi: 10.1042/cs0680215. [DOI] [PubMed] [Google Scholar]
- 25.Mador MJ. Effect of nebulized lidocaine on ventilatory response to CO2 in healthy subjects. J Appl Physiol. 1993;74:1419–24. doi: 10.1152/jappl.1993.74.3.1419. [DOI] [PubMed] [Google Scholar]
- 26.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuaychoo B, Lee MG, Kollarik M, Pullmann R, Jr, Undem BJ. Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol. 2006;575:481–490. doi: 10.1113/jphysiol.2006.109371. [DOI] [PMC free article] [PubMed] [Google Scholar]