Abstract
The albumin D site-binding protein (DBP) governs circadian transcription of a number of hepatic detoxification and metabolic enzymes prior to the activity phase and subsequent food intake of mice. However, the behavior of mice is drastically affected by the photoperiod. Therefore, continuous adjustment of the phase of circadian Dbp expression is required in the liver. Here we describe a direct impact of CRYPTOCHROME1 (CRY1) on the phase of Dbp expression. Dbp and the nuclear receptor Rev-Erbα are circadian target genes of BMAL1 and CLOCK. Surprisingly, dynamic CRY1 binding to the Dbp promoter region delayed BMAL1 and CLOCK-mediated transcription of Dbp compared with Rev-Erbα. Extended presence of CRY1 in the nucleus enabled continuous uncoupling of the phase of Dbp from Rev-Erbα expression upon change from short to longer photoperiods. CRY1 thus maintained the peak of DBP accumulation close to the activity phase. In contrast, Rev-Erbα expression was phase-locked to the circadian oscillator and shaped by accumulation of its own gene product. Our data indicate that fine-tuning of circadian transcription in the liver is even more sophisticated than expected.
Keywords: Circadian transcription, clock, photoperiod, relative phase, output
Circadian clocks provide optimal adaptation to the predictable successions of day and night on Earth. They allow organisms to synchronize daily changes in metabolism, physiology, and behavior to the external light:dark phase (Ko and Takahashi 2006; Yu and Hardin 2006). To function properly, elaborate phase-resetting mechanisms keep their phase in resonance with the environment. They also adjust to seasonal changes of the photoperiod (Schultz and Kay 2003; Hazlerigg and Loudon 2008), but molecular mechanisms that regulate these adjustments remain to be analyzed in detail. Altogether, circadian clocks offer organisms anticipation of, and, hence, preparation for, daily recurring events under regularly changing conditions.
Circadian clocks are based on cell-autonomous, self-sustained oscillators with free-running period lengths of about a day. The circadian oscillator of mammals relies on interconnected feedback loops, in which transcriptional repressors rhythmically challenge transcriptional activators (Ko and Takahashi 2006; Yu and Hardin 2006). MOP3/BMAL1 (Hogenesch et al. 1998; Bunger et al. 2000) and CLOCK (King et al. 1997; Gekakis et al. 1998) form heterodimers (CLOCK/BMAL1) to activate transcription of repressors via E-box motifs. The nuclear receptor REV-ERBα (NR1D1) is part of the stabilizing loop, and instantly represses transcription of the Bmal1 and Clock genes via Rev-Erbα response elements (RREs) (Preitner et al. 2002; Ueda et al. 2002). RORα (NR1F1) counteracts the action of REV-ERBα (TK Sato et al. 2004).
The PERIOD (PER) (Albrecht et al. 1997; Shearman et al. 1997; Sun et al. 1997; Tei et al. 1997) and CRYPTOCHROME (CRY) (Griffin et al. 1999; Kume et al. 1999; van der Horst et al. 1999; Vitaterna et al. 1999) proteins are part of the 24-h rhythm-generating core loop operating nearly in anti-phase to the stabilizing loop. The PER and CRY proteins gradually accumulate until they negatively feed back on their own synthesis. Upon decay of the repressors, a new cycle of CLOCK/BMAL1-mediated transcription can occur. The transcriptional networks are flexible, which may provide the base for adjustments to the light:dark phase and the photoperiod.
The molecular makeup of the circadian oscillator facilitates direct coupling of output genes via CLOCK/BMAL1 and regulatory elements of the E-box type (Jin et al. 1999; Ripperger et al. 2000). A simple way to spread the action of the circadian oscillator to different phases is to involve core clock proteins in rhythmic expression of intermediary transcription factors (Ueda et al. 2005; Yan et al. 2008; Bozek et al. 2009); for example, the albumin D site-binding protein (DBP) (Lopez-Molina et al. 1997; Ripperger and Schibler 2006). DBP and the related thyrotroph embryonic factor (TEF) and hepatic leukemia factor (HLF) activate transcription of a variety of detoxification and metabolic enzymes in the liver before animals start eating, and hence they preadapt the hepatic metabolism for subsequent food intake (Gachon et al. 2006). To achieve this, CLOCK/BMAL1-mediated transcription of Dbp has to be perfectly coordinated, even in different photoperiods. However, mechanisms that offer such capacity to the CLOCK/BMAL1 transcriptional activator are currently unknown.
To gain insights into the regulatory potential of CLOCK/BMAL1, we analyzed circadian transcription of the Rev-Erbα and Dbp genes in mouse liver. Two distinct mechanisms interfered with activation of these genes by CLOCK/BMAL1, separating their phases of expression by ∼2 h. A CRY1-based mechanism delayed Dbp expression, while autorepression prematurely blunted Rev-Erbα expression. Surprisingly, the CRY1-based delaying mechanism does not lock the phase difference between both genes irreversibly, but it provides the circadian oscillator of the liver with flexibility to adjust the phase of Dbp expression according to the photoperiod.
Results
E-boxes determine the phase of Dbp transcription
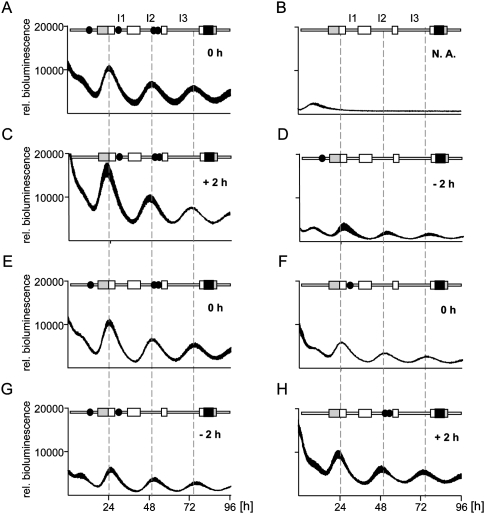
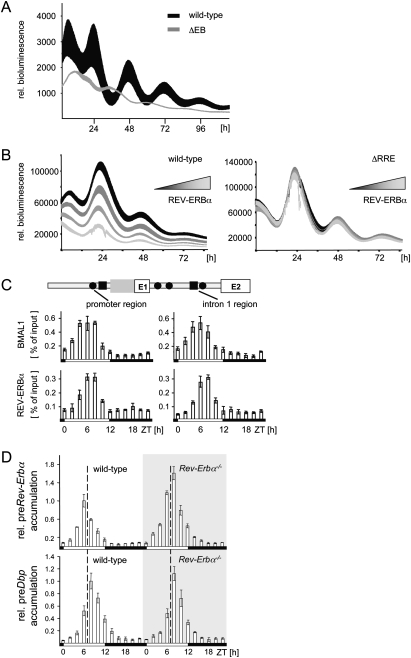
Previously, the major circadian regulatory regions of the Dbp gene were identified (Ripperger et al. 2000; Yamaguchi et al. 2000; Ripperger and Schibler 2006; Kiyohara et al. 2008). These regions contained several DNA-binding elements of the E-box type, which are potential binding sites for BMAL1 and CLOCK. Both transcription factors bound rhythmically to three regions containing these elements in vivo (Ripperger and Schibler 2006). To analyze the functional relevance of the E-box motifs in these regulatory regions, NIH 3T3 fibroblasts were transiently transfected with Dbp reporter constructs. These contained either a wild-type version of the Dbp circadian regulatory regions, or mutant versions affected in one, two, three, or all of the E-box motifs in the three regions (Fig. 1). Transfection of the wild-type reporter construct into NIH 3T3 cells and synchronization of these cells with dexamethasone resulted in three circadian cycles of luciferase expression during the recording period (Fig. 1A). As a control, deletion of all potential E-box motifs completely abolished circadian oscillations (Fig. 1B), as demonstrated previously in Rat-1 fibroblasts with a stable integration of a related mDbp transgene (Ripperger and Schibler 2006).
Figure 1.
Influence of E-box motifs on the phase of Dbp expression. A luciferase reporter construct (black box) was inserted into exon 4 of the mDbp gene. In the different constructs, none, one, or more E-boxes (solid black circles) were inactivated. (A–H) Four individual cultures of mouse NIH 3T3 fibroblasts were transiently transfected with either of the indicated constructs. Shown is the average bioluminescence ± SD measured continuously over 96 h after synchronization with dexamethasone (n = 4). For a better comparison, all curves were adjusted to the same scale. Dashed lines indicate the positions of the peaks of the three circadian cycles of the wild-type construct. The term “rel. luminescence” refers to the measured counts per second normalized to the amount of secreted alkaline phosphatase. The numbers in the panels refer to the observed phase differences. (+) Phase advance; (−), phase delay; (N.A.) not applicable; (gray boxes) Position of the noncoding exons; (white boxes) position of the codons in exons 1–4; (I1–I3) positions of introns 1–3.
Interestingly, we noticed that the phase of Dbp expression was influenced by the promoter E-box and the two intron 2 E-boxes: Deletion of the promoter E-box provoked a phase advance by ∼2 h (Fig. 1C), whereas, conversely, deletion of the two E-boxes in intron 2 resulted in a similar phase delay (Fig. 1G). In contrast, the E-box in intron 1 did not affect the phase of Dbp expression (Fig. 1E,F). By driving luciferase expression from Dbp reporter constructs containing either the promoter or intron 2 E-boxes alone, we obtained the reverse regulatory behavior (Fig. 1, cf. D and C, and cf. H and G). The experimental observations were not due to changed period lengths of rhythmic luciferase activity derived from the different reporter constructs (Supplemental Fig. 1). Taken together, it is tempting to speculate that the precise phase of Dbp expression is determined solely by the action of its E-boxes.
The affinity of an E-box for the CLOCK/BMAL1 heterodimer affects the phase
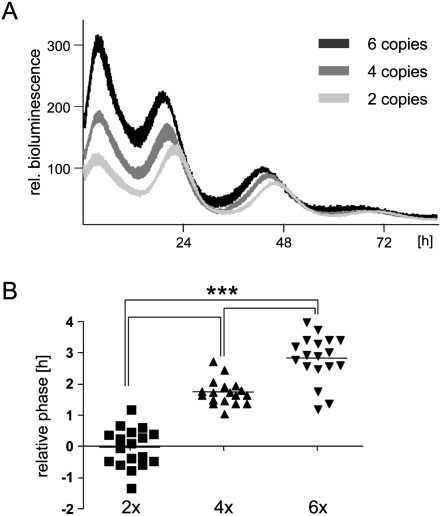
Next, we analyzed the E-box motifs that affect the phase of Dbp expression in further detail. Only one of the intron 2 E-box motifs (+2398) (Ripperger et al. 2000) was capable of driving robust circadian rhythmicity of luciferase expression comparable with the genomic Dbp construct (Fig. 2A). The other two E-boxes, when removed from their genomic context, could not regulate stable circadian luciferase expression from a minimal promoter. The constructs based on the promoter E-box (−163) (Ripperger and Schibler 2006) and the second intron 2 E-box (+2510) (Ripperger et al. 2000) showed very high luciferase activity with severely dampened amplitude (data not shown), probably due to the association of other E-box-binding factors. For the particular intron 2 E-box motif, we noticed that the phase of luciferase expression was dependent on the E-box copy number in this construct: The more copies were inserted, the earlier expression started (Fig. 2A,B). We speculate that increasing the copy number may increase the probability of BMAL1 and CLOCK binding to regulatory elements in a cooperative manner. Hence, the first E-box motif occupied by transcriptionally active CLOCK/BMAL1 should determine the phase of Dbp expression. Our characterization of the various Dbp reporter constructs would indicate that this motif is located in intron 2 (Fig. 1H), and, consequently, Dbp should be expressed 2 h in advance. Since this is obviously not the case, an active mechanism might be in place to delay the phase of Dbp expression.
Figure 2.
The copy number of the intron 2 E-box motif affects the phase. (A) NIH 3T3 fibroblasts were transfected with reporter constructs driven by two, four, or six copies of an E-box motif (+2398 in intron 2 of the Dbp gene). The cells were synchronized and bioluminescence was measured as in Figure 1. (B) Statistical analysis of the relative phases (n = 18). The relative phases were determined from the raw data using the LumiCycle software. For better comparison, the phase of the construct with two copies of the E-box was set to 0. One-way ANOVA with Bonferroni's post-test. (+) Phase advance; (−), phase delay.
The Dbp promoter region contains a CRY1-binding element
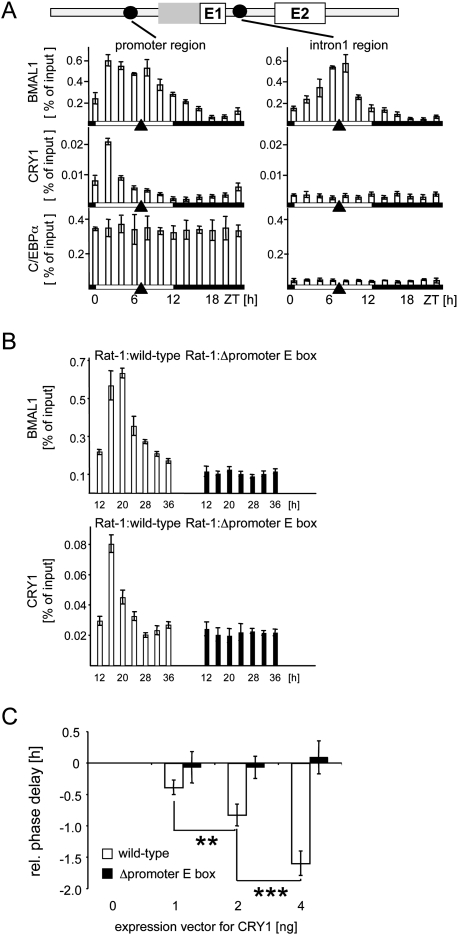
Using chromatin immunoprecipitation on mouse liver chromatin, we scanned the entire Dbp gene for potential regulatory factors that might affect the phase of its expression (Supplemental Fig. 2). We observed two peaks of BMAL1 binding to the promoter region of Dbp, but only one at the intron 1 region (Fig. 3A; Ripperger and Schibler 2006) or the intron 2 region (Supplemental Fig. 2). The first peak of binding occurred ∼6 h before the peak of Dbp transcription (at Zeitgeber time 2 [ZT2], when ZT0 is lights on and ZT12 lights off), and may thus represent part of a mechanism to delay the phase of Dbp expression. The second peak, which was in the same phase as BMAL1 binding to the intron 1 and intron 2 regions, coincided with the peak of transcriptional activation of the Dbp gene (ZT7) (Ripperger and Schibler 2006). Binding of the heterodimerization partner CLOCK in vivo paralleled BMAL1 binding (Supplemental Fig. 2). Thus, different kinds of CLOCK/BMAL1 complexes were identified at the Dbp gene with probably opposing functions.
Figure 3.
Circadian binding of BMAL1 and CRY1 to the promoter region of Dbp. (A) Chromatin immunoprecipitation analysis using antibodies against BMAL1, CRY1, or C/EBPα, and chromatin from mouse liver tissue taken at 2-h intervals. The phases of light or dark are depicted below the graphs. Black triangles show the peak of transcriptional activity of the Dbp gene. The coimmunoprecipitated DNA fragments were quantified with real-time PCR probes specific for the promoter region or the intron 1 region. Shown is the amount of DNA coimmunoprecipitated from the input (% of input). (B) Chromatin immunoprecipitation analysis using antibodies against BMAL1 or CRY1, and chromatin from Rat-1 fibroblasts with a single-copy integration of a wild-type 6.9-kb fragment of the mDbp gene (white bars) or a mutated version without functional promoter E-box motif (black bars). The cells were synchronized with dexamethasone and chromatin prepared at the indicated time points. The coimmunoprecipitated DNA fragments were quantified using real-time PCR probes specific for the promoter region of the mouse transgene. (C) NIH 3T3 fibroblasts were cotransfected with either a wild-type mDbp-luciferase construct or the version with the inactivated promoter E-box motif, and increasing amounts (1, 2, or 4 ng) of an expression vector for mCRY1. Shown are the relative phases of reporter gene expression compared with the transfection without expression vector for CRY1 (n = 4; mean ± SD). One-way ANOVA with Bonferroni's post-test.
Unexpectedly, we detected rhythmic binding of the negative core clock component CRY1 specifically to the promoter region of Dbp (Fig. 3A; Supplemental Fig. 2). The phase of CRY1 recruitment was in concert with the first peak of BMAL1 binding to the same region, but did not match the phase of its proposed function as repressor within the core loop. Therefore, we may have identified an additional function of CRY1 to delay the phase of Dbp expression. As a control, we monitored the constant binding of the transcription factor C/EBPα to the Dbp promoter region (Fig. 3A). Although binding there, genetic experiments suggest that this factor is not crucial for Dbp expression in the liver (Wang et al. 1995).
To pinpoint the binding site of CRY1 in the promoter region of Dbp, we used previously generated Rat-1 fibroblasts with a single-copy integration of a wild-type mouse Dbp gene, or a mutated version with the promoter E-box motif mutated (Ripperger and Schibler 2006). Similar to BMAL1, the rhythmic binding of CRY1 was dependent on the functional E-box motif in the promoter region of the transgene (Fig. 3B). This was not due to a general inability of both regulatory factors to bind to an E-box in the stable cell line, because the binding of either factor to the endogenous gene was not affected (Supplemental Fig. 3). Furthermore, binding of BMAL1, CLOCK, and CRY1 to an oligonucleotide encompassing the promoter E-box of the Dbp gene was observed in vitro (Supplemental Fig. 4). To monitor the effect of CRY1 on the phase of Dbp expression in vitro, we performed cotransfection experiments in NIH 3T3 fibroblasts. Cotransfection of the Dbp genomic reporter construct, but not of the construct without a promoter E-box, together with an expression plasmid for CRY1, provoked a significant phase delay of luciferase activity (Fig. 3C). In summary, our data suggest that, at ZT2, CRY1 is recruited specifically to the promoter E-box of Dbp, and may delay the phase of circadian Dbp expression.
The delaying mechanism relies on functional CRY1
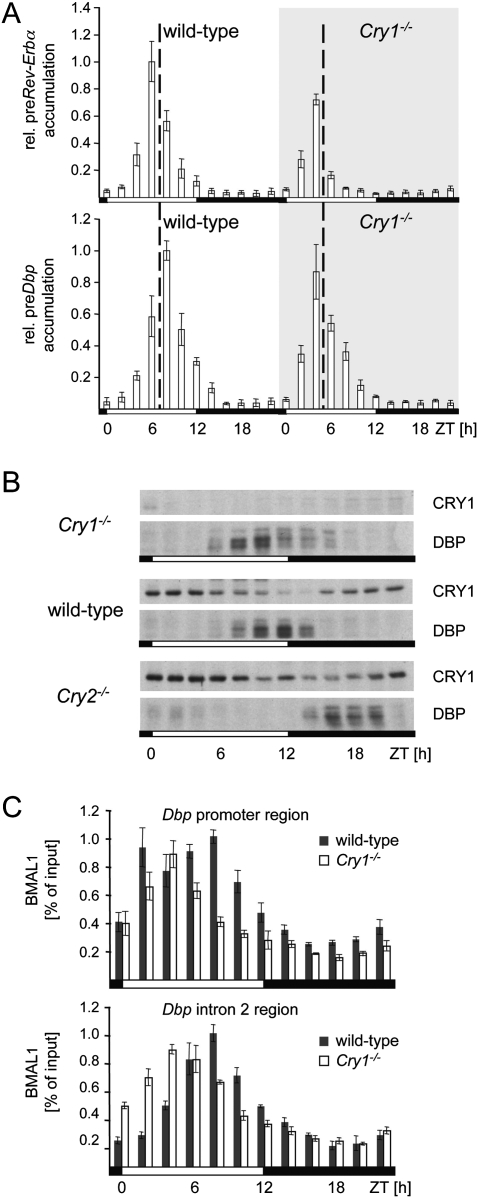
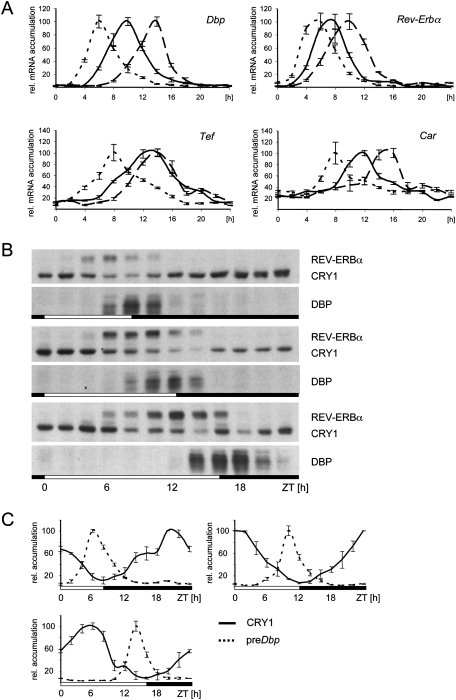
The transcriptional peaks of the two clock-controlled genes Rev-Erbα and Dbp are separated in the liver of mice, with the peak of Dbp lagging ∼2 h behind the peak of Rev-Erbα (Fig. 4A). In order to analyze the importance of CRY1 for the phase-delaying mechanism of Dbp, we measured the accumulation of precursor mRNAs of Rev-Erbα or Dbp in the liver of homozygous Cry1 knockout mice (van der Horst et al. 1999). In these mice, the phase of the circadian oscillator in general is advanced by ∼2 h. In the absence of CRY1, Dbp and Rev-Erbα had a similar phase of expression (Fig. 4A), indicating that the delay of Dbp expression does not occur anymore. To determine the impact of this changed phase of Dbp expression on the accumulation of the DBP protein, we performed an analysis of nuclear extracts obtained from liver nuclei of Cry1−/−, wild-type, or Cry2−/− mice (Fig. 4B). In both Cry-deficient backgrounds, the accumulation of the DBP protein was unnatural. In Cry1-deficient mice, DBP accumulated ∼3 h earlier as compared with wild-type animals, while, in Cry2-deficient mice, the protein mounted up 4 h later. Therefore, in both Cry-deficient backgrounds, the capacity of DBP to regulate expression of a variety of detoxification and metabolic enzymes in the liver precisely at the transition from the light to the dark phase might be severely affected.
Figure 4.
Importance of CRY1 for the phase-delaying complex. (A) Precursor RNA accumulation of Dbp or Rev-Erbα in liver tissue from wild-type or Cry1-deficient mice at 2 h resolution. The values were normalized to constant Gapdh expression, and the peak of mRNA accumulation observed in the wild-type mice was arbitrarily set to 1.0 (n = 3; mean ± SD). (B) Western blot analysis for accumulation of the indicated proteins from liver nuclear extracts of Cry1-deficient, wild-type, or Cry2-deficient mice at 2 h resolution. (C) Chromatin immunoprecipitation analysis for BMAL1 at the same time points as in B using real-time PCR probes specific for the Dbp promoter region or the intron 2 region and chromatin from wild-type (black bars) or Cry1 homozygous knockout mice (white bars).
In the livers of Cry2-deficient mice, CRY1 persisted longer in the nucleus, probably to compensate for the lack of CRY2 protein (Fig. 4B). In coherence with our in vitro data, the expression of Dbp might be delayed in response to the prolonged presence of CRY1. Indeed, the presence of CRY1 at the Dbp promoter region was extended in Cry2-deficient mice (Supplemental Fig. 5). Accordingly, transcription of Dbp was shifted further away from Rev-Erbα. Using chromatin immunoprecipitation, we found that the characteristic two peaks of BMAL1 binding observed at the promoter region of Dbp were absent in Cry1-deficient mice (Fig. 4C). These data indicate that CRY1 is crucial for the formation of the phase-delaying complex or its ability to bind to the promoter E-box motif in a phase preceding Dbp transcription.
The transcription of Rev-Erbα is blunted by autorepression
In the course of our analysis of the Dbp gene, we noticed that, in Cry1-deficient mice, the initiation of transcription of the Dbp and Rev-Erbα genes coincided, but the transcription of the Dbp gene persisted slightly longer (Fig. 4A). Therefore, another regulatory mechanism may exist that restricts specifically the temporal expression of the Rev-Erbα but not the Dbp gene. This mechanism may add to the relative phase difference of both genes in vivo. Previously, regions in the Rev-Erbα gene containing in vivo binding sites for BMAL1 and CLOCK have been mapped by chromatin immunoprecipitation (Ripperger 2006). A luciferase reporter construct encompassing all of these potential regulatory sites was sufficient to drive stable circadian expression in NIH 3T3 cells (Fig. 5A). After inactivation of five potential E-box motifs, the remaining construct displayed weak but stable circadian cycles in anti-phase to the original construct (Fig. 5A; Supplemental Fig. 6). This construct was thus no longer regulated by the core but by the stabilizing loop of the circadian oscillator, implicating activation by RORα and repression by REV-ERBα itself.
Figure 5.
Autorepression of the Rev-Erbα gene. (A) Identification of functional E-boxes within the Rev-Erbα gene. NIH 3T3 fibroblasts were transfected with a reporter gene containing the wild-type regulatory region of the Rev-Erbα gene (black), or a version with all potential E-boxes inactivated (gray). (B) Identification of functional RREs within the Rev-Erbα gene. The genomic Rev-Erbα-luciferase construct, or a version with the two potential REV-ERBα-binding sites mutated, was cotransfected with increasing amounts (22, 66, or 200 ng) of a vector containing the entire Rev-Erbα gene (average from n = 4; mean ± SD). (C) Chromatin immunoprecipitation analysis to monitor the binding of BMAL1 or REV-ERBα to the promoter region or the intron 1 region of the Rev-Erbα gene using the same chromatin as in Figure 3A. Above is a scheme of the regulatory region of the Rev-Erbα gene around exon 1 and exon 2 indicating the positions of potential E-boxes (black circles) or potential RREs (black squares). (D) Precursor RNA accumulation of Dbp or Rev-Erbα in liver tissue from wild-type or Rev-Erbα-deficient mice at 2-h resolutions. The peak of mRNA accumulation observed in the wild-type mice was arbitrarily set to 1.0 (n = 3; mean ± SD). Note that, in Rev-Erbα–deficient mice, we measure the intron 1 of the knockout allele, which is identical to the intron 1 of the Rev-Erbα gene and probably has the same half-life.
For the human Rev-Erbα gene, it was described previously that its regulatory region contained a binding site for REV-ERBα, thereby offering the possibility of autorepression (Adelmant et al. 1996). Furthermore, REV-ERBα was shown to bind to the mouse Rev-Erbα gene in vivo (Schmutz et al. 2010). Indeed, a luciferase reporter gene driven by the circadian regulatory region of Rev-Erbα was repressed by REV-ERBα in a dose-dependent fashion (Fig. 5B). The overexpression of REV-ERBα had an impact on the amplitude as well as on the phase of reporter gene expression (Supplemental Fig. 7). Upon deletion of two potential RREs (Harding and Lazar 1995; Zhao et al. 1998) located in the promoter region and the intron 1 region of the Rev-Erbα gene, this effect was abolished (Fig. 5B; Supplemental Fig. 7). Therefore, REV-ERBα may have a direct impact on the regulation of its own gene.
Using chromatin immunoprecipitation, we observed rhythmic binding of REV-ERBα to Rev-Erbα regions harboring potential RREs, the promoter region, and the intron 1 region (Fig. 5C). The peak of REV-ERBα binding occurred ∼2 h later than the peak of BMAL1 binding (Fig. 5C), consistent with a new function of REV-ERBα as a repressor of CLOCK/BMAL1 activity at the Rev-Erbα gene. We used Rev-Erbα-deficient mice to monitor the regulatory effect in vivo. Due to the construction of the knockout allele (Preitner et al. 2002), we could measure the accumulation of its precursor mRNA with a probe mapping to the first intron. The first intron is identical in the wild-type and knockout allele, and, consequently, the respective nascent precursors are expected to have the same half-life. Hence, it was possible to measure and compare the ongoing transcription efficiency in the presence or absence of the REV-ERBα protein. We found that precursor Rev-Erbα accumulation in the livers of Rev-Erbα-deficient mice was ∼50% increased as compared with wild-type mice, and terminated roughly at the same moment as the precursor Dbp accumulation (Fig. 5D).
Thus, another regulatory mechanism is operative at the Rev-Erbα gene to fine-tune the CLOCK/BMAL1 heterodimer. In contrast to the CRY1-dependent regulatory mechanism of the Dbp gene, this regulatory mechanism does not delay transcription, but causes repression in advance. As a result, accumulation of the REV-ERBα protein shapes the expression of the Rev-Erbα gene. Both mechanisms together separate the peaks of Rev-Erbα and Dbp expression by ∼2 h under normal laboratory conditions (normal photoperiod [NP], 12 h light/12 h dark).
The phase difference of Rev-Erbα and Dbp expression varies with the photoperiod
In our previous experiments, we observed certain flexibility of the phase of Dbp expression. To assign a biological function to this observation, we analyzed the accumulation of Dbp and Rev-Erbα in the liver of mice subjected to either short photoperiods (SPs; 8 h light/16 h dark) or long photoperiods (LPs; 16 h light/8 h dark) (Fig. 6A). The regulatory loops of the hepatic circadian oscillator were modulated in different photoperiods to fit into the time window of the new light or dark phase regarding the expression of Per1, Per2, Cry1, and Bmal1 (Supplemental Fig. 8). In the livers of mice kept in an SP or an LP, the expression of Dbp was shifted closer to or further away from the phase of Rev-Erbα expression, respectively (Fig. 6A). The expression of another circadian output gene in a similar phase, Tef, did not follow the expression of Dbp. The phase of Dbp between the two opposite photoperiods was shifted by 8 h, while the phase of Rev-Erbα was shifted by only 4–5 h compared with 6 hours for most of the other oscillator components (Supplemental Fig. 8).
Figure 6.
Influence of the photoperiod on Dbp expression. (A) mRNA accumulation of the indicated genes in mouse liver. The samples were extracted from liver of wild-type mice every 2 h in SP (dotted lines), NP (solid lines), or LP (dashed lines). For better comparison, all peaks were set to 100%, and there is a time scale starting from the transition from the external light to dark phase (ZT0) in hours depicted below the graphs. The first value is double-plotted at the end of the curve. (B) Western blot analysis for the accumulation of the indicated proteins. The photoperiods are indicated as white and black bars below the panels. (C) Accumulation of the CRY1 protein (solid lines; n = 3; mean ± SD) or the precursor mRNA of Dbp (dotted lines; n = 3; mean ± SD) in three different photoperiods. Protein accumulation was normalized to RNA polymerase II in the same extracts, and precursor mRNA accumulation was normalized to Gapdh. The peaks of each accumulation were set to 100%. The first value is double-plotted at the end of the curve.
The accumulation of the DBP protein appears to be locked close to the transition from the light phase to the dark phase, regardless of the imposed photoperiod (Fig. 6B). As a consequence, the Dbp target gene Constitutive androstane receptor (Car; NR1I3) (Gachon et al. 2006), which is important for the regulation of detoxification enzymes in the liver, was also expressed close to the transition from the inactivity phase to the activity phase (the peak of mRNA accumulation under SP is about ZT8, under laboratory conditions is about ZT12, and under LP is about ZT16) (Fig. 6A). Therefore, the capacity of DBP to regulate detoxification and metabolic enzymes via CAR prior to the activity phase of mice is maintained in different photoperiods.
Under the different light:dark conditions employed here, the phase of Dbp transcription inversely correlated with the levels of CRY1 protein found in the nucleus (Fig. 6B,C). In coherence with these data, an uncoupling of Dbp from Rev-Erbα expression in a long photoperiod was not observed in livers of Cry1-deficient mice (Supplemental Fig. 9). Therefore, CRY1 may be the main factor to drive uncoupling of Dbp from Rev-Erbα expression during lengthening of the photoperiod.
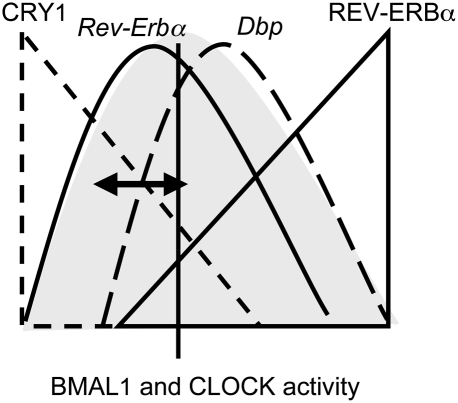
The phase of mRNA accumulation of Dbp can vary relative to the phase of the circadian oscillator, as exemplified by Rev-Erbα (Fig. 6A). Our data suggest a biological significance for this flexibility. The overall phase of the hepatic circadian oscillator shifts according to the photoperiod (Supplemental Fig. 8). As a consequence, DBP accumulation would be shifted too much to activate transcription of some output genes prior to the activity phase of mice. Therefore, there is a mechanism in place to uncouple and to adjust the phase of Dbp expression under those conditions. To sum up, we elucidated here two sophisticated fine-tuning mechanisms that determine the phases of Rev-Erbα and Dbp expression by interfering with the transcription activation potential of CLOCK/BMAL1 (Fig. 7). The CRY1-based mechanism allows flexibility of the phase of Dbp expression to adjust the phase of Dbp expression according to the photoperiod.
Figure 7.
Model of Dbp phase determination in the liver. During a certain time window of the day, BMAL1 and CLOCK may have an intrinsic transcriptional activation potential (gray shading) centered between the peaks of Dbp and Rev-Erbα expression. For Dbp, transcription is delayed in response to the nuclear CRY1 protein levels (dashed lines); for Rev-Erbα, the accumulation of REV-ERBα provokes autorepression (solid lines). Note that, in this simple model, the presence of CRY1 can shift the phase of Dbp expression closer to or further away from Rev-Erbα expression (black arrow).
Discussion
Mechanisms to fine-tune CLOCK/BMAL1 activity
BMAL1 and CLOCK have prominent functions within the mammalian circadian oscillator. On the one hand, they generate circadian transcriptional feedback loops in concert with specialized repressors (Ko and Takahashi 2006; Yu and Hardin 2006). On the other hand, they couple directly rhythmic expression of some output genes to the circadian oscillator (Jin et al. 1999; Ripperger et al. 2000). Mechanisms that affect the regulatory activity of CLOCK/BMAL1 to regulate genes in different phases are not yet elucidated in detail. This is particularly intriguing due to the fact that both proteins are constantly present in the nucleus (Ripperger et al. 2000; Lee et al. 2001).
The DEC proteins can modulate the phase of CLOCK/BMAL1-mediated expression of E-box-containing genes (Honma et al. 2002; F Sato et al. 2004). They were originally identified as competitors of CLOCK/BMAL1 function. Recently, DEC1 was described to generally delay the expression phases of the E-box-containing genes Per1, Dbp, and Rev-Erbα but not of the E′-box-containing genes Per2 and Cry1 (Nakashima et al. 2008).
Different mechanisms may fine-tune the phases of other CLOCK/BMAL1-regulated genes. The Per2 gene includes a phase regulatory element in its promoter region (Akashi et al. 2006). An E-box-like motif of the Cry1 gene also has an impact on the phase of expression (Fustin et al. 2009). In addition, REV-ERBα can determine the phase of Cry1 expression (Etchegaray et al. 2003; Liu et al. 2008). However, it is currently unknown whether any of these mechanisms allows uncoupling of gene expression from the phase of the circadian oscillator.
Here, we analyzed hepatic expression of Rev-Erbα and Dbp, two genes that show expression patterns separated by 2 h (Fig. 4A) in spite of the fact that both are genuine target genes of BMAL1 and CLOCK (Figs. 3A, 5C; Ripperger 2006; Ripperger and Schibler 2006). Two ingenious ways interfered with CLOCK/BMAL1-mediated transcriptional activation of both genes in two distinct phases (Fig. 7).
CRY1 was the main factor to modulate the phase of Dbp expression. This finding was surprising because so far only a function of CRY1 within the core loop had been postulated (Kume et al. 1999; van der Horst et al. 1999; Shearman et al. 2000). The CRY and PER proteins accumulate over the course of the circadian cycle to repress expression of Rev-Erbα, Dbp, Per1, and Per2, as well as many more clock-controlled target genes. Interaction of CRY1 with BMAL1 or PER2 was analyzed in detail (Chaves et al. 2006; Sato et al. 2006; Langmesser et al. 2008), and might involve PER2 acting as adapter (Chen et al. 2009).
In the context of our study, binding of CRY1 to the promoter E-box of Dbp was independent of PER proteins (data not shown). Similar to Drosophila Per (Menet et al. 2010), the mammalian PER proteins bind to regulatory E-box motifs after the peak of transcriptional activation (Schmutz et al. 2010). Therefore, we may have found not only a new function for CRY1 to modulate the phase of Dbp expression, but also a new complex containing CRY1, BMAL1, and CLOCK. However, to understand the composition of this complex and its highly specific DNA-binding properties, purification of this complex from the mouse liver is necessary. Such a complex may contain histone deacetylases (Naruse et al. 2004) to keep the chromatin structure in a condensed state and therefore block access of CLOCK/BMAL1 to the intron 1 and intron 2 E-box sites of the Dbp gene.
The question arises as to how persistence of CRY1 in the nucleus is regulated to modulate Dbp expression. Our data suggest a role for CRY2 in this process, since the presence of high amounts of CRY1 was extended in Cry2-deficient mice (Fig. 4B). Alternatively, REV-ERBα acts as an upstream regulator of the Cry1 gene (Etchegaray et al. 2003; Liu et al. 2008). If REV-ERBα shifts closer to the transition from dark to light, then Cry1 is repressed earlier, CRY1 is rapidly cleared from the nucleus, and, thus, Dbp is expressed earlier. This kind of scenario may occur during the transition from NPs to SPs (Fig. 6C; Supplemental Fig. 8). In the LP, accumulation of CRY1 was uncoupled from Cry1 expression (Figs. 6B; Supplemental Fig. 8). Therefore, there may be a post-translational regulatory mechanism involved.
Autorepression caused early transcriptional termination of Rev-Erbα expression (Fig. 7). The binding of CLOCK/BMAL1 overlapped with REV-ERBα during repression (Fig. 5C,D). Thus, REV-ERBα did not displace CLOCK/BMAL1 from E-box motifs, but probably restricted more indirectly the activity of CLOCK/BMAL1 by affecting the local chromatin structure. REV-ERBα may interact with N-CoR and histone deacetylase 3 (Yin and Lazar 2005) to establish an inactive chromatin conformation at the Rev-Erbα gene. The autorepression separated the peaks of Rev-Erbα and Dbp expression, even in different photoperiods (Fig. 6A). As a consequence, the phase of Rev-Erbα became less shifted than the overall phase of the circadian oscillator (Supplemental Fig. 8). However, the reasoning for this transcriptional fine-tuning is less evident, since REV-ERBα persisted longer in the nucleus than DBP (Fig. 6B). As speculation, REV-ERBα may regulate different target genes in multiple consecutive waves, while the action of DBP has to be restricted to a single action.
Both genes bear functional E-box motifs with very similar sequences (Supplemental Table S1). Two recent studies (Nakahata et al. 2008; Paquet et al. 2008) identified circadian control elements composed of an E-box motif and a related E′-box motif separated by 6 or 7 nucleotides (nt). As a speculation, the distance between the E-box and E′-box may allow or influence the binding of CRY1. This would imply that many genes bearing related E-box motifs with a spacer of 6 nt are expressed in slightly delayed phases. In principle, affinity of an E-box for BMAL1 and CLOCK can affect the phase of gene expression (Fig. 2A). However, in contrast to the CRY1-based mechanism operative at the Dbp promoter E-box, this would determine only the phase of expression, but probably would not provide flexibility.
Flexibility of the mammalian circadian network according to the photoperiod
Organisms adjust their daily life not only to the environmental light:dark cycle, but also to the photoperiod. In nature, the photoperiod is subject to seasonal variation, and (at least in our latitude) varies continuously between 8 h light/16 h dark to 16 h light/8 h dark and back again over the course of a year. Photoperiodic responses are changes according to these conditions, and they are intimately connected with the circadian timing system. In plants, two distinct photoperiodic responses, hypocotyl elongation and flowering, are affected by many mutations that also affect their circadian oscillator (for review, see Schultz and Kay 2003). For example, in Arabidopsis, AtCRY2 is a genuine photoreceptor (only vaguely related to mCRY proteins) impacting on flowering.
Coincidently, in the soybean, the photoreceptor GmCRY1 is also a major regulator of photoperiodic flowering (Zhang et al. 2008). Under LP conditions, accumulation of the GmCry1 mRNA and GmCRY1 protein was tightly coupled, while, under SP conditions, the protein was significantly lagging behind. Therefore, a post-translational mechanism exists that controls accumulation of GmCRY1 protein depending on the photoperiod as well.
Photoperiodic responses are not restricted to plants. A study performed with Neurospora demonstrated uncoupling of frequency transcription and FRQ translation in different photoperiods (Tan et al. 2004). The mRNA accumulation reflected the external light conditions, but FRQ protein accumulation could be uncoupled by up to 6 h and was locked to the middle of the dark period. Concomitantly, asexual spore formation was locked to the same time window. Hence, similar to the phase adjustment of Dbp, this uncoupling might fulfill a biological function.
Specialized brain structures link mammals to the external light:dark phase. These suprachiasmatic nuclei (SCN) show significant responses to the photoperiod in Siberian hamsters (Nuesslein-Hildesheim et al. 2000), mice (Steinlechner et al. 2002), or rats (Sumova et al. 2002). However, similar phenomena observed in peripheral organs such as the liver are less well understood. While the photoperiodic response of the SCN probably is mediated directly by light, the phase of the hepatic circadian oscillator is affected by feeding (Damiola et al. 2000; Stokkan et al. 2001).
The activity of mice and, consequently, the feeding behavior are influenced by the SCN. Therefore, the circadian oscillator of the liver adjusts to the photoperiod. Indeed, both loops of the circadian oscillator were compressed or expanded to fit into either 8 or 16 h (Fig. 6A; Supplemental Fig. 8). Molecular mechanisms governing these modulations remain to be elucidated. Nevertheless, these modulations may have consequences for the detoxification potential of the liver, and specific mechanisms must adjust phases, such as the CRY1-based mechanism for the phase of Dbp. As a net result, the phases of Dbp transcription and DBP accumulation are always close to the beginning of the activity phase to govern rhythmic output gene expression. In this fashion, the circadian oscillator can preadapt the liver for subsequent food intake according to the photoperiod.
Taken together, our experiments provide new insights into the mammalian circadian oscillator. On the one hand, complicated fine-tuning mechanisms restrict the activity of CLOCK/BMAL1 to precise phases; on the other hand, one of these mechanisms offered unexpected flexibility to Dbp expression. Such flexibility should be taken into account while planning new therapeutic treatments based on circadian changes in physiology or metabolism. The success or the side effects of a particular treatment may be dependent on not only the circadian clock, but also the photoperiod. The understanding of the regulatory properties of circadian oscillators in different photoperiods may provide new insights into the link between the circadian clock and potential distortions that render us prone to diseases like depression or cancer.
Materials and methods
Animals
Animals were housed in a 12-h light/12-h dark regimen unless otherwise indicated, with food and water ad libitum. ZT0 is defined as the time when the lights go on. Animals were housed for ∼3 wk under the indicated photoperiods. The age of the animals was between 3 and 4 mo. The Cry1-deficient, Cry2-deficient (van der Horst et al. 1999), and Rev-Erbα-deficient (Preitner et al. 2002) mice have been described. All animal care and handling was performed according to the State of Fribourg's law for animal protection and the Animal Welfare Act of the Dutch government. As required by the Dutch law, animal studies in the Netherlands were approved by an independent Animal Ethical Committee (equivalent to the International Animal Care and Use Committee).
Chromatin immunoprecipitation
Our method of chromatin immunoprecipitation from liver tissue of mice has been described (Ripperger and Schibler 2006). Briefly, livers from mice were extracted at the indicated time points and homogenized in 1× PBS in the presence of 1% formaldehyde. Purified nuclei were obtained by centrifugation through 2.05 M sucrose/10% glycerol cushions, and the nuclei were sonicated in the presence of 1% SDS three times for 30 sec using an ultrasonic homogenizer 300V/T equipped with a cup horn device (BioLogics). The prepared chromatin was adjusted to 0.1% SDS, 1% Triton X-100, 20 mM Tris-Hcl (pH 7.5), 150 mM NaCl, and 2 mM EDTA; precleared by centrifugation; and subjected to immunoprecipitation with the indicated antibodies. The DNA/protein complexes bound to protein A-agarose were washed as described, and the cross-links were reversed overnight at 65°C in 50 μL of 1% SDS, 20 mM Tris (pH 7.5), 150 mM NaCl, and 2 mM EDTA. Forty microliters of the supernatant was mixed with 160 μL of binding buffer (MSB Vario Cleanup), loaded on the corresponding columns, and eluted with 50 μL of water. Five microliters of each reaction was directly used in TaqMan real-time PCRs of 15-μL final volumes on a RotorGene 6200 machine (Corbett Life Science) with 1× Ex Taq PCR master mix (TaKaRa Bio, Inc.) and specific PCR probes (Supplemental Table S2). Antibodies used in these experiments have been described (Ripperger and Schibler 2006).
Plasmid constructions
For the Dbp-luciferase construct, a 6.9-kb BglII fragment of the mouse Dbp gene was inserted into the BclI–BglII sites of the plasmid pCDNA5/FRT (Life Technologies Corporation). As a second step, an IRES-luciferase fragment was inserted into the singular EcoRV site in exon 4. This construct is supposed to contain all regulatory features of the Dbp gene, but does not yield stable DBP protein (Lopez-Molina et al. 1997; Ripperger and Schibler 2006). Potential regulatory binding sites for BMAL1 and CLOCK (Ripperger and Schibler 2006) were exchanged by site-directed mutagenesis to BglII sites (promoter region, intron 1), or were digested with PmlI and religated in the presence of a HindIII linker (intron 2). Double and triple replacements were obtained by the exchange of appropriate restriction fragments between the constructs. Duplicates of the E-box motif (+2398) (Ripperger et al. 2000) of Dbp and its flanking regions, described in Supplemental Table S2, were cloned into the pGL4.24 vector (Promega) with the indicated copy numbers. The plasmid pGL3_Rev-Erbα was obtained by insertion of a 3.8-kb fragment of the mRev-Erbα gene (starting from the EcoRV restriction enzyme site 690 base pairs [bp] upstream of the transcription start site to the beginning of exon 2) into the MluI and NcoI restriction enzyme sites of pGL3 basic (Promega) to create a fusion protein of the first 16 amino acids of REV-ERBα and the firefly luciferase. Site-directed mutagenesis was used to inactivate potential E-box motifs in this DNA fragment: CACGTGAAGCTCTCACGTT (ΔEB1) in the 5′ upstream promoter region, CAGAGCCGGGCCCACGTG (ΔEB2), CACGTGCGAGGGGC ACACGTGGAGTGGGGACGTG (ΔEB3), and CATGTGTCACCTCACACA (ΔEB4) in the intron 1 region. A SphI restriction fragment of the pGL3_Rev-Erbα-ΔEB4 clone was ligated into the pGL3_Rev-Erbα-ΔEB1 clone to create pGL3_Rev-Erbα-ΔEB1_ΔEB4. Three consecutive rounds of site-directed mutagenesis were performed on this clone to obtain pGL3_Rev-Erbα-ΔEB1_ΔEB2_ΔEB3_ΔEB4 without any potential E-box left. The success of mutagenesis was verified by DNA sequencing. Site-directed mutagenesis was also employed to inactivate two potential DR2-RRE-binding motifs: GTGTCACTGGGGCA in the promoter region, and GTGTCAGTGGGTGA in the intron 1 region. The expression vectors bearing an entire mRev-Erbα minigene (Preitner et al. 2002) or a HA-tagged Cry1 (Chaves et al. 2006) have been described.
Real-time luciferase measurements
Induction of rhythms and luciferase measurements were performed according to Schmutz et al. (2010). NIH 3T3 fibroblasts were transfected with 4 μL of JetPEI (Polyplus) and a total of 2 μg of DNA per 3.5-cm dish according to the manufacturer's instructions. Equal transfection efficiencies were verified by determining the enzymatic activity obtained from a cotransfected secreted alkaline phosphatase reporter plasmid in the culture supernatants sampled prior to the induction of the cells (Roche Applied Science). Real-time readouts of the cells transfected with the different reporter constructs were recorded using a LumiCycle machine (Actimetrics).
Tagged reporter cell lines
The stable Rat-1 cell lines (with a single-copy integration of a wild-type mDbp gene, or a version with the promoter-proximal E-box motif inactivated), the synchronization of these cells with dexamethasone, the use of these cells for chromatin immunoprecipitation, and the RT–PCR probes to distinguish between the endogenous gene and the transgene have been described (Ripperger and Schibler 2006).
Expression analysis
The isolation procedure of total RNA from mouse liver, the measurement of mRNA accumulation or precursor mRNA accumulation as a way to determine transcription by RT–PCR (primers are listed in Supplemental Table S2; Preitner et al. 2002; Gachon et al. 2006), the purification of nuclei from the mouse liver, the extraction of nuclear protein extracts, and the detection of proteins on nitrocellulose membranes with specific antibodies have been described (Ripperger and Schibler 2006).
Statistical analysis
The period lengths and phase angles of the bioluminescence records were determined from the raw data with the LumiCycle analysis software (Actimetrics). For some experiments, detrended bioluminescence data were calculated with the LumiCycle software. Data sets were analyzed by one-way ANOVA and a Bonferroni post-test using the Prism4 software (GraphPad Software Inc.). Differences were considered as statistically significant with P < 0.01 (**) or P < 0.001 (***).
Acknowledgments
We thank Ueli Schibler (Geneva) and Urs Albrecht (Fribourg) for their generous supply of reagents, and Isabelle Schmutz (Fribourg) for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation, the Cantons of Fribourg and Geneva, and the EU FP6 integrated project EUCLOCK (018741).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.578810.
Supplemental material is available at http://www.genesdev.org.
References
- Adelmant G, Begue A, Stehelin D, Laudet V 1996. A functional Rev-erbα responsive element located in the human Rev-erbα promoter mediates a repressing activity. Proc Natl Acad Sci 93: 3553–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi M, Ichise T, Mamine T, Takumi T 2006. Molecular mechanism of cell-autonomous circadian gene expression of Period2, a crucial regulator of the mammalian circadian clock. Mol Biol Cell 17: 555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Sun ZS, Eichele G, Lee CC 1997. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Bozek K, Relogio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H 2009. Regulation of clock-controlled genes in mammals. PLoS One 4: e4882 doi: 10.1371/journal.pone.0004882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA 2000. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves I, Yagita K, Barnhoorn S, Okamura H, van der Horst GT, Tamanini F 2006. Functional evolution of the photolyase/cryptochrome protein family: Importance of the C terminus of mammalian CRY1 for circadian core oscillator performance. Mol Cell Biol 26: 1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Schirmer A, Lee Y, Lee H, Kumar V, Yoo SH, Takahashi JS, Lee C 2009. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell 36: 417–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U 2000. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JP, Lee C, Wade PA, Reppert SM 2003. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421: 177–182 [DOI] [PubMed] [Google Scholar]
- Fustin JM, O'Neill JS, Hastings MH, Hazlerigg DG, Dardente H 2009. Cry1 circadian phase in vitro: Wrapped up with an E-box. J Biol Rhythms 24: 16–24 [DOI] [PubMed] [Google Scholar]
- Gachon F, Olela FF, Schaad O, Descombes P, Schibler U 2006. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab 4: 25–36 [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ 1998. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564–1569 [DOI] [PubMed] [Google Scholar]
- Griffin EA Jr, Staknis D, Weitz CJ 1999. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286: 768–771 [DOI] [PubMed] [Google Scholar]
- Harding HP, Lazar MA 1995. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol 15: 4791–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlerigg D, Loudon A 2008. New insights into ancient seasonal life timers. Curr Biol 18: R795–R804 doi: 10.1016/j.cub.2008.07.040 [DOI] [PubMed] [Google Scholar]
- Hogenesch JB, Gu YZ, Jain S, Bradfield CA 1998. The basic-helix–loop–helix–PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci 95: 5474–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K 2002. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419: 841–844 [DOI] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM 1999. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96: 57–68 [DOI] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, et al. 1997. Positional cloning of the mouse circadian clock gene. Cell 89: 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara YB, Nishii K, Ukai-Tadenuma M, Ueda HR, Uchiyama Y, Yagita K 2008. Detection of a circadian enhancer in the mDbp promoter using prokaryotic transposon vector-based strategy. Nucleic Acids Res 36: e23 doi: 10.1093/nar/gkn018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS 2006. Molecular components of the mammalian circadian clock. Hum Mol Genet 15: R271–R277 doi: 10.1093/hmg/ddl207 [DOI] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM 1999. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205 [DOI] [PubMed] [Google Scholar]
- Langmesser S, Tallone T, Bordon A, Rusconi S, Albrecht U 2008. Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol Biol 9: 41 doi: 10.1186/1471-2199-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM 2001. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107: 855–867 [DOI] [PubMed] [Google Scholar]
- Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA 2008. Redundant function of REV-ERBα and β and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet 4: e1000023 doi: 10.1371/journal.pgen.1000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Conquet F, Dubois-Dauphin M, Schibler U 1997. The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behavior. EMBO J 16: 6762–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet JS, Abruzzi KC, Desrochers J, Rodriguez J, Rosbash M 2010. Dynamic PER repression mechanisms in the Drosophila circadian clock: From on-DNA to off-DNA. Genes Dev 24: 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Yoshida M, Takano A, Soma H, Yamamoto T, Yasuda A, Nakatsu T, Takumi T 2008. A direct repeat of E-box-like elements is required for cell-autonomous circadian rhythm of clock genes. BMC Mol Biol 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Kawamoto T, Honda KK, Ueshima T, Noshiro M, Iwata T, Fujimoto K, Kubo H, Honma S, Yorioka N, et al. 2008. DEC1 modulates the circadian phase of clock gene expression. Mol Cell Biol 28: 4080–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse Y, Oh-hashi K, Iijima N, Naruse M, Yoshioka H, Tanaka M 2004. Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol Cell Biol 24: 6278–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuesslein-Hildesheim B, O'Brien JA, Ebling FJ, Maywood ES, Hastings MH 2000. The circadian cycle of mPER clock gene products in the suprachiasmatic nucleus of the siberian hamster encodes both daily and seasonal time. Eur J Neurosci 12: 2856–2864 [DOI] [PubMed] [Google Scholar]
- Paquet ER, Rey G, Naef F 2008. Modeling an evolutionary conserved circadian cis-element. PLoS Comput Biol 4: e38 doi: 10.1371/journal.pcbi.0040038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U 2002. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260 [DOI] [PubMed] [Google Scholar]
- Ripperger JA 2006. Mapping of binding regions for the circadian regulators BMAL1 and CLOCK within the mouse Rev-erbα gene. Chronobiol Int 23: 135–142 [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U 2006. Rhythmic CLOCK–BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet 38: 369–374 [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Shearman LP, Reppert SM, Schibler U 2000. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev 14: 679–689 [PMC free article] [PubMed] [Google Scholar]
- Sato F, Kawamoto T, Fujimoto K, Noshiro M, Honda KK, Honma S, Honma K, Kato Y 2004. Functional analysis of the basic helix–loop–helix transcription factor DEC1 in circadian regulation. Interaction with BMAL1. Eur J Biochem 271: 4409–4419 [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB 2004. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43: 527–537 [DOI] [PubMed] [Google Scholar]
- Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB 2006. Feedback repression is required for mammalian circadian clock function. Nat Genet 38: 312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U 2010. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev 24: 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz TF, Kay SA 2003. Circadian clocks in daily and seasonal control of development. Science 301: 326–328 [DOI] [PubMed] [Google Scholar]
- Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF Jr, Reppert SM 1997. Two period homologs: Circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19: 1261–1269 [DOI] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, et al. 2000. Interacting molecular loops in the mammalian circadian clock. Science 288: 1013–1019 [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Jacobmeier B, Scherbarth F, Dernbach H, Kruse F, Albrecht U 2002. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J Biol Rhythms 17: 202–209 [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M 2001. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493 [DOI] [PubMed] [Google Scholar]
- Sumova A, Sladek M, Jac M, Illnerova H 2002. The circadian rhythm of Per1 gene product in the rat suprachiasmatic nucleus and its modulation by seasonal changes in daylength. Brain Res 947: 260–270 [DOI] [PubMed] [Google Scholar]
- Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC 1997. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Tan Y, Dragovic Z, Roenneberg T, Merrow M 2004. Entrainment dissociates transcription and translation of a circadian clock gene in neurospora. Curr Biol 14: 433–438 [DOI] [PubMed] [Google Scholar]
- Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y 1997. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389: 512–516 [DOI] [PubMed] [Google Scholar]
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, et al. 2002. A transcription factor response element for gene expression during circadian night. Nature 418: 534–539 [DOI] [PubMed] [Google Scholar]
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S 2005. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet 37: 187–192 [DOI] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, et al. 1999. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398: 627–630 [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, et al. 1999. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci 96: 12114–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ 1995. Impaired energy homeostasis in C/EBPα knockout mice. Science 269: 1108–1112 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H 2000. Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol 20: 4773–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Wang H, Liu Y, Shao C 2008. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol 4: e1000193 doi: 10.1371/journal.pcbi.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Lazar MA 2005. The orphan nuclear receptor Rev-erbα recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol 19: 1452–1459 [DOI] [PubMed] [Google Scholar]
- Yu W, Hardin PE 2006. Circadian oscillators of Drosophila and mammals. J Cell Sci 119: 4793–4795 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li H, Li R, Hu R, Fan C, Chen F, Wang Z, Liu X, Fu Y, Lin C 2008. Association of the circadian rhythmic expression of GmCRY1a with a latitudinal cline in photoperiodic flowering of soybean. Proc Natl Acad Sci 105: 21028–21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Khorasanizadeh S, Miyoshi Y, Lazar MA, Rastinejad F 1998. Structural elements of an orphan nuclear receptor–DNA complex. Mol Cell 1: 849–861 [DOI] [PubMed] [Google Scholar]