Abstract
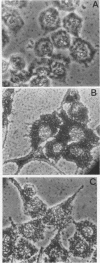
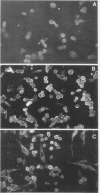
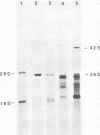
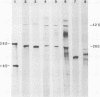
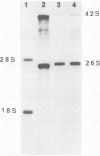
We have established a persistent infection of BHK cells with a preparation of Sindbis virus heavily enriched in defective interfering (DI) particles. The small fraction of cells that survived the initial infection grew out to form a stable population of cells [BHK(Sin-1) cells], most of which synthesized viral RNA and viral antigens. The presence of DI particles in this virus stock was required to establish this persistent state. BHK(Sin-1) cells released a small-plaque, temperature-sensitive virus (Sin-1 virus) as well as DI particles containing DI RNAs larger than those present in the original stock used to establish the persistent state. A cloned stock of Sin-1 virus, free of detectable DI particles, was able to initiate a persistent infection more quickly and with greater cell survival than the original stock of Sindbis virus containing DI particles. About 2 weeks after the Sin-1 virus-infected cells were cultured, DI RNAs arose and soon became the dominant viral RNA species produced by these cells.
Full text
PDF




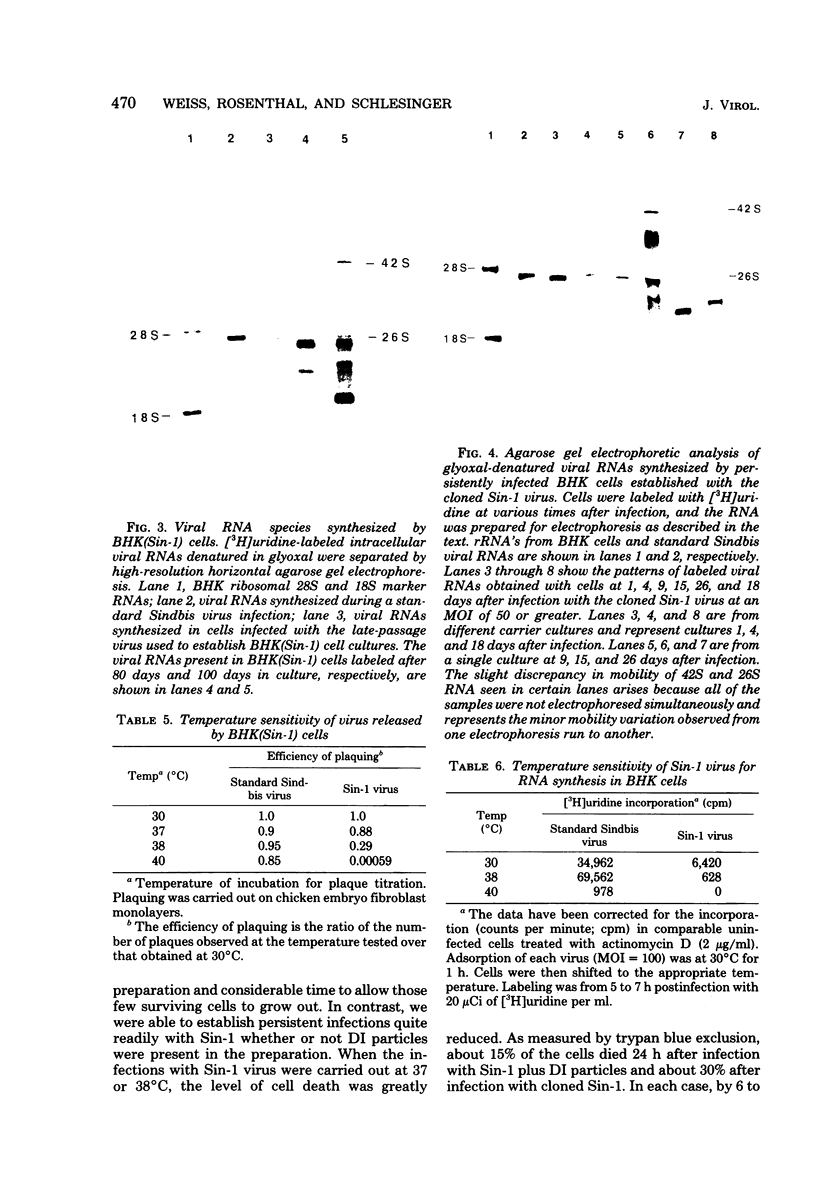




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAMBERS V. C. The prolonged persistence of western equine encephalomyelitis virus in cultures of strain L cells. Virology. 1957 Feb;3(1):62–75. doi: 10.1016/0042-6822(57)90023-5. [DOI] [PubMed] [Google Scholar]
- Eaton B. T. Evidence for the synthesis of defection interfering particles by Aedes albopictus cells persistently infected with Sindbis virus. Virology. 1977 Apr;77(2):843–848. doi: 10.1016/0042-6822(77)90503-7. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Sefton B. M. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell. 1978 Nov;15(3):985–992. doi: 10.1016/0092-8674(78)90282-9. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Inglot A. D., Albin M., Chudzio T. Persistent infection of mouse cells with Sindbis virus: role of virulence of strains, auto-interfering particles and interferon. J Gen Virol. 1973 Jul;20(1):105–110. doi: 10.1099/0022-1317-20-1-105. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Dutko F. J., Pfau C. J. Determinants of spontaneous recovery and persistance in MDCK cells infected with lymphocytic choriomeningitis virus. J Gen Virol. 1979 Jul;44(1):113–122. doi: 10.1099/0022-1317-44-1-113. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Bruton C. J., Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the defective virion RNA. J Virol. 1976 Sep;19(3):1034–1043. doi: 10.1128/jvi.19.3.1034-1043.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan K. B. Generation of defective interfering particles of Semliki Forest virus in a clone of Aedes albopictus (mosquito) cells. J Virol. 1979 Apr;30(1):38–44. doi: 10.1128/jvi.30.1.38-44.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y., Ito Y., Shimokata K. Properties of the viruses selected during persistent infection of L cells with VSV. J Gen Virol. 1978 Aug;40(2):481–484. doi: 10.1099/0022-1317-40-2-481. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y. Studies of L cells persistently infected with VSV: factors involved in the regulation of persistent infection. J Gen Virol. 1977 May;35(2):265–279. doi: 10.1099/0022-1317-35-2-265. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Ramseur J. M., Friedman R. M. Prolonged infection of L cells with vesicular stomatitis virus. Defective interfering forms and temperature-sensitive mutants as factors in the infection. Virology. 1978 Mar;85(1):253–261. doi: 10.1016/0042-6822(78)90429-4. [DOI] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Novel antiviral activity found in the media of Sindbis virus-persistently infected mosquito (Aedes albopictus) cell cultures. J Virol. 1979 Jan;29(1):51–60. doi: 10.1128/jvi.29.1.51-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Holland J. J. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979 Feb;93(1):91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Schwöbel W., Ahl R. Peristence of sindbis virus in BHK-21 cell cultures. Arch Gesamte Virusforsch. 1972;38(1):1–10. doi: 10.1007/BF01241350. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Koshelnyk K. A., Stollar V. Temperature-sensitive virus from Aedes albopictus cells chronically infected with Sindbis virus. J Virol. 1974 Feb;13(2):439–447. doi: 10.1128/jvi.13.2.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Generation of defective virus after infection of newborn rats with reovirus. J Virol. 1976 Oct;20(1):234–247. doi: 10.1128/jvi.20.1.234-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L. THE VIRAL CARRIER STATE IN ANIMAL CELL CULTURES. Prog Med Virol. 1964;6:111–148. [PubMed] [Google Scholar]
- Weiss B., Goran D., Cancedda R., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the intracellular defective viral RNA. J Virol. 1974 Nov;14(5):1189–1198. doi: 10.1128/jvi.14.5.1189-1198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: chemical composition, biological activity, and mode of interference. J Virol. 1973 Oct;12(4):862–871. doi: 10.1128/jvi.12.4.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Oldstone M. B. Inhibition of immunologic injury of cultured cells infected with lymphocytic choriomeningitis virus: role of defective interfering virus in regulating viral antigenic expression. J Exp Med. 1977 Jun 1;145(6):1449–1468. doi: 10.1084/jem.145.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Preble O. T., Jones E. V. Persistent infection of L cells with vesicular stomatitis virus: evolution of virus populations. J Virol. 1978 Oct;28(1):6–12. doi: 10.1128/jvi.28.1.6-13.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants isolated from hamster and canine cell lines persistently infected with Newcastle disease virus. J Virol. 1975 Nov;16(5):1332–1336. doi: 10.1128/jvi.16.5.1332-1336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]