Abstract
Pregnenolone sulfate (PS) is an endogenous neurosteroid synthesized by glial cells, which acts as a potent convulsant when injected intracerebroventricularly and intraperitoneally. PS is found in relatively high concentrations in the hippocampus. But its convulsant action in the hippocampus has not been characterized. A range of PS doses were infused directly into the right hippocampus of 42 rats, which were subsequently monitored for behavioral and electrographic seizures. At the highest dose (4 µmol), PS produced status epilepticus (SE) and severe behavioral convulsions. As the dose of PS was reduced, the fraction of rats having SE diminished (ED50 for SE = 2.7 µmol). At doses lower than 300 nmol, PS infusion produced discrete electrographic seizures (ED50 = 68 nmol) associated with mild behavioral seizures. Both the behavioral seizure score (BSS) and the total number of seizures during the observation period changed in a dose-dependant manner. In separate experiments in cultured hippocampal neurons, PS enhanced NMDA-evoked whole-cell currents (EC50 = 16 µM). The results demonstrate that the hippocampus is highly sensitive to the convulsant effects of PS and that the enhancement of NMDA currents could contribute to the convulsant action of PS.
Keywords: Pregnenolone sulfate, Status epilepticus, Hippocampus, GABAA receptor, NMDA receptor
1. Introduction
Neuroactive steroids (neurosteroids) belong to group of substances endogenously synthesized from precursor sex hormones in gonads and de novo from cholesterol in the brain, which have potent nongenomic effects on inhibitory and excitatory neurotransmission. Many studies suggest that the neurosteroid allopregnanolone plays an important role in modulating seizures. Allopregnanolone blocks seizures induced by cocaine, N-methyl-d-aspartate (NMDA), pilocarpine and kainic acid in a dose-dependent fashion (Gasior et al., 1997; Kokate et al., 1996). Allopregnanolone at least in part mediates anticonvulsant effect of progesterone. When injected peripherally, progesterone and allopregnanolone have a similar efficacy as anticonvulsants. However, when the conversion of progesterone to allopregnanolone is blocked by inhibiting the enzyme 5 alpha-reductase, the anticonvulsant activity of progesterone is lost (Frye et al., 1998; Kokate et al., 1999a). Furthermore, pentylenetetrazol (PTZ)-induced seizures are blocked by direct infusion of allopregnanolone into the pontine reticular formation, but not by progesterone infusion (Frye et al., 2000). Withdrawal from neurosteroids also increases seizure susceptibility (Moran and Smith, 1998; Reddy et al., 2001).
In contrast to allopregnanolone, the effects of the sulfated metabolite of progesterone, pregnenolone sulfate (PS), on seizures and epilepsy are less well understood. PS enhances convulsant potency of intraperitoneally administered NMDA in mice (Maione et al., 1992), chronic subcutaneous injections in rats significantly shifted the pentylenetetrazol dose-percent convulsions and latency curves to the left, and decreased the ED50 of pentylenetetrazol for tonic convulsions (Reddy and Kulkarni, 1998). Intracerebroventricular administration of PS evoked limbic and tonic-clonic seizures in mice and potentiated the action of subconvulsive doses of intraperitoneally injected NMDA and pentylenetetrazol (Kokate et al., 1999b).
While these studies demonstrated the proconvulsant effect of PS, the drug was infused peripherally or in the ventricles and there was no electrographic monitoring. These factors make it difficult to determine the site of seizure generation in these studies. In order to fully characterize PS-induced seizures by a combination of EEG and behavioral analysis, we infused PS into hippocampi of rats. Hippocampus was chosen as the site for PS infusion for several reasons. PS is present in high concentrations in the hippocampus (Wang et al., 1997) and can modulate learning and memory function when infused into this structure (Flood et al., 1995; Vallee et al., 1997). Moreover, the hippocampus has long been implicated in temporal lobe epilepsy and is the focus for investigation into the basic mechanisms of epileptogenesis (McNamara, 1999). Patients with temporal lobe epilepsy have a characteristic pathological lesion in their hippocampus (Sloviter, 1994). Neurons in the hippocampal–parahippocampal circuit can undergo plastic changes that significantly contribute to the development of epileptic seizures (Lothman, 1992). These changes include altered properties of post-synaptic inhibitory GABAA and glutamatergic receptors (Mody, 1998).
PS inhibits GABAA receptors (Jung-Testas et al., 1999) and enhances NMDA receptor-mediated currents (Majewska, 1992; Wu et al., 1991). Inhibition of GABAA receptors can lead to synchronous bursting of hippocampal pyramidal neurons and seizures (Dingledine and Gjerstad, 1980; Heyer et al., 1981; Miles and Wong, 1987; Traub and Wong, 1982). Similarly, activation of NMDA receptors can lead to seizures (Stafstrom and Sasaki-Adams, 2003), and NMDA receptors are activated in various seizure models (Mody, 1998; Sutula et al., 1996).
Given the convulsant effects of PS and the fact that the hippocampus is both a target site for PS activity and also an important structure in the production of seizures, we have characterized the convulsive properties of PS when infused directly into the hippocampus of awake, freely moving rats using both EEG and behavioral measurements. In separate experiments, we studied the effects of PS on NMDA receptor currents elicited from cultured hippocampal neurons.
2. Materials and methods
2.1. PS infusion into the hippocampus
All experimental procedures were performed according to the protocol approved by the University of Virginia Animal Care and Use Committee. The animals were maintained at artificial 12 h/12 h light/dark cycle and had free access to food and water. Procedures for surgery and electrode implantation have been described previously (Lothman et al., 1985). Briefly, the animals were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg). Bipolar electrodes were stereotactically implanted in the left ventral hippocampus (AP −5.3, ML −4.9, DV −5.0 to dura; incisior bar −3.3). A guide cannula (Plastic One, Roanoke, VA) was stereotactically implanted into the right ventral hippocampus (AP −5.3, ML +4.9, DV −5.0 from dura; incisior bar −3.3). The electrodes and cannula were mounted on the skull with jeweler's screws and dental cement. Following a one-week recovery, the electrode headsets were connected to a Grass 7D amplifier and then to a Stellate digital system for electroencephalographic (EEG) recording. PS was suspended in 15% hydroxypropyl-β-cyclodextrin (Sigma, St. Louis, MO) and infused into the ventral hippocampus via a 28-gauge injection needle (extending 1 mm past the cannula guide). The needle was connected to a 1.0 ml syringe, which was driven by an infusion pump (KD Scientific, Portland, OR), at the rate of 1.0 µl/min. The total volume of infusion was 20 µl for all animals except the highest dose, which required an infusion of 40 µl due to the limited solubility of PS. EEG and video (recorded on VHS tape) monitoring began 10 min prior to PS infusion and continued for 2 h after infusion was completed.
EEG recordings were subsequently reviewed for the presence of seizures as well as status epilepticus (SE). Seizures were characterized by the appearance of high frequency (>2 Hz) rhythmic spike wave discharges with amplitudes at least three times that of the baseline EEG. The EEG criterion for SE was occurrence of continuous epileptiform activity for 30 min, during which spike frequencies do not drop below 2 Hz or intermittent seizures lasting 30 min with brief periods of spiking lasting less than 5 min. The termination of these events was recorded when the spike frequency dropped below 2 Hz or disappeared for more than 5 min.
Behavioral seizures (from video recordings) were scored as follows: stage 0, normal behavior; stage 1, wet dog shakes, facial twitches, chewing; stage 2, head bobbing and/or jerking; stage 3, forelimb clonus; stage 4, forelimb clonus and rearing; and stage 5, fore-limb clonus, rearing and loss of balance (Racine, 1972). A sixth stage was also added to indicate the occurrence of “running fits” consisting of bouts of uncontrolled running behavior lasting 10–20 s. Behavioral data were analyzed using a Kruskal–Wallis ANOVA on ranks test.
After the experiments, animals were euthanized by deep anesthesia, and brains were removed and frozen. Brains were sectioned on a microtome, mounted, and nissl stained. Localization of the electrodes and the infusion site was confirmed histologically for each animal.
2.2. Hippocampal culture
Rat hippocampal cultures were prepared according to the method described by Banker (Goslin et al., 1998). Briefly, hippocampi were dissected from 18 day rat embryos, dissociated by trypsin and triturated with a Pasteur pipette. The neurons were plated on cover-slips coated with poly-l-lysine in minimal essential medium (MEM) with 10% horse serum at an approximate density of 25,000/cm2. Once the neurons had attached to the substrate, they were transferred to a dish containing a glial monolayer and maintained for up to 4 weeks in serum-free MEM with N2 supplements. Only pyramidal neurons were used for voltage-clamp recordings. The neurons were visually identified by the shape of the soma and large apical dendrite. All recordings were performed on neurons when they were 14–18 day old in culture.
2.3. Recordings of NMDA receptor currents
Patch electrodes were pulled from borosilicate glass (Sutter Instruments, Novato, CA) on a horizontal Flaming–Brown microelectrode puller (model P-97, Sutter Instruments) using a two-stage pull protocol. Electrode resistances were 4–6 MΩ.
Whole-cell patch-clamp recordings were made from cultured pyramidal neurons (Hamill et al., 1981). For recordings of NMDA elicited currents, patch electrodes were filled with recording solution containing (in mM): Cs-methanosulfonate 125, CsCl 15, HEPES 10, Cs4-BAPTA 5, and MgCl2 3. Na2-ATP salt was added in 2 mM concentration to intracellular solution before the recording session. The osmolarity was 290 mOsm, pH 7.3. The recording medium contained (in mM): NaCl 168, KCl 2.4, HEPES 10, d-glucose 10, glycine 0.01, and CaCl2 1.3. The osmolarity was in the range of 318–325 mOsm and pH was adjusted to 7.4 with 10 N NaOH. Seventy-five percent of series resistance was compensated. For the recordings of NMDA elicited currents, the cells were clamped at −50 mV and application of NMDA produced inward currents. The currents were amplified and recorded with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) and low-pass filtered at 3 kHz with a eight pole Bessel filter prior to digitization, storage, and display using the patch-clamp technique (Hamill et al., 1981). The currents were displayed on-line on a Gould Viper TA11 digital chart recorder (Gould, OH), and were also recorded on a hard drive of Pentium II personal computer using the axoscope 7.0 program (Axon Instruments, Foster City, CA) (digitized at 1000 Hz). Peak currents were measured manually from the computer recordings and confirmed by chart paper recording.
NMDA (Sigma, St. Louis MO) was dissolved in extracellular solution and PS (Steraloids, Newport, RI) was dissolved in dimethylsulphoxide (DMSO). All drugs were prepared as 50 mM stock solutions and were stored at −20 °C. The stock solutions were diluted in extracellular solution on the day of an experiment. The drugs were applied to the neurons with a modified U-tube “multipuffer” application system (Greenfield and Macdonald, 1996) with the tip of application pipette placed 100–200 µm from the cell. A rapid stream of drug solution through a U-shaped tube can be suddenly halted, resulting in extrusion of a drug through a hole in the bottom of the U. The multipuffer combines the “U-tube” approach with a puffer: single glass micropipette pulled to a fine tip (40–60 µm) from which a single drug concentration can be applied by pressure ejection. In order to exclude the possible effect of DMSO, in control assays, cells were exposed to DMSO alone at the same concentration as the one present in the drug-treated cells. The data from each cell were fit to a three parameter logistic equation (equation for a sigmoid curve): I = I(max)/(1 + 10((Log EC50−Log(NMDA)) × Hill slope)), where I is the peak NMDAR current at a given NMDA concentration. The Hill slope, EC50 and I (max) (maximal current) were derived from the equation that best fits the observed data by least squares fit method using the Graphpad Prism 3.0 curve fitting program on a IBM PC compatible computer. All values are reported as means ± S.E.M.
3. Results
3.1. Status epilepticus induced by PS
Only animals in which histology confirmed that the recording electrode and infusion cannula were located within the ventral hippocampus were included in the analysis. Control animals (n= 4) received intrahippocampal infusions of 40 µl of the vehicle solution alone and did not show any behavioral or EEG sign of seizure activity. In experimental animals (n= 37), increasing doses of PS (300 fmol–4 µmol) suspended in cyclodextrin were infused into the right hippocampus and EEG was recorded from the left hippocampus.
The highest dose of PS (4 µmol) was infused over 40 min into the right hippocampus and epileptiform activity appeared during the infusion period in all four rats. Electrographic seizures began 90–540 s into the infusion. Initial seizures were either single seizure events with a return to normal EEG or recurrent seizures separated by spikes. All animals receiving 4 µmol infusions progressed to status epilepticus within 60 min. There was continuous epileptiform activity in which spike frequencies did not drop below 2 Hz and there was post-spike EEG suppression (Fig. 1C). Continuous seizures were seen in three of the four animals, which received the 4 µmol dose of PS. In one animal, discrete seizures occurred repeatedly with brief periods (1–5 min) of 1–2 Hz spikes between seizures.
Fig. 1.
Patterns of hippocampal epileptiform activity with increasing doses of PS. Each panel represents a continuous EEG recording from a ventral hippocampal electrode (contralateral to the injection cannula) during the first 30 min after the PS infusion was completed. (A) A 100 nM dose of PS produces isolated discharges followed by the long periods of relatively normal EEG. (B) Higher dose of PS (2 µmol) produces recurrent a pattern of closely spaced discharges with short periods of low amplitude epileptiform activity between paroxysms. (C) Infusion of a high doses of PS (4.0 µmol) resulted in continuous, high amplitude discharges with some variation in the frequency of epileptiform bursts.
Behavior seizures progressed at a slower pace. Although limbic seizures with (behavioral seizure score (BSS) 1–2) accompanied the initial electrographic seizures, convulsions appeared later, between 10 and 40 min during the infusion period. The severity of the convulsions increased rapidly after the initial appearance, culminating in periodic running fits (BSS = 6) in three out of four animals. This was followed by death in two animals within the first 2 h.
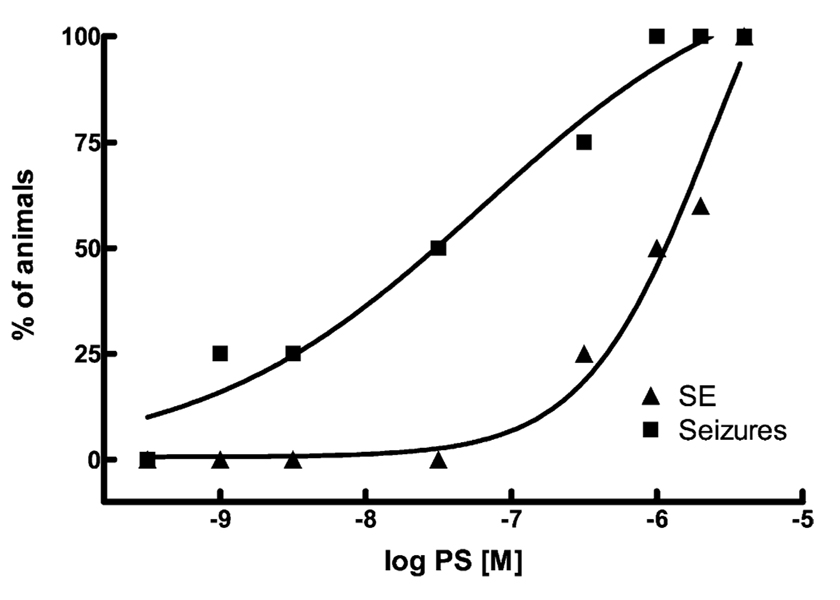
We further characterized the ability of PS to cause status epilepticus by infusing smaller doses of PS into the hippocampus. As doses of PS were reduced, the fraction of animals exhibiting status epilepticus decreased rapidly, such that none of the rats had status epilepticus when 300 nmol PS was infused. The relationship between the PS dose and the percentage of rats going into status epilepticus fit well to a sigmoidal function, with an ED50 of 2.7 µmol (Fig. 2).
Fig. 2.
Relationship between the dose of PS and the appearance of discrete seizures and SE. The abscissa shows the dose of PS infused into the hippocampus, and the ordinate shows the percent fraction of animals exhibiting either discrete seizures (squares) or SE (triangles). The percent fraction of animals that exhibited discrete seizures or SE increased as increasing doses of PS were infused into the hippocampus. Lines represent the best fit to a sigmoidal function. The ED50 for the appearance of SE was 2.7 µM which was significantly higher than that for the production of discrete seizures, 68 nM (68 nM (N = 4,5,4,4,6,4,4,4 for 4.0, 2.0, 1.0 µmol, 300, 30, 3.0, 1.0, 0.3 nmol, respectively).
Behavioral seizures also became less intense as the PS dose was decreased. The BSS of the most intense seizure was recorded in each rat and averaged for each dose. The mean maximum behavioral seizure declined from 5.75 ± 0.25 to 1.0 ± 0.0 (Fig. 3). There was a significant effect of PS dose on BSS (p≤ 0.001, Kruskal–Wallis ANOVA on ranks).
Fig. 3.
Increasing doses of PS resulted in more intense behavioral seizures. The dose of PS infused is displayed on the abscissa while the BSS is shown on the ordinate. The BSS 1–5 were according to Racine criteria (see Materials and methods) with an additional score of 6 for the most severe behavioral seizure consisting of wild running. Doses of PS associated with the production of SE seem to produce a large jump in the severity of behavioral seizures. There was a significant effect of the dose of PS on BSS (p≤ 0.001, Kruskal–Wallis ANOVA on ranks: N= 4,5,4,4,6,4,4,4 for 4.0, 2.0, 1.0 µmol, 300, 30, 3.0, 1.0, 0.3 nmol, respectively).
3.2. Discrete seizures produced by PS
Discrete electrographic seizures occurred when 300 nmol PS was infused into the hippocampus of four rats. Individual seizures were defined by the appearance of high frequency spikes with amplitudes several times baseline EEG and frequencies greater than 5 Hz. The termination of these events was recorded when the spike frequency dropped below 1–2 Hz. Only two animals exhibited periods of spike activity in between seizure events. The periods of spikes lasted only several minutes and did not reach a high enough frequency to qualify as status epilepticus. The first seizure started 5–19 min into the infusion and during the 2-h observation period, the animals had 2–8 seizures. Behavioral manifestations of these events were very mild (BSS 1–2) and did not intensify during the 2-h observation period.
Doses of PS below 300 nm continued to cause discrete seizures in some animals but did not produce inter-event spikes (Fig. 1A). As the dose of infused PS was diminished, the fraction of rats that had seizures also decreased. The relationship between the PS dose and the percent of animals with seizures fit well to a sigmoidal function, with an ED50 of 68 nM (Fig. 2). Individual animals also had fewer total seizures during the 2 h observation period as PS dose decreased (Fig. 4). In contrast, PS dose did not appear to alter the duration of individual seizures, or the time interval between them. Statistical analysis was not performed due to small numbers of events at low doses.
Fig. 4.
Relationship between the dose of PS and the number of seizures during the 2-h observation period following infusion. Low doses (300 nM and less) produced few seizures (between 0 and 8 seizures per animal). Larger doses produces substantial increases in the number of recorded seizures. The 4.0 µmol dose was omitted from analysis due to the appearance of continuous seizures in several animals (N = 5,4,4,5,3,3,1,1 for 2.0, 1.0 µmol, 300, 30, 3.0, 1.0, 0.3 nmol;, respectively).
3.3. PS enhancement of NMDA receptor currents
In order to investigate the cellular mechanism of the convulsant action of PS, we studied the effects of PS on the peak amplitude of NMDA receptor currents in hippocampal neurons in culture. PS is known to modulate both GABAA receptors (Majewska, 1992) and NMDA receptors (Bowlby, 1993). We wished to determine whether the efficacy and potency of PS modulation of NMDA receptor currents and GABAA receptor currents in a principal hippocampal neuron were similar or not. Previous studies in chick spinal chord neurons had suggested that PS had a higher potency at GABAA receptors than at NMDA receptors. In the past, we have characterized the PS modulation of GABAA receptors on hippocampal dentate granule cells (Mtchedlishvili et al., 2001) and in cultured hippocampal neurons (Mtchedlishvili and Kapur, 2003) but we have not investigated PS modulation of NMDA currents.
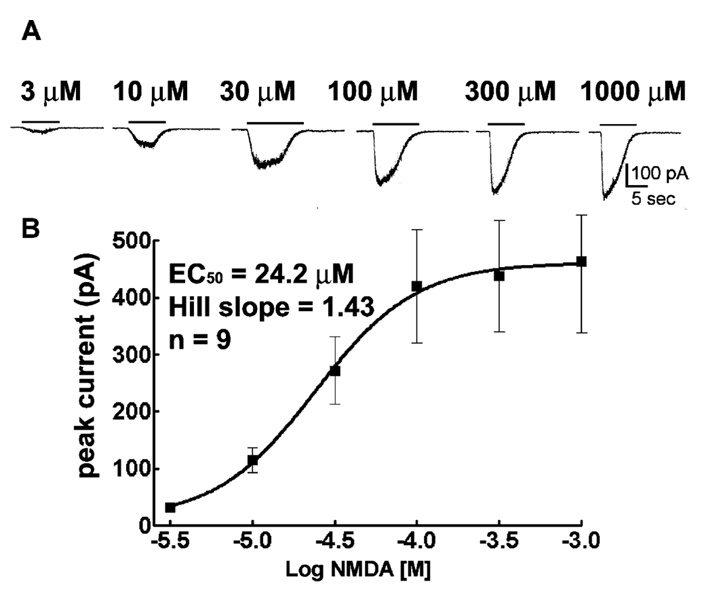
Whole-cell voltage-clamp recordings from these cells demonstrated the presence of spontaneous excitatory postsynaptic currents with an NMDA receptor-mediated component when magnesium was excluded from the extracellular medium (data not shown). Whole-cell NMDA receptor currents were elicited by applying increasing concentrations of NMDA to pyramidal neurons voltage clamped to −65 mV. NMDA (3 µM) elicited slowly activating and desensitizing inward currents (Fig. 5A). The peak amplitudes of NMDA elicited currents increased in a concentration-dependent manner, and saturation occurred at a 300 µM concentration and fit to the sigmoidal function. The maximal amplitude of NMDA elicited current was 423 ± 139 pA (n= 9). The EC50 was 24.2 µM, Hill coefficient = 1.43 (Fig. 5B).
Fig. 5.
Application of micromolar concentrations of NMDA elicited whole-cell currents in hippocampal pyramidal neurons in culture. (A) NMDA elicited whole-cell current traces in concentration-dependent manner. The horizontal bars indicate duration of NMDA application. Note slow activation and decay of currents. (B) NMDA concentration-NMDA receptor current relationships. NMDA concentration response curves were obtained for hippocampal pyramidal neuron 14–18 days in culture rats (n= 9).
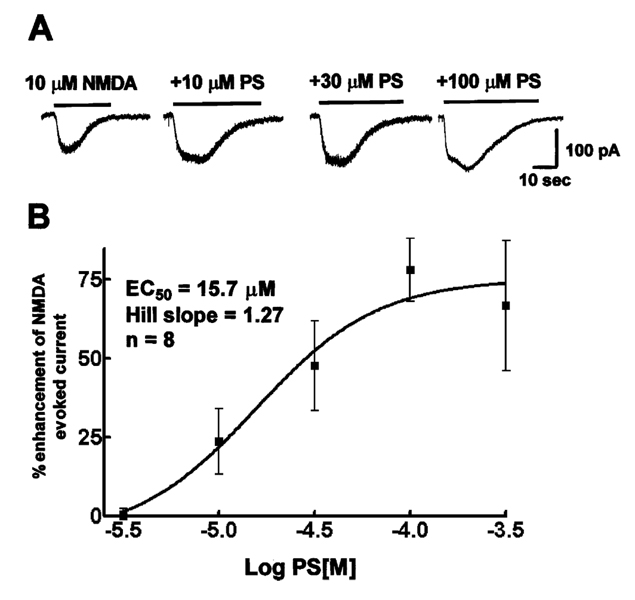
PS (100 µM), co-applied with 10 µM NMDA to a hippocampal neuron, increased the peak amplitude of a current elicited by the application of 10 µM NMDA alone (78.1 ± 9.9%, n= 8). For further study of PS enhancement of NMDA elicited currents, a range of increasing concentrations of PS (3–300 µM) were co-applied with 10 µM NMDA. PS enhanced the peak current amplitude in a concentration-dependent manner (Fig. 6A). Saturation of PS action occurred at 300 µM concentration. The peak current amplitude recorded after application of each concentration of PS was compared to the average of currents elicited by 10 µM NMDA alone before and after application of PS. The PS concentration and peak NMDA receptor current enhancement data were fitted to an equation for a sigmoidal curve and the best fit suggested the EC50 for enhancement was 15.7 µM, and the Hill coefficient was 1.27 (n= 8, Fig. 6B).
Fig. 6.
PS enhanced peak amplitude of currents evoked by the application of 10 µM NMDA in a cultured hippocampal neuron. (A) Traces of currents evoked by 10 µM NMDA and are larger when co-applied with 10, 30 and 100 µM PS. (B) PS concentration–NMDA receptor current relationships. PS concentration response curves were obtained from eight hippocampal neurons 14–18 days in culture.
4. Discussion
The principal findings in this study are that intrahippocampal administration of PS in rats can produce status epilepticus at high doses (1–4 µmol). Discrete seizures appear at much lower doses of PS 10–300 nmol. Furthermore, PS modulates NMDA receptor in cultured hippocampal neurons.
Infusion of high doses of PS caused status epilepticus, consisting of continuous or recurrent electro-graphic seizures, and prolonged limbic and secondarily generalized tonic–clonic seizures. At the highest dose of PS, status epilepticus resulted in the death of animals. During status epilepticus, the EEG consisted of continuous seizures or seizures interrupted by brief periods of spiking. Similar EEG has been described during human status epilepticus (Treiman et al., 1990)and in animal models of status epilepticus (Treiman et al., 1990). In contrast to other models, such as the pilocarpine or the kainic acid model, where neurotoxins generate status epilepticus, this model uses a substance generated by the brain.
As the dose of PS was reduced, the response quickly changed from prolonged status epilepticus to discrete seizures. This transition was not only associated with a rapid decrease in the number of animals in status epilepticus but also a rapid reduction in both the total number of seizures and the associated behavioral response. The reduction reflects the transition from a state of status epilepticus, which is self-sustaining process, to unitary seizure events, which are a direct result of the action of PS on neuronal populations. The mechanisms that transform individual seizures into status epilepticus are not well understood, and in this study, the transition occurred over a 10-fold range of doses. Increased local PS concentration during infusion could assist in the transition from discrete seizures to status epilepticus. The reason larger doses caused status epilepticus could also be related to a larger network (or volume) of neurons being affected by PS. Tissue volume has been hypothesized as a critical factor in seizure production (Schwindt et al., 1997). The lack of an appreciable dose dependent delay in the appearance of individual seizures may also point to a smaller but also critical volume associated with the production of discrete seizures. Thus, two hypothetical concentric spheres could exist around the infusion site, one representing a volume necessary to produce discrete seizures and a larger sphere representing the volume of tissue required for the transition to status epilepticus. In addition to the tissue volume, higher doses would also increase the amount of time that these high PS concentrations persist in a particular volume of tissue. Future experiments with independent manipulation of the concentration of PS and infusion volume may help to characterize the relationship between these parameters and the transition to status epilepticus.
The doses of PS required to produce seizures are lower than those reported in a previous study (Kokate et al., 1999b). In that study, performed on mice with intracerebroventricular infusion of PS, only behavioral seizures were measured and the dose necessary to induce seizures in 50% of the animals (CD50) was 92 nmol and the threshold dose of PS for generating a seizure was 50 nmol. In contrast, the current study used intrahippocampal infusion into the rat hippocampus and found an EC50 of 68 nM, and the threshold dose of 1 nmol. A number of factors including brain size (the mouse brain weighs approximately 25% of the rat brain) make dose comparisons difficult. Besides species differences, it is possible that direct infusion into the hippocampus could produce locally high concentrations near the infusion site. Small population of neurons near the site of infusion would be exposed to transient concentrations significantly higher than those concentrations that would occur after ICV or IP injections. On the other hand, it may simply be that because low doses of PS produce seizures with little or no behavioral manifestation, the sensitivity difference could be explained by reliance on behavior manifestations of seizures in previous studies. Finally, it is possible that PS preferentially affects the hippocampus. Studies on the effect of PS on learning and memory suggested that limbic structures are particular targets of action of PS (Flood et al., 1995; Vallee et al., 1997).
We found that the lowest dose infused into the hippocampus that caused seizures was 1 nmol (419 ng). Since there is 25–30 ng/g of PS in hippocampal tissue homogenates, it appears unlikely that endogenous PS by itself can initiate seizures. However, PS may play a role in determining seizure threshold, especially in the epileptic brain. It is possible that the concentration of PS is higher in the epileptic hippocampus since PS is synthesized in glia, and gliosis is a prominent feature of temporal lobe epilepsy (Du et al., 1993). Coupled with changes in the excitatory/inhibitory systems of the hippocampus, changes in endogenous PS concentrations and/or changes in the potency of PS could lead to a role of this neurosteroid compound in modulating seizure threshold. Thus, questions concerning changes in neurosteroid pharmacology in chronic epilepsy will be important in extending the data presented here and to further characterize the role of PS and other neurosteroids in chronic epilepsy.
4.1. Mechanism of convulsant action
The local concentration of PS resulting from its infusion was not measured in this study. However, even if PS diffused into a tissue volume of 0.1 ml, the local concentration of PS at the infusion site would be in the 10 µM–40 mM range, which clearly exceeds the concentrations required for modulation of both NMDA and GABA receptors on cultured pyramidal neurons. In the past, it was suggested that PS antagonism of GABAA receptors was more significant than enhancement of NMDA receptor function (Kokate et al., 1999b). This conclusion was based on the finding that systemic administration of benzodiazepines or neurosteroids blocked PS-induced seizures, while they are weak or ineffective against NMDA-induced seizures. Additionally, PS effects on GABAA receptor and NMDA receptor actions in cultured chick spinal cord neurons (Wu et al., 1991) were cited. The current study did not compare the potencies of GABAA receptor agonist with that of NMDA receptor antagonists, so it does not directly address the mechanism of convulsant action of PS. However, since PS was infused into the hippocampus for characterizing its convulsant action, it is important that a comparison of PS potencies on GABAA receptor and NMDA receptors be performed in hippocampal neurons.
Wu and colleagues found that, in cultured chick spinal neurons, the EC50 for PS enhancement NMDA receptor currents was reported to be 57 M. In contrast, we found that in cultured rat hippocampal neurons, PS was more potent at modulating NMDA receptor currents, with an EC50 of 16 µM. The difference in potency of PS in modulating NMDA receptors on hippocampal neurons may result from species difference and also from the type of neuron studies. NMDA receptors are composed of NR1 and NR2A, B, C and D subunits. It is reported that whereas PS potentiates NR1/NR2A and NR1/NR2B combinations, it inhibits NR1/NR2C and NR1/NR2D combinations of NMDA receptors (Malayev et al., 2002). Thus, it is possible that the chick spinal cord neurons in culture express different complement of NMDA receptor sub-units than cultured rat hippocampal neurons. The potency of PS in modulating NMDA receptors in rat hippocampal neurons is likely to be more relevant to its convulsant effect, especially when it is infused into the hippocampus.
A comparison of PS effects on NMDA and GABA receptor currents recorded from cultured pyramidal neurons supported the notion that PS exerts its convulsant action primarily by acting on GABAergic synapse. Emerging evidence suggests that PS has both pre and postsynaptic effects on gabaergic and glutamatergic synaptic transmission in hippocampal neurons. These studies suggest that PS inhibits GABAergic synaptic transmission with greater potency and efficacy than it enhances glutamatergic neurotransmission. PS effects on GABAergic synapses were characterized in detail recently (Mtchedlishvili and Kapur, 2003). PS blocks GABA release in a very potent manner, with an IC50 of 26 nM, and GABA release is suppressed 80–90% by 1–10 µM PS. PS inhibited GABAA receptors with an IC50 of 4 µm and had maximal effect of 45%. In contrast, PS enhanced NMDA receptor currents in cultured hippocampal neurons, with an EC50 of 16 µM and maximal effect of 60%. It was recently demonstrated that PS enhances glutamate release from hippocampal neurons, but this effect occurs at 10 µM concentration PS (Meyer et al., 2002). These studies suggest that GABAergic neurotransmission is more sensitive to the effects of PS than glutamatergic neurotransmission and may account for the convulsant action of PS (Kokate et al., 1999b).
Acknowledgements
This work was supported by the NINDS grants KO2-NS 02081 and RO1 NS40337 and by a grant from the National Alliance on Research on Schizophrenia and Depression (NARSAD foundation) foundation. We thank Cassie Gregory for the expert technical assistance and Wei Wei Yen and Josh Labrie for their contribution to PS infusion studies.
References
- Bowlby M. Pregnenolone sulfate potentiation of N-methyl-d-aspartate receptor channels in hippocampal neurons. Molecular Pharmacology. 1993;43:813–819. [PubMed] [Google Scholar]
- Dingledine R, Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. Journal of Physiology (London) 1980;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Williamson J, Bertram E, Lothman E, Okuno E, Schwarcz R. Kynurenine pathway enzymes in a rat model of chronic epilepsy: immunohistochemical study of activated glial cells. Neuroscience. 1993;55:975–989. doi: 10.1016/0306-4522(93)90312-4. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proceedings of the National Academy of Sciences, United States of America. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Manjarrez J, Camacho-Arroyo I. Infusion of 3alpha,5alpha-THP to the pontine reticular formation attenuates PTZ-induced seizures. Brain Research. 2000;881:98–102. doi: 10.1016/s0006-8993(00)02897-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ, Bayon LE. Finasteride blocks the reduction in ictal activity produced by exogenous estrous cyclicity. Journal of Neuroendocrinology. 1998;10:291–296. doi: 10.1046/j.1365-2826.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Goldberg SR, Witkin JM. Anticonvulsant and behavioral effects of neuroactive steroids alone and in conjunction with diazepam. Journal of Pharmacology and Experimental Therapeutics. 1997;282:543–553. [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cellular and molecular neuroscience. Cambridge: The MIT Press; 1998. pp. 339–370. [Google Scholar]
- Greenfield LJ, Macdonald RL. Whole-cell and single-channel α1β1γ2S GABAA receptor currents elicited by a “multipuffer” drug application device. Pflugers Archieves. 1996;432:1080–1090. doi: 10.1007/s004240050238. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archieves. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heyer EJ, Nowak LM, Macdonald RL. Bicuculline: a convulsant with synaptic and nonsynaptic actions. Neurology. 1981;31:1381–1390. doi: 10.1212/wnl.31.11.1381. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Do TA, Koenig H, Desarnaud F, Shazand K, Schumacher M, Baulieu EE. Progesterone as a neurosteroid: synthesis and actions in rat glial cells. Journal of Steroid Biochemistry and Molecular Biology. 1999;69:97–107. doi: 10.1016/s0960-0760(98)00149-6. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and Kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5alpha-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. Journal of Pharmacology and Experimental Therapeutics. 1999a;288:679–684. [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neurosteroid pregnenolone sulfate in mice. Brain Research. 1999b;831:119–124. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Lothman EW. Basic mechanisms of the epilepsies. Current Opinions in Neurological Neurosurgery. 1992;5:216–223. [PubMed] [Google Scholar]
- Lothman EW, Hatlelid JM, Zorumski CF, Conry JA, Moon PF, Perlin JB. Kindling with rapidly recurring hippocampal seizures. Brain Research. 1985;360:83–91. doi: 10.1016/0006-8993(85)91223-5. [DOI] [PubMed] [Google Scholar]
- Maione S, Berrino L, Vitagliano S, Leyva J, Rossi F. Pregnenolone sulfate increases the convulsant potency of N-methyl-d-aspartate in mice. European Journal of Pharmacology. 1992;219:477–479. doi: 10.1016/0014-2999(92)90493-n. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Progress in Neurobiology. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. British Journal of Pharmacology. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399:A15–A22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela CF. Neurosteroids Enhance Spontaneous Glutamate Release in Hippocampal Neurons. Journal of Biological Chemistry. 2002;277(32):28725–28732. doi: 10.1074/jbc.M202592200. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. Journal of Physiology (London) 1987;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I. Ion channels in epilepsy. International Review of Neurobiology. 1998;42:199–226. doi: 10.1016/s0074-7742(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith SS. Progesterone withdrawal I: pro-convulsant effects. Brain Research. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. Diminished allopregnanolone enhancement of GABA(A) receptor currents in a rat model of chronic temporal lobe epilepsy. Journal of Physiology. 2001;537:453–465. doi: 10.1111/j.1469-7793.2001.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. A Presynaptic Action of the Neurosteroid Pregnenolone Sulfate on GABAergic Synaptic Transmission. Molecular Pharmacology. 2003;64:857–864. doi: 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and Clinical Neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Proconvulsant effects of neurosteroids pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. European Journal of Pharmacology. 1998;345:55–59. doi: 10.1016/s0014-2999(98)00034-x. [DOI] [PubMed] [Google Scholar]
- Schwindt W, Nicholson C, Lehmenkuhler A. Critical volume of rat cortex and extracellular threshold concentration for a pentylenetetrazol-induced epileptic focus. Brain Research. 1997;753:86–97. doi: 10.1016/s0006-8993(96)01495-3. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. On the relationship between neuropathology and pathophysiology in the epileptic hippocampus of humans and experimental animals. Hippocampus. 1994;4:250–253. doi: 10.1002/hipo.450040304. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Sasaki-Adams DM. NMDA-induced seizures in developing rats cause long-term learning impairment and increased seizure susceptibility. Epilepsy Research. 2003;53:129–137. doi: 10.1016/s0920-1211(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Sutula T, Koch J, Golarai G, Watanabe Y, McNamara JO. NMDA receptor dependence of kindling and mossy fiber sprouting: evidence that the NMDA receptor regulates patterning of hippocampal circuits in the adult brain. Journal of Neuroscience. 1996;16:7398–7406. doi: 10.1523/JNEUROSCI.16-22-07398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Research. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Darnaudery M, Corpechot C, Young J, Koehl M, Le Moal M, Baulieu EE, Robel P, Simon H. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proceedings of the National Academy of Sciences, United States of America. 1997;94:14865–14870. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MD, Wahlstrom G, Backstrom T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. Journal of Steroid Biochemistry and Molecular Biology. 1997;62:299–306. doi: 10.1016/s0960-0760(97)00041-1. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-d-aspartate receptor. Molecular Pharmacology. 1991;40:333–336. [PubMed] [Google Scholar]