Abstract
Expression of the immediate early gene, c-fos, was used to investigate changes in neuronal activity in forebrain regions involved in male sexual behavior following social, hormonal and/or seasonal manipulations in the male green anole. These factors all influence behavior, yet it is unclear how they interact to modify neuronal activity in forebrain regions, including the preoptic area (POA) and ventromedial nucleus of the amygdala (AMY). These regions are involved in the display of sexual behaviors in male green anoles as in many other vertebrates. To determine the effects of seasonal, hormonal and social cues on these brain areas, we investigated c-fos under environmental conditions typical of the breeding or non-breeding season in adult male green anoles that were castrated and implanted with either testosterone (T) or blank (Bl) capsules. We also manipulated social cues by exposing only half of the animals in each group to females. T enhanced courtship and copulatory behaviors, but decreased c-fos expression in the AMY. A similar, although not statistically significant, pattern was observed in the POA, and the density of c-fos+ cells was negatively correlated in that region with the number of extensions of a throat fan (dewlap) used during courtship. Therefore, it appears that in the male green anole, T may diminish c-fos expression (likely in inhibitory neurons) in the POA and AMY to create a permissive environment in which the appropriate behavioral response can be displayed.
Keywords: Immediate early gene, Courtship, Copulation, Reptile, Reproductive behavior
1. Introduction
Limbic forebrain regions, including the preoptic area (POA) and amygdala (AMY), are involved in the expression of male sexual behaviors. Changes in neuronal activity in these forebrain regions, indicated by expression of immediate early genes such as c-fos, are associated with the display of male behaviors in a diverse array of vertebrates (Greco et al., 1996, 1998; Heeb and Yahr, 1996; Kollack-Walker and Newman, 1995, 1997; Mello, 2002; Schaefer and Zakon, 1996; Shimura et al., 1994). These areas also express steroid receptors in numerous vertebrate species (Ball et al., 2004; Hull et al., 2002; Wade, 2005), and testosterone (T), and/or its metabolites, estradiol and dihydrotestosterone, activate courtship and copulatory behaviors in males of many species, often on a seasonal basis (Ball et al., 2004; Ball and Balthazart, 2004; Cooke, 2006; Cooke et al., 1999; Lovern et al., 2004; Prendergast et al., 2002; Romeo et al., 2001; Sakata et al., 2003; Wood and Swann, 2000). Environmental conditions can interact with hormone levels to affect the expression of immediate early genes. For example, following exposure to females, c-fos expression in the POM (the avian homologue of a portion of the mammalian POA) of male starlings, is positively correlated with behavior during the breeding season (BS; when T levels are high), but not non-breeding season (NBS, Heimovics and Riters, 2005, 2006). Social cues can also directly influence neuronal activity; c-fos is greater in reproductive forebrain nuclei in rodents and songbirds following social contact (Mello, 2002; Schwab et al., 2004). While many studies have examined the effects of seasonal, hormonal and social cues on c-fos expression separately, few have investigated potential interactions among them, fewer have examined c-fos in reptilian brains (Bertolucci et al., 2000) and none to our knowledge have done so in green anole lizards.
Green anoles are ideal for conducting this type of work. They breed seasonally and exhibit naturally occurring behavioral plasticity. During the BS, males extend a bright red throat fan (dewlap) to court females, and copulation involves intromission of one of two bilateral hemipenes (Crews, 1980; Greenberg and Nobel, 1944; Jenssen et al., 2000). Lesion studies document that the AMY (also known as the ventromedial nucleus of the posterior dorsal ventricular ridge) and POA facilitate male reproductive behaviors in lizards, as in other vertebrates (Bass and Zakon, 2005; Crews and Moore, 2005; Greenberg et al., 1984; Morgantaler and Crews, 1978; Murphy and Hoffman, 2001; Panzica et al., 1996; Wade, 2005; Wilczynski et al., 2005; Wood and Swann, 2000; Yahr and Gregory, 1993). Courtship and copulatory behaviors in green anoles are facilitated by environmental conditions typical of the BS and are greatly diminished during the NBS. T, rather than its metabolites, is the primary activator of these behaviors in males (Adkins and Schlesinger, 1979; Crews et al., 1978; Greenberg and Nobel, 1944; Jenssen et al., 2000; Lovern et al., 2004; Neal and Wade, 2007; O’Bryant and Wade, 1999; Rosen and Wade, 2000; Wade, 2005; Winkler and Wade, 1998). T and seasonal environmental conditions also affect forebrain morphology in the anole. Soma size of POA and AMY neurons is increased by T, and an interaction exists in the AMY, such that the hormone enhances soma size more in the BS than NBS (Neal and Wade, 2007; O’Bryant and Wade, 2002). Androgen receptors are present in the POA and AMY (Rosen et al., 2002), suggesting that T may act directly in these regions, although it is currently unknown whether that is in fact the case. While at least visual exposure to females is required for males to display courtship behaviors, neither visual nor direct female contact affects POA or AMY morphology (Neal and Wade, 2007). However, in intact males, larger soma size in the AMY is associated with higher rates of dewlap extensions (Neal and Wade, in press).
To determine whether T, season and female contact alter neuronal activity, expression of c-fos was examined in the POA and AMY. Males were exposed to T or vehicle under either BS or NBS temperatures and photoperiods, as well as to one of two levels of female contact (exposed to a female, or not).
2. Results
2.1. Behavior
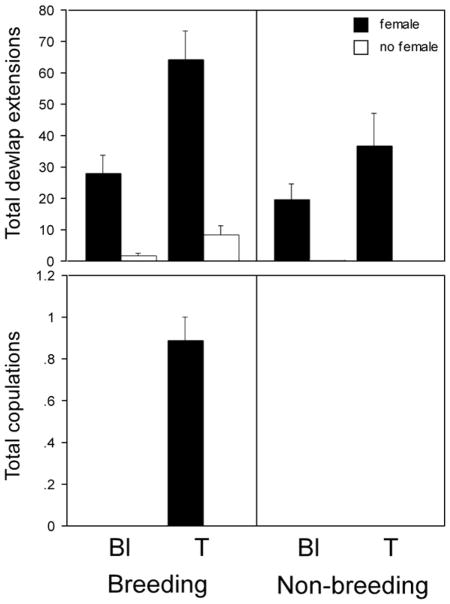
As expected (see Introduction), T increased dewlap extensions (F=13.34, p=0.0005), as did BS conditions (F=7.82, p=0.0068) and exposure to females (F=70.62, p<0.0001). A significant interaction existed between hormone treatment and social contact such that the effects of T were greater in males presented with females than those who were not (F=8.05; p=0.0061; Fig. 1, top). No other interactions were detected. For copulatory behavior, only males exposed to females were included in the analysis, and only T-treated males in the BS displayed this behavior. Main effects of treatment and season, and the interaction between them, were all statistically significant (each F=64.0; p<0.0001 Fig. 1, bottom).
Fig. 1.
Number of dewlap extensions (top) and copulations (bottom) during the 1.5-h behavior test. Males were castrated and implanted with either testosterone (T) or blank (Bl) capsules during the breeding and non-breeding seasons, and either exposed to females or not. For dewlap extensions, main effects of season, hormone treatment and female exposure condition existed, as well as an interaction between hormone treatment and female exposure (top). Main effects of season and T treatment and an interaction between seasonal and hormonal manipulations were also detected for total copulations (bottom).
2.2. AMY
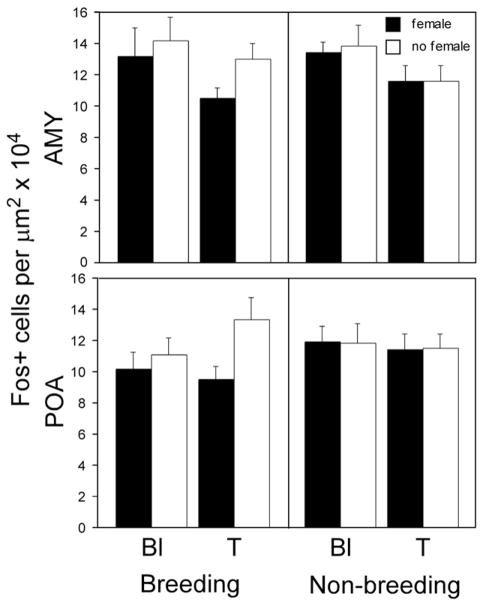
T decreased the density of c-fos+ cells in the AMY (F=5.13; p=0.027; Fig. 2, top and Fig. 3). No main effects of season or female exposure or interactions between variables existed (all F≤1.29; all p≥0.26). The density of c-fos+ cells in the AMY was not significantly correlated with the number of dewlap extensions (R2=0.01; p=0.617; data not shown).
Fig. 2.
Average density of c-fos+ cells in the AMY (top) and the POA (bottom) under environmental conditions typical of the breeding and non-breeding seasons in gonadectomized males implanted with either testosterone (T) or blank (Bl) capsules, and exposed to females or not. A main effect of hormone treatment was detected in the AMY.
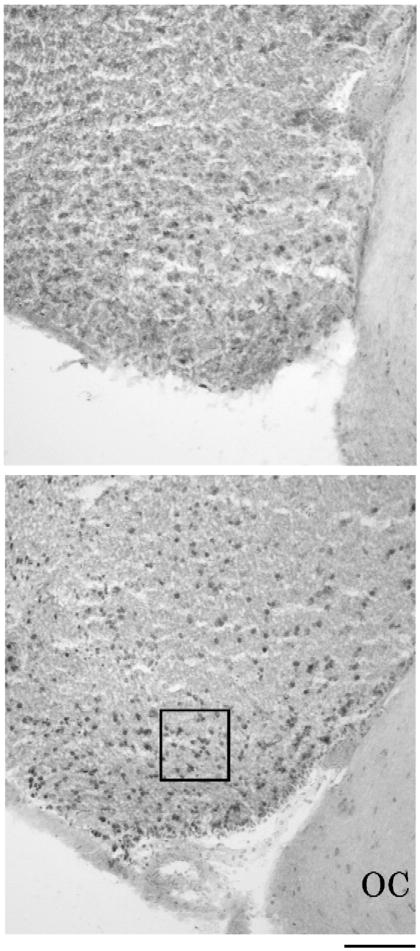
Fig. 3.
Photomicrographs of c-fos expression in the AMY of males given testosterone (top) or blank (bottom) capsules during the breeding season. OC=optic chiasm. The 100 μm×100 μm box depicts the area of the AMY that was analyzed. Scale bar=100 μm.
2.3. POA
The pattern of expression in the POA was generally similar to that of the AMY, but the density of c-fos+ cells in the POA did not differ significantly between groups, and no interactions were detected (all F≤2.42; all p≥0.125; Fig. 2, bottom). In the POA, however, a negative correlation between the number of dewlap extensions and the density of c-fos+ cells existed in males exposed to females (R2=0.20; p=0.006; Fig. 4). When this correlation was broken down by group, it was detected only in T-treated males in the BS (R2=0.55; p=0.022; Figs. 4 and 5).
Fig. 4.
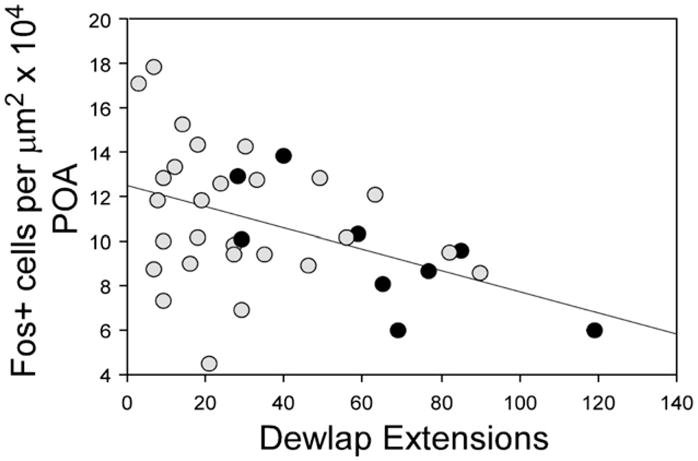
Relationships between courtship behaviors (dewlap extensions) and the density of c-fos+ cells in the POA. Black circles represent testosterone-treated males exposed to females in the breeding season and gray circles indicate all other males exposed to females.
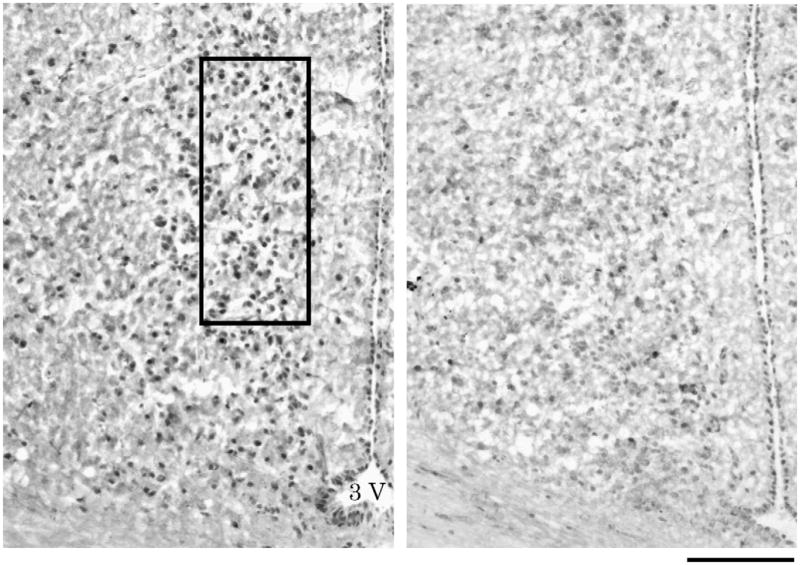
Fig. 5.
Photomicrographs of c-fos immunoreactivity in the POA of males displaying high (left; 119 dewlap extensions) and low (right; 33 extensions) levels of behavior. The 250 μm×100 μm box depicts the area of the POA that was analyzed. 3V=third ventricle. Scale bar=100 μm.
2.4. Renal sex segments
The height of epithelial cells in the kidneys (renal sex segments) was measured as an indication of androgen exposure (Holmes and Wade, 2004; Neal and Wade, 2007; Winkler and Wade, 1998). Main effects of T and season existed such that cell height was larger in T- than Bl-treated males (F=201.09, p<0.0001) and during the BS than NBS (F=103.28; p<0.0001). Also, a significant interaction was detected such that the effect of T was greater in the BS than NBS (F=100.75; p<0.0001). These data (not shown) are consistent with those in other experiments (Holmes and Wade, 2004, 2005; Neal and Wade, 2007; Winkler and Wade, 1998) and indicate that the hormone released from the capsule affected androgen-sensitive tissues as expected.
3. Discussion
3.1. Summary
In the present experiment, T activated behavior yet decreased the density of c-fos+ cells in the AMY. This result on immediate early gene expression was independent of season and female presence, suggesting that the hormone may relatively directly decrease c-fos expression in inhibitory neurons. A similar pattern was revealed in the POA, although it was not statistically significant. Courtship behavior was, however, negatively correlated with the density of c-fos+ cells in this region, which is consistent with the idea that either enhanced behavior causes fewer cells to express c-fos or that activity of particular cells inhibits courtship displays. The latter idea fits well with the AMY data (see below).
3.2. Relationship to previous studies in the green anole
The anole POA and AMY have a variety of characteristics associated with behavioral functions. For example, they are critical to the display of male courtship and copulation (Crews and Moore, 2005; Greenberg et al., 1984; Morgantaler and Crews, 1978; Wade, 2005). T enhances both the behaviors and soma size in these areas, and the effects are greater in the BS than NBS on both courtship and copulatory behavior and AMY soma size (Neal and Wade, 2007; O’Bryant and Wade, 1999). As cells in the POA and AMY express AR (Rosen et al., 2002), T could act directly in these forebrain areas to facilitate both behavioral and morphological change, but this idea has not yet been tested. In the present study, with the exception of males in the BS that were not exposed to females, the pattern of T effects in the POA and AMY were the same. In this group (BS males without females), T seemed to on average increase the density of c-fos+ cells in the POA of Bl-treated males, but it had the opposite effect in the AMY. Also, although social contact with females does not appear to affect mean group differences in soma size (Neal and Wade, 2007) or c-fos expression in the POA or AMY (present study), the rate of dewlap extensions is positively correlated with AMY, but not POA, soma size in intact adult males during the BS (Neal and Wade, in press). These data, combined with the fact that courtship displays are negatively correlated with c-fos expression in the POA (present study), suggest that individual differences in neuronal changes in the POA (c-fos expression) and AMY (soma size) may partially facilitate (or be facilitated by) the neural processing of social cues and/or the display of courtship behaviors. Collectively, these differences between the two regions in response to both T and degree of behavioral displays might reflect more specialized functions of these forebrain areas. They are reciprocally interconnected and both contribute to sexual motivation and behavior. However, in mammals, the POA is most likely the primary forebrain site for initiating male reproductive behaviors, whereas the AMY may be more involved in motivation (Wood, 1997). Thus, T may diminish neuronal activity more in the AMY (integrates sensory information) than the POA (initiates male sexual behavior), as these regions may have different roles in the display of male green anole sexual behavior.
3.3. Relationship to other species
The expression of immediate early genes, such as c-fos, has been widely used to identify brain regions involved in the control of sexual behavior, including those composing the “social behavior network” (Goodson, 2005; Newman, 1999). For example, c-fos expression is enhanced in the POA and AMY of male rodents, quail and house sparrows following exposure to a sexual stimulus and/or reproductive behavior (Heeb and Yahr, 1996; Pfaus and Heeb, 1997; Riters et al., 2004; Taziaux et al., 2006). T and season interact to produce changes in immediate early gene expression associated with male sexual behavior in starlings; following contact with a female, c-fos expression in the POA positively correlates with behavior during the BS when T levels are high, but not NBS when T is low (Heimovics and Riters, 2005, 2006). Also, c-fos expression is increased in rodents when males and females are housed together, thus experiencing social contact, compared to isolated counterparts (Schwab et al., 2004). In contrast to the present results, immediate early gene expression is increased in specific regions of the hypothalamus and suprachiasmatic nucleus of the female frog forebrain after hearing a socially relevant call compared to those hearing no sound or irrelevant calls (Hoke et al., 2005). However, similar to our results, immediate early gene expression in the POA in these frogs did not differ between groups receiving social stimuli compared to those that did not.
In fact, negative relationships can exist between social stimulation and the expression of immediate early genes. For example, c-fos expression is decreased in some limbic regions of the brain in mated compared to unmated male primates. However, as in the present research, in other brain regions including the POA and portions of the AMY, female exposure did not affect c-fos expression (Michael et al., 1999). Similarly, in several avian species (ranging from a highly territorial species to one that is very gregarious), immediate early gene expression in the extended medial AMY is negatively correlated with group size of the species (Goodson, 2005; Goodson et al., 2005a,b).
3.4. Potential functional explanations of differential c-fos expression
The present data suggest that T acts as a physiological cue, which may convey information about environmental stimuli, to release inhibitory cells under conditions in which behavior should be enhanced. This idea is compatible with work in some other species. For example, in male gerbils, c-fos and the inhibitory neurotransmitter GABA are co-localized in half of POA and AMY neurons, whereas only about one-fourth of the neurons co-express c-fos and the excitatory neurotransmitter glutamate, following mating (Simmons and Yahr, 2003). In addition, hormonal manipulations can modulate GABA receptor function (Clark and Henderson, 2003). For example, in mice, T treatment decreases the levels of individual GABAa receptor subunit mRNAs in the POA and AMY (McIntyre et al., 2002). The present results are consistent with these from mammals and suggest that T treatment might decrease the activity (as indicated by c-fos expression) of GABAergic neurons in the POA and AMY, thus creating a permissive environment for displaying reproductive behavior regardless of whether a female was present or not.
This conclusion may appear to contradict other studies, however, for example those in which immediate early gene expression can be enhanced with T treatment in birds (Heimovics and Riters, 2005, 2006) or in rodents in which increases in c-fos expression can be directly attributed to T or estradiol exposure (e.g., Cattaneo and Maggi, 1990; Insel, 1990; Nagypal and Wood, 2007). It will be important in the future to more fully characterize the phenotype of the steroid-responsive cells, as well as to investigate additional mechanisms across these vertebrate groups, such as co-activators, which affect the manner in which particular cells respond to steroid signals (MacLean et al., 1997; O’Bryant and Jordan, 2005). Also, studies in mice suggest that activation of specific serotonergic receptors (such as 5HT1A, 5HT1B and 5HT2C) inhibits sexual motivation (Popova and Amstislavskaya, 2002a,b). So, diminished c-fos expression in the anole may indicate decreased changes in activity of neurons containing serotonergic receptors.
It is also possible that the decrease in c-fos expression due to T relates to functions other than sexual behavior. For example, this brain region is involved in fear responses in some species (e.g., rodents: Aikey et al., 2002). Perhaps more likely, though, is a connection to aggression. In anole lizards, subordinate (less aggressive) males have higher baseline serotonergic activity in the AMY than their more aggressive counterparts (Summers et al., 2005). Plasma T increases with aggressive encounters in anoles (Yang and Wilczynski, 2002). Thus, it is possible that T diminishes activity of serotonergic neurons to allow the male to engage in aggressive behaviors.
3.5. Conclusions and future directions
Collectively, the data are consistent with the idea that T may decrease the activity of inhibitory neurons, making it more likely that given the appropriate context, the relevant behaviors will be displayed. For example, if a female is present, reproductive behaviors would be facilitated in breeding males. It will now be important to determine whether GABA and 5HT expression are influenced by T and/or female exposure in the male green anole. In particular, co-localization studies of GABA and/or 5HT with c-fos could reveal whether these neurotransmitters influence the display of masculine sexual behavior. In addition, it is possible that other types of POA and AMY neurons are active. They may express proteins other than c-fos (Bailey and Wade, 2003; Hoffman and Lyo, 2002; Hoke et al., 2005), or other biochemical markers of changes in neuronal activity (Tlemçani et al., 2000) might show relationships between that were not detected in the present study. This and previous work in the green anole POA and AMY suggest that T, and potentially AR, increases motivation and expression of masculine sexual behaviors by enhancing soma size and diminishing c-fos expression.
4. Experimental procedure
4.1. Animals and housing
Wild-caught, adult male green anoles were purchased in the season in which they were tested (BS or NBS) from Charles Sullivan, Co. (Nashville, TN). Prior to use in the experiment, males were individually housed in 10 gallon glass aquaria, and adult females were group housed in 29 gallon aquaria for 1.5 weeks. Environmental conditions typical of the BS (14 h of light; room temperatures ranging from 28 °C during the day to 19 °C at night) or NBS (10 h of light; temperatures varying from 24 °C during the day to 15 °C at night) were maintained with fluorescent, full-spectrum and incandescent lights. Basking temperatures were up to 10 °C warmer than ambient from incandescent spotlights placed on top of each cage. Aquaria were sprayed daily with water, and the humidity was consistently set at 70%. Sphagnum peat moss bedding, wooden dowels for perching, a water dish and basking rocks were provided in each aquarium. During the BS, animals were fed crickets or mealworms three times a week and during the NBS, twice each week. All procedures were performed in accordance with Michigan State University Institutional Animal Care and Use Committee and NIH guidelines.
4.2. Endocrine manipulations
Males from the BS and NBS were anesthetized by hypothermia, gonadectomized, and subcutaneously implanted with a Silastic capsule (7 mm×0.7 mm ID×1.65 mm OD). Implants were either packed with testosterone propionate (5 mm of hormone), or left empty (blank; Bl). Capsules containing 10% estradiol benzoate (5 mm long) were made from a slurry of Silastic sealant (Dow Corning; Midland, MI) and 2 mg estradiol benzoate extruded through a 10 cc syringe. They were subcutaneously implanted in ovariectomized females to facilitate receptivity.
4.3. Behavior testing
Two weeks after receiving implants, males were randomly assigned to one of two female exposure conditions. They either received a stimulus female placed directly in their cage or no female. The number of dewlap extensions and copulations by each male was recorded for 1.5 h by an observer blind to hormone manipulation during the active period of their day (1.5 to 2 h after lights-on).
4.4. Tissue collection
Immediately following behavior testing, males were rapidly decapitated. Brains and kidneys were removed and frozen in isopentane, and stored at −80 °C. The Silastic capsule’s presence and condition were confirmed at this time. Brain and kidney tissues were sectioned in six series on a cryostat at 20 μm and stored at −80 °C. Brains were processed for c-fos immunohistochemistry and kidneys were stained with hematoxylin and eosin.
4.5. Immunohistochemistry
All tissue was processed for c-fos immunohistochemistry in one run (as in Bailey et al., 2002; Bailey and Wade, 2003, 2005). Briefly, after warming slides to room temperature, sections were rinsed, fixed in 4% paraformaldehyde and incubated in 0.5% hydrogen peroxide for 30 min followed by a 1-h incubation in 4% normal donkey serum in 0.1 M PBS with 0.3% Triton X-100. Tissue was incubated for 72 h at 4 °C in c-fos primary antibody (1:1000; Santa Cruz Biotech, Santa Cruz, CA, #sc-253) in 0.1 M PBS with 0.3% Triton X-100 and 30% glycerol. Following a series of rinses, tissue was incubated in biotinylated donkey anti-rabbit secondary antibody (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA). The Elite ABC kit solution (Vector; Burlingame, CA), and nickel-enhanced diaminobenzidine (Sigma; St. Louis, MO) were used to visualize c-fos immunoreactivity. To ensure that non-specific labeling had not occurred, the primary antibody was omitted, and no labeling was detected.
C-fos+ cells were quantified at 300× in the forebrain of every individual in a 250 μm×100 μm (POA) or 100 μm×100 μm (AMY) box placed in 3–5 sections (depended on quality of tissue) from each brain area on both sides. This procedure resulted in 6–10 values per individual. The long edge of the box was placed 40 μm lateral to the third ventricle, and the short edge of the box approximately 225 μm dorsal to the optic chiasm just above the suprachiasmatic nucleus in the POA. The AMY box was placed in the center of the medial–lateral extent of the nucleus and 50 μm dorsal to the ventral edge of the brain. The number of fos+ cells was averaged within each brain region in each animal for use in statistical analyses. Very little, or no, c-fos expression was observed in a variety of other brain regions, including the anterior dorsal ventricular ridge, medial cortex, and in the rostral nucleus accumbens, indicating relative specificity in the expression of this protein.
In ten tubules randomly selected from the two kidneys, the height of four epithelial cells was measured for a total of 40 measurements. These values provide an indication of relative androgen exposure (see Holmes and Wade, 2004; Neal and Wade, 2007; Winkler and Wade, 1998).
4.6. Statistical analysis
Sample sizes for all groups were 9, except that the tissue of one Bl-treated male exposed to a female during the NBS was damaged (n=8) and was not included in the final analyses. The total number of dewlap extensions, average kidney epithelial cell height and density of c-fos+ cells in the POA and AMY were separately analyzed with a three-way ANOVA (hormone×season×female exposure condition). A two-way ANOVA was performed on the total number of copulations in males directly exposed to a female (hormone×season). Simple regressions were conducted to determine the degree to which the number of dewlap extensions and c-fos expression in the POA and AMY were correlated. All statistical analyses were computed with StatView (SAS Institute, Inc., Cary, NC).
Acknowledgments
We thank Laurel Beck, Shannon Jackson, Stephany Latham and Camilla Peabody for their technical assistance. This work was supported by NSF (IBN-0234740) and NIH (K02-MH065907).
References
- Adkins E, Schlesinger L. Androgens and the social behavior of male and female lizards (Anolis carolinensis) Horm Behav. 1979;13 (2):139–152. doi: 10.1016/0018-506x(79)90053-9. [DOI] [PubMed] [Google Scholar]
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42 (4):448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J Neurobiol. 2002;52 (1):43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Mol Brain Res. 2003;116 (1–2):147–154. doi: 10.1016/s0169-328x(03)00288-2. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. FOS and ZENK responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav Brain Res. 2005;162 (1):108–115. doi: 10.1016/j.bbr.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann NY Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83 (2):329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Bass AH, Zakon HH. Sonic and electric fish: at the crossroads of neuroethology and behavioral neuroendocrinology. Horm Behav. 2005;48 (4):360–372. doi: 10.1016/j.yhbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bertolucci C, Sovrano VA, Magnone MC, Foa A. Role of suprachiasmatic nuclei in circadian and light-entrained behavioral rhythms of lizards. Am J Physiol Regul Integr Comp Physiol. 2000;279 (6):R2121–R2131. doi: 10.1152/ajpregu.2000.279.6.R2121. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, Maggi A. c-fos induction by estrogen in specific rat brain areas. Eur J Pharmacol. 1990;188 (2–3):153–159. doi: 10.1016/0922-4106(90)90050-8. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic–androgenic steroids. Neurosci Biobehav Rev. 2003;27 (5):413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138 (3):997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96 (13):7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Interrelationships among ecological, behavioral, and neuroendocrine processes in the reproductive cycle of Anolis carolinensis and other reptiles. Adv St Behav. 1999;11:1–74. [Google Scholar]
- Crews D, Moore MC. Historical contributions of research on reptiles to behavioral neuroendocrinology. Horm Behav. 2005;48 (4):384–394. doi: 10.1016/j.yhbeh.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Crews D, Traina V, Wetzel FT, Muller C. Hormonal control of male reproductive behavior in the lizard, Anolis carolinensis: role of testosterone, dihydrotestosterone, and estradiol. Endocrinology. 1978;103 (5):1814–1821. doi: 10.1210/endo-103-5-1814. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48 (1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc Lond, B Biol Sci. 2005a;272 (1560):227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Saldanha CJ, Hahn TP, Soma KK. Recent advances in behavioral neuroendocrinology: insights from studies on birds. Horm Behav. 2005b;48 (4):461–473. doi: 10.1016/j.yhbeh.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Edwards DA, Michael RP, Clancy AN. Androgen receptor immunoreactivity and mating-induced Fos expression in forebrain and midbrain structures in the male rat. Neuroscience. 1996;75 (1):161–171. doi: 10.1016/0306-4522(96)00183-2. [DOI] [PubMed] [Google Scholar]
- Greco B, Edwards DA, Zumpe D, Clancy AN. Androgen receptor and mating-induced fos immunoreactivity are co-localized in limbic and midbrain neurons that project to the male rat medial preoptic area. Brain Res. 1998;781 (1–2):15–24. doi: 10.1016/s0006-8993(97)01136-0. [DOI] [PubMed] [Google Scholar]
- Greenberg B, Nobel GK. Social behavior of the American chameleon (Anolis carolinensis Voigt) Physiol Behav. 1944;17:392–439. [Google Scholar]
- Greenberg N, Scott M, Crews D. Role of the amygdala in the reproductive and aggressive behavior of the lizard, Anolis carolinensis. Physiol Behav. 1984;32 (1):147–151. doi: 10.1016/0031-9384(84)90088-x. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. c-Fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neuroscience. 1996;72 (4):1049–1071. doi: 10.1016/0306-4522(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65 (3):207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50 (5):726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? J Neuroendocrinol. 2002;14 (4):259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci U S A. 2005;102 (30):10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Wade J. Seasonal plasticity in the copulatory neuromuscular system of green anole lizards: a role for testosterone in muscle but not motoneuron morphology. J Neurobiol. 2004;60 (1):1–11. doi: 10.1002/neu.10334. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wade J. Testosterone regulates androgen receptor immunoreactivity in the copulatory, but not courtship, neuromuscular system in adult male green anoles. J Neuroendocrinol. 2005;17 (9):560–569. doi: 10.1111/j.1365-2826.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- Hull E, Meisel RL, Sachs BD. Male sexual behavior. In: Rubin RT, editor. Hormones, Brain and Behavior. Vol. 1. Academic Press; New York: 2002. pp. 3–137. [Google Scholar]
- Insel TR. Regional induction of c-fos-like protein in rat brain after estradiol administration. Endocrinology. 1990;126 (4):1849–1853. doi: 10.1210/endo-126-4-1849. [DOI] [PubMed] [Google Scholar]
- Jenssen TA, Orrell KS, Lovern MB. Sexual dimorphisms in aggressive signal structure and use by a polygynous lizard, Anolis carolinensis. Copeia. 2000;1:140–149. [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66 (3):721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32 (5):481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Lovern MB, Holmes MM, Wade J. The green anole (Anolis carolinensis): a reptilian model for laboratory studies of reproductive morphology and behavior. Ilar J. 2004;45 (1):54–64. doi: 10.1093/ilar.45.1.54. [DOI] [PubMed] [Google Scholar]
- MacLean HE, Warne GL, Zajac JD. Localization of functional domains in the androgen receptor. J Steroid Biochem Mol Biol. 1997;62 (4):233–242. doi: 10.1016/s0960-0760(97)00049-6. [DOI] [PubMed] [Google Scholar]
- McIntyre KL, Porter DM, Henderson LP. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABA(A) receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43 (4):634–645. doi: 10.1016/s0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. J Comp Physiol. 2002;188 (11–12):943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- Michael RP, Clancy AN, Zumpe D. Effects of mating on c-fos expression in the brains of male macaques. Physiol Behav. 1999;66 (4):591–597. doi: 10.1016/s0031-9384(98)00329-1. [DOI] [PubMed] [Google Scholar]
- Morgantaler A, Crews D. Role of the anterior hypothalamus-preoptic area in the regulation of reproductive behavior in the lizard, Anolis carolinensis: implantation studies. Horm Behav. 1978;11 (1):61–73. doi: 10.1016/0018-506x(78)90058-2. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438 (2):191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Nagypal A, Wood RI. Region-specific mechanisms for testosterone-induced Fos in hamster brain. Brain Res. 2007;1141:197–204. doi: 10.1016/j.brainres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JK, Wade J. Courtship and copulation in the adult male green anole: effects of season, hormone and female contact on reproductive behavior and morphology. Behav Brain Res. 2007;177 (2):177–185. doi: 10.1016/j.bbr.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JK, Wade J. Androgen receptor expression and morphology of forebrain and neuromuscular systems in male green anoles displaying individual differences in sexual behavior. Horm Behav. doi: 10.1016/j.yhbeh.2007.04.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- O’Bryant EL, Jordan CL. Expression of nuclear receptor coactivators in androgen-responsive and -unresponsive motoneurons. Horm Behav. 2005;47 (1):29–38. doi: 10.1016/j.yhbeh.2004.08.010. [DOI] [PubMed] [Google Scholar]
- O’Bryant EL, Wade J. Sexual dimorphisms in a neuromuscular system regulating courtship in the green anole lizard: effects of season and androgen treatment. J Neurobiol. 1999;40 (2):202–213. doi: 10.1002/(sici)1097-4695(199908)40:2<202::aid-neu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- O’Bryant EL, Wade J. Seasonal and sexual dimorphisms in the green anole forebrain. Horm Behav. 2002;41 (4):384–395. doi: 10.1006/hbeh.2002.1778. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17 (1):51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44 (4):397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Popova NK, Amstislavskaya TG. 5-HT2A and 5-HT2C serotonin receptors differentially modulate mouse sexual arousal and the hypothalamo-pituitary–testicular response to the presence of a female. Neuroendocrinology. 2002a;76 (1):28–34. doi: 10.1159/000063681. [DOI] [PubMed] [Google Scholar]
- Popova NK, Amstislavskaya TG. Involvement of the 5-HT(1A) and 5-HT(1B) serotonergic receptor subtypes in sexual arousal in male mice. Psychoneuroendocrinology. 2002b;27 (5):609–618. doi: 10.1016/s0306-4530(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Rubin RT, editor. Hormones, Brain and Behavior. Vol. 2. Academic Press; New York: 2002. pp. 93–156. [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155 (2):307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Cook-Wiens E, Richardson HN, Sisk CL. Dihydrotestosterone activates sexual behavior in adult male hamsters but not in juveniles. Physiol Behav. 2001;73 (4):579–584. doi: 10.1016/s0031-9384(01)00499-1. [DOI] [PubMed] [Google Scholar]
- Rosen G, O’Bryant E, Matthews J, Zacharewski T, Wade J. Distribution of androgen receptor mRNA expression and immunoreactivity in the brain of the green anole lizard. J Neuroendocrinol. 2002;14 (1):19–28. doi: 10.1046/j.0007-1331.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, Wade J. The role of 5alpha-reductase activity in sexual behaviors of the green anole lizard. Physiol Behav. 2000;69 (4–5):487–498. doi: 10.1016/s0031-9384(00)00207-9. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Woolley SC, Gupta A, Crews D. Differential effects of testosterone and progesterone on the activation and retention of courtship behavior in sexual and parthenogenetic whiptail lizards. Horm Behav. 2003;43 (5):523–530. doi: 10.1016/s0018-506x(03)00060-6. [DOI] [PubMed] [Google Scholar]
- Schaefer J, Zakon HH. Opposing actions of androgen and estrogen on in vitro firing frequency of neuronal oscillators in the electromotor system. J Neurosci. 1996;16 (8):2860–2868. doi: 10.1523/JNEUROSCI.16-08-02860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab TM, Solomon NG, Isaacson LG, Callahan P. Reproductive activation of pine voles (Microtus pinetorum): examination of physiological markers. Brain Res. 2004;1021 (2):256–263. doi: 10.1016/j.brainres.2004.06.067. [DOI] [PubMed] [Google Scholar]
- Shimura T, Yamamoto T, Shimokochi M. The medial preoptic area is involved in both sexual arousal and performance in male rats: re-evaluation of neuron activity in freely moving animals. Brain Res. 1994;640 (1–2):215–222. doi: 10.1016/0006-8993(94)91875-9. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. GABA and glutamate in mating-activated cells in the preoptic area and medial amygdala of male gerbils. J Comp Neurol. 2003;459 (3):290–300. doi: 10.1002/cne.10605. [DOI] [PubMed] [Google Scholar]
- Summers CH, Korzan WJ, Lukkes JL, Watt MJ, Forster GL, Overli O, Hoglund E, Larson ET, Ronan PJ, Matter JM, Summers TR, Renner KJ, Greenberg N. Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol Biochem Zool. 2005;78 (5):679–694. doi: 10.1086/432139. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur J Neurosci. 2006;23 (7):1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- Tlemçani O, Ball GF, D’Hondt E, Vandesande F, Sharp PJ, Balthazart J. Fos induction in the Japanese quail brain after expression of appetitive and consummatory aspects of male sexual behavior. Brain Res Bull. 2000;52 (4):249–262. doi: 10.1016/s0361-9230(00)00233-1. [DOI] [PubMed] [Google Scholar]
- Wade J. Current research on the behavioral neuroendocrinology of reptiles. Horm Behav. 2005;48 (4):451–460. doi: 10.1016/j.yhbeh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Lynch KS, O’Bryant EL. Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm Behav. 2005;48 (4):440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler SM, Wade J. Aromatase activity and regulation of sexual behaviors in the green anole lizard. Physiol Behav. 1998;64 (5):723–731. doi: 10.1016/s0031-9384(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32 (1):40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM. Reproduction in Context. MIT Press; Boston: 2000. [Google Scholar]
- Yahr P, Gregory JE. The medial and lateral cell groups of the sexually dimorphic area of the gerbil hypothalamus are essential for male sex behavior and act via separate pathways. Brain Res. 1993;631 (2):287–296. doi: 10.1016/0006-8993(93)91547-6. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Wilczynski W. Relationships between hormones and aggressive behavior in green anole lizards: an analysis using structural equation modeling. Horm Behav. 2002;42 (2):192–205. doi: 10.1006/hbeh.2002.1811. [DOI] [PubMed] [Google Scholar]