Summary
Cancer stem cells (CSC) have been identified in hematological malignancies and several solid cancers. Similar to physiological stem cells, CSC are capable of self-renewal and differentiation and have the potential for indefinite proliferation, a function through which they may cause tumor growth. Although conventional anti-cancer treatments might eradicate most malignant cells in a tumor, they are potentially ineffective against chemoresistant CSC, which may ultimately be responsible for recurrence and progression. Human malignant melanoma is a highly aggressive and drug-resistant cancer. Detection of tumor heterogeneity, undifferentiated molecular signatures, and increased tumorigenicity of melanoma subsets with embryonic-like differentiation plasticity strongly suggest the presence and involvement of malignant melanoma stem cells (MMSC) in the initiation and propagation of this malignancy. Here, we review these findings in the context of functional properties ascribed to melanocyte stem cells and CSC in other cancers. We discuss the association of deregulated signaling pathways, genomic instability, and vasculogenic mimicry phenomena observed in melanoma subpopulations in light of the CSC concept. We propose that a subset of MMSC may be responsible for melanoma therapy-resistance, tumor invasiveness, and neoplastic progression and that targeted abrogation of a MMSC compartment could therefore ultimately lead to stable remissions and perhaps cures of metastatic melanoma.
Keywords: melanoma, cancer stem cells, tumorigenicity, self-renewal, differentiation, progression, chemoresistance
Characteristics and epidemiology of malignant melanoma
Human malignant melanoma is a highly metastatic cancer that is markedly resistant to conventional therapy (Chin et al., 2006; Helmbach et al., 2003; Li and Mcclay, 2002; Soengas and Lowe, 2003). Although the survival rate of patients diagnosed with melanoma has improved considerably over the past decades (Beddingfield, 2003), the lifetime risk and overall mortality because of melanoma escalate yearly (Thompson et al., 2005) and increase more rapidly than in most adult onset cancers (Jemal et al., 2001). While primary melanomas are largely (>95%) curable using surgical excision when diagnosed early (Breslow, 1978), the 5-yr survival of patients with regional lymph node infiltrations decreases to 50% (Balch et al., 2001). Melanoma prognosis is particularly poor for patients with visceral metastases as evidenced by a median survival rate of only a few months (Balch et al., 2001). The minimal therapeutic benefit of current treatment regimens in this group of patients (Soengas and Lowe, 2003) indicates the urgency for novel strategies to overcome chemoresistance.
Melanoma risk may correlate with distinct skin pigmentation phenotypes (Gandini et al., 2005a), for example those linked to melanocortin-1 receptor polymorphic variants (Valverde et al., 1995). In addition, epidemiological factors, such as intermittent ultraviolet radiation exposure concomitant with sunburns, particularly during childhood, significantly promote the susceptibility to melanoma (Gandini et al., 2005b; Thompson et al., 2005). Patients with strong family history of the disease hereby constitute 8–12% of all melanoma cases (Fountain et al., 1990). Common germline mutations, described for the cyclin-dependent kinase inhibitor 2A (CDKN2A) or the cyclin-dependent kinase 4 genes (Chin et al., 2006), are, however, infrequent in familial melanomas (Thompson et al., 2005).
Clinically, melanomas are classified according to the total thickness in millimeters, mitotic rate, presence of ulceration, penetration depth, and location of existing metastases (Chin, 2003). Histologically, five distinct stages of melanoma progression can be distinguished: benign nevi without dysplastic changes, dysplastic nevi, radial-growth phase (RGP), vertical-growth phase (VGP), and metastatic melanoma (Chin, 2003). Of note, RGP melanoma cells are mainly confined to the epidermis, are non-tumorigenic in experimental animal models and unable to metastasize in patients (Hsu et al., 2002). In contrast, VGP melanoma lesions are comprised of expansive, anchorage-independent nodules and are highly tumorigenic and metastatic in immunodeficient mice and patients (Hsu et al., 2002).
It is important to recognize that there are diverse cell types located within or surrounding melanoma lesions, including endothelial cells, immune cells, keratinocytes, and fibroblasts (Lee and Herlyn, 2007). Prior to malignant transformation, melanocytes are interspersed at a constant ratio of ~1:35 among keratinocytes, forming the ‘epidermal melanin unit’ (Hsu et al., 2002). Keratinocytes likewise serve as the key regulators of early stage melanoma cell homeostasis and proliferation (Lee and Herlyn, 2007). Mechanistically, this homeostatic state involves E-cadherin-mediated keratinocyte-melanoma cell bonding (Hsu et al., 2002). During melanoma progression, advanced melanoma cells escape keratinocyte control by various mechanisms, including downregulation of E-cadherin, upregulation of molecules important for melanoma–melanoma cell-, melanoma–endothelium-and/or melanoma–fibroblast interactions such as N-cadherin or MelCAM, and loss of anchorage to the basement membrane because of an altered profile of matrix adhesion molecules (Haass et al., 2005). Upon appropriate activation, cellular components within the malignant microenvironment, namely N-cadherin-expressing endothelial cells or fibroblasts, promote these processes through the secretion of proliferative growth factors, such as insulin-like growth factor 1, fibroblast growth factor 2, transforming growth factor-beta, or VEGF (vascular endothelial growth factor) (Lee and Herlyn, 2007). These paracrine mediators can modulate the microenvironment to cause proliferation, invasion, and migration of deregulated melanoma cells (Hsu et al., 2002). Hence, distinct changes of the microenvironmental milieu regulate the malignant transformation from melanocyte to melanoma.
Melanomagenesis and progression are commonly described as ‘de-differentiation’ processes of transformed, mature melanocytes, enabling the stepwise metamorphosis from nevus to RGP to VGP melanoma and, ultimately, to disseminated disease (Clark et al., 1984; Herlyn et al., 1985, 1987). Strikingly, the majority of melanomas emerge in normal appearing skin or unexpected sites along the neural crest migratory route, not in dysplastic nevi (Kelly et al., 1997; Lucas et al., 2003). Based on these observations and recent findings of melanoma heterogeneity and plasticity (Fang et al., 2005; Frank et al., 2005; Grichnik et al., 2006; Monzani et al., 2007; Topczewska et al., 2006; Wang et al., 2006) an alternative hypothesis has been put forth in light of the cancer stem cell (CSC) concept, suggesting mutated melanocyte stem cells or immature progenitor cells present in skin as precursors to melanoma.
Normal adult stem cells and tumor stem cells
Normal adult stem cells reside in most somatic tissues, where they form the cellular basis for tissue homeostasis, maintenance, and repair, as has been shown, for example, in the skin (Fuchs, 2007). These relatively rare, tissue-specific stem cells are defined, firstly, by their ability to replicate themselves through self-renewal, and secondly, by their ability to give rise to progenitor or transiently amplifying cells which further differentiate into the mature cell types that constitute the tissue of origin (Reya et al., 2001). In addition, recent reports provide evidence that some postnatal stem cells bear multi-lineage differentiation plasticity (Guan et al., 2006; Jiang et al., 2002; Toma et al., 2001). A capacity of stem or progenitor cells for cell fusion, including fusion with more differentiated cell types (Frank et al., 2003; Spees et al., 2003; Wang et al., 2003), may also account for apparent plasticity. For example, it has been shown that fusion of human mesenchymal stem cells (MSC) with more mature cells may contribute to the observed expression by fusion hybrids of surface epitopes characteristic of more differentiated cell types (Spees et al., 2003). Thirdly, alongside their ability to self-renew and differentiate, adult stem cells can proliferate extensively (Reya et al., 2001).
Of note, normal stem cells tend to be more resistant to cytotoxic agents than mature cell types, which may relate to their relative quiescence (Harrison and Lerner, 1991), activation of anti-apoptotic mechanisms (Peters et al., 1998), or high-expression levels of ATP-binding cassette (ABC) transporters (Zhou et al., 2001). The ‘stemness’ of these long-lived tissue cells is maintained through self-renewal-associated pathways, such as Wnt, Notch, and hedgehog (Molofsky et al., 2004). The interplay between these pathways and integrating signals from the stem cell niche (Scadden, 2006) provides a crucial regulatory balance between self-renewal and differentiation (Morrison and Kimble, 2006).
Tumors differ from physiological tissues in that they demonstrate a loss of these homeostatic mechanisms (Leong and Karsan, 2006; Taipale and Beachy, 2001). Thus, oncogenesis can be viewed as a deregulation of self-renewal. However, striking parallels have been acknowledged for some time between stem cells and tumorigenic cancer cells: both have extensive proliferative capacities, normal tissues and tumors are comprised of phenotypically heterogeneous cell populations with varying clonogenic potentials, and many of the characteristics of stem cell systems, including stem cell plasticity, are also relevant to tumor growth (Bruce and Van Der Gaag, 1963; Fialkow, 1976; Fidler and Hart, 1982; Hamburger and Salmon, 1977). Taken together, these findings have led to the recent development of the CSC theory, which postulates that only a fraction of the cells in a tumor, the CSC, are essential for its propagation (Reya et al., 2001).
Cancer stem cells were first identified by the pioneering work of Dick and others in cancers of the hematopoietic lineage (Bonnet and Dick, 1997; Lapidot et al., 1994). In addition, several recent reports have demonstrated the existence of CSC in solid tumors, including breast cancers (Al-Hajj et al., 2003), medullo-blastoma and glioblastoma tumors of the brain (Singh et al., 2004), and colon cancer (O’Brien et al., 2007; Ricci-Vitiani et al., 2007). In breast cancer, prospectively identified and isolated tumorigenic human cancer cells of a CD44+CD24−/lowLineage− phenotype were found to possess the exclusive capacity to form xenograft tumors in mice, as opposed to breast cancer cells with alternate phenotypes, which failed to form tumors in vivo (Al-Hajj et al., 2003). The tumorigenic cancer subpopulation could be serially passaged in these studies, generating new tumors containing additional CD44+CD24−/lowLineage− tumorigenic cells, as well as the phenotypically diverse-mixed populations of non-tumorigenic cells present in the initial tumor (Al-Hajj et al., 2003). In human brain and colon cancer, CSC capable of self-renewal and differentiation were shown to be exclusively contained within tumor cell subsets characterized by expression of the CD133 (AC133) stem cell marker, whereas CD133− tumor cells were found to lack tumorigenicity (O’Brien et al., 2007; Ricci-Vitiani et al., 2007; Singh et al., 2004). Thus, the term ‘cancer stem cell’, as employed by investigators in the cancer field (Al-Hajj et al., 2003; Bonnet and Dick, 1997; Lapidot et al., 1994; O’Brien et al., 2007; Reya et al., 2001; Ricci-Vitiani et al., 2007; Singh et al., 2004) and adopted in this review characterizes cancer subpopulations, which contain a cancer component exhibiting increased in vivo tumorigenicity, the capacity for indefinite potential for in vivo self-renewal, and the ability to generate phenotypically diverse populations including non-tumorigenic cancer cells present in the initial tumor in serial xenotransplantation experiments.
It should be noted that this definition is distinct from the generally accepted, more rigorous stem cell definition used by investigators studying physiological hematopoietic stem cells, which reconstitute the full hierarchical population from a single cell. In this regard, it is important to recognize that physiological stem cells can not only divide asymmetrically but also symmetrically (Morrison and Kimble, 2006), a feature that may potentially also be operative in cancer through deregulation of pathways associated with normal stem cell development (Leong and Karsan, 2006; Taipale and Beachy, 2001). This aspect of stem cell biology offers a potential explanation for the observed higher relative frequencies of CSC in human tumors compared with the relative rarity of physiological stem cells in physiological tissues.
Another potential explanation for the observed high-relative frequency of CSC in human tumors emerges out of recent data obtained in breast cancer, suggesting that cancer bulk components may also contribute to tumor progression and metastasis (Shipitsin et al., 2007). In solid malignancies, optimal tumor growth and aggressive disease characteristics might therefore emanate from interactions between elevated levels of CSC (Massague, 2007) with non-tumorigenic tumor components, rather than from rare CSC alone.
In a manner reminiscent of normal stem cells, stemlike cancer cells have been found to be highly resistant to drugs and toxins, for example through the expression of MDR1 P-glycoprotein (ABCB1) or related ABC transporters, particularly during relapse (Dean et al., 2005; Frank et al., 2005; Gottesman et al., 2002; Wulf et al., 2001). In addition to their tumor-initiating properties, CSC might therefore likewise compose the small reservoir of drug-resistant cells that survives chemotherapy and subsequently drives tumor recurrence and metastatic disease. Thus, therapeutic strategies that specifically target and eradicate the CSC compartment could potentially increase the efficacy of current treatment regimens and might reduce the risk of relapse and metastasis.
Given the striking similarities between physiological and malignant stem cells, it is plausible that CSC may arise by cumulative genetic alterations from long-lived normal stem cells that already contain the machinery for indefinite self-renewal (Reya et al., 2001). Indirect evidence supports the notion that distinct populations of epithelial stem cells are indeed the target of malignant transformation in certain types of lung cancer (Kim et al., 2005). However, recent results obtained in the hematopoietic system indicate that CSC can also arise through oncogenic transformation of lineage-restricted progenitor or differentiated cell types (Jamieson et al., 2004; Krivtsov et al., 2006). In addition, CSC may also evolve through epigenetic transformation of normal stem cells by a dysfunctional environmental niche. In support of this theory, Houghton et al. (2004) reported that bone marrow-derived cells could give rise to gastric adenocarcinoma when recruited into gastric glands upon chronic injury. Similarly, in Kaposi’s sarcoma endothelial cells transdifferentiate into cancer cells (Nickoloff and Foreman, 2002). Finally, it is also conceivable that mutated somatic cells can fuse with normal stem cells, thereby generating malignant CSC (Bjerkvig et al., 2005).
With regard to malignant melanoma, emerging evidence now supports the CSC concept in the initiation and propagation of the disease. Here, we will discuss important clues on MMSC from their putative cells of origin, melanocyte stem cells, and the CSC model and its clinical implications as it relates to malignant melanoma.
Melanocyte stem cells
It is currently unknown whether and how putative MMSC may relate to physiological stem cells of the melanocytic lineage. A more detailed understanding of the distinctive characteristics of melanocyte stem cells in the context of regulatory signals from surrounding niches could, however, provide important insights into the process of melanomagenesis. During development, melanocyte precursors arise in the neural crest from where they migrate over substantial distances, for example, to the basal layer of the epidermis or the hair follicle, where they differentiate into pigment-synthesizing progeny (Mayer, 1973). It is plausible that such migratory skills reflect the strong propensity of malignant melanoma cells to metastasize, a feature thought to be inherent to the tumor stem cell compartment (Reya et al., 2001).
Immature melanocytes, termed melanoblasts, have been identified for some time (Cock and Cohen, 1958). In primary murine cultures, these small cells devoid of pigment can be induced to differentiate into large, pigmented, and highly proliferative melanocytes (Sviderskaya et al., 1995). In addition, the selective pressure of exogenous factors, such as stem cell factor (SCF) or leukemia inhibitory factor, permits long-term growth of transiently dominant melanoblast populations (Sviderskaya et al., 1995). During development, melanoblasts arise in the neural crest from where they migrate through the epidermis towards emerging hair follicles (Mackenzie et al., 1997). Once localized to the hair follicle, melanoblasts can either differentiate into mature melanocytes responsible for hair pigmentation or, alternatively, give rise to melanocyte stem cells responsible of maintaining the melanocyte system (Yonetani et al., 2007).
Using melanocyte-tagged transgenic mice, Nishimura et al., (2002) first described the identification of such melanocyte stem cells. These phenotypically immature and slow-cycling cells were found capable of self-maintenance throughout the hair cycle and were fully competent of differentiating into melanin-producing progeny upon pharmacological disruption of hair follicle pigmentation (Nishimura et al., 2002). In malignant melanoma, a slow-cycling, non-pigmented phenotype may thus be suggestive of candidate MMSC.
Melanocyte stem cells reside within a specialized stem cell niche called the bulge (Cotsarelis et al., 1990; Fuchs, 2007), located in the lower permanent portion of the hair follicle known as the outer root sheath (Nishimura et al., 2002). The hair follicle represents an attractive system for studying stem cell function and activity because it continuously passes through cycles of tissue regeneration. Hair growth occurs in alternating phases of proliferation (anagen), regression (catagen), and quiescence (telogen) (Nishimura et al., 2002). In early anagen, regulating signals from the bulge region can activate dormant melanocyte stem cells to differentiate into transiently amplifying progeny. The latter cell population proliferates and further differentiates into mature, melanin-producing melanocytes, which migrate to the base of the hair follicle (hair bulb) and provide pigment for the growing hair matrix. During catagen, mature and transiently amplifying melanocytes undergo apoptosis. At least one resting melanocyte stem cell remains in the bulge region throughout the hair cycle, including telogen, ensuring the production of melanocytes at the beginning of a new hair cycle (Nishimura et al., 2002).
Of note, transiently amplifying melanocytes can escape the niche and migrate to the epidermis, where they can further differentiate into pigmented melanocytes. Moreover, some of these transiently amplifying melanocytes can return to a quiescent state and eventually revert to stem cells by colonizing vacant niches (Nishimura et al., 2002). In addition, neural crest derivatives have been shown to differentiate into neurons, Schwann cells, or smooth muscle cells in a niche-dependent fashion (Amoh et al., 2005; Dupin et al., 2000; Toma et al., 2001). In summary, the data by Nishimura et al. (2002) identify a melanocyte stem cell pool within the bulge region as the source of differentiated melanocytes not only in the hair follicle, but also in the basal epidermis. These findings further emphasize that integrating signals from the niche play a dominant role in stem cell fate.
Molecularly, melanocyte stem cells can be distinguished from their more differentiated progeny by a Dct+, Pax3+, Tyr−, Si−, Tyrp1−, Kit−, Mitf−, Sox10−, Lef1−, and Mki67− phenotype (Osawa et al., 2005). Among these molecules, expression or absence of Pax3, Sox10, and Mitf have been shown to be particularly critical for melanocyte stem cell maintenance, differentiation, and quiescence (Nishikawa and Osawa, 2007). Sox10 and its downstream target gene Mitf (micophthalmia-associated transcription factor), both of which have been implicated as master regulators of melanocyte development (Vance and Goding, 2004), were found downregulated in melanocyte stem cells (Nishikawa and Osawa, 2007). On the contrary, the transcription factor Pax3, which competes with Mitf for occupancy of the Dct (dopachrome tautomerase) promoter (Lang et al., 2005), was found expressed by melanocyte stem cells (Osawa et al., 2005). Thus, a Pax3+, Sox10−, and Mitf− phenotype can maintain an undifferentiated state and promotes quiescence of these lineage-restricted stem cells (Nishikawa and Osawa, 2007). Importantly, elevated levels of Wnt signaling molecules, including activated β-catenin, antagonize the regulatory balance between Pax3, Sox10, and Mitf towards terminal differentiation (Lang et al., 2005; Schepsky et al., 2006; Takeda et al., 2000). On the other hand, Notch signaling promotes the maintenance of melanocyte stem cells (Moriyama et al., 2006). A central role in regulating melanocyte stem cell fate has also been assigned to keratinocytes, which represent the only cellular component in direct proximity (Nishikawa and Osawa, 2007). In this regard, Mak et al. (2006) recently showed that the depletion of melanocyte stem cells in B-cell lymphoma 2 (Bcl2) null mice achieved via abrogation of c-kit (SCF receptor) signaling could be reversed by continuous expression of SCF by epidermal keratinocytes. An additional mechanism perpetuating melanocyte stem cell maintenance and quiescence is the general suppression of housekeeping functions on the transcriptional level (Nishikawa and Osawa, 2007), a feature that has also enabled the isolation of melanocyte stem cells from murine skin (Osawa et al., 2005).
The existence of self-renewing and differentiation-capable melanocyte stem cells in humans has yet to be conclusively proven in genetic lineage tracking experiments. However, findings of repigmentation processes in vitiligo, a depigmentation disorder resulting from the destruction of functional melanocytes, strongly suggest the presence of an immature cell pool that replenishes the skin with functional melanocytes upon disease control (Yu, 2002). Like in rodent skin, different melanocyte phenotypes are distinguishable in the human epidermis and hair follicle, including amelanotic, non-proliferative cell types, and pigmented, more proliferative melanocyte populations (Grichnik et al., 1996; Tobin and Bystryn, 1996). The existence of melanocyte stem cells in the human hair follicle, as compared with murine melanocyte-tagged models, was additionally indicated by a recent study demonstrating that hair graying is linked to the selective depletion and defective self-renewal mechanisms of melanocyte stem cells (Nishimura et al., 2005). On the molecular level, the observed aging process of melanocyte stem cells was associated with a deficiency in Bcl2, which causes selective apoptosis of the stem cell component, and distinct mutations of the melanocyte master transcriptional regulator Mitf (Nishimura et al., 2005).
The deregulation of key molecules balancing melanocyte stem cell quiescence, differentiation and self-maintenance, such as Mitf, Slug, Pax3, or Twist, has also been linked to melanoma progression after malignant transformation (Garraway et al., 2005; Gupta et al., 2005; Lang et al., 2005; Yang et al., 2004). Thus, transformed melanocyte stem cells together with a disrupted niche environment might be involved in the genesis of malignant melanoma and may represent melanoma-initiating, stem-like cells.
CSC and malignant melanoma
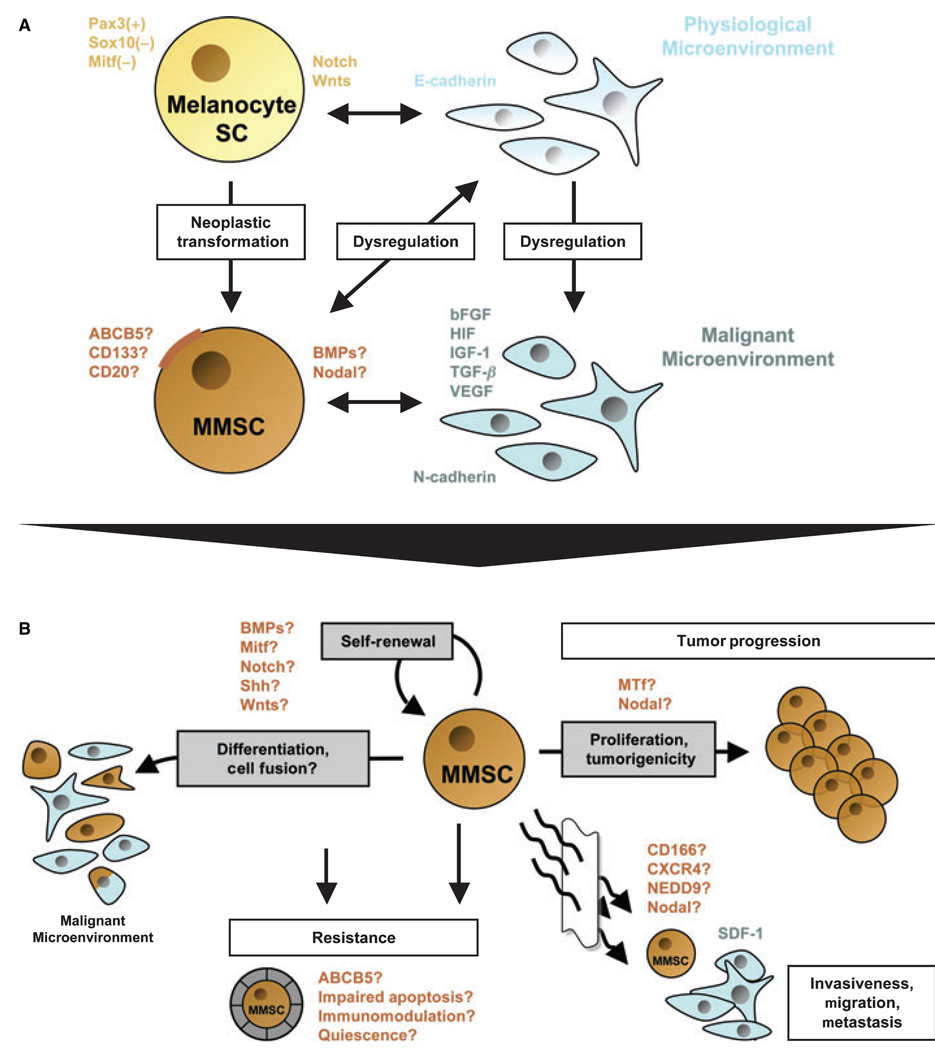
Accumulating evidence supports the presence and involvement of CSC in tumor initiation and progression, chemoresistance and therapeutic failure in human malignant melanoma (Fang et al., 2005; Frank et al., 2005; Grichnik et al., 2006; Monzani et al., 2007; Topczewska et al., 2006; Wang et al., 2006). Similar to physiological tissues, melanomas consist of phenotypically heterogeneous cell populations (Fang et al., 2005; Frank et al., 2005; Hendrix et al., 2003). Highly aggressive melanoma cell subsets have been associated with molecular signatures that resemble those of pluripotent stem cells (Bittner et al., 2000; Hendrix et al., 2003). In addition, stem and progenitor cell-associated proteins have been detected in melanoma, such as cancer testis antigens (Simpson et al., 2005), bone morphogenetic proteins (BMP) (Rothhammer et al., 2007), Notch receptors (Balint et al., 2005), Wnt proteins (Weeraratna et al., 2002), or the ABCB5, CD133, CD166, CD34, nestin, or c-kit stem cell antigens (Frank et al., 2005; Hendrix et al., 2003; Klein et al., 2007; Van Kempen et al., 2000). Recent findings of melanoma subsets with embryonic-like differentiation plasticity and increased tumorigenic potential (Fang et al., 2005; Monzani et al., 2007; Topczewska et al., 2006) further suggest the existence of MMSC, which may contribute to natural progression and therapeutic failure in this disease (a hypothetical model summarizing defining characteristics of putative MMSC populations in the context of molecular networks and distinct microenvironmental clues that may foster MMSC-driven melanoma initiation, progression, and resistance, discussed in this review, is given in Figure 1).
Figure 1.
Schematic model of a potential relationship between melanocyte stem cells (Melanocyte SC) and putative malignant melanoma stem cells (MMSC) in the context of distinct physiological or malignant microenvironments, and overview of defining features and functional properties ascribed to putative MMSC. (A) Physiological melanocyte stem cells are characterized by a Pax3+, Sox10− and Mitf− phenotype, which promotes immaturity and quiescence. The balance between melanocyte SC self-renewal and differentiation, quiescence and proliferation in the hair follicle is further regulated by Notch and/or Wnt signaling molecules. It is thought that the homeostasis of these lineage-restricted SC is maintained through interactions with the physiological microenvironment, mainly composed of E-cadherin-expressing keratinocytes. MMSC might arise by cumulative oncogenic mutations and/or loss of tumor suppressors from long-lived melanocyte stem cells, which already contain the machinery for indefinite self-renewal. Surface markers that have been associated with melanoma stem-like cells include ABCB5, CD133, or CD20. Moreover, morphogens such as Nodal or BMP4 may be secreted by putative MMSC populations. In addition to the neoplastic transformation of the melanocyte stem cell itself, distinct changes in the microenvironment may facilitate the formation of MMSC and subsequent MMSC-driven melanomagenesis. A dysregulated, malignant microenvironment described for melanocytic tumors is characterized by the production of large quantities of proliferative growth factors, including bFGF, HIF, IGF-1, TGF-β, and VEGF. These paracrine mediators, produced mainly by N-cadherin-expressing endothelial cells or surrounding fibroblasts, may drive MMSC proliferation and hence melanoma progression. On the other hand, MMSC might actively alter their surrounding microenvironment through secreted factors (i.e., Nodal or BMP), thus sustaining a niche hospitable to tumor initiation and growth. (B) Defining characteristics of putative MMSC populations and their potential involvement in melanoma initiation, progression, and resistance. Analogous to the cancer stem cell (CSC) definition, MMSC should exhibit (1) increased in vivo tumorigenicity, (2) in vivo self-renewal capacity, and (3) the competence to differentiate into phenotypically diverse populations, including non-tumorigenic cancer cells present in the original tumor. Regulatory signaling networks operative in physiological stem cells that have been shown to be dysregulated in malignant melanoma, such as BMP, Mitf, Notch, Shh, or Wnts, may likewise modulate the balance between self-renewal and differentiation of MMSC. Molecules that have been associated with the tumorigenic potential of malignant melanoma, and which might be involved in MMSC-driven tumor growth, include melanotransferrin (MTf) and Nodal. Transdifferentiation potential, which has been ascribed to distinct melanoma subsets, might likewise involve MMSC. In addition MMSC fusion with more differentiated, including physiological cell types, might represent a possible mechanism by which putative MMSC could mimic a physiological phenotype and function. The differentiation plasticity of MMSC may mask malignant populations from immune surveillance and could thus contribute to melanoma resistance. The mechanisms by which normal stem cells tend to be more resistant than mature cell types, including increased expression of ABC transporters, relative quiescence, immunomodulatory functions, or impairment of apoptotic pathways, may likewise contribute to the refractoriness of putative MMSC. Finally, candidate factors, such as CD166, CXCR4, or NEDD9, might also play a role in putative MMSC-driven melanoma cell invasion, migration, and metastasis, as a correlate of their known physiological and pathophysiological functions.
Despite the suggestive evidence, however, a molecular marker for the prospective isolation of MMSC subpopulations that exhibit increased in vivo tumorigenicity, the capacity for in vivo self-renewal, and the ability to generate phenotypically diverse populations of non-tumorigenic cancer cells present in the initial tumor in serial xenotransplantation experiments (Reya et al., 2001), has not been identified in the scientific literature to date.
Melanoma heterogeneity and tumorigenic melanoma subpopulations
Increased in vivo tumorigenicity compared with tumor bulk components is one of the hallmarks of CSC (Al-Hajj et al., 2003; Bonnet and Dick, 1997; Lapidot et al., 1994; O’Brien et al., 2007; Reya et al., 2001; Ricci-Vitiani et al., 2007; Singh et al., 2004). As in many other cancers, malignant subpopulations of increased tumor formation capacity in immunodeficient mice are also found in human malignant melanoma (Fang et al., 2005; Monzani et al., 2007; Topczewska et al., 2006).
Fang et al. (2005) described melanoma cell subsets capable of forming non-adherent spheres when cultured in growth medium for human embryonic stem cells. The sphere formation approach was originally established for physiological stem cells of the brain, which selectively grow under these culture conditions, whereas differentiated cells rapidly die in response to mitogens present in the medium (Reynolds and Weiss, 1992). The sphere-forming subpopulations of human melanoma cells, which differentiated under appropriate conditions into multiple cell lineages and preferentially expressed the CD20 marker, were more tumorigenic when grafted to mice than their adherent counterparts with lesser differentiation plasticity (Fang et al., 2005). Sphere-forming melanoma cells persisted after dissociation and re-plating of in vivo-formed xenografts and in long-term cultures in vitro, indicating their competence to self-renew. Based on these findings, the authors proposed that melanomas contain a subpopulation of stem-like cells that contribute to heterogeneity and tumorigenesis (Fang et al., 2005). Although xenografted sphere-derived cells gave rise to significantly larger tumors, the difference in tumor formation capacity compared with adherent populations was modest (3/5 versus 1/5 tumor xenografts, respectively) (Fang et al., 2005). Furthermore, some melanoma spheres lost their ability to differentiate into certain cell lineages over time, indicating potential limitations of the sphere formation assay in efficiently enriching for CSC subsets. While the results by Fang et al. (2005) do not establish the presence of prospectively and molecularly defined MMSC, they provide a rationale to further examine CD20-sorted or other minority populations present in more tumorigenic melanoma spheres in serial xenotransplantation experiments to identify MMSC.
Our group recently cloned and characterized ABCB5 (Frank et al., 2003, 2005), a novel human MDR P-glycoprotein family member, which mediates chemoresistance in melanoma via its function as a drug efflux transporter (Frank et al., 2005). Remarkably, ABCB5 expression is closely co-regulated with melanoma tumor antigen p97 [melanotransferrin (MTf)], a molecule associated with melanoma growth (Suryo Rahmanto et al., 2007). In addition, we found ABCB5 to be specifically expressed on CD133+ tumor stem cell phenotype (O’Brien et al., 2007; Ricci-Vitiani et al., 2007; Singh et al., 2004)-expressing minority populations among heterogeneous malignant melanoma cultures and within clinical human malignant melanomas of both primary and metastatic origin (Frank et al., 2005).
As tumor cell subsets defined by CD133 enrich for CSC in colonic cancers (O’Brien et al., 2007; Ricci-Vitiani et al., 2007) and tumors of the brain (Singh et al., 2004), in a subsequent study Monzani et al. (2007) compared the abilities of CD133+ versus CD133− melanoma cells to initiate tumor formation in vivo. Importantly, primary tumor initiating properties were exclusively contained within melanoma cell subsets characterized by expression of CD133, whereas CD133− melanoma cells were found to lack tumorigenicity (Monzani et al., 2007). In contrast to the findings by Fang et al. (2005), more tumorigenic CD133+ melanoma subsets were confined to adherent cell populations, whereas melanoma sphere-associated cells were devoid of CD133 (Monzani et al., 2007). In tumor xenografts in vivo, and under standard culture conditions in vitro, a melanoma cell line was found to be comprised of a majority of CD133+ cells expressing markers of melanoma cell plasticity (Hendrix et al., 2003) and low levels of the side-population determinant ABCG2 (Zhou et al., 2001). Based on their findings, Monzani et al. (2007) proposed that MMSC could be defined by a CD133+/ABCG2+ phenotype, but serial xenotransplantation of purified populations prospectively identified by these markers was not performed in this study. Indeed, when others examined ABCG2 as a candidate prospective marker of tumorigenic cancer subpopulations, the molecule was not found to serve such a function (Patrawala et al., 2005). In addition, CD133+ subpopulations may not be consistently present in all primary and metastatic melanomas according to earlier findings (Klein et al., 2007). Hence, more extensive studies, including secondary tumor formation and stringent self-renewal and differentiation experiments are needed to draw more definitive conclusions about CD133 or ABCG2 as candidate MMSC markers.
In a separate study, Grichnik et al. (2006) isolated melanoma cell subsets using the flow cytometry-based side population (SP) technique, which defines cell populations based on their ability to efflux the Hoechst dye (Goodell et al., 1996). Importantly, the SP phenotype has been proposed as a surrogate marker of physiological stem cells (Goodell et al., 1996) and has also served as a determinant of cancer subpopulations with increased tumorigenic capacity (Patrawala et al., 2005). Grichnik and colleagues described melanoma SP cells, which were unmelanized and small in size and had the capacity to give rise to larger, pigmented cells upon clonal purification (Grichnik et al., 2006). Interestingly, the residual proliferative capacity of melanoma cultures treated with the cytotoxic agent Ara-C (β-d-arabinofuranoside hydrochloride) was concentrated in this cell fraction (Grichnik et al., 2006), which may relate to the expression of ABC transporters associated with the SP phenotype (Zhou et al., 2001). However, the molecular and cytological profiles of SP subsets in normal and malignant tissues are not well defined, and the evidence that these flow cytometry-sorted subpopulations are enriched for stem cells is only correlative.
Further, potential markers of stem-like melanoma subpopulations are indicated by the study of Topczewska et al. (2006) who showed that aggressive melanoma cells secrete the embryonic morphogen Nodal, a member of the TGF-β superfamily. Most notably, inhibition of Nodal signaling reduced both colony formation in soft agar and melanoma tumorigenicity in an orthotopic mouse model (Topczewska et al., 2006). In addition, Nodal knockdown also promoted melanoma cell differentiation, as evidenced by an increase in tyrosinase activity and decreased vascular endothelial cadherin (VE-cadherin) and keratin levels (Topczewska et al., 2006). Given the heterogeneous expression of Nodal in clinical melanoma specimen and its requirement for optimal tumor growth, the authors suggested that Nodal might be a marker of the melanoma stem cell phenotype (Topczewska et al., 2006).
In summary, several recent studies have identified tumorigenic melanoma subpopulations in human to immunodeficient mouse xenotransplantation experiments (Fang et al., 2005; Monzani et al., 2007; Topczewska et al., 2006). While extensions of these findings might ultimately lead to the molecular identification of MMSC, clearly, enhanced tumorigenicity of a particular melanoma subpopulation alone is not sufficient to define cancer ‘stemness’.
The microenvironmental niche, a prominent regulator of tumor growth
Cancer stem cells, like normal stem cells, may depend on cellular interactions with differentiated, physiological cell types or on their own non-tumorigenic cancer bulk populations to sustain stem cell features (Scadden, 2006). In addition, secreted factors or other niche components may regulate tumor establishment, growth, and progression (Scadden, 2006). Tumorigenicity as determined in xenotransplantation experiments may further vary with the applied experimental conditions, such as the species-specific host milieu, microenvironmental clues at the tissue site of injection, or the activation status of immune effector mechanisms in recipient animals. In support of this hypothesis, Kelly et al. (1997) showed that a large proportion of mouse tumor cells could sustain myeloid and lymphoid neoplasms in histocompatible recipient mice. These findings stand in contrast to those obtained using human to immunodeficient mouse xenotransplantation models, which identified a rare subset of human CD34+CD38− leukaemia initiating cells exclusively capable of tumor development and growth (Bonnet and Dick, 1997; Lapidot et al., 1994).
A pertinent role of the host milieu and/or the micro-environment in tumorigenesis has also been recognized for malignant melanoma (Hendrix et al., 2007). For example, exposure to embryonic skin in utero could inhibit the tumor formation capacity of murine B16 melanoma cells (Gerschenson et al., 1986). Likewise, the chick or zebrafish embryonic microenvironments were able to prevent tumorigenesis when human metastatic melanoma cells were implanted (Kulesa et al., 2006; Lee et al., 2005). On the other hand, endothelial cell-and/or fibroblast-secreted growth factors can create a malignant microenvironment that propagates the switching of cadherin subtypes and the decoupling of melanocytes or early stage melanoma cells from the basement membrane, thus accelerating malignant melanocyte transformation and ultimately melanoma cell proliferation and progression (Haass et al., 2005; Hsu et al., 2002; Lee and Herlyn, 2007). Another milieu furthering tumorigenicity and advanced stage disease might be a microenvironment devoid of molecular oxygen, which results in the induction of HIF-1 (hypoxia-inducible factor), increased expression of VEGF, and decreased E-cadherin levels (Lee and Herlyn, 2007). Taken together, these findings establish that the tumorigenic potential of malignant cells, including CSC, may be altered by distinct microenvironments.
Putative CSC might also reciprocally modulate the surrounding niche through the secretion of paracrine factors or direct cell–cell contact. For example, Nodal signaling by aggressive melanoma cells injected into zebrafish blastula resulted in the formation of ectopic embryonic axes, presumably by influencing endogenous progenitor cell fate (Topczewska et al., 2006). Similarly, Nodal and other signaling molecules may sustain cancer niche environments that enable tumorigenic cancer populations to initiate and promote tumor growth. For instance, the melanoma tumor microenvironment may inhibit the activation of immune effector mechanisms (Gajewski, 2006). Strategies by which tumorigenic melanoma cells may evade immune surveillance include the secretion of soluble immunoregulatory mediators, such as TGF family members, the induction of regulatory T cells or plasmocytoid dendritic cells (Baumgartner et al., 2007; Mccarter et al., 2007; Viguier et al., 2004), or the expression of negative costimulatory molecules, such as programmed cell death 1 ligand (Dong et al., 2002). These mechanisms parallel those described for bone marrow-derived MSC, which have also been shown to exert immunoregulatory effects (Frank and Sayegh, 2004; Le Blanc et al., 2004). Taken together, the available evidence allows for the possibility that a subpopulation of immunoprivileged MMSC may foster tumor formation at least in part via attenuation of the immune response.
These findings further highlight that distinct features of the niche environment may complicate the interpretation of tumorigenicity experiments with sorted, isolated cell subsets, particularly in xenotransplantation models. Thus, the identification of MMSC may prove challenging, when pursued outside the context of tumor bulk populations and/or requisite human support cells. Ideally, assessment of enhanced tumorigenicity, self-renewal and differentiation capacity of tumor subpopulations, and thus the identification of CSC, would therefore need to occur in a dynamic tumor environment by co-grafting genetically labeled putative MMSC with non-tumorigenic bulk populations.
Differentiation plasticity of malignant melanoma cells
During development, a distinct microenvironmental milieu can govern the specification of pluripotent embryonic stem cells (ESC) (Lotem and Sachs, 2006). Multi-lineage differentiation plasticity is one of the hallmarks of ESC (Reya et al., 2001). While differentiation in light of the CSC concept refers to the ability of tumor cells to give rise to phenotypically diverse populations (including non-tumorigenic cancer cells) that recapitulate the histological features of the initial tumor in serial xenotransplantation experiments (Reya et al., 2001), a multipotent, plastic, embryonic-like phenotype has also been proposed as a defining characteristic of putative MMSC populations (Fang et al., 2005; Hendrix et al., 2003; Monzani et al., 2007).
In fact, aggressive melanoma cells display elevated levels of genes associated with embryonic development and pluripotency when compared with their poorly aggressive counterparts (Bittner et al., 2000). For instance, the spectrum of overexpressed genes was found to include some that are characteristic of hematopoietic, neuronal, muscle, endothelial, and additional progenitor cell types (Bittner et al., 2000). Furthermore, endothelium-associated genes have been found expressed by aggressive melanoma cells, including VE-cadherin, tyrosine kinase receptor 1, or ephrin receptor A2, which are functionally involved in physiological angiogenesis and vasculogenesis (Hendrix et al., 2003).
While it is thought that the tumor vasculature is mostly composed of non-malignant endothelial cell populations originating from host angiogenesis (Folkman, 1995; Risau, 1997), there exists now evidence that melanoma cells with high degrees of differentiation plasticity may contribute to the de novo formation of tumor blood vessels (vasculogenesis) via a process termed vasculogenic mimicry (Hendrix et al., 2003). These extracellular matrix (ECM)-rich vasculogenic tumor cell networks were shown to conduct fluid, contained fibrin-ogen and anti-coagulant factors, and might thus provide for surrogate, melanoma cell-driven methods of tumor perfusion, nutritional exchange, and dissemination (Hendrix et al., 2003; Maniotis et al., 1999). Interestingly, addition of angiogenesis inhibitors to melanoma cell cultures in vitro did not affect tubular network formation, while angiogenesis by human vascular endothelial cells was abrogated under similar conditions (Van Der Schaft et al., 2004). These findings suggest that vasculogenic mimicry might represent an important survival mechanism contributing to the failure of currently available angiogenesis inhibitors to fully effect tumor eradication (Folkman, 2007).
Importantly, BMP, secreted morphogen members of the TGF-β superfamily that play critical roles in early embryonic vascular development, have also been linked to melanoma cell-driven vasculogenesis (Rothhammer et al., 2007). Furthermore, Rothhammer et al. (2007) described that impaired BMP4-activity resulted in reduced tumor growth concomitant with large necrotic areas in an in vivo mouse model. Of note, BMP4-responsive BMPR1a+ cells coincide with undifferentiated progenitors in a physiological stem cell niche (Frank et al., 2006). Moreover, the BMP4-BMPR1a signaling system can regulate the size of the CSC population in brain tumors (Piccirillo et al., 2006). Based on these findings, it is conceivable that the CSC compartment within a tumor may be responsible for tumor vasculogenesis, and that such a potential function of CSC might represent one role by which CSC may drive neoplastic formation and growth.
A reservoir of stem-like plasticity of malignant melanoma is further suggested by the presence of lipoblast-like (Cruz et al., 2003), osteoid (Hoorweg et al., 1997), or cartilaginous (Grunwald and Rothem, 1987) differentiation patterns in some melanoma patient biopsies. In addition, melanoma cells grown as floating spheres can be induced to give rise to multiple cell lineages, including melanocytic, adipocytic, osteocytic, chondrocytic, and neural lineages (Fang et al., 2005; Monzani et al., 2007). The differentiation plasticity of certain types of melanoma cells could contribute to the failure of current therapeutic regimens by masking malignant target populations (Hendrix et al., 2003).
However, the cellular and molecular events underlying tumor cell plasticity are not well understood and the possible relationship of phenomena such as vasculogenic mimicry to putative MMSC is currently unknown. MMSC could potentially truly transdifferentiate into endothelial and other cell types to serve stromal functions ultimately fostering a tumorigenic microenvironment hospitable to metastatic progression. Another possibility is that differentiation plasticity ascribed to aggressive melanoma cells could be based in part on MMSC-mediated recruitment of or MMSC fusion with surrounding physiological cells resembling cell fusion of physiological stem cells with more differentiated cells (Frank et al., 2003; Spees et al., 2003; Wang et al., 2003). Genetic lineage tracking experiments of candidate MMSC would represent a critical first step towards elucidating the possible relationship of stem-like cancer cells to melanoma transdifferentiation. Such assays could identify the potential contribution of MMSC to tumor vasculogenesis, and MMSC-targeted therapies directed at the tumor blood supply could provide for potential novel therapeutic strategies.
Self-renewal and differentiation pathways operative in physiological stem cells – insights into melanomagenesis
Regulatory signaling pathways, such as Sonic hedgehog (Shh), Wnt, or Notch, modulate the differentiation plasticity of physiological stem cells and promote stem cell self-renewal (Golan-Mashiach et al., 2005; Lotem and Sachs, 2006; Reya and Clevers, 2005; Reya et al., 2001; Taipale and Beachy, 2001). There is evidence that several key mechanisms that control physiological stem cell function may also regulate melanomagenesis and progression (Chin et al., 2006). These melanoma signaling pathways have been reviewed in detail (Chin, 2003; Chin et al., 2006; Haluska et al., 2006; Herlyn, 2006). Here we provide a brief overview of some of the most relevant pathways in light of the CSC concept.
In physiological settings, the hedgehog family, including Shh, is essential for embryonic skin development and adult, stem cell-driven skin regeneration (Levy et al., 2005; Taipale and Beachy, 2001). Expression and localization of Shh varied within the hair cycle (Oro and Higgins, 2003) and was found to modulate hair-follicle formation (Levy et al., 2005) and the proliferation of human melanocytes (Stecca et al., 2007). Notably, constitutive activation of the hedgehog signaling pathway drove the development of basal cell carcinomas (Hutchin et al., 2005). In addition, Shh signaling was also found to regulate the proliferation and metastatic potential of human melanomas (Stecca et al., 2007).
Another network of secreted signaling molecules that has been implicated both in the regulation of self-renewal of normal stem cells and in malignant proliferation of cancer cells is the Wnt family (Dorsky et al., 1998; Gat et al., 1998; Reya and Clevers, 2005; Rubinfeld et al., 1997). For instance, Wnt5a signaling directly correlated with increasing tumor grade and invasion of metastatic melanoma (Weeraratna et al., 2002) and inhibition of the Wnt2 ligand induced apoptosis in vitro and suppressed tumor growth in a melanoma xenograft model in vivo (You et al., 2004). In addition, activation of β-catenin, a key effector of the canonical Wnt cascade, was shown to promote pigment cell formation (Dorsky et al., 1998). Furthermore, enforced β-catenin signaling was linked to higher proliferative capacity, impaired differentiation, and increased self-perpetuation of epidermal and hematopoietic stem cells (Reya et al., 2001; Zhu and Watt, 1999) and the de novo formation of hair follicles (Gat et al., 1998). Aberrant β-catenin activation in transgenic mice led to the development of hair tumors (Gat et al., 1998). In the context of melanoma, mutational events resulting in constitutively elevated β-catenin levels, for example through the deregulation of proteins involved in its bio-degradation, were associated with disease progression (Larue and Delmas, 2006; Rubinfeld et al., 1997).
Similarly, overexpression of Notch receptors and their ligands Jagged-1, Jagged-2 or Delta-like 1 proteins correlated with melanocytic tumor growth and progression (Hoek et al., 2004; Massi et al., 2006). In addition, ectopically enforced Notch1 signaling enhanced melanoma cell proliferation and metastatic potential via activation of the β-catenin or the mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt (MAPK/PI3K–Akt) pathways (Balint et al., 2005; Liu et al., 2006). In normal tissue homeostasis, Notch activation fostered physiological stem cell self-renewal, for example in the neural or hematopoietic systems (Shen et al., 2004; Varnum-Finney et al., 2000).
A potential link between normal stem cells and CSC is further indicated by the existence of common self-maintenance mechanisms involving the PTEN (phosphatase and tensin homolog) tumor-suppressor pathway (Yilmaz et al., 2006). Importantly, loss of PTEN function promoted melanoma development (Stahl et al., 2003). Additional tumor-suppressor pathways that regulate normal stem cell self-renewal (Molofsky et al., 2003) and also control melanomagenesis when inactivated include two distinct proteins encoded by the CDKN2A locus, namely p16 (also known as INK4A) and ARF (the alternative reading frame of the CDKN2A locus) (Chin et al., 2006).
Finally, mutations of the melanocyte master regulator Mitf have been implicated in self-renewal deficiency of melanocyte stem cells (Nishimura et al., 2005). Importantly, the melanoma oncogene Mitf (Garraway et al., 2005) was recently shown to control melanoma cell proliferation and invasiveness via regulation of the diaphanous-related formin Dia1 (Carreira et al., 2006). Mitf amplification was also associated with tumorigenicity, disease progression, and decreased melanoma patient survival (Garraway et al., 2005).
In summary, genetic alterations of the signal-transduction machinery of physiological stem cell maintenance and differentiation significantly contribute to malignant melanoma development and progression. The convergence of self-renewal mechanisms between normal stem cells and CSC in other tissues (Reya and Clevers, 2005; Reya et al., 2001; Sanai et al., 2005) suggests the involvement of MMSC in the pathogenesis of melanocytic tumors. Therefore, studying self-renewal pathways and their deregulation in malignant melanoma may help to identify MMSC populations and could provide novel insights into the cellular events responsible for MMSC-driven tumorigenic growth.
Genomic instability and cancer – clues on putative MMSC populations
Genomic instability and substantially altered cancer genomes are hallmark features of malignant neoplasms including melanoma (Futreal et al., 2004; Storchova and Pellman, 2004). Specifically, melanomas manifest extensive chromosomal rearrangements, such as translocations, chromosomal amplifications, or deletions, and, in addition, germline or somatic point mutations, some of which may govern disease development and progression (Chin et al., 2006; Curtin et al., 2005). Malignant transformation and the acquisition of metastatic features represent complex, multi-step processes (Hahn and Weinberg, 2002). While such cumulative genomic alterations may lead to diverse melanoma populations with differential cytogenetic abnormalities, uniform genetic characteristics between primary tumors and metastases from the same patient would suggest a clonal progression of melanoma (Bevilacqua et al., 1998). Moreover, melanocytic tumors would thus originate from a common, transformed ancestor.
Indeed, similar patterns of chromosomal aberrations were identified by comparative genomic hybridization between primary VGP and RGP melanomas (Bastian et al., 1998) and between primary and metastatic melanomas (Balazs et al., 2001). In a recent report, Wang et al. (2006) cytogenetically compared primary melanoma material and biopsies from a series of recurrent lesions collected from a single patient, who experienced repeated clinical remissions and relapses over a decade of observation. In spite of marked phenotypic variations, consistent genetic traits and chromosomal imbalances persisted among the distinct tumor specimens examined, indicating that all metastases were clonally derived from the same primary tumor (Wang et al., 2006). Interestingly, a rare point mutation of the β-catenin gene was identified in each of the metastatic samples (Wang et al., 2006). Although some genetic changes were shared in this series of patient biopsies, not all of the aberrations were perpetuated (Wang et al., 2006).
Taken together, these findings suggest that melanomas may arise from mutations and chromosomal rearrangements that accumulate in a common precursor cell pool, which subsequently drives the establishment of primary lesions upon cancerogenous transformation. The data by Wang et al. further indicate, that the potential to metastasize is confined to the very same minority population, while its genomically less stable progeny is unable to spread the disease (Grichnik, 2006). This hypothesis is in line with the CSC concept, which postulates that it is the CSC subset that drives both primary tumor formation and metastasis (Reya et al., 2001).
Tumor progression and metastasis – processes driven by MMSC?
Metastasis is defined as the process by which cancer cells escape the primary neoplasm and establish new tumor colonies at distant organ or tissue sites. This process requires tumor cell migration into the lymphatic system and/or dissemination through the blood circulation (Fidler, 2003). Melanoma is a highly metastatic cancer and the 5-yr survival rate of melanoma patients with lung or other visceral metastasis is <10% (Balch et al., 2001). In spite of its clinical significance, the cellular and molecular mechanisms underlying metastatic melanoma progression remain inadequately understood.
Competing theories have been proposed for the evolution of metastases (Bernards and Weinberg, 2002). One theory, the clonal selection model, postulates that the continuous accumulation of mutations and chromosomal imbalances may lead to foci of cancerous cells with metastatic properties in advanced stages of disease. However, an alternative hypothesis has been put forth whereby the tendency to metastasize may be determined early in tumorigenesis (Fidler and Kripke, 1977). This hypothesis is supported by the recent identification of metastatic signatures present in primary tumor cells or their transformed precursors prior to neoplastic progression (Gupta et al., 2005; Ramaswamy et al., 2003). Of note, functional genomic studies further suggest that distinct cancer cell subsets within primary lesions are preordained to metastasize to specific organs (Minn et al., 2005). In light of the CSC concept, it is plausible that such minority populations may coincide with stem-like cancer cells with the capacity to initiate tumor formation. In support of this notion, some characteristics ascribed to physiological stem cells and putative CSC populations show striking parallels to cellular attributes underlying the metastatic process. Moreover, niche constituent cells or signaling pathways, that function as key modulators of normal stem cell proliferation and migration (Scadden, 2006), also play pivotal roles in tumor cell invasion, dissemination, and engraftment (Fidler, 2003).
Specifically, cellular niche components have been found to provide anchoring sites for somatic stem cells, for example in the bone marrow, where haematopoietic stem cells (HSC) are physically attached to osteoblastic cells (Calvi et al., 2003; Zhang et al., 2003). This attachment process is orchestrated by the interplay of distinct signaling molecules including β-catenin, the main downstream effector of the evolutionary conserved Wnt cascade (Zhang et al., 2003). Aberrant activation of the Wnt pathway, a feature associated with melanoma progression (Larue and Delmas, 2006), results in the translocation of β-catenin from adherens junctions to the nucleus (Reya and Clevers, 2005). Disruption of such intercellular bonds formed by β-catenin/E-cadherin complexes has been causally linked to the breakdown of epithelial-cell homeostasis during cancer cell invasion termed epithelial to mesenchymal transition (EMT) (Thiery, 2003). Interestingly, some of the key molecules associated with melanocyte stem cell maintenance and melanoma metastasis, such as Slug or Twist (Gupta et al., 2005; Yang et al., 2004), also act as EMT-inducing, transcriptional repressors of E-cadherin expression (Nieto, 2002). Thus, interrelations between Wnt signaling factors and the cadherin-catenin adhesion system govern both the localization of normal stem cells in their niche and, when dysregulated, invasive melanoma cell growth. Other molecules involved in local melanoma invasion (Seftor et al., 1999) that also regulate normal stem cell adhesion include integrins (Hirsch et al., 1996) and matrix metalloproteinases (Heissig et al., 2002). Most notably, a recent comparative oncogenomics study led to the identification of a novel melanoma metastasis gene, NEDD9, which is required for melanoma cell invasion and dissemination as determined in a series of loss-of-function and gain-of-function experiments (Kim et al., 2006). Moreover, NEDD9 expression in patient biopsies correlated with human melanoma progression (Kim et al., 2006). Interestingly, NEDD9 was also found overexpressed in physiological CD133+ human cord blood progenitor cells exposed to hypoxia (Martin-Rendon et al., 2007), an environmental milieu that likewise enhances the metastatic potential of cancer cells (Pouyssegur et al., 2006).
An additional parallel between stem cells and metastatic melanoma subsets is given by their potential to migrate to distinct target organs or tissues. This migratory capacity, which is crucial both for tissue homeostasis and metastatic progression, is based on common regulatory mechanisms. For example, stromal cell derived factor 1 (SDF-1)-signaling through the C-X-C chemokine receptor type 4 (CXCR4) was shown to regulate HSC migration, homing, and engraftment (Peled et al., 1999). Similarly, the SDF-1/CXCR4 system was found to direct the dissemination of tumor subpopulations in melanoma (Murakami et al., 2002) and other cancers (Muller et al., 2001). Inhibition of interactions between CXCR4, expressed by melanoma cells, and its ligand SDF-1, produced at sites of tumor cell engraftment, suppressed melanoma metastasis to murine lungs (Cardones et al., 2003; Takenaga et al., 2004). In addition, enhanced expression levels of CXCR4 correlated with poor prognosis in patients with malignant melanoma (Scala et al., 2005). Another molecular enhancer of melanoma migration is the ECM protein osteopontin (Hayashi et al., 2007), which was also implicated as a negative regulator of the HSC pool size (Stier et al., 2005).
In a recent study, Kaplan et al. (2005) demonstrated how melanoma cell-conditioned media could program hematopoietic progenitor cells to home to pre-metastatic sites prior to tumor cell engraftment. Through the disruption of molecular networks sustaining the given microenvironment, the recruited progenitor cell clusters ultimately generated permissive, pre-metastatic niches for incoming tumor cells (Kaplan et al., 2005). Thus, yet to be determined melanoma cell-secreted factors triggered a complex cascade of cellular interactions underlying the establishment of a metastatic tumor focus.
Whether CSC populations may facilitate the formation of pre-metastatic environments and hence enable neoplastic progression remains an open question. In support of this theory, however, the melanoma cell-secreted morphogen Nodal, which was also found to orchestrate physiological progenitor cell function, was proposed as a candidate marker of MMSC and its expression correlated with melanoma progression in patients (Topczewska et al., 2006). Putative MMSC populations may hence establish their own microenvironment through the secretion of Nodal or other soluble mediators that may foster surrounding physiological cells to co-operate in establishing a metastatic tumor focus. An increased expression in metastatic melanoma biopsies compared with primary lesions and/or benign nevi was also noted for the stem cell markers CD133, CD166, nestin (Klein et al., 2007; Van Kempen et al., 2000), and members of the Notch family (Balint et al., 2005; Massi et al., 2006). This correlation of putative CSC determinants with advanced stages of disease, further suggests a potential role of MMSC in the metastatic process.
The MMSC concept could for example explain why the majority of melanoma cells detectable in the blood circulation remain unable to initiate metastatic lesions (Fidler and Talmadge, 1986). The clonal origin of heterogeneously diverse melanoma metastases (Fidler and Talmadge, 1986) might relate to the recruitment of putative tumorigenic MMSC to the pre-metastasis niche, where they could again give rise to more differentiated progeny. Moreover, the inherent plasticity associated with putative MMSC populations (Fang et al., 2005; Monzani et al., 2007) paired with epigenetic and/or genetic alterations might hereby provide for a selective advantage in adapting to a foreign microenvironment. Finally, tissue-specific metastasis and a migratory phenotype may be a function of the molecular networks operative in the physiological precursor cell pool, from which putative MMSC originate.
Perspectives – translational relevance of the MMSC concept
Findings of melanoma subpopulations of primitive molecular phenotype, which display an embryonic-like differentiation plasticity and increased tumorigenic potential strongly suggest the presence of MMSC in melanocytic tumors (Fang et al., 2005; Frank et al., 2005; Grichnik et al., 2006; Hendrix et al., 2003; Monzani et al., 2007; Topczewska et al., 2006). In addition, distinct sets of molecules and pathways which have been shown to orchestrate normal stem cell self-renewal and differentiation (Reya et al., 2001), are likewise operative in certain melanoma subsets (Chin et al., 2006). To study MMSC and to further dissect their biologic role in neoplastic formation and growth, however, a molecular marker is critically required to prospectively isolate such melanoma stem-like subpopulations. The identification of molecularly defined MMSC should shed light on the cellular events driving melanomagenesis and may provide improved experimental systems for the discovery of MMSC-targeted novel therapeutic strategies.
Chemotherapeutic refractoriness of advanced malignant melanoma may be attributable to several resistance mechanisms that have been implicated in tumor stem cell biology (Dean et al., 2005), including the impairment of cancer apoptotic pathways, a relative mitotic quiescence, and reduced drug accumulation because of high-expression levels of ABC drug efflux transporters (Gottesman et al., 2002). In particular, tumor cell dormancy and melanoma recurrence following initial remission may be related to the inability of current therapeutic regimens to eradicate the putative MMSC compartment.
Moreover, MMSC might also drive metastatic melanoma progression, as indicated by overlapping signaling networks that regulate both stem cell migration and malignant melanoma dissemination. The identification and molecular characterization of MMSC subsets could thus lead to the development of improved biomarkers for diagnosis, disease staging, and clinical management of melanoma. If MMSC populations are indeed associated with chemoresistance and malignant melanoma progression in human patients, specific targeting of MMSC via a molecular marker could provide more potent and selective means for melanoma therapy.
Acknowledgments
We apologize to those colleagues whose studies were not cited in this review because of space limitations. Our research is supported by the NCI/NIH (grant 1RO1CA113796-01A1 to M.H.F.) and the NCI/NIH Specialized Program of Research Excellence (SPORE) in Skin Cancer (grant 2P50CA093683-05S2 to Thomas S. Kupper).
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. U. S. A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc. Natl Acad. Sci. U. S. A. 2005;102:5530–5534. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs M, Adam Z, Treszl A, Begany A, Hunyadi J, Adany R. Chromosomal imbalances in primary and metastatic melanomas revealed by comparative genomic hybridization. Cytometry. 2001;46:222–232. doi: 10.1002/cyto.1131. [DOI] [PubMed] [Google Scholar]
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J. Clin. Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J. Clin. Invest. 2005;115:3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian BC, Leboit PE, Hamm H, Brocker EB, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–2175. [PubMed] [Google Scholar]
- Baumgartner J, Wilson C, Palmer B, Richter D, Banerjee A, Mccarter M. Melanoma induces immunosuppression by up-regulating FOXP3(+) regulatory T cells. J. Surg. Res. 2007;141:72–77. doi: 10.1016/j.jss.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddingfield FC., III The melanoma epidemic: res ipsa loquitur. Oncologist. 2003;8:459–465. doi: 10.1634/theoncologist.8-5-459. [DOI] [PubMed] [Google Scholar]
- Bernards R, Weinberg RA. A progression puzzle. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- Bevilacqua RA, Nunes DN, Stroun M, Anker P. The use of genetic instability as a clinical tool for cancer diagnosis. Semin. Cancer Biol. 1998;8:447–453. doi: 10.1006/scbi.1998.0122. [DOI] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat. Rev. Cancer. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Breslow A. The surgical treatment of stage I cutaneous melanoma. Cancer Treat. Rev. 1978;5:195–198. doi: 10.1016/s0305-7372(78)80013-9. [DOI] [PubMed] [Google Scholar]
- Bruce WR, Van Der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–6757. [PubMed] [Google Scholar]
- Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS, Testori A, Larue L, Goding CR. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006;20:3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat. Rev. Cancer. 2003;3:559–570. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- Clark WH, Jr, Elder DE, Guerry DT, Epstein MN, Greene MH, Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum. Pathol. 1984;15:1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Cock AG, Cohen J. The melanoblast reservoir available to a feather papilla. J. Embryol. Exp. Morphol. 1958;6:530–545. [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Cruz J, Reis-Filho JS, Lopes JM. Primary cutaneous malignant melanoma with lipoblast-like cells. Arch. Pathol. Lab. Med. 2003;127:370–371. doi: 10.5858/2003-127-0370-PCMMWL. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- Dupin E, Glavieux C, Vaigot P, Le Douarin NM. Endothelin 3 induces the reversion of melanocytes to glia through a neural crest-derived glial-melanocytic progenitor. Proc. Natl Acad. Sci. U. S. A. 2000;97:7882–7887. doi: 10.1073/pnas.97.14.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Fialkow PJ. Clonal origin of human tumors. Biochim. Biophys. Acta. 1976;458:283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Talmadge JE. Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res. 1986;46:5167–5171. [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N. Engl. J. Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Fountain JW, Bale SJ, Housman DE, Dracopoli NC. Genetics of melanoma. Cancer Surv. 1990;9:645–671. [PubMed] [Google Scholar]
- Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004;363:1411–1412. doi: 10.1016/S0140-6736(04)16134-5. [DOI] [PubMed] [Google Scholar]
- Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH, Frank MH. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J. Biol. Chem. 2003;278:47156–47165. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- Frank NY, Kho AT, Schatton T, Murphy GF, Molloy MJ, Zhan Q, Ramoni MF, Frank MH, Kohane IS, Gussoni E. Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin. J. Cell. Biol. 2006;175:99–110. doi: 10.1083/jcb.200511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, Rahman N, Stratton MR. A census of human cancer genes. Nat. Rev. Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin. Cancer Res. 2006;12 doi: 10.1158/1078-0432.CCR-05-2517. 2326s–2330s. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer. 2005a;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer. 2005b;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- Gat U, Dasgupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Graves K, Carson SD, Wells RS, Pierce GB. Regulation of melanoma by the embryonic skin. Proc. Natl Acad. Sci. U. S. A. 1986;83:7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan-Mashiach M, Dazard JE, Gerecht-Nir S, et al. Design principle of gene expression used by human stem cells: implication for pluripotency. FASEB J. 2005;19:147–149. doi: 10.1096/fj.04-2417fje. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Grichnik JM. Genomic instability and tumor stem cells. J. Invest. Dermatol. 2006;126:1214–1216. doi: 10.1038/sj.jid.5700240. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Ali WN, Burch JA, Byers JD, Garcia CA, Clark RE, Shea CR. KIT expression reveals a population of precursor melanocytes in human skin. J. Invest. Dermatol. 1996;106:967–971. doi: 10.1111/1523-1747.ep12338471. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Burch JA, Schulteis RD, Shan S, Liu J, Darrow TL, Vervaert CE, Seigler HF. Melanoma, a tumor based on a mutant stem cell? J. Invest. Dermatol. 2006;126:142–153. doi: 10.1038/sj.jid.5700017. [DOI] [PubMed] [Google Scholar]
- Grunwald MH, Rothem A. Desmoplastic cartilaginous formation in malignant melanoma. J. Cutan. Pathol. 1987;14:255. doi: 10.1111/j.1600-0560.1987.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat. Genet. 2005;37:1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass NK, Smalley KS, Li L, Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–159. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA. Rules for making human tumor cells. N. Engl. J. Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin. Cancer Res. 2006;12:2301s–2307s. doi: 10.1158/1078-0432.CCR-05-2518. [DOI] [PubMed] [Google Scholar]
- Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Lerner CP. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78:1237–1240. [PubMed] [Google Scholar]
- Hayashi C, Rittling S, Hayata T, Amagasa T, Denhardt D, Ezura Y, Nakashima K, Noda M. Serum osteopontin, an enhancer of tumor metastasis to bone, promotes B16 melanoma cell migration. J. Cell. Biochem. 2007;101:979–986. doi: 10.1002/jcb.21298. [DOI] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmbach H, Sinha P, Schadendorf D. Human melanoma: drug resistance. Recent Results Cancer Res. 2003;161:93–110. doi: 10.1007/978-3-642-19022-3_9. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat. Rev. Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- Herlyn M. Molecular targets in melanoma: strategies and challenges for diagnosis and therapy. Int. J. Cancer. 2006;118:523–526. doi: 10.1002/ijc.21605. [DOI] [PubMed] [Google Scholar]
- Herlyn M, Thurin J, Balaban G, et al. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985;45:5670–5676. [PubMed] [Google Scholar]
- Herlyn M, Clark WH, Rodeck U, Mancianti ML, Jambrosic J, Koprowski H. Biology of tumor progression in human melanocytes. Lab. Invest. 1987;56:461–474. [PubMed] [Google Scholar]
- Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hoek K, Rimm DL, Williams KR, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- Hoorweg JJ, Loftus BM, Hilgers FJ. Osteoid and bone formation in a nasal mucosal melanoma and its metastasis. Histopathology. 1997;31:465–468. doi: 10.1046/j.1365-2559.1997.2890882.x. [DOI] [PubMed] [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70:522–536. doi: 10.1046/j.1432-0436.2002.700906.x. [DOI] [PubMed] [Google Scholar]
- Hutchin ME, Kariapper MS, Grachtchouk M, Wang A, Wei L, Cummings D, Liu J, Michael LE, Glick A, Dlugosz AA. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes Dev. 2005;19:214–223. doi: 10.1101/gad.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J. Natl Cancer Inst. 2001;93:678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JW, Yeatman JM, Regalia C, Mason G, Henham AP. A high incidence of melanoma found in patients with multiple dysplastic naevi by photographic surveillance. Med. J. Aust. 1997;167:191–194. doi: 10.5694/j.1326-5377.1997.tb138843.x. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kim M, Gans JD, Nogueira C, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod. Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl Acad. Sci. U. S. A. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Lee JT, Herlyn M. Microenvironmental influences in melanoma progression. J. Cell. Biochem. 2007;101:862–872. doi: 10.1002/jcb.21204. [DOI] [PubMed] [Google Scholar]
- Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Mcclay EF. Systemic chemotherapy for the treatment of metastatic melanoma. Semin. Oncol. 2002;29:413–426. doi: 10.1053/sonc.2002.35237. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–4190. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- Lotem J, Sachs L. Epigenetics and the plasticity of differentiation in normal and cancer stem cells. Oncogene. 2006;25:7663–7672. doi: 10.1038/sj.onc.1209816. [DOI] [PubMed] [Google Scholar]
- Lucas CR, Sanders LL, Murray JC, Myers SA, Hall RP, Grichnik JM. Early melanoma detection: nonuniform dermoscopic features and growth. J. Am. Acad. Dermatol. 2003;48:663–671. doi: 10.1067/mjd.2003.283. [DOI] [PubMed] [Google Scholar]
- Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev. Biol. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- Mak SS, Moriyama M, Nishioka E, Osawa M, Nishikawa S. Indispensable role of Bcl2 in the development of the melanocyte stem cell. Dev. Biol. 2006;291:144–153. doi: 10.1016/j.ydbio.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Rendon E, Hale SJ, Ryan D, et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–1012. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- Massague J. Sorting out breast-cancer gene signatures. N. Engl. J. Med. 2007;356:294–297. doi: 10.1056/NEJMe068292. [DOI] [PubMed] [Google Scholar]
- Massi D, Tarantini F, Franchi A, Paglierani M, Di Serio C, Pellerito S, Leoncini G, Cirino G, Geppetti P, Santucci M. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Mod. Pathol. 2006;19:246–254. doi: 10.1038/modpathol.3800526. [DOI] [PubMed] [Google Scholar]
- Mayer TC. The migratory pathway of neural crest cells into the skin of mouse embryos. Dev. Biol. 1973;34:39–46. doi: 10.1016/0012-1606(73)90337-0. [DOI] [PubMed] [Google Scholar]
- Mccarter MD, Baumgartner J, Escobar GA, Richter D, Lewis K, Robinson W, Wilson C, Palmer BE, Gonzalez R. Immunosuppressive dendritic and regulatory T cells are upregulated in melanoma patients. Ann. Surg. Oncol. 2007;14:2854–2860. doi: 10.1245/s10434-007-9488-3. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur. J. Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Osawa M, Mak SS, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J. Cell Biol. 2006;173:333–339. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, Hwang ST. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- Nickoloff BJ, Foreman KE. Etiology and pathogenesis of Kaposi’s sarcoma. Recent Results Cancer Res. 2002;160:332–342. [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Osawa M. Generating quiescent stem cells. Pigment Cell Res. 2007;20:263–270. doi: 10.1111/j.1600-0749.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev. Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Osawa M, Egawa G, Mak SS, Moriyama M, Freter R, Yonetani S, Beermann F, Nishikawa S. Molecular characterization of melanocyte stem cells in their niche. Development. 2005;132:5589–5599. doi: 10.1242/dev.02161. [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Peters R, Leyvraz S, Perey L. Apoptotic regulation in primitive hematopoietic precursors. Blood. 1998;92:2041–2052. [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007;26:4158–4170. doi: 10.1038/sj.onc.1210182. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N. Engl. J. Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin. Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- Schepsky A, Bruser K, Gunnarsson GJ, Goodall J, Hallsson JH, Goding CR, Steingrimsson E, Hecht A. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol. Cell. Biol. 2006;26:8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Hendrix MJ. Molecular role(s) for integrins in human melanoma invasion. Cancer Metastasis Rev. 1999;18:359–375. doi: 10.1023/a:1006317125454. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc. Natl Acad. Sci. U. S. A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003;63:2881–2890. [PubMed] [Google Scholar]
- Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz IAA. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc. Natl Acad. Sci. U. S. A. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell. Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- Suryo Rahmanto Y, Dunn LL, Richardson DR. Identification of distinct changes in gene expression after modulation of melanoma tumor antigen p97 (melanotransferrin) in multiple models in vitro and in vivo. Carcinogenesis. 2007;28:2172–2183. doi: 10.1093/carcin/bgm096. [DOI] [PubMed] [Google Scholar]
- Sviderskaya EV, Wakeling WF, Bennett DC. A cloned, immortal line of murine melanoblasts inducible to differentiate to melanocytes. Development. 1995;121:1547–1557. doi: 10.1242/dev.121.5.1547. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- Takenaga M, Tamamura H, Hiramatsu K, Nakamura N, Yamaguchi Y, Kitagawa A, Kawai S, Nakashima H, Fujii N, Igarashi R. A single treatment with microcapsules containing a CXCR4 antagonist suppresses pulmonary metastasis of murine melanoma. Biochem. Biophys. Res. Commun. 2004;320:226–232. doi: 10.1016/j.bbrc.2004.05.155. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Bystryn JC. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res. 1996;9:304–310. doi: 10.1111/j.1600-0749.1996.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat. Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Van Der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW, Hendrix MJ. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J. Natl Cancer Inst. 2004;96:1473–1477. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- Van Kempen LC, Van Den Oord JJ, Van Muijen GN, Weidle UH, Bloemers HP, Swart GW. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am. J. Pathol. 2000;156:769–774. doi: 10.1016/S0002-9440(10)64943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance KW, Goding CR. The transcription network regulating melanocyte development and melanoma. Pigment Cell Res. 2004;17:318–325. doi: 10.1111/j.1600-0749.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Wang E, Voiculescu S, Le Poole IC, El-Gamil M, Li X, Sabatino M, Robbins PF, Nickoloff BJ, Marincola FM. Clonal persistence and evolution during a decade of recurrent melanoma. J. Invest. Dermatol. 2006;126:1372–1377. doi: 10.1038/sj.jid.5700193. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, Andreeff M, Goodell MA. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yonetani S, Moriyama M, Nishigori C, Osawa M, Nishikawa SI. In vitro expansion of immature melanoblasts and their ability to repopulate melanocyte stem cells in the hair follicle. J. Invest. Dermatol. 2007 doi: 10.1038/sj.jid.5700997. In press; doi: 10.1038/sj.jid.5700997. [DOI] [PubMed] [Google Scholar]
- You L, He B, Xu Z, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64:5385–5389. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- Yu HS. Melanocyte destruction and repigmentation in vitiligo: a model for nerve cell damage and regrowth. J. Biomed. Sci. 2002;9:564–573. doi: 10.1159/000067283. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Watt FM. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]