Abstract
Angiogenesis is a fundamental biological process that is regulated by a fine balance between pro- and antiangiogenic molecules, and is deranged in various diseases. Historically, angiogenesis was only implicated in few diseases, such as, cancer, arthritis, and psoriasis. However, in recent years, it has been increasingly evident that excessive, insufficient or abnormal angiogenesis contributes to the pathogenesis of many more disorders. Research in angiogenesis offers a potential to cure a variety of diseases such as Alzheimer's and AIDS. Modulation of angiogenesis may have an impact on diseases in the twenty-first century similar to that which the discovery of antibiotics had in the twentieth century.
Keywords: Angiogenesis, antiangiogenic drugs, vascular endothelial growth factor
The process of forming new blood vessels is known as angiogenesis. Blood vessel formation is of two types. Vasculogenesis is the generation of blood vessels from endothelial cell progenitors (hemangioblasts). It is responsible for the formation of the primary vasculature of the body during early embryonic development.[1] Angiogenesis is a complex, highly regulated process, involving the sprouting, splitting, and remodeling of the existing vessels. Physiologically angiogenesis occurs under tight regulation in the female reproductive system and during wound healing. Many pathological conditions such as ischemic tissue injury are also benefited by revascularization. On the other spectrum, excessive angiogenesis, may result in different diseases including cancer, atherosclerosis, rheumatoid arthritis, Crohn's disease, diabetes, psoriasis, endometriosis, and adiposity.[2] These diseases may benefit from the therapeutic inhibition of angiogenesis.[2]
History
The term angiogenesis was coined in 1787 by Dr. John Hunter, a British surgeon.[3] Professor Judah Folkman laid the foundation of angiogenesis research, when he put forward the idea that the growth of a tumor depended on their blood supply.[3] It was postulated that the tumor secretes some diffusible substance that stimulates the growth of new capillaries. Soon the basic fibroblast growth factor (bFGF) was shown to be capable of inducing an angiogenic response in vitro. Subsequently, many other angiogenic growth factors have been isolated, including the vascular endothelial growth factor (VEGF), which is a major mediator in tumor angiogenesis. In recent years, the angiopoietins have emerged as important regulators of angiogenesis. Several naturally occurring angiogenic inhibitors have also been discovered, such as, interferon-a/b, thrombospondin-1, angiostatin, and endostatin.
Angiogenic Process
The vascular network formation consists of multiple coordinated, sequential, and interdependent steps mediated by a wide range of angiogenic factors, including growth factors, chemokines, angiogenic enzymes, endothelial specific receptors, and adhesion molecules. The process of neovascularization is halted due to the downregulation of angiogenic factors or the increase of inhibitors in the local concentration. Thus, angiogenesis requires a finely balanced equilibrium between pro-angiogenic and antiangiogenic factors.
Steps of the Angiogenic Process
The angiogenic process is divided broadly into three major steps including the initiation of the angiogenic response, endothelial cell (EC) migration, proliferation and tube formation, and finally the maturation of the neovasculature [Figure 1].
Figure 1.
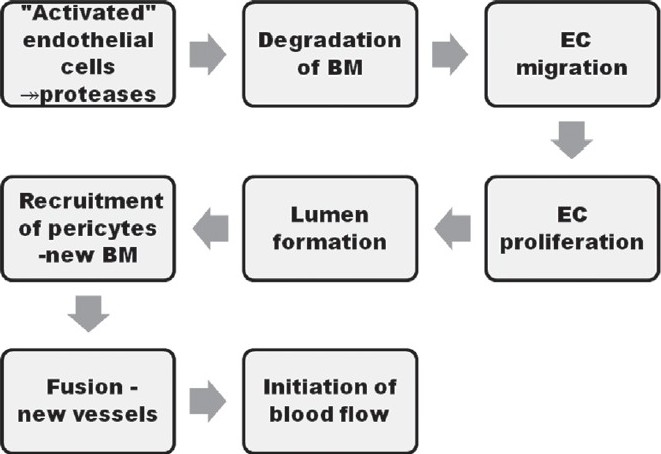
Steps of angiogenesis
1. Initiation of the angiogenic response
Angiogenesis is initiated in response to hypoxia, by the release of hypoxia inducible factors (HIF), which facilitate the release of angiogenic stimulators, which in turn lead to EC activation. In both physiological and pathological angiogenesis, EC activation is the first process that takes place.[4] Activated EC secrete proteases, which degrade the extracellular tissue to facilitate endothelial penetration. Proteases may be broadly divided into matrix metalloproteases (MMPs) and the plasminogen activator (PA) / plasmin system. The MMPs are capable of degrading different protein types. PAs activate the plasminogen into plasmin, which degrades several components of extracellular matrix (ECM). Both PAs and MMPs are secreted together with their inhibitors, plasminogen activator inhibitors, and tissue inhibitors of metalloproteases, respectively, ensuring a stringent control of local proteolytic activity.
2. Endothelial cell migration, proliferation, and tube formation
Extracellular matrix degradation results in an increased concentration of various growth factors, which stimulate EC migration and proliferation. The ‘leader’ EC, followed by more EC, starts to migrate through the degraded matrix, thus forming small sprouts.[5] After the initial period of migration, rapid EC proliferation begins, thus increasing the rate of sprout elongation. These processes are also mediated by cell adhesion molecules.[6] Cell adhesion molecules can be classified into four families and neovascularization is facilitated by the member of each family.[6] Integrin αvβ3and αvβ5, vascular endothelial cadherin, vascular cell adhesion molecule-1, P-selectin, and E-selectin are implicated in angiogenesis.
3. Maturation of the neovasculature
The final phase of the angiogenic process involves maturation of the neovasculature. The new basement membrane is synthesized by the newly forming capillaries. During this process extracellular proteolysis is locally inhibited to permit the deposition and assembly of the ECM components. After the formation of the capillary sprout, degradation of the newly formed ECM occurs again at the tip of the sprout, to allow further invasion. Interaction between the EC and ECM and the mesenchymal cells is a prerequisite for the formation of a stable vasculature. The polarity of the endothelial cells is established by cell adhesion molecules in order to form a lumen.[6] The platelet-derived growth factor (PDGF) regulates the recruitment of pericytes and smooth muscle cells required for further stabilization of the new capillaries. Finally, when sufficient neovascularization has occurred, the angiogenic factors are downregulated or the local concentration of the inhibitors increases. As a result, the endothelial cells become quiescent.
Modulators of angiogenesis
Angiogenesis is a dynamic balance between the positive and negative regulators [Figure 2]. Both physiological and pathological angiogenesis are mediated by numerous ‘classic’ factors. Various angiogenic inhibitors have also been described, which inhibit the angiogenic process. Some of the factors have both pro- and antiangiogenic functions. A summary of the more important angiogenic activators and inhibitors is given in Table 1, although this list is by no means exhaustive.[7,8] Pharmacological manipulation of these factors may lead to the desired angiogenic response. Besides these, other mediators, such as, erythropoietin, angiotensin -II, endothelins, urotensin, leptin, adiponectin, resistin, neuropeptide Y, vasoactive intestinal peptide, and substance P were found to stimulate angiogenesis, whereas, somatostatin, ghrelin, and natriuretic peptides were found to inhibit it.[9] Of the long list of growth factors involved in the angiogenic process, VEGF, FGF-2, and angiopoietin-1 are considered to be the most important mediators of tumor angiogenesis. The characteristic of these factors is discussed here.
Figure 2.
Angiogenesis: A dynamic balance between positive and negative regulators
Table 1.
| Positive regulators | Negative regulators |
|---|---|
| Growth factors | Protein fragments |
| VEGF | Angiostatin |
| Placental growth factor | Endostatin |
| Fibroblast growth factors 1 and 2 | Antiangiogenic antithrombin III |
| Pleiotrophin | Prolactin |
| Platelet derived growth factor | Soluble mediators |
| Transforming growth factor-α | Thrombospondin-1 |
| Transforming growth factor-β | Troponin 1 |
| Epidermal Growth Factor | Interferons |
| Insulin-like growth factor | Interleukins |
| Hepatocyte growth factor | Pigment epithelial derived factor |
| Angiopoietins | Platelet factor-4 |
| Angiopoietin-1 | Plasminogen activator inhibitor |
| Angiopoietin-2 | Tissue inhibitors of metalloproteinaise |
| Cytokines and chemokines | Matrix derived |
| Tumor necrosis factor- α | Arrestin |
| Interleukin - 8 and 3 | Canstatin |
| Prostaglandin E1, E2 | Tumstatin |
| Enzymes | Fibulin |
| Thymidine phosphorylase | Endorepellin |
| Cycloxygenase-2 | Other angiostatics |
| Angiogenin | 2-methoxyestradiol |
| Cell adhesion molecules | PEX |
| Integrins | Vasostatin |
| Vascular cell adhesion molecule -1 | |
| E - Selectin | |
| Vascular endothelial cadhedrin | |
| Miscellaneous | |
| Plasminogen activators | |
| Matrix metalloproteinase | |
| Plasminogen activator inhibitor | |
| Oestrogens | |
| Proliferin | |
| Leptin | |
| Erythropoetin | |
| Granulocyte colony stimulating factor | |
| Granulocyte-monocyte colony | |
| stimulating factor |
Nitric oxide
VEGF (VEGF-A)
VEGF (VEGF-A) is one of the most potent proangiogenic factors. It belongs to the VEGF family, which includes other structurally related growth factors, such as, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and PlGF.[10] VEGF is expressed in various tissues, including the brain, kidney, and liver.[10] VEGF stimulates most steps of angiogenesis and promotes angiogenesis. One of the most important inducer of VEGF is hypoxia.[11] Besides, many other growth factors including PDGF, EGF, TNF-α, TGF-β, and IL-1β mediate their angiogenic action by inducing VEGF expression.[10] VEGF mediates its action by an interaction with two VEGF-specific tyrosine kinase receptors, VEGFR-1 and VEGFR-2. VEGF signals mainly through VEGFR-2, whereas, the role of VEGFR-1 is not very clear in angiogenesis. VEGF plays a crucial role in physiological angiogenesis, as well as, has a role in several human cancers, diabetic retinopathy, rheumatoid arthritis, and atherosclerosis.
Fibroblast growth factor (FGF-2)
The FGF family consists of nine structurally related polypeptides, all having a high affinity for heparin. FGF-2 was one of the first angiogenic factors to be characterized.[12] High concentrations of FGF-2 are found in the brain and pituitary gland. FGF-2 shows angiogenic activity in vivo.[12] The effects of FGFs are mediated via high-affinity tyrosine kinase receptors, of which at least four members have been described. FGF-2 is implicated in both physiological and pathological angiogenesis. FGF-2 is thought to play a role in the growth and neovascularization of solid tumors. High levels of FGF-2 are present in the EC of Kaposi's sarcoma and in the serum and urine of patients with many types of advanced cancinomas.[13]
Angiopoietins
Angiopoietins are endogenously secreted glycoproteins that include angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2). Both angiopoietins bind to Tie-2 (for tyrosine kinase with immunoglobulin and EGF-like domains), present on the endothelium, but only the binding of Ang-1 results in angiogenesis.[14] Therefore, Ang-2 is a natural antagonist of Ang-1.[15] VEGF and angiopoietins have complementary roles in angiogenesis.[16] During embryogenesis, VEGF promotes differentiation and proliferation of EC and the formation of immature vessels. Ang-1 induces the remodeling and stabilization of blood vessels, which involves interactions with the ECM.
Models to study the angiogenic process
In vitro models
Various in vitro models are available for studying most of the steps in the angiogenic cascade, including EC proliferation, migration, and differentiation.[17] The proliferation studies are based on cell counting, thymidine incorporation or immunohistochemical staining. Cell migration can be studied by determining chemotaxis using the Boyden chamber.[17] Cells are added to the top well, chemotactic solutions are placed in the lower well, and after a period of incubation the cells that have migrated toward the chemotactic stimulus are counted. Differentiation can be induced in vitro by culturing EC in different ECM components, including fibrin clots, collagen gels, and matrigel. Advantages of in vitro models include the ability to study the individual steps of angiogenesis, the possibility to control the different parameters involved, and lower costs and effort. However, these models have inherent limitations that their effect may not be reciprocated in in vivo models. Therefore, the in vitro efficacy of a compound has to be substantiated by in vivo models.
In vivo models
Chick chorioallantoic membrane (CAM), rabbit cornea assay, sponge implant models, matrigel plugs, and conventional tumor models are the classical in vivo angiogenesis models.[18]
Chick chorioallantoic membrane assay: The early chick embryo lacks a mature immune system and is therefore suitable for the study of tumor-induced angiogenesis. Tissue grafts are placed on the CAM and increase in vessel number and a typical radial rearrangement is seen. Blood vessels entering the graft are counted under a stereomicroscope. It is the most widely used assay for screening purposes as it is relatively simple and inexpensive. However, as the CAM already contains a well-developed vascular network, it is difficult to discriminate between new capillaries and already existing ones.
Rabbit Cornea Assay: This is very reliable method as the cornea presents an avascular site. Any vessel penetrating from the limbus into the corneal stroma is identified as newly formed. Slow release polymer pellets containing an angiogenic substance are implanted in ‘pockets’ created in the corneal stroma of the rabbit, to induce an angiogenic response. Computer image analysis after perfusion of the cornea with India ink is used to quantify the vascular response. This method is technically more demanding and more expensive.
Sponge Implant Models: Angiogenesis is frequently studied by the subcutaneous implantation of artificial sponges in animals. Sponge implantation is associated with non-specific immune responses, which produce significant angiogenic responses. An angiogenic response is detected either histologically, morphometrically (vascular density), biochemically (hemoglobin content) or by measuring the blood flow rate using a radioactive tracer. Direct data comparison becomes difficult due to the differences in sponge materials, shape, and size.
Matrigel Plugs: Matrigel is a matrix of a mouse basement membrane neoplasm. It is a complex mixture of basement membrane proteins and various growth factors. Subcutaneous injection of matrigel containing growth factors can be injected in mice. The matrigel plugs are removed after 10 days and angiogenesis is quantified histologically or morphometrically in the plug sections. Although it is expensive, matrigel provides a more natural environment compared to artificial sponges, to initiate an angiogenic response.
Conventional Tumor Models: Numerous animal tumor models like C6 rat glioma, B16BL6 melanoma, lewis lung carcinoma (LLC), and Walker 256 carcinoma have been developed to test the antiangiogenic and anti-cancer activity of potential drugs. Subcutaneous implantation of tumor cells is done and the tumor size is determined at regular time intervals. The efficacy of potential antiangiogenic agents can be evaluated on strongly vascularized tumors.
Pathological Angiogenesis
Insufficient angiogenesis
Both angiogenesis insufficiency as well as excess can lead to various disorders. Insufficient angiogenesis is characteristic of many disorders, including ischemic tissue injury or cardiac failure, where angiogenesis should be enhanced to improve the disease condition. The delayed healing of gastric ulcers has been attributed to the ability of the Helicobacter pylori to produce angiogenic inhibitors, whereas, reduced VEGF levels are responsible for recurrent aphthous ulcerations.[19] Organ dysfunction occurring in pre-eclampsia is associated with lower levels of VEGF.[20] Many age-related diseases such as nephropathy[21] and bone loss[22] have been found to be associated with progressive loss of the microvasculature. Age-dependent reductions in vessel density in the skin cause vessel fragility leading to the development of purpura, telangiectasia, palor, angioma, and venous lake formation.[23] Respiratory system disorders such as pulmonary fibrosis[24] and emphysema[25] are also associated with reduced angiogenic signaling. The extent of angiogenesis has also been correlated with survival in stroke patients[26] and insufficient VEGF levels are associated with motor neuron degeneration and are reminiscent of amyotrophic lateral sclerosis.[27] Amyloid-β, which has a role in the pathogenesis of Alzheimer's disease has adverse effects on the cerebral vasculature.[28]
Excessive angiogenesis
Excessive vascular growth contributes to numerous disorders, and the list is growing rapidly. Cancer, arthritis, psoriasis, and blinding retinopathy are already known disorders associated with excess angiogenesis. However, numerous other disorders are characterized by excessive vessel growth. These include many inflammatory, allergic, infectious, traumatic, metabolic or hormonal diseases, such as, atherosclerosis, restenosis, transplant arteriopathy, warts, scar keloids, synovitis, osteomyelitis, asthma, nasal polyps, choroideal and intraocular disorders, retinopathy of prematurity, diabetic retinopathy, AIDS, endometriosis, and uterine bleeding.[29] In recent times, studies have shown that a high-fat diet induces an angiogenic gene program in fat tissues, which stimulates adipogenesis, while treatment of obese mice with antiangiogenic agents results in weight reduction.[30] Infectious diseases are also angiogenic. Viral and bacterial pathogens carry angiogenic genes or induce the expression of angiogenic genes in the host.[31] The human herpesvirus 8 transforms EC and causes Kaposi's sarcoma in HIV-1 infected AIDS patients. Similar to diabetic retinopathy, there is increased glomerular expression of vascular growth factors that contribute to diabetic nephropathy by promoting vessel leakage.[32]
Therapeutic Angiogenesis (Induction of Angiogenesis)
Therapeutic angiogenesis would be beneficial in a variety of diseases. Table 2 tabulates a list of various conditions, which are likely to be benefited by the clinical manipulation of angiogenesis.[7] Becaplermin is an already approved topical recombinant PDGF preparation indicated for diabetic neuropathic lower extremity ulcers. Various angiogenic approaches to treat ischemic diseases are already in clinical trials.[33]
Table 2.
Clinical modulation of angiogenesis[7]
| Inhibition of angiogenesis | Stimulation of angiogenesis |
|---|---|
| Approved indications | Approved indications |
| Advanced cancer | Chronic wound - diabetic ulcer, |
| Ocular neovascularization | Experimental indications |
| Kaposi Sarcoma | Myocardial ischemia |
| Experimental indications | Peripheral ischemia |
| Hemangiomas | Cerebral ischemia |
| Psoriasis | Reconstructive surgery |
| Rheumatoid arthritis | Gastroduodenal ulcers |
| Endometriosis | |
| Atherosclerosis |
Adapted from Liekens et al. Angiogenesis: regulators and clinical applications[7]
Angiogenesis can be induced mainly by two methods. Either angiogenic proteins or endothelial progenitor cells synthesizing angiogenic growth factors can be injected directly into a site to stimulate blood vessel growth, or the right genes can be activated to induce a signaling cascade that would lead to angiogenesis. The delivery of VEGF or bFGF to the ischemic tissue is the most tried intervention. Transfer of a protein molecule is advantageous as it provides more controlled delivery, has established safety, and predictable pharmacokinetics. However, as these proteins degrade once they are injected into the body, they require sustained delivery. The alternative approach to overcome these drawbacks is to activate or inhibit specific genes through gene therapy. As hypoxia-inducible factors initiate an entire angiogenic response, they have been considered for angiogenic (gene) therapy.[34] However, caution is warranted as these hypoxia-inducible factors can also control cell death.[35] There are still many challenges before gene therapy can be applied to clinical settings. Gene expression can be manipulated by introducing therapeutic DNA, which must reach the target cells and transcribed. Transfer vectors are being investigated to deliver DNA to the cells.
Challenges of Angiogenic Therapy
However, there are many challenges in administering these treatments in patients. VEGF forms leaky and tortuous vessels, which may be controlled by combining it with Ang1. Whether this abnormal vascular morphology can lead to impaired microcirculation is not known.[36] Furthermore, the adverse effects of increased levels of angiogenic factors such as triggering of dormant tumors and acceleration of atherosclerosis is not known. Another question is whether a single or a combination of angiogenic molecules will be required to stimulate ‘functional and sustainable’ angiogenesis. For example, genetic studies have shown that the VEGF120 isoform alone is able to initiate, but not complete, the angiogenic program.[37]
Antiangiogenic Therapy
There are several diseases that will benefit from the inhibition of excessive angiogenesis [Table 2].[7] The approved antiangiogenic drugs for various indications are highlighted in Table 3.[3] Besides these, several other antiangiogenic agents are in various phases of clinical trials. In general, five strategies are being used as antiangiogenic therapies by the investigators:
Inhibitors of activated EC
Inhibitors of EC intracellular signaling
Inhibitors of ECM remodeling
Inhibitors of adhesion molecules
Inhibitors of angiogenic mediators or their receptors
Table 3.
Approved antiangiogenic agents for various indications[3]
| Agent | Mechanism | Indication |
|---|---|---|
| Monoclonal antibody therapies | ||
| Bevacizumab | Humanized VEGF antibody | Metastatic colorectal cancer (mCRC), |
| Non-small cell lung cancer (NSCLC), | ||
| Advanced breast cancer, | ||
| Neovascular (wet) Age-related macular | ||
| degeneration | ||
| Cetuximab | Chimeric IgG1 EGFR antibody | mCRC, head and neck cancer |
| Panitumumab | Humanized IgG2 anti-EGFR antibody. | mCRC |
| Trastuzumab | Humanized IgG1 HER-2 antibody | Breast cancer |
| Ranizumab | Recombinant Fab fragment of anti-VGF antibody | Ocular neovascularization |
| Tyrosine Kinase Inhibitors (TKIs) | ||
| Erlotinib | Small molecule TKI-EGFR | NSCLC, pancreatic cancer |
| Sorafenib | TKI of VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, Raf-1 | Advanced renal cell carcinoma (RCC), advanced hepatocellular carcinoma |
| Sunitinib | TKI of VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, RET | Advanced RCC, GIST |
| Other anticancer Angiogenic Agents | ||
| Temsirolimus | Small molecule inhibitor of mTOR (mammalian target of rapamycin), part of PI3 kinase / AKT pathway involved in tumor cell proliferation and angiogenesis | Advanced renal cell carcinoma |
| Everolimus | Small molecule inhibitor of mTOR | Advanced renal cell carcinoma |
| Bortezomib | Proteasome inhibitor-indirect antiangiogenic action | Multiple myeloma (MM), mantle cell lymphoma |
| Thalidomide | Immunomodulatory, anti-inflammatory, antiangiogenic | MM |
| Lenalidomide | Immunomodulatory, anti-inflammatory, antiangiogenic | Myelodysplastic Syndrome, MM |
| rhEndostatin (available only in China) | Endogenous angiogenesis inhibitor; blocks VEGF and down regulates MMP-2/9 | NSCLC |
| Approved agents have antiangiogenic properties dermatology | ||
| Alitretinoin | Topical retinoid-downregulation of VEGF | AIDS-related Kaposi's sarcoma |
| Imiquimod | Toll-Like Receptor 7 agonist-downregulation of FGF-2 and MMP-9 | Benign genital warts |
| Malignant skin cancers | ||
| Interferon α | Endogenous antiangiogenic cytokine | Hemangiomas, giant cell tumor |
| Approved agent for use in ophthalmologic indication | ||
| Ranibizumab | Recombinant humanized IgG1 kappa VEGF-A antibody | Neovascular (wet) age-related macular degeneration. |
| Pegaptanib | Pegylated oligonucleotide (aptamer) - binds to extracellular VEGF. | Neovascular (wet) age-related macular degeneration. |
Adapted from Understanding angiogenesis (document on the internet) -http://www.angio.org/ua.php[3]
1. Inhibitors of Activated EC
Several natural inhibitors of angiogenesis have been described [Table 2]. TSP-1 is constitutively produced by normal cells and is the main physiological inhibitor of angiogenesis. TSP-1 is downregulated, while the angiogenic activity is increased, during tumorigenesis. Accordingly, mutation of the tumor suppressor p53 gene results in the loss of TSP-1 production and a switch to the angiogenic phenotype. Decrease in angiogenesis and inhibition of tumor growth occurs with overexpression of TSP-1.[38] The other most promising antiangiogenic drugs, angiostatin and endostatin, are derived from the tumor cells themselves. Angiostatin suppresses the growth of a number of human tumors and their metastases, in mice.[39] Endostatin, a fragment of collagen, derived through elastase-mediated cleavage,[40] has been isolated from the media of hemangioendothelioma (EOMA) cells. Endostatin specifically suppresses endothelial cell proliferation and shows potent inhibitory activity against many tumor cell lines. In recent times, a potent antiangiogenic agent aaAT (antiangiogenic antithrombin) has been purified from small cell lung cancer.[41] TNP-470 (AGM-1470), a synthetic derivative of the antibiotic fumagillin, is the most studied angiogenic inhibitor. TNP-470 has been shown to inhibit type 2 methionine aminopeptidase, resulting in the abrogation of the processing of methionine, which may lead to the inactivation of unidentified proteins essential for EC growth.[42] TNP-470 is effective in the treatment of a wide variety of tumors. Thalidomide also has antiangiogenic activity. It demonstrates marked responses in patients with multiple myeloma, including those who relapsed after high-dose chemotherapy. The tubulin-binding drug Combretastatin A-4, exhibits a selective toxicity for proliferating EC by induction of apoptosis.
Inhibitors of EC intracellular signaling
Some compounds like Genistein and Lavendustin A have the ability to block the activity of the angiogenic factors by inhibiting the protein tyrosine kinase, which is the second messenger system of these factors. Apart from this Ang-2 Inhibits Tie-2.
2. Inhibitors of ECM Remodeling
Metalloproteinase inhibitors (MMPI) are theoretically the most promising antiangiogenic agents. MMPI research has focused on synthetic, orally available inhibitors. Several MMPIs ranging from broad spectrum inhibitors, which block most of the MMPs, to selective inhibitors, which selectively inhibit a particular MMP, have been developed. Despite significant activity in the preclinical models, MMPIs failed to demonstrate a significant effect in the advanced stage clinical trials in most human malignancies. Batimastat was the first MMPI to be evaluated in humans, but the trials were suspended due to its low oral bioavailability. MMPIs that have entered clinical trials for an oncologic indication include prinomastat, BAY 12-9566, BMS-275291, marimastat, MMI270(B), and Metastat.
3. Inhibition of Cell Adhesion Molecules
αvβ3 integrin, an adhesion receptor for ECM components is present selectively on activated endothelial cells, and therefore, is an attractive target for antiangiogenic therapy. Both the peptide antagonist of αvβ3 and an anti-αvβ3 monoclonal antibody, inhibit adhesion-dependent signal transduction by angiogenic factors, leading to apoptosis of the activated endothelium. These compounds have been found to inhibit angiogenesis. Currently the clinical potential of an anti-αvβ3 antibody, Etaracizumab, is being evaluated in various phases of clinical trials, for many patients with late-stage cancer.[43]
4. Inhibitors of Angiogenic Mediators or their Receptors
Angiogenesis is very sensitive to small changes in factors such as VEGF and FGF-2. Strategies to inhibit the production or release of these growth factors or to interfere with their receptor interactions have been developed. Anti-VEGF antibodies, soluble VEGF receptors, or dominant negative VEGFR-2 have proven successful in the treatment of various conditions such as wet ocular neovascularization and many types of cancers. Synthetic low molecular weight inhibitors of tyrosine kinase receptors have been designed to interfere with growth factor receptor signaling. As tumor cells may depend on different cytokines, the inhibition of one single growth factor may cause only a partial control of tumor growth. This has led to the development of a nonselective tyrosine kinase inhibitor, which inhibits VEGF, FGF-2, and PDGF tyrosine kinase receptors. Various tyrosine kinase inhibitors have already been approved for various anticancer indications. Various exogenous heparin analogs, such as, suramin, are being developed for their capacity to inhibit FGF-2-induced angiogenesis.[7]
Challenges of antiangiogenic therapy
The long-term side effects of many antiangiogenic therapies are not known. Interference with VEGF signaling may cause tumour-dependent toxicity. Although vessel count is considered as a successful prognostic factor, it does not accurately predict vascular function. Furthermore, many tumors do not ‘shrink’ during various antiangiogenic therapies. Thus, new imaging methods are needed to monitor vascular function and a therapeutic response in patients. Finally, the angiogenic response may depend on the individual genetic constitution; hence, the administration of agents should be based on the biology of the individual, to generate maximum therapeutic benefit.
Conclusion
The study of angiogenesis is making a profound impact on the biological and medical world. The hope of being able to build new, functional, and durable blood vessels in ischemic tissues, or conversely, to prevent their further growth in malignant and inflamed tissues is becoming more realistic every day. However, efforts to therapeutically stimulate new blood vessels have significantly lagged behind those to inhibit angiogenesis. Better understanding of the underlying process will enable the scientist to develop new drugs and therapies that will significantly enhance our ability to treat intractable diseases, such as, cancer, diabetes, and heart disease.
References
- 1.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neuro Oncol. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.The angiogenesis foundation. Understanding angiogenesis (document on the internet) Cambridge: The organization; 1994. [updated on 2009 Jul 10] [cited on 2009 Jul 12] Available from http://www.angio.org/ua.php. [Google Scholar]
- 4.Papetti M, Herman IM. Mechanisms of normal and tumour-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–70. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 5.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff J. Cell adhesion and angiogenesis. J Clin Invest. 1997;100:S37–9. [PubMed] [Google Scholar]
- 7.Liekens S, Clereq ED, Neyts J. Angiogenesis: Regulators and clinical applications. Biochem Pharmacol. 2001;61:253–70. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 8.Dorrell M, Jarvinen HU, Aguilar E, Friedlander M. Ocular neovascularisation: Basic mechanism and therapeutic advances. Surv Ophthalmol. 2007;52:S3–19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Domenico R, Maria TC, Gastone G. Nonclassic endogenous novel regulators of angiogenesis. Pharmacol Rev. 2007;59:185–205. doi: 10.1124/pr.59.2.3. [DOI] [PubMed] [Google Scholar]
- 10.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–20. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–81. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 12.Presta M, Moscatelli D, Joseph-Silverstein J, Rifkin DB. Purification from a human hepatoma cell line of a basic fibroblast growth factor-like molecule that stimulates capillary endothelial cell plasminogen activator production, DNA synthesis, and migration. Mol Cell Biol. 1986;6:4060–6. doi: 10.1128/mcb.6.11.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen M. Angiogenic factors as tumor markers. Invest New Drugs. 1997;15:29–37. doi: 10.1023/a:1005766511385. [DOI] [PubMed] [Google Scholar]
- 14.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, et al. Requisite role of angiopoietin-1: A ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 15.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 16.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: Dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–62. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 17.Montesano R, Pepper MS, Vassalli JD, Orci L. Modulation of angiogenesis in vitro. EXS. 1992;61:129–36. doi: 10.1007/978-3-0348-7001-6_21. [DOI] [PubMed] [Google Scholar]
- 18.Ribatti D, Vacca A. Models for studying angiogenesis in vivo. Int J Biol Markers. 1999;14:207–13. doi: 10.1177/172460089901400403. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson L, Bardhan KD, Atherton J, Kalia N. Helicobacter pylori prevents proliferative stage of angiogenesis in vitro: Role of cytokines. Dig Dis Sci. 2002;47:1857–62. doi: 10.1023/a:1016469217449. [DOI] [PubMed] [Google Scholar]
- 20.Maynard SE, Min J, Merchan J, Lim KH, Li J, Mondal S, et al. Excess Placental sFlt-1 may contribute to endothelial dysfunction, hypertension and proteinuria in preeclampsia. J Clin Invest. 2004;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, et al. Impaired angiogenesis in the aging kidney: VEGF and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37:601–11. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 22.Martinez P, Esbrit P, Rodrigo A, Alvarez-Arroyo MV, Martínez ME. Age-related changes in parathyroid hormone-related protein and VEGF in human osteoblastic cells. Osteoporos Int. 2002;13:874–81. doi: 10.1007/s001980200120. [DOI] [PubMed] [Google Scholar]
- 23.Chang E, Yang J, Nagavarapu U, Herron GS. Aging and survival of cutaneous microvasculature. J Invest Dermatol. 2002;118:752–8. doi: 10.1046/j.1523-1747.2002.01714.x. [DOI] [PubMed] [Google Scholar]
- 24.Koyama S, Sato E, Haniuda M, Numanami H, Nagai S, Izumi T. Decreased level of VEGF in bronchoalveolar lavage fluid of normal smokers and patients with pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:382–5. doi: 10.1164/rccm.2103112. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–9. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 27.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the VEGF promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–8. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 28.De La Torre JC. Alzheimer's disease: How does it start? J Alzheimers Dis. 2002;4:497–512. doi: 10.3233/jad-2002-4606. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 30.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99:10730–5. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada K, Lu S, Chisholm DM, Syrjänen S, Schor AM. Angiogenesis and vasodilation in skin warts: Association with HPV infection. Anticancer Res. 2000;20:4519–23. [PubMed] [Google Scholar]
- 32.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 33.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–6. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Post M, Volk R, Gao Y, Li M, Metais C, et al. PR39, a peptide regulator of angiogenesis. Nat Med. 2000;6:49–55. doi: 10.1038/71527. [DOI] [PubMed] [Google Scholar]
- 35.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, et al. Role of HIF-1a in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 36.Jain RK, Munn LL. Leaky vessels. Call Ang1! Nat Med. 2000;6:131–2. doi: 10.1038/72212. [DOI] [PubMed] [Google Scholar]
- 37.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the VEGF isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 38.Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, et al. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441–52. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y. Therapeutic potentials of angiostatin in the treatment of cancer. Haematologica. 1999;84:643–50. [PubMed] [Google Scholar]
- 40.Wen W, Moses MA, Wiederschain D, Arbiser JL, Folkman J. The generation of endostatin is mediated by elastase. Cancer Res. 1999;59:6052–6. [PubMed] [Google Scholar]
- 41.O'Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science. 1999;285:1926–8. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 42.Turk BE, Griffith EC, Wolf S, Biemann K, Chang YH, Liu JO. Selective inhibition of amino-terminal methionine processing by TNP-470 and ovalicin in endothelial cells. Chem Biol. 1999;6:823–33. doi: 10.1016/s1074-5521(99)80129-x. [DOI] [PubMed] [Google Scholar]
- 43.Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, et al. Phase I and pharmacokinetic study of etaracizumab: A humanized monoclonal antibody against αvβ3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]


