Abstract
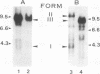
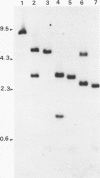
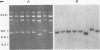
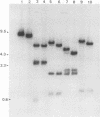
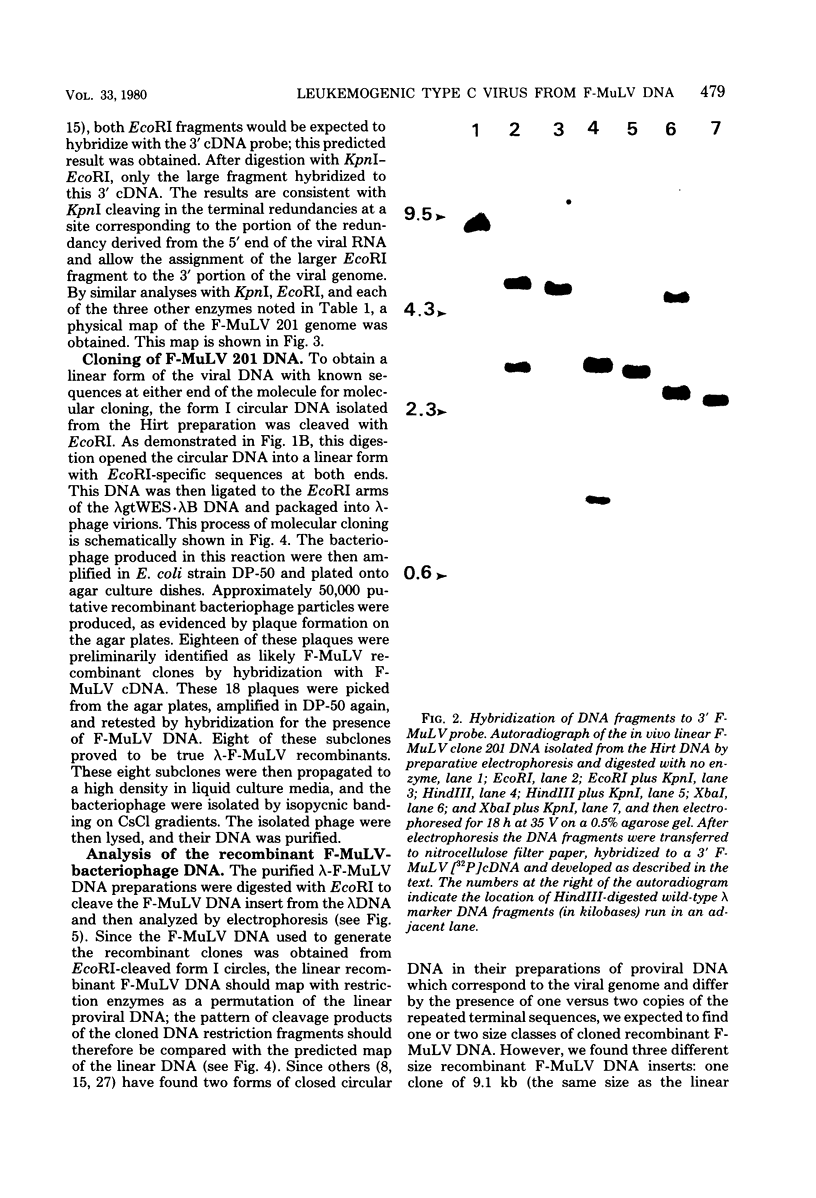
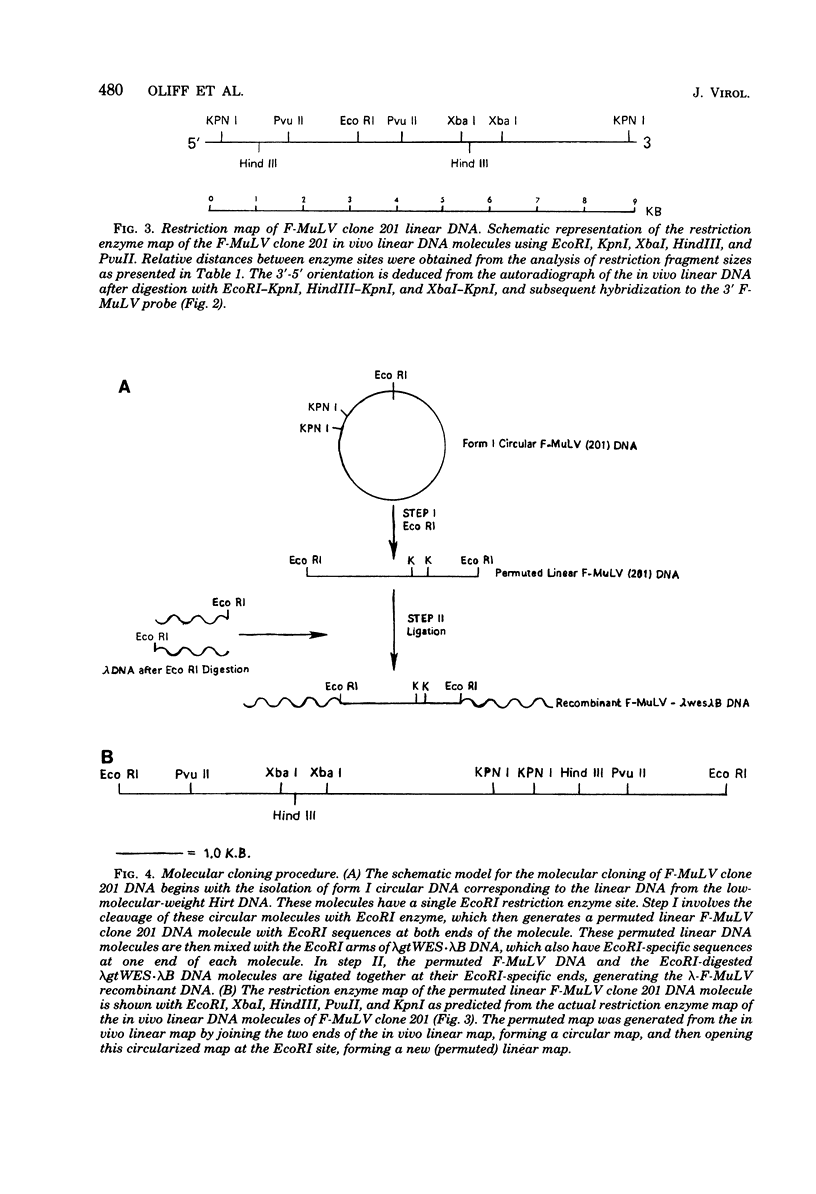
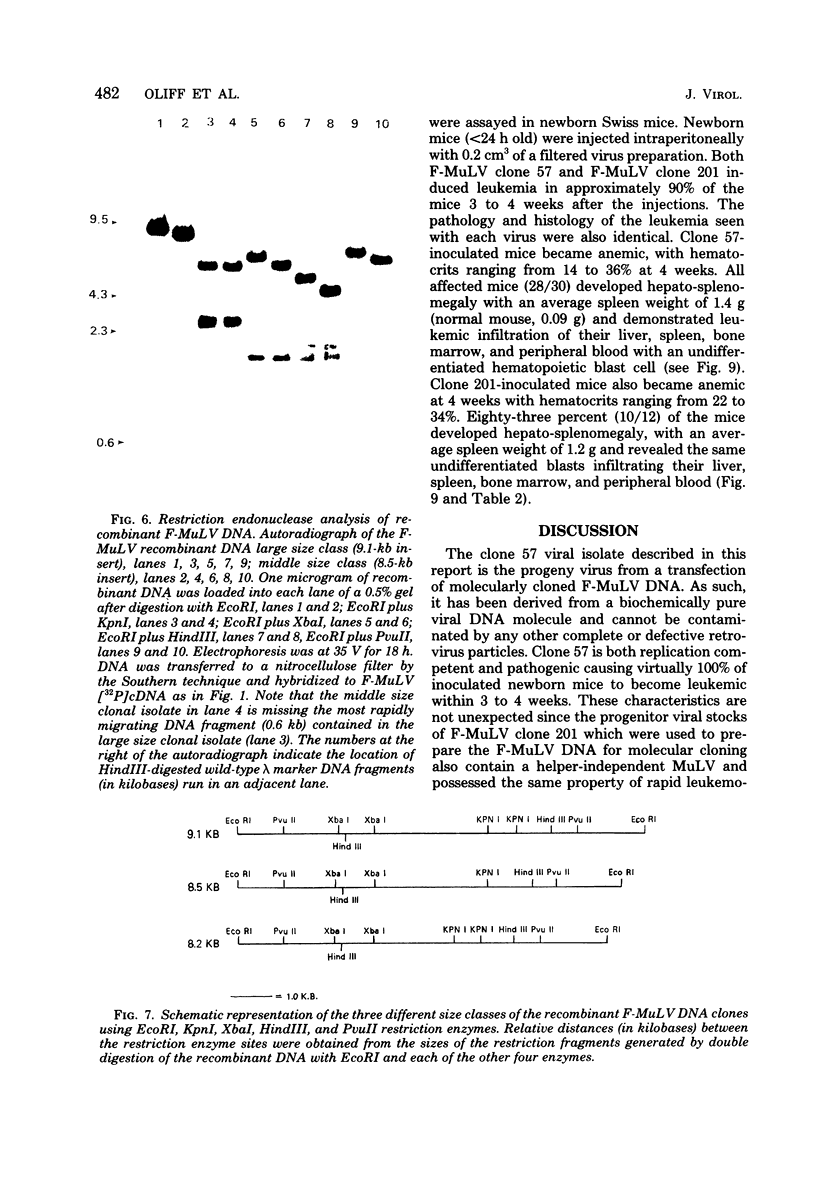
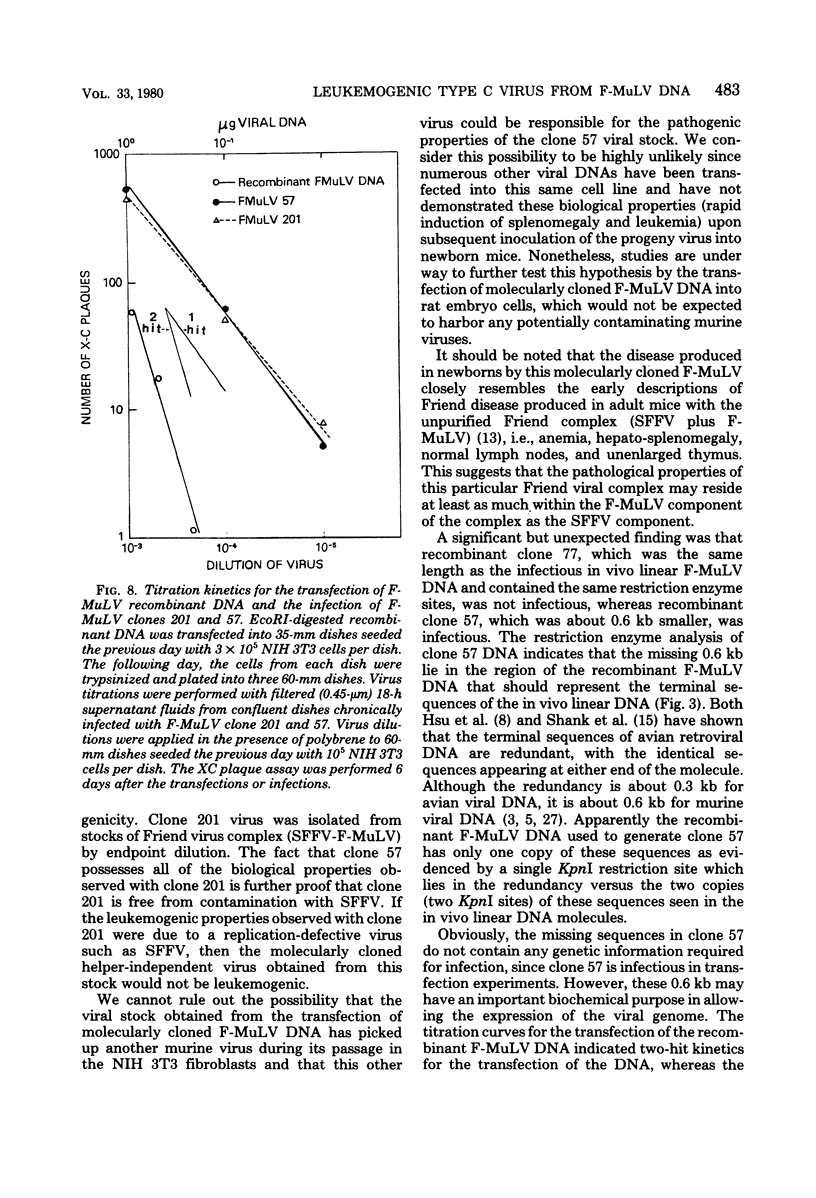
Unintegrated viral DNA was isolated via the Hirt procedure from mouse fibroblasts newly infected with Friend murine leukemia virus (F-MuLV) clone 201, a biologically cloned helper virus isolated from stocks of F-MuLV complex. A physical map of the unintegrated in vivo linear viral DNA was generated for several restriction endonucleases. The supercoiled viral DNA was digested with EcoRI, which cleaved the viral DNA at a unique site. The linearized viral DNA was then inserted into lambda gtWES.lambda B at the EcoRI site and cloned in an approved EK2 host. Eight independent lambda-mouse recombinants were identified as containing F-MuLV DNA inserts by hybridization with F-MuLV 32P-labeled complementary DNA. One of the F-MuLV DNA inserts was 9.1 kilobases (kb) and had the same restriction enzyme sites as the unintegrated linear F-MuLV DNA. Six inserts were 8.5 kb; each lacked a single copy of the terminally redundant sequences of the unintegrated linear viral DNA. One insert was 8.2 kb and contained a 0.9-kb deletion. After digestion with EcoRI, one recombinant DNA preparation containing an 8.5-kb insert was infectious for NIH 3T3 cells. Undigested recombinant DNA was not infectious. The infectivity of the EcoRI-digested DNA followed multihit kinetics, indicating that more than one molecule was required to register as an infectious unit. The virus isolated from this transfection (F-MuLV-57) was NB-ecotropic, helper-independent, and formed XC plaques. Inoculation of this virus into newborn NIH Swiss mice induced leukemia and splenomegaly in greater than 90% of animals within 3 to 4 weeks. The gross and microscopic abnormalities induced by F-MuLV clone 57 were identical to those seen with the original parent stocks of F-MuLV clone 201. These results indicate that this helper-independent F-MuLV can induce a rapid nonthymic leukemia in the absence of the spleen focus-forming virus.
Full text
PDF




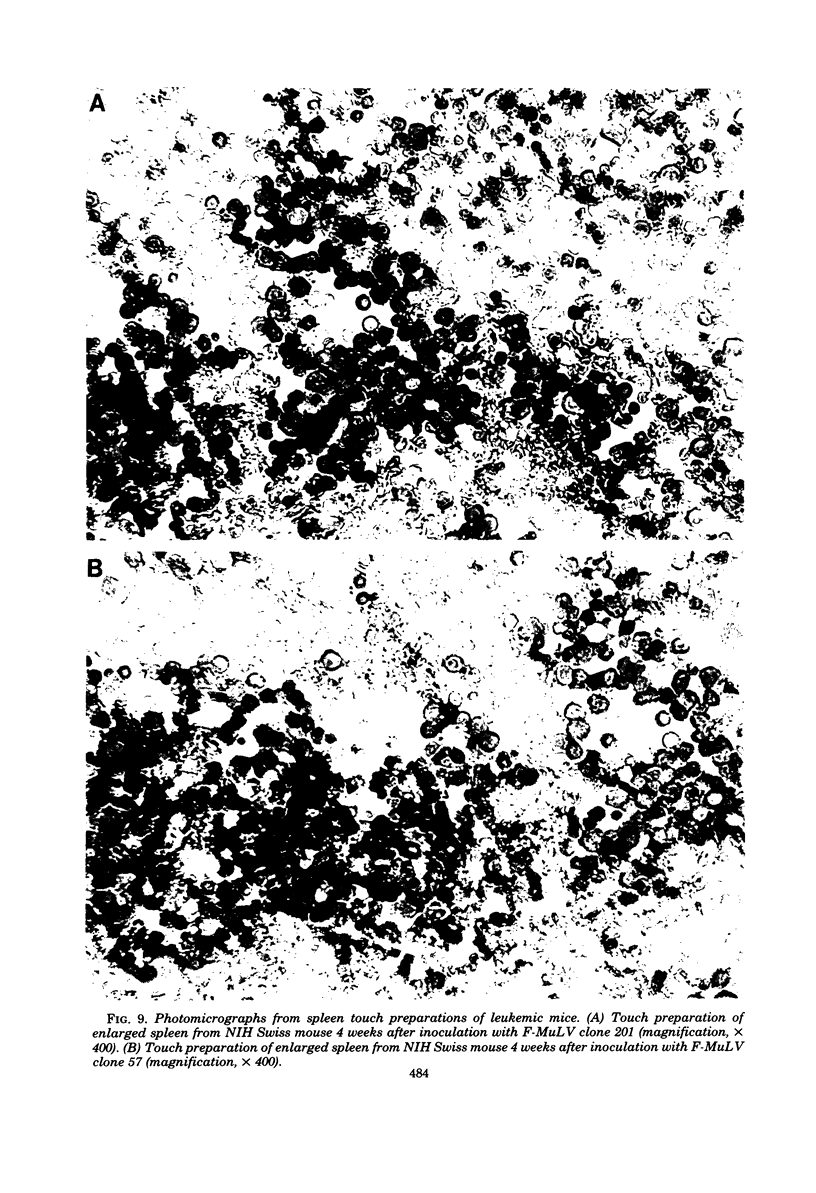

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper G. M., Castellot S. B. Assay of noninfectious fragments of DNA of avian leukosis virus-infected cells by marker rescue. J Virol. 1977 May;22(2):300–307. doi: 10.1128/jvi.22.2.300-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METCALF D., FURTH J., BUFFETT R. F. Pathogenesis of mouse leukemia caused by Friend virus. Cancer Res. 1959 Jan;19(1):52–58. [PubMed] [Google Scholar]
- Polsky F., Edgell M. H., Seidman J. G., Leder P. High capacity gel preparative electrophoresis for purification of fragments of genomic DNA. Anal Biochem. 1978 Jul 1;87(2):397–410. doi: 10.1016/0003-2697(78)90689-9. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Gianni A. M., Rozenblatt S., Weinberg R. A. Infectious viral DNA of murine leukemia virus. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4910–4913. doi: 10.1073/pnas.72.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steeves R. A. Editorial: Spleen focus-forming virus in Friend and Rauscher leukemia virus preparations. J Natl Cancer Inst. 1975 Feb;54(2):289–297. doi: 10.1093/jnci/54.2.289. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Parks W. P., Vass W. C., Scolnick E. M. Isolation of a fibroblast nonproducer cell line containing the Friend strain of the spleen focus-forming virus. Virology. 1977 Feb;76(2):602–615. doi: 10.1016/0042-6822(77)90242-2. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura F. K., Weinberg R. A. Restriction endonuclease cleavage of linear and closed circular murine leukemia viral DNAs: discovery of a smaller circular form. Cell. 1979 Feb;16(2):323–332. doi: 10.1016/0092-8674(79)90009-6. [DOI] [PubMed] [Google Scholar]