Abstract
Background
Genetic factors interact with environmental stressors to moderate risk for human psychopathology, but sex may also be an important mediating factor. Different strategies for coping with environmental stressors have evolved in males and females, and these differences may underlie the differential prevalence of certain types of psychopathology in the two sexes. In this study, we investigated the possibility of sex-specific gene–environment interactions in a nonhuman primate model of response to social threat.
Methods
Rhesus macaques (77 males and 106 females) were exposed to an unfamiliar conspecific. Using factor analysis, we identified three behavioral factors characterizing the response to social threat. Monkeys were genotyped for the serotonin transporter–linked polymorphism (5-HTTLPR), and the effects of genotype, early life stress, and sex on behavioral responses were evaluated.
Results
Factor analysis produced five factors: High-Risk Aggression, Impulsivity/Novelty-Seeking, Gregariousness/Boldness, Harm Avoidance, and Redirected Aggression. Overall, males displayed higher levels of High-Risk Aggression and Gregariousness/Boldness than females. Levels of High-Risk Aggression in males carrying the s allele were significantly higher if they were also exposed to early adversity in the form of peer rearing.
Conclusions
Our findings support those from studies in humans suggesting that males are more vulnerable to externalizing or aggression-related disorders. The results highlight the importance of interactions that exist among behavior, genes, and the environment and suggest that sex differences in vulnerability to psychopathology may be grounded in our evolutionary history.
Keywords: Aggression, serotonin transporter, gene–environment interaction, Macaca mulatta, sex differences, social intrusion
The interaction between environmental adversity and genetic variation is a key factor in the development of psychopathology (1-3). From an evolutionary perspective, the roots of psychopathology lie in the various strategies that have evolved for coping with environmental challenges (4), be they aggressive and bold or anxious and harm avoidant (5). Interestingly, males and females often adopt such opposing strategies (aggressive/bold vs. nonaggressive/cautious) in response to stress, presumably because of different selection pressures acting on the two sexes (6,7). This differentiation of response suggests that males and females may be at risk for developing different types of psychopathology. Indeed, evidence suggests that males are more likely to develop externalizing disorders (e.g., antisocial behavior and substance use disorders), whereas females are more prone to internalizing disorders such as anxiety and depression (8,9). These observations underscore the notion of psychopathology as an outcome of the response to stress and suggest that in addition to genetic and environmental variables, an individual's sex is likely to play an important role.
Surprisingly, investigations of gene by environment (G×E) interactions in the development of psychopathology often do not test for sexually dichotomous effects. Some recent studies investigating the serotonin transporter polymorphism (5-HTTLPR) are the exception and provide some evidence for differential effects in females and males. Interactions between the loss-of-function 5-HTTLPR short (s) allele and environmental adversity that are associated with depression severity appear to occur more frequently in females (10-13). Although a recent meta-analysis failed to find an interactive effect of 5-HTTLPR genotype and stressful life events on risk for depression whether the sexes were combined or were analyzed separately (14), it is of interest that several studies have demonstrated sexually dichotomous effects, with females showing increased risk for depression when carrying the s allele and males showing increased risk if homozygous for the long (l) allele (15,16). Emerging evidence for sex differences in G×E interactions is also seen is studies of aggressive behavior, a trait often linked to externalizing disorders such as antisocial behavior and antisocial personality disorder (ASPD). Males homozygous for the 5-HTTLPR s allele, when exposed to an environmental stressor, show an increase in aggressive behavior, whereas females do not (17). Also, among males, carriers of the s allele are more prone to violent criminal behavior if also exposed to an adverse childhood environment (18). Considered together, the findings of sex-specific G×E interactions for depression and aggressive behavior suggest that genetic variants related to serotonin system dysfunction may manifest differently in the two sexes: as depressive symptoms in females and as behavioral dyscontrol in males (17).
The study of G×E interactions in the development of psychopathology in humans can be challenging, mainly because of difficulties in accurately quantifying environmental exposure (19). The rhesus macaque (Macaca mulatta) model has led the way as a controlled experimental system that provides a consistent measure of early adversity (via peer-rearing). In the peer-rearing model, animals are reared without a mother under standardized conditions in a nursery environment. Peer-rearing results in a number of behavioral and neurophysiologic alterations that often persist into adulthood (20-22). Using this model, we have repeatedly demonstrated G×E interactions that translate to the human condition (23-28). In this study, we investigate the possibility of sexually dichotomous G×E interactions using a challenge that mimics a stressor faced by nonhuman primates in their natural environments—the presence of an unfamiliar conspecific. On the basis of similar studies in vervet monkeys (29), we predicted that males and females would respond differently to this form of social threat, with males more likely to exhibit an aggressive response. Because human males carrying the s allele exhibit higher levels of aggression following provocation in the laboratory (17), we examined whether rh5-HTTLPR genotype would predict aggression in rhesus macaques exposed to social threat. We then examined whether rh5-HTTLPR genotype interacted with early adversity (peer rearing) to predict behavioral responses to social threat and whether these interactions differed between males and females.
Methods and Materials
Subjects
The subjects were 183 rhesus macaques (106 females and 77 males) maintained at the National Institutes of Health Animal Center (NIHAC) in Poolesville, Maryland (Table S1 in Supplement 1). Of these subjects, 45 were not included in previous analyses investigating the effects of rh5-HTTLPR. At the time of testing, all subjects were housed in social groups of 8 to 12 animals. About one third of the social groups were breeding groups consisting of 8 to 10 adult females and 1 to 2 adult males, whereas the remaining groups were nonbreeding, same-sex groups of younger subadult monkeys. Groups were housed in large indoor–outdoor runs, the indoor and outdoor portions of which could be separated by a guillotine door.
Infants were randomly assigned to one of three rearing conditions at birth: mother-reared (MR), peer-reared (PR), or surrogate/peer-reared (SPR). MR infants remain with their mothers in their natal social group of 8 to 10 adult females, two adult males, and other similar-aged infants. PR and SPR infants are raised by human caregivers in the neonatal nursery, and these rearing procedures have been described in detail elsewhere (22). Briefly, PR and SPR infants were removed from their mothers in the first 1 to 2 days following birth and taken to the nursery, where for the first 37 days they were housed individually with a rocking “surrogate” mother and were bottle fed a special formula mixture. After 37 days, PR infants were placed in permanent social groups of four age-matched infants, and SPR infants continued to be housed individually with the exception of a daily “play period” during which they were placed in groups of four age-matched infants for 2 hours/day, 5 days/week. All infants, including MR infants, remained in their rearing condition for the first 6 months of life.
Intruder Challenge Test
Behavioral responses to an unfamiliar conspecific were measured via a modified version of the Intruder Challenge Test developed by Fairbanks (30). In this test, subjects are exposed to an unfamiliar conspecific in a controlled manner by placing the “intruder” animal into a transfer cage, which is then positioned adjacent to the test subjects' home enclosure. Subjects may approach the intruder and interact through the mesh of the cage and enclosure, but full contact is prevented to avoid injury to any of the animals.
All intruder animals were completely unfamiliar to the test subjects and were selected to match the test subjects' age, sex, and relative body size. Before the test, the intruder animal was placed into an individual transfer cage, measuring .76 m wide × .63 m deep × .91 m high, for a 30-min acclimation period. Also, before the test, three randomly selected test subjects were separated from the larger social group into the outdoor portion of their home enclosure, a space measuring 2.64 m wide × 3m long × 2.44 m high, and given a 10-min acclimation period. The test began when the intruder animal's cage was placed directly at the front of the enclosure. Test subjects' behavior was recorded for 30 min, with one observer assigned to each subject. Recording of behavior was performed using handheld computers equipped with Observer software (Noldus, Lessburg, Virginia). The software allows each observer to record the frequency and duration of various behaviors performed by the test subject. The behaviors recorded were based on a standard behavioral etho-gram developed by our laboratory, which has been used in many contexts (e.g., social behavior in breeding groups) and across a relatively large number of birth cohorts (Table S2 in Supplement 1). Interobserver reliability was established at greater than r = .85, and all observers were blind to the subjects' rearing condition and genotype at the time of data collection.
Genotyping
See Supplement 1.
Statistical Analyses
Behavioral data from the intruder challenge test were subjected to factor analysis, and factor scores for each subject were extracted using the principal components method with varimax normalized rotation. Preliminary analyses revealed no differences between PR and SPR subjects in the resulting factors (analysis of variance [ANOVA], all ps > .05). Therefore, these groups were combined into one category: nursery-reared (NR).
For the first step in the analyses, we tested for effects of age and sex on the behavior factors using ANOVA, with age summarized into subadult (<5 years of age) and adult (≥5 years of age) categories. Data for two of the extracted factors were found to be nonnormally distributed (Kolmogorov–Smirmov test, p < .01), and attempts were made to correct for this by transforming the data using both a rank transformation and a log transformation. However, neither transformation resulted in normally distributed data. Both of these extracted factors were based on observational data of aggression, which in this study included a large number of zero values. As a result, the distributions of both the raw aggression scores and the resultant factor scores were skewed. Because ANOVA is quite robust against nonnormality, we elected to retain untransformed factor scores for use in further analyses. Age and sex were found to influence the behavior factors; consequently, we included these variables in a second round of analyses testing for the effects of genotype and rearing condition. In this step, we used analyses of covariance (ANCOVA), with 5-HTTLPR genotype (l/l vs. l/s) and rearing condition (MR vs. NR) as independent variables, age as a continuous covariate, and the behavior factors as dependent variables. Initially, we also included social dominance rank (low vs. high) as a coindependent variable, because dominance rank has long been known to influence behavior in nonhuman primates, especially during times of conflict (31). However, because no main effects or interactions with genotype were found and because the inclusion of dominance rank did not reduce the residual variance, it was ultimately removed from the analyses. Preliminary analysis also showed that we were underpowered to detect three-way interactions among genotype, rearing, and sex, and consequently we performed separate ANCOVAs for males and females. The number of subjects homozygous for the s allele was small (Table S1 in Supplement 1). Analyses involving genotype were performed both with these subjects combined with the l/s animals and then with these subjects excluded. Because results did not differ between the two methods, we present the results with the s/s and l/s subjects combined. All analyses were conducted using Statistica (Statasoft, Tulsa, Oklahoma), and the general linear models procedure was used for all ANOVA tests. Threshold p values were adjusted for multiple testing using the Bonferroni method.
Results
Factor Analysis
The factor analysis produced five factors that together explained 52.0% of the variance (Table S3 in Supplement 1). The factors we obtained in this study vary slightly from those reported in a previous analysis of behavior from the Intruder Challenge (32), most likely because we included some behaviors (receiving aggression from the intruder and from cagemates, and latency to approach the intruder) that were not included in prior analyses. The decision to include behaviors performed by the intruder in this factor analysis stemmed from preliminary analyses indicating that aggression on the part of the test subject was correlated with aggressive behavior on the part of the intruder. Therefore, we wanted to account for this relationship in characterizing the response of the test subject. Even with the addition of these behaviors, the High-Risk Aggression factor, as in the previous report, explained the highest proportion of the variance (17.2% in the present case).
Effects of Age and Sex
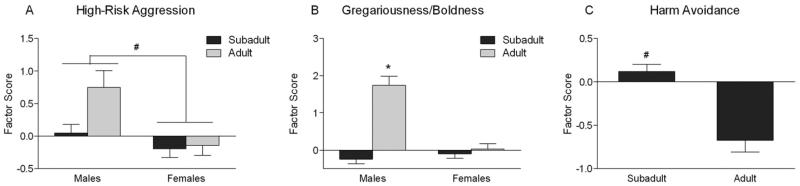
Results for the ANOVAs testing for effects of age and sex are presented in Table 1. There was a main effect of sex [F(1,179) = 10.34, p = .002] on High-Risk Aggression. Males showed significantly higher levels of High-Risk Aggression than females (Figure 1A). For the Gregariousness/Boldness factor, there was an interaction between age and sex [F(1,179) = 31.77, p < .001], with adult males scoring significantly higher on this factor compared with subadult males and females of both age groups (Figure 1B). There was a main effect of age [F(1,179) = 24.76, p < .001] on Harm Avoidance, with subadult subjects scoring higher for this factor than adult subjects (Figure 1C). There were no significant effects of sex or age on either Impulsivity/Novelty-Seeking or Redirected Aggression.
Table 1.
Summary of the Results for Effects of Sex and Age Group on Behavioral Responses to Social Intrusion
| Factor | Effect | df | F | p |
|---|---|---|---|---|
| High-Risk Aggression | Sexa | 1, 179 | 10.34 | .002 |
| Age | 1, 179 | 4.61 | .033 | |
| Sex × Age | 1, 179 | 3.38 | .070 | |
| Impulsivity/Novelty-Seeking | Sex | 1, 179 | .05 | .820 |
| Age | 1, 179 | .01 | .936 | |
| Sex × Age | 1, 179 | 2.01 | .158 | |
| Gregariousness/Boldness | Sexa | 1, 179 | 22.58 | <.001 |
| Agea | 1, 179 | 41.14 | <.001 | |
| Sex × Agea | 1, 179 | 31.77 | <.001 | |
| Harm Avoidance | Sex | 1, 179 | .07 | .786 |
| Agea | 24.76 | <.001 | ||
| Sex × Age | 1, 179 | .12 | .728 | |
| Redirected Aggression | Sex | 1, 179 | 2.04 | .155 |
| Age | 1, 179 | 1.68 | .196 | |
| Sex × Age | 1, 179 | .00 | .949 | |
| Breakdown of Individual Behaviors for High-Risk Aggression | ||||
|
| ||||
| Contact Aggression to Intruder | Sexb | 1, 179 | 6.79 | .010 |
| Age | 1, 179 | 4.16 | .043 | |
| Sex × Age | 1, 179 | 1.73 | .190 | |
| Receive Contact Aggression from Intruder | Sexb | 1, 179 | 8.66 | .004 |
| Ageb | 1, 179 | 6.32 | .013 | |
| Sex × Age | 1, 179 | 3.10 | .080 | |
| Receive Noncontact Aggression from Intruder | Sexb | 1, 179 | 17.65 | <.001 |
| Ageb | 1, 179 | 11.01 | .001 | |
| Sex × Ageb | 1, 179 | 9.54 | .002 | |
Significant effect at p < .01 (Bonferroni correction: .05/5 factors tested = .01).
Significant effect at p < .017 (Bonferroni correction: .05/3 behaviors = .017).
Figure 1.
Age and sex effects on behavioral responses to social intrusion. The bars depict least square means and standard errors (# indicates a significant main effect after Bonferroni correction from the analysis of variance, and * indicates a significant difference at p < .05 using Fisher's least significant difference post hoc tests). (A) High-risk Aggression factor. (B) Gregariousness/Boldness, characterized by spending time in social contact with the intruder animal. (C) Harm Avoidance.
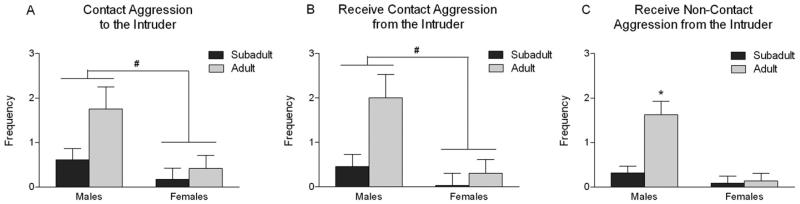
Because the High-Risk Aggression factor included aggressive behaviors directed toward the test subject as well as aggression on the part of the test subject, we could not be certain as to whether a high score was attributable to the behavior of the intruder, to that of the test subject, or both. Therefore, we extended the analysis to look at the effects of age and sex on the individual behavioral components (Table 1). There was a main effect of sex [F(1,179) = 6.79, p = .01] on contact aggression by the test subject, as well as on the receipt of contact aggression from the intruder [sex: F(1,179) = 8.66, p = .004; age: F(1,179) = 6.32, p = .01]. Males displayed as well as received more instances of contact aggression than females (Figure 2A and 2B). Age and sex also interacted to influence the rate at which noncontact aggression was received from the intruder [F(1,179) = 9.54, p = .002], with adult males receiving the highest incidence of this behavior (Figure 2C).
Figure 2.
Age and sex effects on the individual behavioral components contributing to the High-Risk Aggression factor. The bars depict least square means and standard errors (# indicates a significant main effect after Bonferroni correction from the analysis of variance, and * indicates a significant difference at p < .05 using Fisher's least significant difference post hoc tests). (A) Contact aggression by the test subject to the intruder. In addition to the sex difference indicated in the graph, there was also a main effect of age, with adults displaying more contact aggression than subadults. (B) Receive contact aggression from the intruder. In addition to the sex difference indicated in the graph, there was also a main effect of age, with adults receiving more contact aggression from the intruder than subadults. (C) Receive noncontact aggression from the intruder.
Effects of Genotype and Rearing Condition
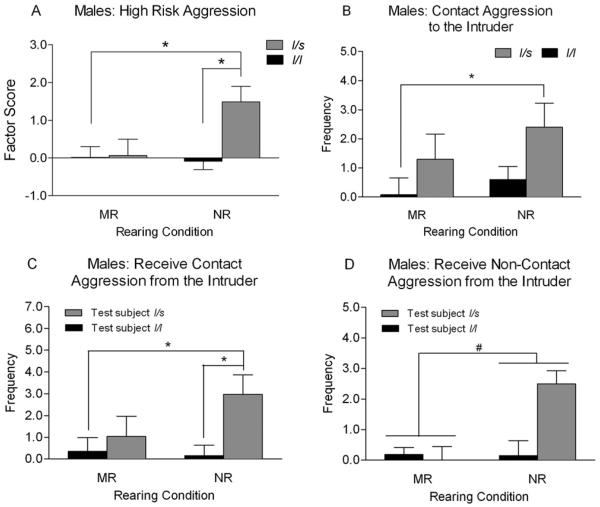
The frequency of the s allele was 16%, and genotype frequencies did not deviate from Hardy–Weinberg equilibrium. Results for the ANCOVAs for males and females including genotype and rearing condition are summarized in Table 2. There was a main effect of rearing condition [F(1,69) = 11.43, p = .001] and an interaction of genotype and rearing condition [F(1,69) = 10.20, p = .002] on High-Risk Aggression in males. NR males carrying the s allele scored significantly higher on the High-Risk Aggression factor than males homozygous for the l allele (Figure 3A). As before, we broke down the High-Risk Aggression factor into the individual behavioral components and analyzed them separately using the same method we used with the behavioral factor (i.e., ANCOVA; Table 3). There was a main effect of rearing condition [F(1,69) = 7.56, p = .008] and an interaction between genotype and rearing condition [F(1,69) = 7.62, p = .007] on contact aggression to the intruder. These variables also predicted the receipt of contact aggression [rearing condition: F(1,69) = 9.47, p = .003; genotype by rearing: F(1,69) = 9.27, p = .003] and noncontact aggression [rearing condition: F(1,69) = 9.80, p = .003] from the intruder. NR males carrying the s allele exhibited the highest frequencies of contact aggression toward the intruder and received the highest frequencies of both contact and noncontact aggression from the intruder (Figure 3B–3D).
Table 2.
Summary of the Results for Effects of Rh5-HTTLPR Genotype and Rearing Condition on Behavioral Responses to Social Intrusion
| Males |
Females |
||||||
|---|---|---|---|---|---|---|---|
| Factor | Effect | df | F | p | df | F | p |
| High-Risk Aggression | Genotype | 1, 69 | .57 | .454 | 1, 98 | .00 | .952 |
| Rearing | 1, 69 | 11.43 | .001a | 1, 98 | .17 | .682 | |
| Genotype*Rearing | 1, 69 | 10.20 | .002a | 1, 98 | .01 | .919 | |
| Impulsivity/Novelty-Seeking | Genotype | 1, 69 | .89 | .349 | 1, 98 | 2.96 | .090 |
| Rearing | 1, 69 | .05 | .817 | 1, 98 | .31 | .581 | |
| Genotype*Rearing | 1, 69 | .58 | .449 | 1, 98 | 2.54 | .114 | |
| Gregariousness/Boldness | Genotype | 1, 69 | .45 | .504 | 1, 98 | .09 | .764 |
| Rearing | 1, 69 | 1.80 | .184 | 1, 98 | .95 | .331 | |
| Genotype*Rearing | 1, 69 | .74 | .394 | 1, 98 | .21 | .650 | |
| Harm Avoidance | Genotype | 1, 69 | .26 | .613 | 1, 98 | .04 | .846 |
| Rearing | 1, 69 | .04 | .846 | 1, 98 | .31 | .577 | |
| Genotype*Rearing | 1, 69 | .76 | .386 | 1, 98 | .07 | .788 | |
| Redirected Aggression | Genotype | 1, 69 | .71 | .403 | 1, 98 | 4.23 | .042 |
| Rearing | 1, 69 | .08 | .782 | 1, 98 | .89 | .347 | |
| Genotype*Rearing | 1, 69 | .07 | .785 | 1, 98 | .00 | .993 | |
Significant effect at p < .005 [Bonferroni correction: .05/10 (5 behavior factors × 2 sexes) = .005].
Figure 3.
Interaction of genotype and rearing condition on aggressive responses to social intrusion in male rhesus macaques. The bars depict least square means (adjusted for the covariate, age) and standard errors (# indicates a significant main effect after Bonferroni correction from the analysis of variance, and * indicates a significant difference at p < .05 using Fisher's least significant difference post hoc tests). (A) High-Risk Aggression factor. (B) Contact aggression by the test subject to the intruder. (C) Receive contact aggression from the intruder. (D) Receive noncontact aggression from the intruder. Although the graph appears to indicate an interaction of rearing condition and genotype, this effect was not significant after the Bonferroni correction for multiple testing (see Table 3). In addition to the main effect of rearing condition depicted in the graph, there was a main effect of genotype, with l/s subjects receiving more noncontact aggression from the intruder than l/l subjects. MR, mother-reared; NR, nursery-reared.
Table 3.
Summary of the Results for Effects of Rh5-HTTLPR Genotype and Rearing Condition on the Individual Behaviors from the High-Risk Aggression Factor in Males
| Behavior | Effecta | df | F | p |
|---|---|---|---|---|
| Contact Aggression to Intruder | Genotype | 1, 69 | .00 | .990 |
| Rearingb | 1, 69 | 7.56 | .008 | |
| Genotype × Rearingb | 1, 69 | 7.62 | .007 | |
| Receive Contact Aggression from Intruder | Genotype | 1, 69 | .02 | .885 |
| Rearingb | 1, 69 | 9.47 | .003 | |
| Genotype × Rearingb | 1, 69 | 9.27 | .003 | |
| Receive Noncontact Aggression from Intruder | Genotypeb | 1, 69 | 10.11 | .002 |
| Rearingb | 1, 69 | 9.80 | .003 | |
| Genotype × Rearing | 1, 69 | 4.12 | .046 |
The effects of genotype and rearing condition refer only to characteristics of the test subject and not the intruder subject.
Significant effect at p < .017 (Bonferroni correction: .05/3 behaviors = .17).
There were no significant effects of rearing condition or genotype on Impulsivity/Novelty-Seeking, Gregariousness/Boldness, or Harm Avoidance in either males or females. A main effect of genotype on Redirected Aggression in females [F(1,98) = 4.23, p = .04] was initially indicated. However, this effect did not remain significant following correction for multiple testing (Table 2).
Discussion
In nonhuman primates, the measurement of behavioral responses to social intrusion by an unfamiliar conspecific, assessed under controlled conditions in the laboratory environment, has proved useful for assessing individual variation in temperament traits that relate to differences in sex, age, and genetic background (29,32). In our analysis, we uncovered five behavioral dimensions characterizing the response of rhesus macaques to an unfamiliar conspecific. Of these, the High-Risk Aggression factor explained the highest amount of variance. This factor is labeled “high risk” because contact aggression directed toward an intruder, which is inherently risky, was exhibited even in the presence of an aggressive intruder. The fact that this type of behavior accounted for so much of the variance (17.2%) is not surprising when one considers that aggression is common in the context of intergroup encounters between unfamiliar animals in the natural habitat, especially among Old World primates. For the most part, this aggression is carried out by males targeting other males, although aggression between females is not uncommon (33,34). As predicted, levels of High-Risk Aggression in this study were significantly higher in male test subjects compared with females. Further analysis of this factor also showed that male test subjects received more aggression from the intruder than did female test subjects.
In addition, adult males scored significantly higher on the Gregariousness/Boldness factor compared with all other age and sex groups. The fact that adult males spent significantly more time in social contact with the intruder suggests that adult males are more apt to engage the intruder in general, even in a nonaggressive manner. Females and subadult males, in contrast, are more likely to maintain a safe distance from the intruder. Together, our results support the notion that when faced with an environmental threat, males are more likely than females to adopt an aggressive or bold strategy. Interestingly, parallel findings have emerged in investigations of human defensive behaviors. When males and females are presented with scenarios involving an unfamiliar person, males are more likely to choose an attack response in highly threatening situations, whereas females are more likely to yell, scream, or call for help (35,36). Furthermore, females tend to feel more threatened when faced with these scenarios. Considered together, the data from human and nonhuman primates highlight some of the similarities between species in defensive behavior and suggest that sex differences in response to social threat may have been preserved as part of our evolutionary history.
What is novel about the current findings is that the effects of genotype that were observed on aggressive responses were limited to individuals who also had a prior history of stress. Males carrying the s allele who were exposed to early adversity in the form of nursery rearing showed significantly higher levels of High-Risk Aggression toward the intruder. This result was mirrored by a similar G×E interaction for contact aggression toward the intruder, one of the components of the High-Risk Aggression factor. Early life stress or maltreatment has been linked to adolescent and adult aggression in humans (37-40) as well as in some animal models, including data from our own laboratory (41-43). Interestingly, studies linking early-life maltreatment to aggression in rodents have observed these effects primarily in males (41,42). Our findings for male rhesus macaques exposed to stress in the form of nursery rearing provides additional evidence of an association between early-life stress and later aggression that is limited to males. This effect of early adversity was mediated, however, by genetic variation in the serotonin transporter regulatory region. Variation in this region in humans has been related to aggression and violent behavior in some studies, but results for a main genotype effect have not been consistent (44-50). Two recent studies have reported G×E effects involving 5-HTTLPR. Verona et al. (17) observed increased aggression (i.e., delivering shocks to a putative “employee” in a laboratory environment) in males homozygous for the serotonin transporter s allele when they were exposed to an acute stressor (intermittent blasts of compressed air to the throat). Reif et al. (18) found that males carrying the s allele were more prone to violent criminal behavior if they also had an adverse childhood environment. Our results complement and expand on these human findings in several ways. First, whereas similar studies in humans typically rely on retrospective assessments of both childhood environment and aggression or violence, we were able to assess aggressive behavior directly in a sample of monkeys with carefully controlled early environments. Second, our finding of a G×E effect in males, but not females, with a history of early adversity underscores the need to consider sex differences in investigations of G×E interactions. Lastly, whereas our results parallel those of Verona and colleagues, both in the analysis of directly observable aggressive behavior and in the finding of male-limited G×E effect, the latter study used an acute stressor and did not take into account effects of early-life history. Our results also add to human studies of another functional variant that influences serotonin system functioning, monoamine oxidase (MAOA), that has been shown to interact with early maltreatment to predict various indexes of aggression and related clinical outcomes (51-55). Although many of these studies also are limited to males, there is emerging evidence that MAOA interacts with maltreatment in females as well (56).
The strategies adopted by males and females when faced with an environmental stressor in many ways parallel those described in the Hawk–Dove model, first proposed by Maynard Smith and Price (57) and expanded on by Korte and colleagues (5). In this model, an aggressive/bold strategy (Hawk) is opposed by a nonaggressive, cautious strategy (Dove), with natural selection maintaining a balance of traits preserving genes for these strategies within a population (5). Similarly, Taylor and colleagues have proposed a model in which males have evolved to exhibit the classic “fight-or-flight” response to stress, whereas females have evolved to exhibit a “tend-and-befriend” response, protecting themselves as well as their offspring from environmental threat (58). Both of these models map well onto the proposed genetic structure of externalizing and internalizing human psychiatric disorders (59). It is important to note, however, that considerable individual differences exist within each sex when it comes to coping with environmental variation (5). To an extent, our findings are consistent with these models. In general, male rhesus macaques adopted a more aggressive strategy when faced with the social threat of an intruder. In our report, we show that individual variation in this aggressive strategy is related to genetic variation influencing serotonin system functioning and exposure to early-life stress. The apparent lack of aggression toward intruder animals on the part of females also fits with these models, with a couple of caveats. First, female test subjects were exposed to female intruder animals, who were less aggressive and thus potentially less likely to elicit an aggressive response. Second, there is some evidence that females may act aggressively in the context of social intrusion, even if not toward the intruder animal. Redirected Aggression (i.e., aggression toward own group members in the context of threat from an unfamiliar animal) was slightly more common in females than males, although the difference was not significant (mean ± SEM for females, .083 ± .097; males, = −/−.158 ± .138). Furthermore, the data suggest an effect of rh5-HTTLPR on this behavior in females, with s allele carriers displaying higher levels of Redirected Aggression than l/l subjects (Figure S1 in Supplement 1). Aggression in this context could potentially be “protective,” that is, aimed at other group members to correct or modify their behaviors. Other forms of aggression (i.e., “displaced” or “redirected” aggression) occur in a variety of other animal species as well as humans and is argued by some to be a postconflict interaction that functions to reduce anxiety or stress (60-65). In any case, it may be that among females, genetic variation that influences aggression is more likely to be reflected in intragroup relations than intergroup encounters.
Evolutionary psychology predicts that during evolutionary history, males and females will come to differ in those domains that have evolved in response to sex-differentiated adaptive problems (66). On the basis of this viewpoint, one would expect that genetic and environmental factors, as well as interactions between the two, would have different effects on behavior and other outcomes in males and females. Responding to social threat is one domain in which males and females are likely to adopt different adaptive solutions. We have shown in this study that the rhesus macaque 5-HTTLPR polymorphism differentially influences aggressive responses to social threat in males and females. Overall, our results lend further support to the notion that males are more vulnerable to externalizing disorders and that genetic variation that affects serotonin system function may play a role in this differential susceptibility (15,17,59). These findings highlight the importance of interactions that exist among behavior, genes, and the environment and suggest that sex differences in vulnerability to psychopathology may be grounded in our evolutionary history.
Supplementary Material
Acknowledgments
We thank Michelle Becker, Courtney Lindell, and the research, animal care, and veterinary staff of the National Institutes of Health Animal Center for their assistance in conducting this study. All procedures were reviewed and approved by the Animal Care and Use Committees of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute of Child Health and Development (NICHD) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. This research was supported by the intramural programs of the NIAAA and NICHD.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Rutter M. Gene–environment interdependence. Dev Sci. 2007;10:12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 2.Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: Multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 3.Thapar A, Harold G, Rice F, Langley K, O'Donovan M. The contribution of gene–environment interaction to psychopathology. Dev Psychopathol. 2007;19:989–1004. doi: 10.1017/S0954579407000491. [DOI] [PubMed] [Google Scholar]
- 4.Darwin C. The Origin of Species. John Murray; London: 1859. [Google Scholar]
- 5.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Eme RF. Sex differences in child-onset, life-course-persistent conduct disorder. A review of biological influences. Clin Psychol Rev. 2007;27:607–627. doi: 10.1016/j.cpr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Wood W, Eagly AH. A cross-cultural analysis of the behavior of women and men: Implications for the origins of sex differences. Psychol Bull. 2002;128:699–727. doi: 10.1037/0033-2909.128.5.699. [DOI] [PubMed] [Google Scholar]
- 8.Cale EM, Lilienfeld SO. Sex differences in psychopathy and antisocial personality disorder: A review and integration. Clin Psych Review. 2002;22:1179–1207. doi: 10.1016/s0272-7358(01)00125-8. [DOI] [PubMed] [Google Scholar]
- 9.Williams JB, Spitzer RL, Linzer M, Kroenke K, Hahn SR, deGruy FV, et al. Gender differences in depression in primary care. Am J Obstet Gynecol. 1995;173:654–659. doi: 10.1016/0002-9378(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 10.Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene–environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 11.Grabe HJ, Lange M, Wolff B, Völzke H, Freyberger HJ, John U, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- 12.Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 14.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjöberg RL, Nilsson KW, Nordquist N, öhrvik J, Leppert J, Lindström L, et al. Development of depression: Sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- 16.Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, et al. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behav Genet. 2008;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verona E, Joiner TE, Johnson F, Bender TW. Gender specific gene–environment interactions on laboratory-assessed aggression. Biol Psychol. 2006;71:33–41. doi: 10.1016/j.biopsycho.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Reif A, Rösler M, Freitag CM, Schneider M, Eujen A, Kissling C, et al. Nature and nurture predispose to violent behavior: Serotonergic genes and adverse childhood environment. Neuropsychopharmacology. 2007;32:2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- 19.Caspi A, Moffitt TE. Gene–environment interactions in psychiatry: Joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 20.Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: Effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci USA. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, et al. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry. 2005;162:1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- 23.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 24.Barr CS, Becker ML, Suomi SJ, Higley JD. Relationships among CSF monoamine metabolite levels, alcohol sensitivity, and alcohol-related aggression in rhesus macaques. Aggress Behav. 2003;29:288–301. [Google Scholar]
- 25.Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, et al. Early experience and sex interact to influence limbic-hypothalamic-pituitary-adrenal-axis function after acute alcohol administration in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2004;28:1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- 26.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ, et al. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol. 2007;19:977–987. doi: 10.1017/S095457940700048X. [DOI] [PubMed] [Google Scholar]
- 28.Champoux M, Bennett AJ, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 29.Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, et al. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry. 2004;55:642–647. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Fairbanks LA. Individual differences in response to a stranger: Social impulsivity as a dimension of temperament in vervet monkeys (Cercopithecus aethiops sabaeus) J Comp Psychol. 2001;115:22–28. doi: 10.1037/0735-7036.115.1.22. [DOI] [PubMed] [Google Scholar]
- 31.Walters JR, Seyfarth RM. Conflict and cooperation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 306–317. [Google Scholar]
- 32.Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, et al. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65:934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higley JD. Aggression. In: Maestripieri D, editor. Primate Psychology. Harvard University Press; Cambridge, MA: 2003. pp. 17–40. [Google Scholar]
- 34.Smuts BB. Gender, aggression, and influence. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 400–412. [Google Scholar]
- 35.Blanchard CD, Hynd AL, Minke KA, Minemoto T, Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear-and anxiety-related defense patterns of non-human mammals. Neurosci Biobehav Rev. 2001;25:761–770. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 36.Shuhama R, Del-Ben CM, Loureiro SR, Graeff FG. Defensive responses to threat scenarios in Brazilians reproduce the pattern of Hawaiian Americans and non-human mammals. Braz J Med Biol Res. 2008;41:324–332. doi: 10.1590/s0100-879x2008000400011. [DOI] [PubMed] [Google Scholar]
- 37.Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- 38.Lansford JE, Miller-Johnson S, Berlin LJ, Dodge KA, Bates JE, Pettit GS. Early physical abuse and later violent delinquency: A prospective longitudinal study. Child Maltreat. 2007;12:233–245. doi: 10.1177/1077559507301841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis DO. From abuse to violence: Psychophysiological consequences of maltreatment. J Am Acad Child Adolesc Psychiatry. 1992;31:383–391. doi: 10.1097/00004583-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Lee V, Hoaken PN. Cognition, emotion, and neurobiological development: Mediating the relation between maltreatment and aggression. Child Maltreat. 2007;12:281–298. doi: 10.1177/1077559507303778. [DOI] [PubMed] [Google Scholar]
- 41.Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- 42.Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- 43.Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcohol Clin Exp Res. 1996;20:643–650. doi: 10.1111/j.1530-0277.1996.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 44.Beitchman JH, Baldassarra L, Mik H, De Luca V, King N, Bender D, et al. Serotonin transporter polymorphisms and persistent, pervasive childhood aggression. Am J Psychiatry. 2006;163:1103–1105. doi: 10.1176/ajp.2006.163.6.1103. [DOI] [PubMed] [Google Scholar]
- 45.Rujescu D, Giegling I, Sato T, Moeller HJ. A polymorphism in the promoter of the serotonin transporter gene is not associated with suicidal behavior. Psychiatr Genet. 2001;11:169–172. doi: 10.1097/00041444-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Zalsman G, Frisch A, Bromberg M, Gelernter J, Michaelovsky E, Campino A, et al. Family-based association study of serotonin transporter promoter in suicidal adolescents: No association with suicidality but possible role in violence traits. Am J Med Genet. 2001;105:239–245. doi: 10.1002/ajmg.1261. [DOI] [PubMed] [Google Scholar]
- 47.Courtet P, Baud P, Abbar M, Boulenger JP, Castelnau D, Mouthon D, et al. Association between violent suicidal behavior and the low activity allele of the serotonin transporter gene. Mol Psychiatry. 2001;6:338–341. doi: 10.1038/sj.mp.4000856. [DOI] [PubMed] [Google Scholar]
- 48.Lesch KP. Variation of serotonergic gene expression: Neurodevelopment and the complexity of response to psychopharmacologic drugs. Eur Neuropsychopharmacol. 2001;11:457–474. doi: 10.1016/s0924-977x(01)00123-7. [DOI] [PubMed] [Google Scholar]
- 49.Patkar AA, Berrettini WH, Hoehe M, Thornton CC, Gottheil E, Hill K, et al. Serotonin transporter polymorphisms and measures of impulsivity, aggression, and sensation seeking among African-American cocaine-dependent individuals. Psychiatry Res. 2002;110:103–115. doi: 10.1016/s0165-1781(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 50.Liao DL, Hong CJ, Shih HL, Tsai SJ. Possible association between serotonin transporter promoter region polymorphism and extremely violent crime in Chinese males. Neuropsychobiology. 2004;50:284–287. doi: 10.1159/000080953. [DOI] [PubMed] [Google Scholar]
- 51.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 52.Craig IW. The role of monoamine oxidase A, MAOA, in the aetiology of antisocial behaviour: The importance of gene-environment interactions. Novartis Found Symp. 2005;268:227–237. doi: 10.1002/0470010703.ch16. Discussion:237–241:242–253. [DOI] [PubMed] [Google Scholar]
- 53.Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, et al. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 54.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene–environment interaction predicting children's mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 55.Jaffee SR, Moffitt TE, Caspi A, Taylor A. Physical maltreatment victim to antisocial child: Evidence of an environmentally mediated process. J Abnorm Psych. 2004;113:44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- 56.Widom CS, Brzustowicz LM. MAOA and the “cycle of violence”: childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 57.Maynard SJ, Price GR. The logic of animal conflict. Nature. 1973;246:15–18. [Google Scholar]
- 58.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 59.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 60.Aureli F, van Schaik CP. Post-conflict behaviour in long-tailed macaques (Macaca fascicularis). II. Coping with the uncertainty. Ethology. 1991;89:101–114. [Google Scholar]
- 61.Aureli F, Veenema HC, van Panthaleon van Eck CJ, van Hooff JARAM. Reconciliation, consolation, and redirection in Japanese macaques (Macaca fuscata) Behaviour. 1993;124:1–21. [Google Scholar]
- 62.Cheney DL, Seyfarth RM. Redirected aggression and reconciliation among vervet monkeys, Cercopithecus aethiops. Behaviour. 1989;110:258–275. [Google Scholar]
- 63.Denson TF, Pedersen WC, Miller N. The displaced aggression questionnaire. J Pers Soc Psychol. 2006;90:1032–1051. doi: 10.1037/0022-3514.90.6.1032. [DOI] [PubMed] [Google Scholar]
- 64.Marcus-Newhall A, Pedersen WC, Carlson M, Miller N. Displaced aggression is alive and well: A meta-analytic review. J Pers Soc Psychol. 2000;78:670–689. doi: 10.1037//0022-3514.78.4.670. [DOI] [PubMed] [Google Scholar]
- 65.Watts DP. Post-conflict social events in wild mountain gorillas. II. Redirection, side direction, and concolation. Ethology. 1995;100:158–174. [Google Scholar]
- 66.Buss DM. Psychological sex differences. Origins through sexual selection. Am Psychol. 1995;50:164–168. doi: 10.1037/0003-066x.50.3.164. Discussion:169–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.