Abstract
New treatments are needed to control prolonged status epilepticus given the high failure rate of current therapies. In an animal model of status epilepticus based on electrical stimulation of the hippocampus, rats demonstrate at least 5 five-hours of seizure activity following stimulation. Phenobarbital (70 mg/kg) administered 15 min after stimulation effectively controlled seizures in 66% of animals (n = 6). When phenobarbital (70 mg/kg) was administered 60 min after stimulation, seizures were controlled in 25% of animals (n = 4). Ketamine (100 mg/kg) administered 15 min after stimulation did not control seizures in any animal (n = 4). But when ketamine was administered one hour after stimulation it effectively controlled seizures in all animals (n = 4). Increasing doses of ketamine were administered 60 min after stimulation to generate a dose-response curve. The ketamine dose response (fraction of seizure free rats) data were fit to a sigmoid curve to derive an ED50 of 58 mg/kg. These findings suggest that prolonged status epilepticus becomes refractory to phenobarbital but can be effectively controlled by ketamine. For patients experiencing prolonged status epilepticus that is refractory to phenobarbital, ketamine may be an alternative to general anesthesia.
Keywords: Phenobarbital, Ketamine, Refractory seizure
1. Introduction
Status epilepticus is a neurological emergency characterized by persistent seizures and high morbidity and mortality (Lowenstein and Alldredge, 1998). Prolonged status epilepticus has a higher mortality rate than brief episodes (Towne et al., 1994; DeLorenzo et al., 1995), and is refractory to many currently used medications (Yaffe and Lowenstein, 1993). In clinical testing, lorazepam was more effective than phenytoin in controlling brief overt convulsive status epilepticus(Treiman et al., 1998). Other agents such as diazepam when used in combination with phenytoin and phenobarbital were as effective as lorazepam. Lorazepam, diazepam, and phenobarbital exert their anticonvulsant effects by enhancing activation of GABAA receptors by GABA. In contrast, phenytoin exerts its anticonvulsant effect by stabilizing the inactivated state of sodium channels thereby limiting sustained repetitive firing of neurons (Macdonald and Kelly, 1994). Therefore, current guidelines for the treatment of status epilepticus target increased activation of GABAA receptors. While drugs acting at the GABAA receptor can control brief status epilepticus, they are less effective in prolonged status epilepticus. In a Veterans Administration co-operative study, less than 25% of patients with prolonged (5.4 h) subtle status epilepticus were controlled by phenobarbital, lorazepam, diazepam, and phenytoin or phenytoin (Treiman et al., 1998; Lowenstein, 1998; Mazarati et al., 1998).
General anesthesia is a common treatment of refractory status epilepticus but it is expensive and carries the risk of hypotension and infection. This suggests that a new class of anticonvulsants is needed for treatment of status epilepticus resistant to GABAergic drugs. We tested the ability of ketamine, a clinically available N-methyl D-Aspartate (NMDA) receptor antagonist, to control status epilepticus refractory to GABAergic drugs.
2. Methods
Bipolar insulated stainless steel electrodes were placed in the left posterior ventral hippocampi (−5.3 mm AP, ±4.9 mm ML, −5.0 DTP, incisor bar at −3) of adult male (250–350 g) Sprague-Dawley rats that had received balanced ketamine/xylazine anesthesia. Another pair of electrodes was placed over the cortex. Amphenol connectors held the electrode pins and the assembly was attached to the skull with stainless steel jeweler’s screws and dental acrylic. Following a 7-day recovery period, limbic status epilepticus was induced by continuous stimulation of the hippocampus (Lothman et al., 1989) with 1 ms biphasic square wave electrical pulses (50 Hz, 400 mA) in 10 s trains applied every 11 s for 90 min. After 90 min of stimulation, the animals were monitored continuously with EEG and behavioral observations to determine the effect of treatment. Following stimulation, animals in behavioral and electrographic status epilepticus were treated at predetermined time points (15, 30, 60 min post-stimulation) by intraperitoneal administration of phenobarbital or ketamine.
Five-minute epochs of electroencephalographic activities were scored at 15-min intervals with a scoring system described previously (Lothman et al., 1989; Treiman et al., 1990). The EEG was scored based on the presence of the following: (grade 1) normal EEG or irregular spikes occurring at a frequency of less than 1 Hz; (grade 2) intermittent seizures separated by normal EEG; (grade 3) continuous seizures; (grade 4) intermittent seizures separated by periodic epileptiform discharges or spikes; (grade 5) periodic discharges only. Animals displaying intermittent seizures interrupted by normal activity (grade 2), continuous seizures (grade 3), or intermittent seizures separated by periodic discharges (grade 4) were considered to be in electrographic status epilepticus. As described in the past, periodic discharges occurred after seizure activity had lasted more than 5 h. The current study focused on the treatment of status epilepticus after 1 h of seizures and periodic discharges were not present in any animal prior to treatment or after treatment.
Phenobarbital sodium was dissolved in distilled water. A commercial preparation of ketamine in an aqueous solution was used. We treated four animals 15 min after the termination of continuous hippocampal stimulation with 1-ml normal saline injected intraperitoneally. Status epilepticus was not controlled by normal saline.
3. Results
3.1. Phenobarbital treatment of seizures
Only animals having continuous seizures at the end of 90 min of stimulation were included in the study. Seven animals were observed with continuous electroencephalographic and visual monitoring for 5 h after the end of stimulation. All of the animals demonstrated continuous status epilepticus for at least 5 h, as reported previously (Lothman et al., 1989; Bertram and Lothman, 1990). Initially, electrical seizures consisted of a rapid spiking pattern that decreased in frequency in the next hour. Later, the EEG changed to rapid repeated bursting (Fig. 1). Status epilepticus ended with conversion of these bursts to periodic discharges. After the termination of stimulation animals demonstrated behavioral seizures characterized by continuous wet dog shakes, facial twitching, and head bobbing (stage 2, Racine score) interrupted by rearing and falls (stage 4 and 5).
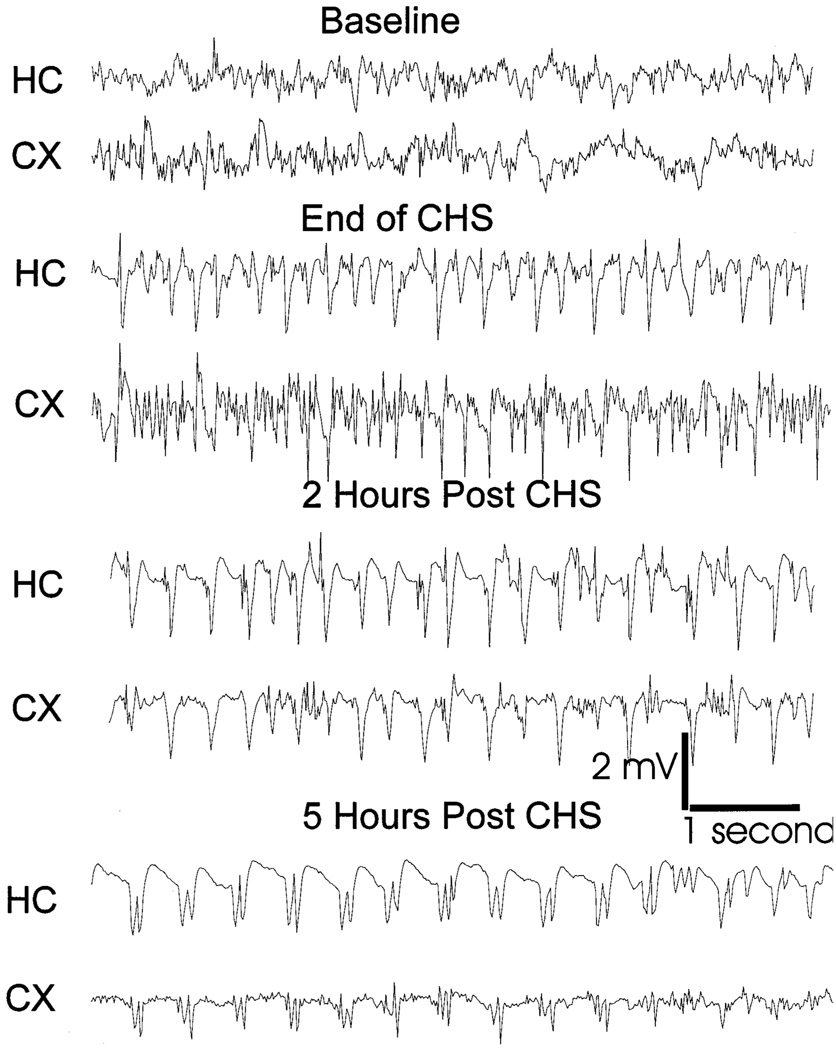
Fig. 1.
EEG recording from the cortex and hippocampus of an animal subjected to continuous hippocampal stimulation. Ten second samples of the continuous record are displayed from a period just before stimulation (baseline), at the beginning of spontaneous seizures, and after 2 and 5 h of spontaneous seizures. Note that a persistent electrographic seizure (status epilepticus) lasted five hours after termination of stimulation. The pattern of electrographic seizure changed with time as demonstrated by a reduction in frequency of the discharges and changes in their morphology.
Animals in status epilepticus were administered phenobarbital (70 mg/kg) after 15 (n = 6), 30 (n = 3), and 60 (n = 4) min of spontaneous seizures (see Fig. 2). All animals treated with phenobarbital (70 mg/kg) were sedated and no Racine stage 4 or 5 seizures occurred for 60 min after the administration of the drug. Some of the rats sedated with phenobarbital had subtle facial twitches and head bobs, thereby preventing confident determination of seizure activity by observation of behavior alone. Therefore, electrographic criteria were used to determine seizure activity. Phenobarbital (70 mg/kg) was administered after 15 min of spontaneous seizures (Fig. 2) and controlled status epilepticus in 4/6 animals (66%). When administered after 30 min of spontaneous seizures it controlled status epilepticus in 1/3 animals (33%); when administered after 60 min of spontaneous seizures, it controlled status epilepticus in 1/4 animals (25%). There was a significant correlation (P < 0.0001, χ2 test) between the time of treatment with phenobarbital and the percentage of seizure-free animals.
Fig. 2.
Prolonged status epilepticus renders phenobarbital less effective. Phenobarbital (70 mg/kg, i.p.) was administered after 15, 30, and 60 min of spontaneous seizures. Arrows mark the time of administration of phenobarbital. Animals were considered seizure-free based on EEG criteria. In all animals behavioral sedation preceded termination of electrographic seizures. The percentage of animals not having electrographic seizures was plotted over time.
3.2. Ketamine treatment of status epilepticus
Ketamine (100 mg/kg) was administered to 4 animals 15 min after the end of stimulation. Following treatment, all animals were sedated and no stage 4 or 5 seizures were observed, but electrographic seizures continued in all animals for 60 min. When ketamine (200 mg/kg) was administered to 3 animals at the same time point, it caused profound sedation, unresponsiveness and respiratory arrest in all of the animals.
In contrast to its failure to control early status epilepticus, ketamine effectively controlled prolonged status epilepticus. Ketamine (100 mg/kg) was administered to 4 animals 60 min after the end of stimulation, that resulted in sedation and in the electrographic seizures changing to slow bursts before terminating (see Fig. 3). The drug controlled seizures within 60 min of administration in 3/4 animals (75%) and the remaining animal became seizure free in the following hour.
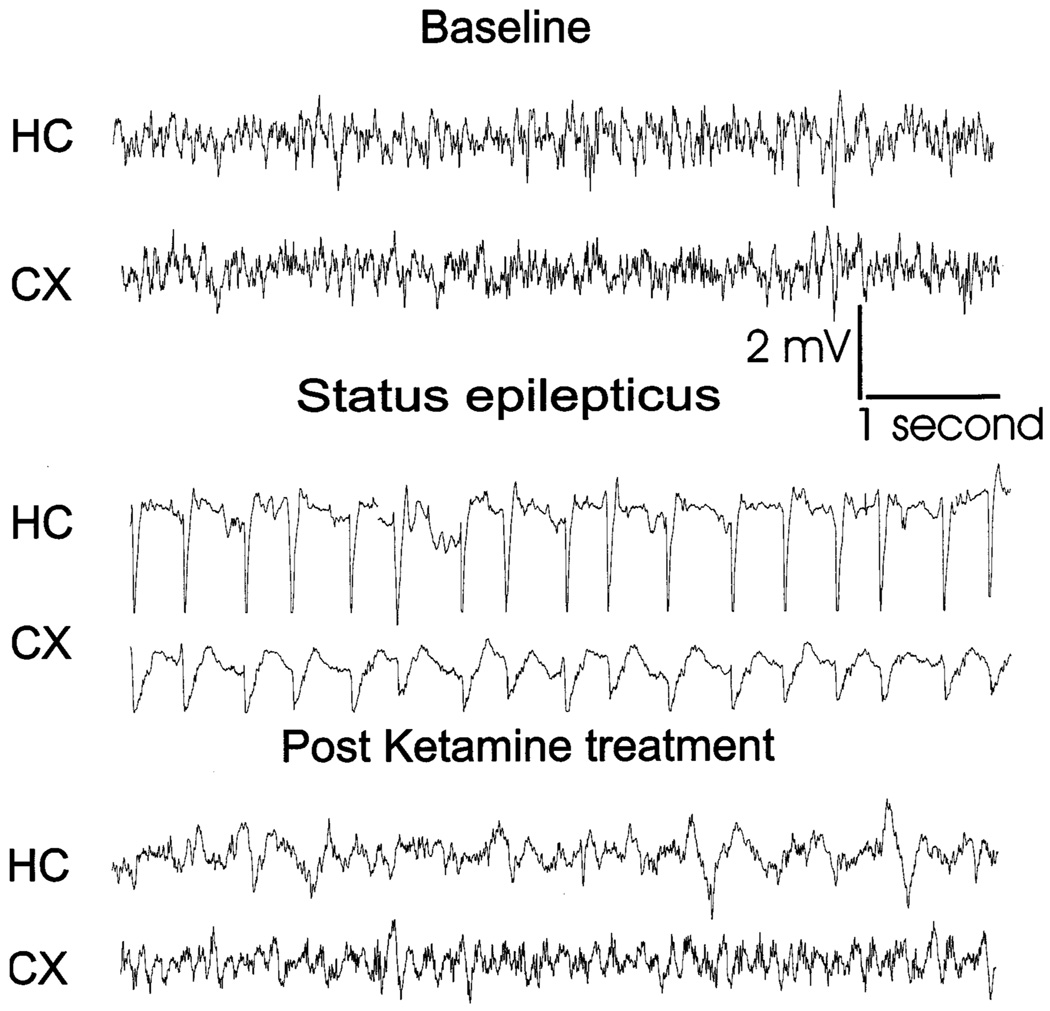
Fig. 3.
EEG recording from the cortex and hippocampus of a rat in status epilepticus and treated with ketamine (100 mg/kg). Samples (10 s) of the continuous EEG record are displayed from a period just before stimulation (baseline), during spontaneous status epilepticus, and after ketamine administration. Ketamine was administered after 60 min of status epilepticus.
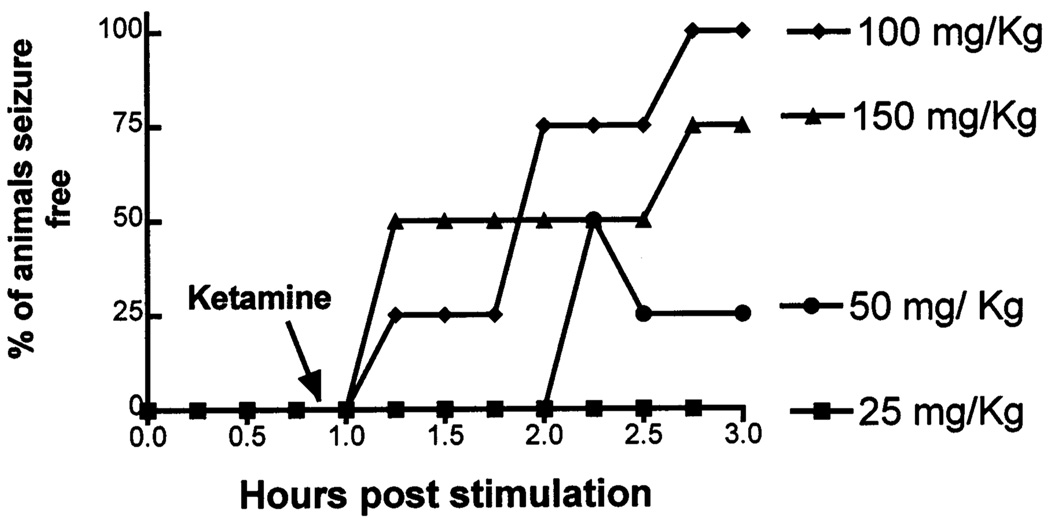
Ketamine treatment of prolonged status epilepticus was studied further by determining a dose-response relationship. Animals were administered Ketamine 25 (n = 4), 50 (n = 4) and 150 (n = 5) mg/kg intraperitoneally following 60 min of seizures. In 4 animals treated with ketamine (25 mg/kg) there was no change in behavioral or EEG seizures. Higher dose of ketamine (50 mg/kg) caused sedation in 2 animals. Electrographic seizures were controlled transiently in 2 animals and in a sustained fashion in 1 animal (Fig. 4). Ketamine (150 mg/kg) was administered to 5 animals resulting in sedation, and 1 animal dying of respiratory arrest. Electrographic seizures were controlled in 3/4 surviving animals (75%). The data for the logarithm of drug doses and the percentage of animals seizure-free were fit to an equation for a sigmoidal curve with a variable slope; and the maximum and minimum responses were fixed to 100% and 0%, respectively. The best fit to the equation suggested an ED50 of 58 mg/kg of ketamine for treatment of status epilepticus. When data for the animal that died of respiratory depression was included in the analysis, the ED50 of ketamine was 75 mg/kg.
Fig. 4.
Ketamine treatment of prolonged (60 min) status epilepticus. Increasing doses of ketamine were administered after 60 min of spontaneous seizures (arrow). The percentage of animals not having electrographic seizures was plotted over time for each dose. One animal that received ketamine (150 mg/kg) died and was not included in the analysis.
4. Discussion
There are three major findings of this study: (1) resistance to phenobarbital develops over prolonged status epilepticus; (2) prolonged status epilepticus can be treated with ketamine, a clinically available NMDA receptor antagonist; (3) ketamine is more effective in treatment of prolonged status epilepticus, compared with early status epilepticus.
Development of resistance to benzodiazepines during status epilepticus was demonstrated in the past (Walton and Treiman, 1988; Kapur and Macdonald, 1997). Development of resistance to phenobarbital during status epilepticus supports the hypothesis that GABAA receptor-mediated inhibition diminishes during status epilepticus (Kapur, 1999; Kapur and Coulter, 1995; Kapur and Macdonald, 1997). Despite differences in the mechanism of action of benzodiazepines and barbiturates on GABAA receptors (Macdonald and Olsen, 1994), development of resistance to both drugs suggests that prolonged seizures alter the structure and/or function of GABAA receptors.
This study found that the NMDA receptor antagonist ketamine did not control seizures when administered in the early phase of status epilepticus. This finding confirmed the reports of other laboratories using different animal models. The NMDA receptor antagonist MK 801 administered prior to or shortly after the onset of seizures, did not control kainate-induced or lithium/pilocarpine-induced status epilepticus (Fariello et al., 1989; Walton and Treiman, 1991; Clifford et al., 1990; Fujikawa et al., 1994; Rice and DeLorenzo, 1998). While some authors reported that seizures were less intense after administration of an NMDA receptor antagonist (Rice and DeLorenzo, 1998), others reported that these drugs worsened electrographic seizures (Fariello et al., 1989; Bertram and Lothman, 1990). It is likely that NMDA receptor antagonists failed to control seizures in these studies because the drugs were administered too early in the course of status epilepticus as suggested by the findings of Williamson and Lothman (1989). They demonstrated that NMDA receptor antagonists have a “use dependent” action i.e repeated seizures must occur before these agents were effective anticonvulsants. In their study (Williamson and Lothman, 1989), fully kindled rats with stable stage 5 behavioral seizures and 50 to 60 s after-discharge durations were administered MK 801 and given 5 kindling stimuli 30 min apart. The behavioral scores and after-discharges in response to the first 2 stimuli showed no change from baseline. In contrast, the behavioral and electrographic seizures declined in a dose-dependent manner in response to the third and fourth stimuli. These findings suggested that NMDA receptor antagonists would be useful in prolonged, persistent, or recurrent seizures. The use-dependent action of MK 801 as an anticonvulsant is in keeping with the known properties of NMDA receptors. During normal low frequency excitatory neurotransmission, NMDA receptors are not activated but prolonged high frequency neuronal activity during sustained status epilepticus is likely to create a suitable environment for activation of NMDA receptors.
Two studies in the past demonstrated the effectiveness of NMDA receptor antagonists in the treatment of prolonged status epilepticus. In the perforant path stimulation model of status epilepticus, seizures that rapidly become refractory to benzodiazepines were controlled by NMDA receptor antagonists (Mazarati and Wasterlain, 1999). Similarly, the NMDA receptor antagonists MK 801 and ketamine controlled prolonged status epilepticus (Bertram and Lothman, 1990).
The finding that ketamine can effectively control status epilepticus resistant to the barbiturate phenobarbital is clinically significant. Anesthetic agents are used currently to treat refractory status epilepticus but these drugs carry a risk of high morbidity and mortality. In the present study ketamine was efficacious in doses unlikely to cause respiratory depression, with an ED50 of 58 mg/kg. Ketamine toxicity in the form of respiratory depression and arrest, occurred in 1/5 animals at a dose of 150 mg/kg and in 3/3 animals at a dose of 200 mg/kg. Thus it is possible that the doses of ketamine necessary to control status epilepticus may not cause respiratory depression.
Ketamine offers an additional advantage in the treatment of refractory status epilepticus in that it is potentially neuroprotective. Patients dying during or shortly after an episode of status epilepticus show loss of CA1 pyramidal neurons, Purkinje cells of the cerebellum and cortical neurons(DeGiorgio et al., 1992). In the animal models of kainic acid and lithium/pilocarpine-induced status epilepticus the non-competitive NMDA receptor antagonists ketamine, phencyclidine and MK-801 were demonstrated to protect against neuronal damage (Clifford et al., 1990; Fujikawa et al., 1994; Wasterlain et al., 1993).
Acknowledgements
Public Health Service Grants (KO8 NS01748 and KO2 NS NS02081) supported this work. We thank Dr. Kevin Kelly for editing the manuscript.
References
- Bertram EH, Lothman EW. NMDA receptor antagonists and limbic status epilepticus: a comparison with standard anticonvulsants. Epilepsy Res. 1990;5:177–184. doi: 10.1016/0920-1211(90)90036-u. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Olney JW, Benz AM, Fuller TA, Zorumski CF. Ketamine, phencyclidine, and MK-801 protect against kainic acid-induced seizure-related brain damage. Epilepsia. 1990;31:382–390. doi: 10.1111/j.1528-1157.1990.tb05492.x. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Tomiyasu U, Gott PS, Treiman DM. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23–27. doi: 10.1111/j.1528-1157.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J. Clin. Neurophysiol. 1995;12:316–325. [PubMed] [Google Scholar]
- Fariello RG, Golden GT, Smith GG, Reyes PF. Potentiation of kainic acid epileptogenicity and sparing from neuronal damage by an NMDA receptor antagonist. Epilepsy Res. 1989;3:206–213. doi: 10.1016/0920-1211(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG, Daniels AH, Kim JS. The competitive NMDA receptor antagonist CGP 40116 protects against status epilepticus induced neuronal damage. Epilepsy Res. 1994;17:207–219. doi: 10.1016/0920-1211(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Kapur J. Status epilepticus in epileptogenesis. Curr. Opin. Neurol. 1999;12:191–195. doi: 10.1097/00019052-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Kapur J, Coulter DA. Experimental status epilepticus alters GABAA receptor function in CA1 pyramidal neurons. Ann. Neurol. 1995;38:893–900. doi: 10.1002/ana.410380609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J. Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by ‘continuous’ hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH. Status epilepticus. Western J. Med. 1998;168:263. [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Alldredge BK. Current concepts-status epilepticus. New England J. Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kelly KM. Mechanisms of action of currently prescribed and newly developed antiepileptic drugs. Epilepsia. 1994;35 Suppl 4:S41–S50. doi: 10.1111/j.1528-1157.1994.tb05955.x. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu. Rev. Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814:179–185. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Wasterlain CG. N-methyl-D-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci. Lett. 1999;265:187–190. doi: 10.1016/s0304-3940(99)00238-4. [DOI] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res. 1998;782:240–247. doi: 10.1016/s0006-8993(97)01285-7. [DOI] [PubMed] [Google Scholar]
- Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35:27–34. doi: 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Calabrese VP, Uthman BM, Ramsay RE, Mamdani MB. A Comparison of four treatments for generalized convulsive status epilepticus. New England J. Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- Walton NY, Treiman DM. Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp. Neurol. 1988;101:267–275. doi: 10.1016/0014-4886(88)90010-6. [DOI] [PubMed] [Google Scholar]
- Walton NY, Treiman DM. Motor and electroencephalographic response of refractory experimental status epilepticus in rats to treatment with MK-801, diazepam, or MK-801 plus diazepam. Brain Res. 1991;553:97–104. doi: 10.1016/0006-8993(91)90235-n. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34 Suppl 1:S37–S53. doi: 10.1111/j.1528-1157.1993.tb05905.x. [DOI] [PubMed] [Google Scholar]
- Williamson JM, Lothman EW. The effect of MK-801 on kindled seizures: implications for use and limitations as an antiepileptic drug. Ann. Neurol. 1989;26:85–90. doi: 10.1002/ana.410260113. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lowenstein DH. Prognostic factors of pentobarbital therapy for refractory generalized status epilepticus. Neurology. 1993;43:895–900. doi: 10.1212/wnl.43.5.895. [DOI] [PubMed] [Google Scholar]