Abstract
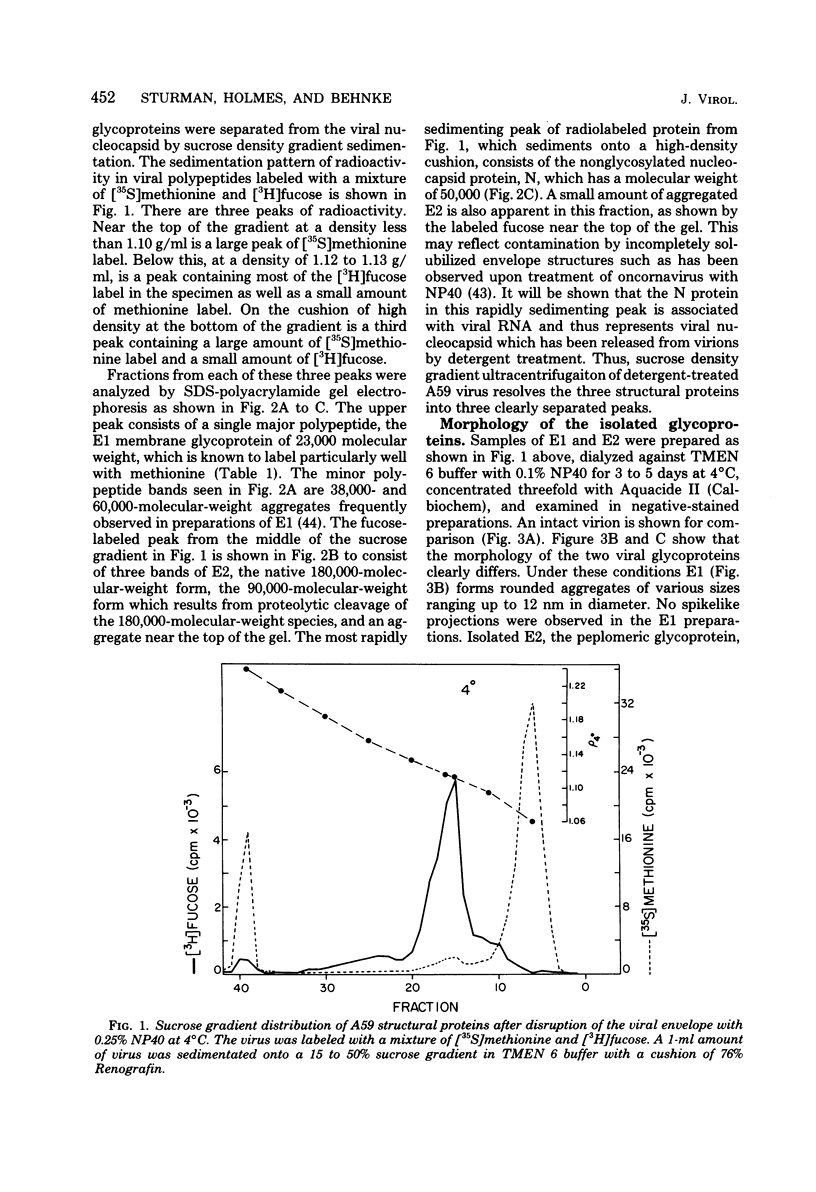
The two envelope glycoproteins and the viral nucleocapsid of the coronavirus A59 were isolated by solubilization of the viral membrane with Nonidet P-40 at 4 degrees C followed by sucrose density gradient sedimentation. Isolated E2 consisted of rosettes of peplomers, whereas E1, the membrane glycoprotein, was irregular and amorphous. Under certain conditions significant interactions occurred between components of Nonidet P-40-disrupted virions. Incubation of the Nonidet P-40-disrupted virus at 37 degrees C resulted in formation of a complex between one of the viral glycoproteins, E1, and the viral nucleocapsid. This was caused by a temperature-dependent conformational change in E1, resulting in aggregation of E1 and interaction with the viral RNA in the nucleocapsid. E1 also bound rRNA. The E1-nucleocapsid complexes can be distinguished on sucrose and Renografin density gradients from native viral nucleocapsids. The separation of the membrane glycoprotein E1 from the peplomeric glycoprotein E2 permitted preparation of antisera against these isolated proteins. A model is proposed for the arrangement of the three major structural proteins in the coronavirus A59 virion in relation to the viral envelope and RNA.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham R. W. The polypeptide composition of avian infectious bronchitis virus. Arch Virol. 1975;49(2-3):207–216. doi: 10.1007/BF01317539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F. Dissection of vesicular stomatitis virus into the infective ribonucleoprotein and immunizing components. J Gen Virol. 1970 Apr;7(1):19–32. doi: 10.1099/0022-1317-7-1-19. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Vogel S. N., Teramoto A. Y., Russell P. K. Antigenic components of group A arbovirus virions. J Virol. 1973 Nov;12(5):1034–1042. doi: 10.1128/jvi.12.5.1034-1042.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H. Cross-linking of the spike glycoproteins in Semliki Forest virus with dimethylsuberimidate. Virology. 1974 Dec;62(2):385–392. doi: 10.1016/0042-6822(74)90400-0. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Pocock D. H., Pike B. V. Isolation of subviral components from transmissible gastroenteritis virus. J Gen Virol. 1976 Aug;32(2):283–294. doi: 10.1099/0022-1317-32-2-283. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Pocock D. H. The polypeptide structure of transmissible gastroenteritis virus. J Gen Virol. 1975 Oct;29(1):25–34. doi: 10.1099/0022-1317-29-1-25. [DOI] [PubMed] [Google Scholar]
- György E., Sheehan M. C., Sokol F. Release of envelope glycoprotein from rabies virions by a nonionic detergent. J Virol. 1971 Nov;8(5):649–655. doi: 10.1128/jvi.8.5.649-655.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Söderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta. 1973 May 11;307(2):287–300. doi: 10.1016/0005-2736(73)90096-5. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Palmer E. L., Whitfield S. G., Kaye H. S., Dowdle W. R. Protein composition of coronavirus OC 43. Virology. 1972 May;48(2):516–527. doi: 10.1016/0042-6822(72)90062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C. Purification and biophysical properties of human coronavirus 229E. Virology. 1976 Nov;75(1):155–165. doi: 10.1016/0042-6822(76)90014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Stohlman S. A. RNA of mouse hepatitis virus. J Virol. 1978 May;26(2):236–242. doi: 10.1128/jvi.26.2.236-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The polypeptide composition of avain infectious bronchitis virus particles. Arch Virol. 1977;55(1-2):47–54. doi: 10.1007/BF01314478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Reynolds J. A., Tanford C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem. 1973 Jul 25;248(14):4926–4932. [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Stevens R. H., Simpson R. W. Presence of infectious polyadenylated RNA in coronavirus avian bronchitis virus. Virology. 1977 Apr;77(2):772–782. doi: 10.1016/0042-6822(77)90498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K. Surface-active agents for isolation of the core component of avian myeloblastosis virus. J Virol. 1972 Apr;9(4):684–697. doi: 10.1128/jvi.9.4.684-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Müller A., ter Meulen V. Genomic RNA of the murine coronavirus JHM. J Gen Virol. 1978 Nov;41(2):217–227. doi: 10.1099/0022-1317-41-2-217. [DOI] [PubMed] [Google Scholar]
- Wege H., Wege H., Nagashima K., ter Meulen V. Structural polypeptides of the murine coronavirus JHM. J Gen Virol. 1979 Jan;42(1):37–47. doi: 10.1099/0022-1317-42-1-37. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]