Abstract
Donor antigen-reactive CD4 and CD8 T cell production of interferon (IFN)-γ is a principal effector mechanism promoting tissue injury during allograft rejection. The CXCR3-binding chemokines CXCL9 and CXCL10 recruit donor-reactive T cells to the allograft, but their role during priming of donor-reactive T cells to effector function is unknown. Using a murine model of MHC-mismatched cardiac transplantation, we investigated the influence of CXCL9 and CXCL10 during donor-reactive T cell priming. In allograft recipient spleens, CXCL9 and CXCL10 were expressed as early as 24 hours post-transplant and increased with similar kinetics, concurrently with CXCR3 expression on T cells. CXCL9, but not CXCL10, expression required natural killer cell production of IFN-γ. The absence of CXCL9 in donor allografts, recipients, or both significantly lowered the frequency of donor-reactive CD8 T cells producing IFN-γ and increased the frequency of donor-reactive CD8 T cells producing IL-17A. In contrast, the absence of CXCL10 increased the frequency of IFN-γ-producing CD8 T cells in a CXCL9-dependent manner. These data provide novel evidence that donor-reactive CD8 T cells utilize the CXCR3 chemokine axis as a costimulation pathway during priming to allografts where CXCL9 promotes the development of IFN-γ producing CD8 T cells and CXCL10 antagonizes this skewing.
INTRODUCTION
Solid organ transplantation is the sole treatment option for patients suffering end-stage organ failure. MHC-mismatched allografts induce a vigorous anti-donor T cell response that requires aggressive immunosuppression to prevent rejection. Acute rejection of allografts is initiated by the emigration of passenger dendritic cells (DCs) from the transplanted organ to the recipient spleen where they prime donor antigen-specific T cells to express the effector functions, including cytolytic activities and cytokine production, that mediate graft tissue injury. The principal cytokine produced by CD4 and CD8 effector T cells in response to allografts is IFN-γ (1–4). The mechanisms that lead to a preferential skewing of the donor-reactive CD4 and CD8 T cell repertoire to predominantly IFN-γ-producing effectors following allograft transplantation is unknown. Whereas CD4 T cell development to an IFN-γ-producing phenotype requires antigen-presenting cell (APC) production of IL-12 (5), CD8 T cell development to IFN-γ-producing cells often occurs independently of IL-12 (6).
Following priming in the spleen, allograft-reactive T cells migrate through the recipient blood stream to the graft where they are activated to express the effector functions, including IFN-γ production, that mediate graft injury. Extensive studies have established that the CXCR3- and CCR5-binding chemokines play prominent roles in the recruitment of effector T cells into allografts (7–10), and correlates to these findings have been found in clinical transplantation (11–13). Studies from this and other laboratories have shown a role for CXCL9/MIG (monokine induced by IFN-γ), CXCL10/IP-10 (IFN-γ-inducible protein 10), and CXCR3 expression in accelerating acute rejection of MHC-mismatched allografts (7, 10, 14–16). In cardiac allografts, graft vascular endothelial cells and infiltrating neutrophils and macrophages produce these T cell chemoattractants (14, 17). CXCL9 and CXCL10 are also produced by dendritic cells (DCs), B cells, and macrophages (18) and bind the G-protein-coupled receptor CXCR3 which is expressed on multiple cell types but predominantly on memory phenotype cells and primed effector T cells producing IFN-γ (19).
In addition to directing leukocyte trafficking to inflammatory sites, many chemokines are produced at sites of T and B cell activation in primary and secondary lymphoid tissues (20). A recent in vitro study suggested that CXCL9 might influence the proliferation and development of alloantigen-reactive T cells in mixed lymphocyte cultures (21). Several models of inflammation have also suggested that CXCR3-binding chemokines may influence the functional development of T cells during antigen priming (22–24). CD4 and CD8 T cells express CXCR3 early during priming in response to MHC-disparate allografts and CXCR3 expression is most pronounced on effector cells that produce IFN-γ. This raises the possibility that downstream signaling from CXCR3 early during CD8 T cell priming may promote preferential polarization of donor-reactive T cells to an IFN-γ-producing phenotype.
In this report, we investigated a potential role for CXCL9 during recipient T cell priming to MHC-mismatched cardiac allografts. We demonstrate that CXCL9 and CXCR3 are coincidentally expressed in the graft-draining lymphoid tissue as early as 24 hours following transplant and that CXCL9 is induced by natural killer (NK) cell-derived IFN-γ. The absence of CXCL9 depresses the number of IFN-γ-producing, donor-reactive CD8 and CD4 T cells, but this does not prolong graft survival, potentially due to increased frequencies of donor-specific CD8 T cells producing IL-17A. Finally, we provide evidence that CXCL9 and CXCL10 antagonize each other as costimulatory molecules during T cell priming to alloantigen. Taken together, these reports implicate an important and unrecognized role for CXCR3-binding chemokines in the acute rejection process. Moreover, we propose that the predominant effector mechanism in complete MHC-mismatched allograft rejection, CD8 T cell production of IFN-γ, is the consequence of high levels of CXCL9 produced in the allograft recipient spleen during priming of these T cells and this is regulated in part by the coincident production of CXCL10 in the priming site.
MATERIALS AND METHODS
Mice
The following mice were used: C57BL/6 (H-2b) and A/J (H-2a) from Charles River Laboratories (Wilmington, MA); DBA/1 (H-2q), Balb/c (H-2d) and B6.Thy1.1 mice from The Jackson Laboratory (Bar Harbor, ME); B6.2C TCR transgenic, B6.CXCL9−/−, A/J.CXCL9−/−, B6.Rag1−/−, B6.CXCL10−/−, Balb/c.CXCL10−/− were bred at our facility. All experiments used 8–12 week-old male mice and the Cleveland Clinic Institutional Animal Care and Use Committee approved all procedures.
Heterotopic cardiac transplantation
Standard methods of murine heterotopic intra-abdominal cardiac transplantation were adapted from the method of Corry and coworkers (25). Total operative times averaged 30–35 minutes, and graft survival was monitored by abdominal palpation with cessation of beating confirmed by laparotomy.
Antibody treatments
For CD8 depletion, 0.2 mg of a 1:1 mixture of anti-CD8 mAbs YTS169 and TB-105 (BioExpress, West Lebanon NH) was administered i.p. on days −3, −2, −1, +4, and every 4 days until rejection. NK cell-depleting anti-NK1.1 mAb (PK136, BioExpress) was given i.p., 0.25 mg on days −3, −2, −1, +2, +4, every 2 days. In all cases, equal amounts of normal rat IgG (Sigma, St. Louis MO) were administered to control groups, and cell depletion was confirmed by flow cytometry analysis of peripheral blood samples. Mouse anti-mouse CXCL9 mAb was administered to graft recipients (150 ug i.p.) on days 1, 3, and 5 post-transplant.
Bone marrow chimera
An established protocol for radiosensitive mice was employed (26). A/J.CXCL9−/− mice received a split dose of 2 × 3.5 Gy of gamma irradiation delivered at 1.21 Gy/min with a four-hour interval between treatments. One day after irradiation, 10–15 × 106 wild-type A/J bone marrow cells were given i.p. Recipients were treated with 0.25 mL gentamycin (Sigma Aldrich) i.p. on days 0 and +2 after bone marrow transplantation (BMT) and were maintained on pH 2.5 acid water for 2 weeks post-BMT. After resting for 8 weeks, chimerism was confirmed using standard PCR analysis of DNA purified from peripheral blood mononuclear cells.
Flow cytometry
Graft-infiltrating leukocytes were analyzed using a modified method of Afanasyev and colleagues (27). Following harvest, graft tissue was incubated for 1 hr at 37°C in RPMI plus Type II collagenase (Sigma Aldrich). After incubation, graft tissue was gently crushed and passed through a 40 μm filter and washed with RPMI. For flow cytometric analysis of splenocytes, single cell suspensions were made after RBC lysis, and 5 × 106 cells were stained. In all cases, surface markers were stained using standard methods and commercially available antibodies (eBioscience, San Diego CA and BD Biosciences, San Jose CA). Flow cytometry was performed using a FACSCalibur (BD Biosciences) cytometer and FlowJo analysis software (Tree Star Inc., Ashland OR).
Intracellular cytokine staining
For intracellular cytokine staining, purified cells were incubated in complete RPMI with 0.01 μg/mL PMA and 1 nM Ionomycin at 37°C for 2 hours. Monensin (2 μM) was then added for another 2 hours of culture. Following restimuation, cells were surface stained, fixed with 4% paraformaldehyde, and then permeabilized with 0.1% saponin (perm buffer). Cells were washed and stained for intracellular cytokines using commercially available antibodies in perm buffer.
In vitro culture assays
Naïve 2C transgenic CD44loCD8+ T cells were flow sort purified and were cultured in 48-well plates with T cell-depleted CXCL9−/− syngeneic stimulators pulsed with 10 uM Ld peptide (SIYRYYGL; a generous gift from A. Morelli, University of Pittsburgh) at a 10:1 (responder:stimulator) ratio for 3 days. In some experiments, recombinant murine CXCL9 protein or anti-CXCL10 mAb (both from R&D Biosystems, Minneapolis MN) was added to the cultures on day 0. Following culture, cells were restimulated with PMA and ionomycin for 4 hr with monensin for the final 2 hr as above and were processed and stained for measurement of intracellular IFN-γ by flow cytometry.
qRT-PCR
Snap-frozen graft pieces were crushed, homogenized using Qiashredders (Qiagen, Valencia CA), and RNA was isolated using Fibrous Tissue Kits (Qiagen) according to the manufacturer's instructions. For analysis of mRNA expression in recipient spleens, spleen pieces were snap-frozen in liquid nitrogen, homogenized as above, and RNA was isolated using RNeasy Mini Kits (Qiagen). For RNA purification from flow-sorted cells, cell pellets were homogenized using Qiashredders, and RNA purification was performed using RNeasy Plus Micro Kits (Qiagen).
Commercially available reagents and probes were used for reverse transcription, and real-time PCR was performed on a 7500 Fast Real-Time thermocycler, all from Applied Biosystems (Foster City, CA). For quantification of message expression, target gene expression was normalized to Mrpl32 gene expression. All samples were plated in triplicate and results are expressed as mean fold increase ± SEM in gene expression over controls.
ELISPOT
Responder CD8 or CD4 T cells were column-purified (R&D Systems) from recipient spleens, and self, donor, and third-party stimulator splenocytes were depleted of T cells using magnetic beads (Invitrogen, Carlsbad CA). Stimulator and responder cell populations were co-cultured in serum-free HL-1 media (BioWhittaker, Walkersville MD) supplemented with 1 mM L-glutamine and 1 mM antibiotic for 24 hours at 37°C in 96-well plates coated with anti-IFN-γ (R4-6A2) or anti-IL17 (TC11-18H10, BD Biosciences) capture Abs. Cells were then washed from the plate, and biotinylated anti-IFN-γ (XMG1.2) or biotinylated anti-IL-17 (TC11-8H4.1, BD Biosciences) was added followed by anti-biotin alkaline phosphatase. Spots were developed with 5-bromo-4-chloro-3-indolyl phosphate (BCIP, Bio-Rad Laboratories, Hercules CA) and nitroblue tetrazolium (NBT, Bio-Rad Laboratories), and total spots per well were quantified using an ImmunoSpot Series 2 Analyzer (Cellular Technology Ltd., Shaker Heights OH).
Statistics
All data was analyzed using GraphPad Prism Pro (GraphPad Software Inc, San Diego CA). Replicates were used as indicated. Log-rank testing was performed to determine differences in survival data and Students' t-test was used to determine significance throughout as indicated with p < 0.05 being considered a significant difference. Error bars throughout indicate SEM.
RESULTS
CXCL9 is required for maximum generation of donor-reactive, IFN-γ-producing T cells
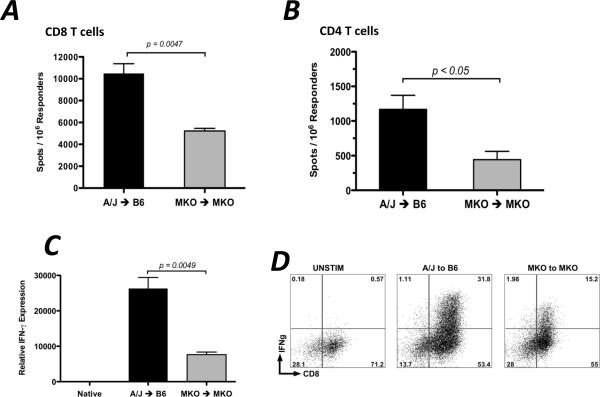
To directly test if the presence of CXCL9 influences the functional development of donor-reactive T cells in vivo, groups of wild-type or CXCL9-deficient C57BL/6 mice received wild-type or CXCL9-deficient A/J heart allografts. CD8 or CD4 T cells were purified from recipient spleens on day 7 post-transplant, and the numbers of donor-reactive, IFN-γ-producing cells were enumerated by ELISPOT. Wild-type cardiac allografts placed in wild-type recipients induced more than twice the number of donor-reactive, IFN-γ-producing CD8 and CD4 T cells than CXCL9−/− allografts placed in CXCL9−/− recipients, and in both cases, there were approximately 10-fold greater number of IFN-γ-producing CD8 T cells when compared to CD4 T cells (Figure 1A and B).
Figure 1. CXCL9 is required for maximum generation of donor-reactive, IFN-γ-producing CD8 T cells.
Wild-type or CXCL9−/− B6 mice received wild-type or CXCL9−/− A/J heart allografts. Recipient spleens were harvested on day 7 post-transplant and purified CD8 (A) or CD4 (B) T cells producing IFN-γ were enumerated by ELISPOT assay. Data is representative of at least 3 independent experiments. (C) mRNA was purified from total graft homogenates on day 7 post-transplant. Quantitative RT-PCR analysis was performed on 5–6 samples/group. Expression of Mrpl32 was used as the endogenous control, and expression of IFN-γ in each sample was normalized to the expression in a random native heart sample. (D) Graft-infiltrating cells were purified on day 7 post-transplant and were re-stimulated in vitro with PMA/Ionomycin for 4 hr with monensin for the last 2 hr. Intracellular cytokine staining was performed using standard techniques and reagents. Numbers in each plot are percentages of total lymphocytes, and plots represent 4–5 samples per group. (E) Allografts were harvested at the times indicated and the number of graft-infiltrating CD8 T cells was quantitated using flow cytometry. Bars in each panel represent mean ± SEM; n = 5–6/group, * p < 0.05, ** p < 0.01.
In concurrence with the decreased frequency of IFN-γ-producing CD8 and CD4 T cells in the spleens of CXCL9−/− allograft recipients, IFN-γ mRNA expression in CXCL9−/− allografts retrieved from CXCL9−/− recipients on day 7 post-transplant was one-third the level of expression in wild-type grafts retrieved from wild-type recipients (Figure 1C). Additionally, when graft-infiltrating cells were purified and re-stimulated in vitro with PMA and ionomycin, fewer CD8 T cells produced IFN-γ when recovered from CXCL9−/− allograft combinations as compared to wild-type allograft combinations (Figure 1D). Taken together, these data show that alloimmune responses generated in the complete absence of CXCL9 induce significantly fewer numbers of donor-reactive, IFN-γ-producing T cells as compared to alloimmune responses in the presence of CXCL9.
The data also suggests that there are fewer graft-infiltrating CD8 T cells in the CXCL9−/− allografts retrieved from CXCL9−/− recipients as compared to wild-type allografts. At day 5 post-transplant, the number of CD8 T cells was significantly reduced in CXCL9-deficient allografts as compared to wild-type allografts, and the trend continued at day 7 post-transplant but was not statistically significant (Figure 1E). The number of graft-infiltrating CD4 T cells in CXCL9-deficient allograft combinations was only marginally reduced at days 5 and 7 post-transplant as compared to wild-type allograft combinations (data not shown).
Both donor- and recipient-derived CXCL9 contribute to CD8 T cell priming
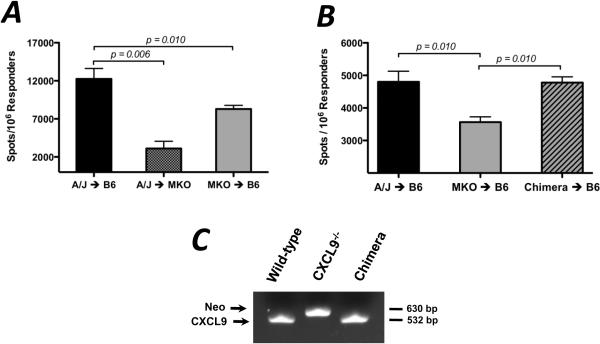
Since the complete absence of CXCL9 resulted in depressed frequencies of donor-reactive T cells producing IFN-γ, the relative contribution of donor- and recipient-derived CXCL9 was tested. Groups of reciprocal allograft combinations were performed with wild-type and CXCL9−/− donors and recipients, and the number of IFN-γ-producing, donor-reactive CD8 T cells was enumerated by ELISPOT on day 7 post-transplant. While CXCL9−/− recipients of wild-type allografts demonstrated significantly decreased numbers of IFN-γ-producing CD8 T cells as compared to wild-type recipients (3105 ± 951 vs. 12248 ± 1377 spots/106 responders, Figure 2A), CXCL9−/− grafts placed in wild-type recipients induced a 32% decrease in IFN-γ-producing CD8 T cells as compared to wild-type grafts. These data indicate that both donor- and recipient-derived CXCL9 influence priming of CD8 T cells, but the primary contribution comes from recipient-derived CXCL9.
Figure 2. Donor- and recipient-derived CXCL9 influence the development of donor-reactive IFN-γ-producing CD8 T cells.
(A) Wild-type or CXCL9−/− B6 mice received wild-type or CXCL9−/− A/J heart allografts as shown. Recipient spleens were harvested on day 7 post-transplant and purified CD8 T cells producing IFN-γ were enumerated by ELISPOT assay. Data is representative of at least 3 independent experiments; n = 4–5/group, * p < 0.05, ** p < 0.01. (B) Chimerism of donor grafts in B was confirmed by PCR analysis of DNA from PBMCs collected from mice just prior to transplant. Data is representative of four mice per group. (C) CXCL9−/− A/J mice were irradiated and wild-type A/J bone marrow was adoptively transferred; mice were then rested for 8 weeks. These chimeric mice were then used as heart donors to wild-type B6 mice. CD8 T cells purified from recipient spleens on day 7 post-transplant, and IFN-γ-producing cells were enumerated using ELISPOT. Data is representative of 2 independent experiments; n = 3–4/group, ** p < 0.01.
CXCL9 can be produced by the cardiac graft endothelium, interstitial/passenger antigen-presenting cells (APCs), and infiltrating leukocytes (14, 18). To determine which population of graft cells (endothelium or passenger APCs) is responsible for producing the CXCL9 that influences CD8 T cell priming in the recipient spleen, bone marrow chimeras were generated in which CXCL9−/− A/J mice were lethally irradiated and received wild-type A/J bone marrow. After resting 8 weeks, chimerism was confirmed using PCR to assess CXCL9 expression in peripheral blood mononuclear cells (Figure 2B). B6 recipients of CXCL9−/− allografts had approximately 26% fewer CD8 T cells producing IFN-γ when compared to recipients of wild-type grafts, but use of a chimeric CXCL9−/− donor graft containing wild-type bone marrow-derived passenger leukocytes restored the number of IFN-γ-producing CD8 T cells to wild-type levels (Figure 2C).
CXCL9 and CXCR3 are expressed in the graft recipient spleen with similar kinetics
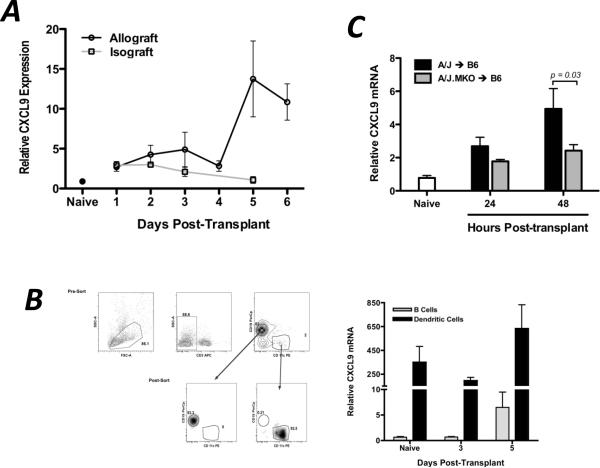
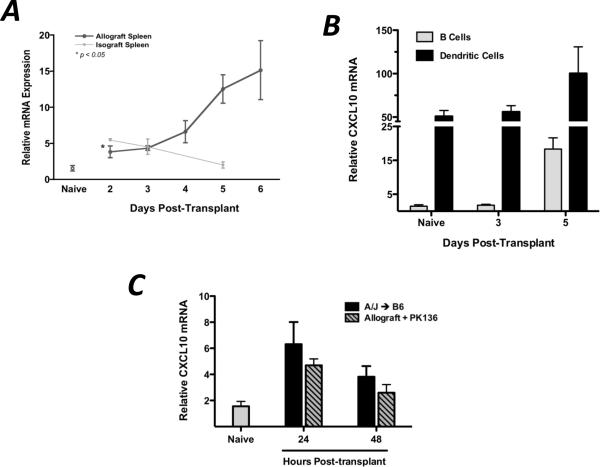
Since CXCL9 plays a clear role in the functional development of donor-reactive T cells to IFN-γ-producing effector cells within the allograft recipient spleen, CXCL9 and its receptor must be expressed in the spleen during this priming. The expression profiles of CXCL9 and its receptor, CXCR3, were measured by qRT-PCR and flow cytometry in wild-type cardiac allograft recipients. CXCL9 mRNA expression in the allograft recipient spleen was biphasic, with increased levels as early as day 2–3 post-transplant and greatly increased levels at 5–6 days post-transplant, and CXCL9 mRNA was expressed in isograft recipient spleens 24 hrs post-transplant but declined therafter (Figure 3A). The early expression of CXCL9 in the allograft recipient spleen was due to constitutively high expression by CD11c+ dendritic cells (DCs, Figure 3B), and the contribution of donor-derived CXCL9 was evident since use of a CXCL9-deficient donor reduced the level of CXCL9 mRNA in the recipient spleen by 50% at 24 and 48 hrs post-transplant (Figure 3C). The late burst in CXCL9 expression was due to a significant increase in the mRNA expression by splenic CD19+ B cells which outnumbered DC 40:1 (Figure 3B).
Figure 3. CXCL9 is produced in the graft recipient spleen as early as 48 hr post-transplant, primarily by CD11c+ DCs.
(A) Wild-type isograft and allograft recipient spleens were harvested at the times post-transplant shown. Total mRNA was purified from spleen homogenates, and expression of IFN-γ mRNA was normalized to a random naïve spleen sample. n = 5–6/group, representative of three independent experiments. (B) B cells (CD19+CD11c−) or dendritic cells (CD19−CD11c+) were flow sorted from recipient spleens at various times post-transplant. mRNA was prepared from the purified cell populations, and IFN-γ expression was normalized to a random naïve B cell sample. n = 5–6/group; mean ± SEM. (C) Total recipient spleen homogenates were analyzed for CXCL9 mRNA expression by qRT-PCR. Bars represent mean ± SEM; n = 4–5/group, * p < 0.05.
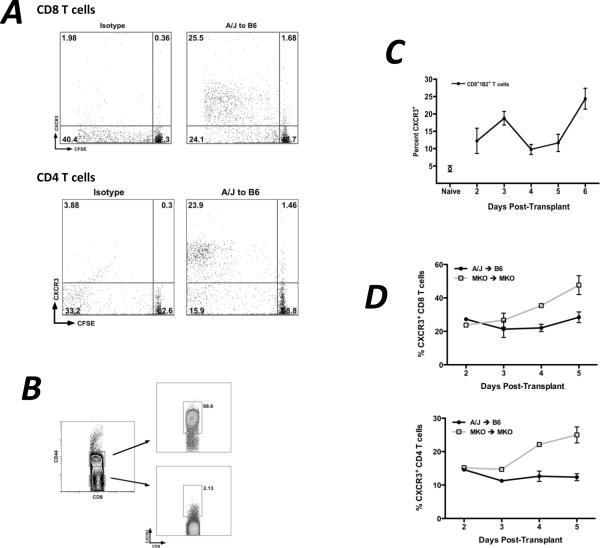
When Ld-reactive 2C transgenic CD8 T cells were adoptively transferred to wild-type recipients one day prior to transplant and CXCR3 expression on the transferred cells was measured at various times post-transplant, CXCR3 expression on the graft-reactive 2C cells in the spleen closely approximated the kinetics of CXCL9 mRNA expression (Figure 4A). CXCR3 expression on polyclonal CD4 and CD8 T cells in the recipient spleens of wild-type and CXCL9-deficient allograft combinations was measured by flow cytometry. While the percent of CD4+ and CD8+ T cells expressing CXCR3 in the wild-type allograft recipient spleen is relatively constant (20–25% of CD8 T cells and 10–15% of CD4 T cells; Figure 4B) over time post-transplant, the percent of CD4 and CD8 T cells expressing CXCR3 in CXCL9-deficient allograft recipients continuously increases with time post-transplant (40–45% of CD8 T cells and 20–25% of CD4 T cells on day 5 post-transplant). Since CXCR3 is internalized upon ligand binding, these data suggest that in the wild-type allograft recipient spleen, CXCL9 binds to CXCR3 on CD4 and CD8 T cells, maintaining a constant frequency of CXCR3-expressing T cells in the spleen.
Figure 4. CXCR3 is expressed in the recipient spleen by proliferating, activated T cells with kinetics similar to CXCL9 expression.
(A) 2.5×106 2C CD8 T cells were adoptively transferred to wild-type B6 recipients of A/J heart allografts one day prior to transplant. Recipient spleens were harvested on the days indicated and CXCR3 expression on 2C CD8 T cells was measured by flow cytometry. Data represents percent of total 2C CD8 T cells expressing CXCR3; n = 3–4/time point; mean ± SEM. (B) The percentage of endogenous polyclonal CD8 (top panel) or CD4 (bottom panel) T cells expressing CXCR3 was determined at the times post transplant shown. Data are representative of two independent experiments; n = 3–4/time point; mean ± SEM. (C) 2.5×106 CD8 or CD4 T cells were CFSE labeled and were transferred to wild-type B6 recipients of A/J heart allografts one day prior to transplant. Recipient spleens were harvested on day 5 post-transplant, and CXCR3 expression on CFSE labeled proliferating cells was measured by flow cytometry. Numbers represent percentage of total transferred cells in each quadrant; plots are representative of three mice per group. (D) Spleens from wild-type B6 recipients of wild-type A/J cardiac allografts were harvested on day 5 post-transplant, and cells were processed using standard techniques for flow cytometry. Numbers represent percentage of total CD8 T cells expressing CXCR3; plots are representative of 4 individual mice.
In the allograft recipient spleen, approximately 50–60% of divided CD8 and CD4 T cells expressed CXCR3 whereas only 2% of undivided T cells expressed CXCR3 (Figure 4C). A significant majority of CD8+CD44hi effector T cells recovered from wild-type allograft recipient spleens on day 5 post-transplant expressed CXCR3, but very few naïve CD44lo T cells expressed CXCR3 (Figure 4D).
NK cell IFN-γ production induces early CXCL9 production in the recipient spleen
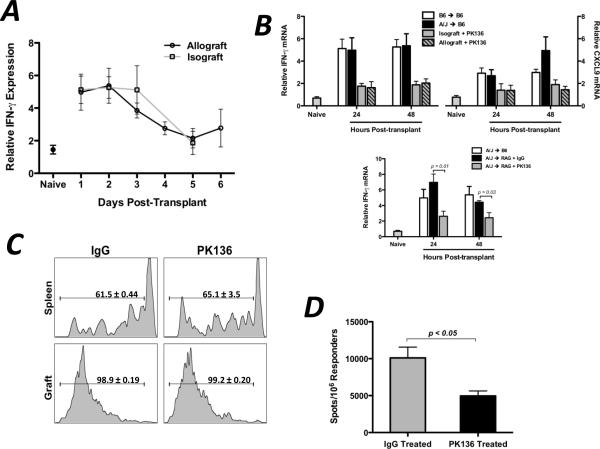
In murine and human models of inflammation and disease, CXCL9 is specifically induced by IFN-γ (28–30). Thus, since CXCL9 was expressed in the allograft recipient spleen and we have shown that its presence influences the functional phenotype of donor-reactive T cells, the IFN-γ requirements for induction were investigated. In both isograft and allograft recipient spleens, IFN-γ mRNA was expressed at elevated levels during the first 3 days post-transplant (Figure 5A). Given that splenic IFN-γ mRNA levels were elevated in isograft and allograft recipients and that use of an IFN-γ−/− donor did not alter recipient splenic IFN-γ mRNA expression (data not shown), it was hypothesized that an innate immune cell of recipient origin was responsible for IFN-γ production after being activated by danger signals or the systemic mediators of ischemia/reperfusion injury. When isograft and allograft recipients were treated with anti-NK1.1 depleting mAb, spleen levels of IFN-γ and CXCL9 at 24 and 48 hours post-transplant were significantly reduced compared to controls (Figure 5B), and these levels were also reduced in NK1.1-depleted RAG1−/− allograft recipients (Figure 5C), indicating that the early IFN-γ driving early CXCL9 production was produced by NK cells and not NKT cells.
Figure 5. Recipient NK cells produce IFN-γ during the first 48hr post-transplant in the recipient spleen.
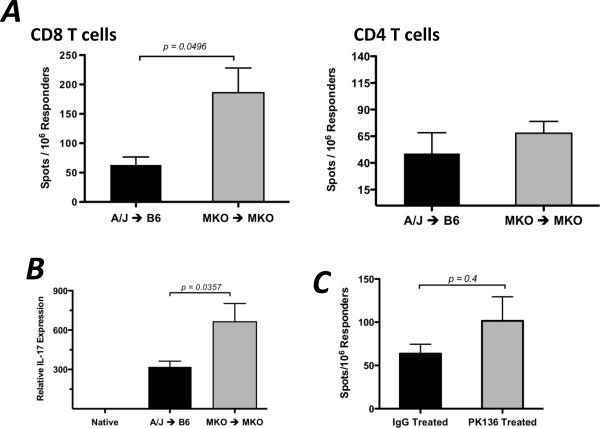
(A) Wild-type isograft or allograft recipient spleens were harvested at the times post-transplant shown. Total mRNA was purified from spleen homogenates and IFN-γ mRNA expression was determined by qRT-PCR; n = 4–6/group, mean ± SEM. (B and C) Wild-type (B) or RAG1−/− (C) isograft or allograft recipients were treated with control IgG or with NK-depleting mAb (PK136, 250ug d −3, −2, −1, 1, i.p.). Total mRNA was purified from spleen homogenates at the times shown. qRT-PCR analysis was performed as in (A); n = 4–5/group, mean ± SEM, * p < 0.05, ** p < 0.01. (D) Wild-type B6 allograft recipients (n = 3/group) were treated with control IgG or PK136 (250ug d −3, −2, −1, 1, 3, 5; i.p.). Recipient spleens were harvested on day 7 post-transplant and the number of purified CD8 T cells producing IFN-γ were enumerated by ELISPOT assay. Data is representative of two independent experiments, mean ± SEM, * p < 0.05.
Since the presence of CXCL9 was important for optimum generation of donor-reactive, IFN-γ-producing T cells and NK cells were required to induce early CXCL9 in the recipient spleen, the effect of NK cell depletion on donor-reactive T cell development was tested. When wild-type cardiac allograft recipients were depleted of NK cells, the frequency of IFN-γ-producing CD8 T cells in the recipient spleen at day 7 post-transplant was significantly lower (approximately 50%) than in IgG-treated controls (Figure 5D). Taken together, these data indicate that NK cells produce IFN-γ in the allograft recipient spleen within 2 days post-transplant, and this subsequently induces the CXCL9 production that influences donor-reactive T cell development in the spleen.
CXCL10 antagonizes CXCL9 during T cell priming
CXCL9, CXCL10, and CXCL11 all bind the same receptor, CXCR3. Since the presence of CXCL9 in the allograft recipient spleen clearly influenced the functional phenotype of donor-reactive T cells, the role of CXCL10/IP10 in this process was tested. Similar to CXCL9 expression in the wild-type allograft recipient spleen, CXCL10 mRNA is expressed at increasing levels with time post-transplant, principally by DC early and by B cells only by day 5 post-transplant (Figure 6A and 6B). Interestingly, CXCL10 mRNA expression was relatively insensitive to NK cell depletion with anti-NK1.1 mAb (Figure 6C).
Figure 6. CXCL10/IP10 is produced in the allograft recipient spleen by DCs and B cells, independent of NK cells.
(A) mRNA expression of CXCL10 was determined in allograft and isograft recipient spleens at various times post-transplant (n = 3–4/group). Data was analyzed as in 3A. (B) B cells and DCs were flow-sort purified as in 3B and CXCL10 mRNA expression was determined as described. (C) Wild-type allograft recipients were depleted of NK cells as described, and total spleen homogenates were taken at the times indicated for qRT-PCR analysis of CXCL10 expression. Data represent two individual experiments, n = 3–4/group; mean ± SEM.
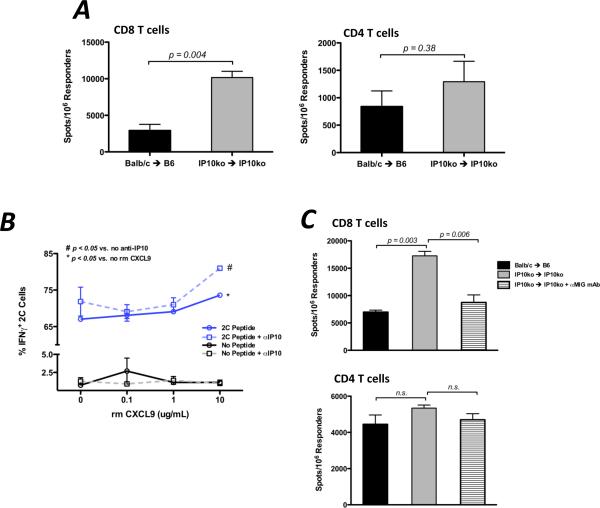
Because CXCL10 is expressed in the allograft recipient spleen in a similar manner as CXCL9 and CXCL9 influences the functional development of donor-reactive T cells, the effect of CXCL10 deficiency on T cell priming was tested. Unexpectedly, when CD8 and CD4 T cells were purified from CXCL10−/− recipients of CXCL10−/− complete MHC-mismatched cardiac allografts on day 7 post-transplant, there was a marked increase in the number of IFN-γ-producing CD8 T cells as compared to wild-type allograft recipients (Figure 7A).
Figure 7. CXCL10/IP10 antagonizes the action of CXCL9 during priming of donor-reactive, IFN-γ-producing CD8 T cells.
(A) Spleens from wild-type or CXCL10−/− B6 recipients of wild-type or CXCL10−/− Balb/c heart allografts were harvested on day 7 post-transplant. CD8 or CD4 T cells were purified and the number of donor-specific cells producing IFN-γ was quantified using ELISPOT. (B) Flow sort purified naïve 2C CD8 T cells were cocultured with B6 spleen cells pulsed or not pulsed with 2C peptide for 3 days in the presence of anti-CXCL10 mAb (5 ug/mL) or control Ab and increasing amounts of recombinant CXCL9 as shown. 2C cells producing IFN-γ were then determined by intracellular cytokine staining. Data represents two independent experiments, 3 replicates/group; mean ± SEM. (C) CXCL10−/− allograft combinations were treated with control IgG or with anti-CXCL9 mAb (150ug, i.p., d1, 3, 5). The number of IFN-γ-producing CD4 or CD8 T cells was enumerated by ELISPOT on day 7 post-transplant. Data represents two independent experiments, n = 3/group; mean ± SEM, * p < 0.05, ** p < 0.01.
To further investigate if and how CXCL9 and CXCL10 strike a balance as antagonistic costimulatory molecules during the functional polarization of donor-reactive CD8 T cells, we attempted to recapitulate our in vivo findings using an in vitro approach. When increasing amounts of recombinant CXCL9 was added to 2C CD8 T cells stimulated with CXCL9−/− syngeneic splenocytes pulsed with Ld peptide (SIYRYYGL), the percent of IFN-γ-producing cells increased concomitantly (Figure 7B). The magnitude of increase over baseline was heightened when anti-IP10 mAb was added to the cultures (Figure 7B).
To extend these findings in vivo, the number of donor-reactive CD4 and CD8 T cells producing IFN-γ was determined at day 7 post-transplant in wild-type allograft recipients, CXCL10-deficient allograft recipients, and CXCL10-deficient allograft recipients treated with anti-CXCL9 mAb. In agreement with previous findings, the number of donor-reactive, IFN-γ-producing CD8 T cells was significantly higher in CXCL10-deficient recipients of CXCL10−/− allografts as compared to wild-type recipients, but treatment of CXCL10−/− recipients with anti-CXCL9 mAb reduced the frequency of IFN-γ-producing CD8 T cells to wild-type levels (Figure 7C, upper panel). These data suggest that CXCL9 promotes the development of IFN-γ-producing CD8 T cell effectors and that CXCL10 impedes this process. Of note, CD4 T cells were relatively insensitive to the presence or absence of CXCL10 (Figure 7C, lower panel). Taken together with the findings of Figure 1, these data suggest that CXCL9 and CXCL10 are antagonistic in providing costimulation to influence the effector phenotype of donor-reactive CD8 T cells to IFN-γ-producing cells during priming.
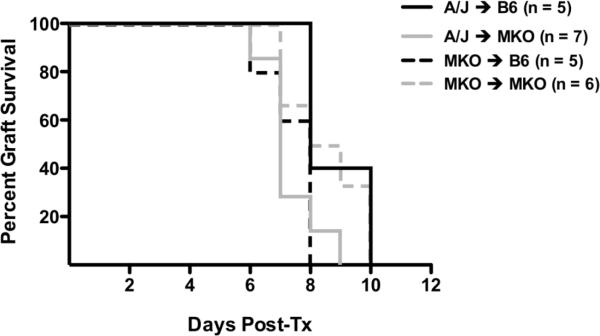
Acute rejection in the absence of CXCL9
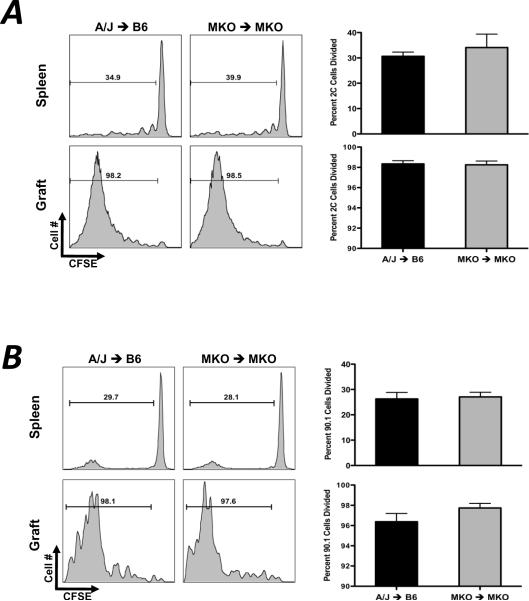
Graft survival studies using CXCL9−/− donors, recipients, or both indicated no prolongation in graft survival in these combinations (CXCL9−/− to CXCL9−/− allograft MST = 8.5 days, wild-type control allograft MST = 8 days; data not shown) despite the marked decrease in T cell priming to IFN-γ-producing effector cells. These results raised the possibility that in the absence of CXCL9, donor-reactive T cell development was skewed to another functional phenotype that was effective in mediating allograft rejection. In such a case, the absence of CXCL9 in the T cell priming environment would not be expected to affect the clonal proliferation of the donor-reactive T cells during priming. To determine whether CXCL9 influences proliferation in response to alloantigen in vivo, Ld-reactive 2C CD8 T cells or polyclonal CD90.1+ CD4 T cells were labeled with CFSE and were adoptively transferred to wild-type or CXCL9−/− CD90.2 B6 recipients of wild-type or CXCL9−/− A/J heart grafts one day prior to transplant. Grafts and recipient spleens were recovered on day 5 post-transplant, and proliferation of transferred cells was quantified by CFSE dilution using flow cytometry. Equivalent percentages of CD8 and CD4 T cells (Figure 8A and 8B, respectively) proliferated in recipient spleens and grafts regardless of the presence or absence of CXCL9. Moreover, NK cell depletion did not affect donor-reactive CD8 T cell proliferation (Figure 8C). These data indicate that the depression in the number of donor-reactive, IFN-γ-producing T cells resulting from CXCL9 deficiency is not due to a defect in clonal expansion of donor-reactive cells in allograft recipients.
Figure 8. CXCL9 does not influence the proliferation of donor-reactive CD8 or CD4 T cells in vivo.
(A) 5×106 Ld-reactive naïve 2C transgenic CD8 T cells were labeled with CFSE and were transferred to wild-type or CXCL9−/− B6 mice one day prior to transplant with wild-type or CXCL9−/− A/J heart grafts. Grafts and recipient spleens were harvested on day 5 post-transplant, and CFSE dilution was measured by standard flow cytometry techniques. Histograms are representative of 4–6 mice per group, and graphs represent percent of 2C CD8 T cells that have divided. (B) 10×106 CD90.1 CD4 T cells were labeled with CFSE and were adoptively transferred to CD90.2 mice one day prior to transplant. Proliferation of CD4 T cells was determined at day 5 post-transplant as in (A). (C) 5×106 CFSE labeled 2C CD8 T cells were adoptively transferred to wild-type B6 allograft recipients treated with control IgG or PK136 one day prior to transplant. Proliferation of 2C cells in the recipient spleens and grafts was determined by flow cytometry on day 5 post-transplant. Histograms are representative of 3 mice per group; numbers represent mean ± SEM percent of total 2C cells that have divided.
Taken together, the above findings suggest that graft rejection in a CXCL9-deficient environment may rely on other effector mechanisms. Initial testing by ELISPOT of CD8 T cells recovered from wild-type and CXCL9-deficient allograft recipients demonstrated no difference in the frequency of donor-reactive cells producing granzyme B or IL-4 (data not shown). However, when CD8 T cells were purified from the spleens of CXCL9−/− recipients of CXCL9−/− cardiac allografts, the frequency of IL-17A-producing cells was significantly higher than CD8 T cells recovered from wild-type allograft recipients, but there was no difference in the frequency of CD4 T cells producing IL-17A (Figure 9A). Consistent with these results, intragraft cytokine mRNA expression in wild-type or CXCL9-deficient allograft combinations at day 7 post-transplant revealed that IL-17A mRNA expression (and not IL-4, IL-5, perforin, or granzyme B; data not shown) was substantially increased in CXCL9−/− allografts placed in CXCL9−/− recipients as compared to wild-type allografts (Figure 9B). Thus, donor-specific production of IL-17 by CD8 T cells may be an alternative mechanism leading to graft rejection in the absence of CXCL9.
Figure 9. In the absence of CXCL9, donor-specific production of IL-17 by CD8 T cells is increased.
(A) Wild-type or CXCL9−/− B6 mice received wild-type or CXCL9−/− A/J heart allografts, and CD8 or CD4 T cells were purified from recipient spleens on day 7 post-transplant. The number of donor-specific cells producing IL-17 was enumerated using ELISPOT. Data represents 3 independent experiments. n = 5–6/group, mean ± SEM, * p < 0.05. (B) Total mRNA was purified from graft homogenates at day 7 post-transplant. IL-17 mRNA expression was normalized to endogenous Mrpl32 expression using a random native heart sample as the baseline. Data represents 2 independent experiments.
DISCUSSION
The inflammatory environment within allografts during rejection is composed of a complex organization of infiltrating cells, cytokines, and other mediators contributing to graft tissue injury. In clinical and experimental transplantation, graft-infiltrating T cell production of IFN-γ is a principal mechanism mediating tissue injury. IFN-γ has both direct and indirect effects on the donor graft, including stimulation of macrophages and neutrophils to produce enzymes that generate toxic molecular products and upregulated expression of integrins, MHC molecules, and interferon-inducible chemokines and chemokine receptors (importantly CXCL9, CXCL10, and CXCR3), all of which increase the potency of the alloimmune response. Despite the prominent role of IFN-γ in graft rejection, the factors directing donor-reactive T cell development to IFN-γ-producing cells remain poorly defined.
In the current study, the potential role of CXCR3-binding chemokines in the skewing of donor-reactive T cells to IFN-γ-producing effector cells was directly tested in recipients of MHC-mismatched heart allografts. We report the following novel findings: 1) that CXCL9 is produced in the allograft recipient spleen within 24 hrs post-transplant in a NK cell-dependent manner; 2) in the absence of NK cells or CXCL9, the development of donor-reactive, IFN-γ-producing CD4 and CD8 T cells is impaired; 3) the effector CD8 T cell population is skewed to an IL-17A-producing phenotype in the absence of CXCL9; and, 4) a balance between CXCL9 and CXCL10 influences the development of the donor-reactive effector T cell repertoire to IFN-γ-producing effector cells. These results are important in light of recent emphasis on CXCR3 blockade for clinical application to prolong allograft survival (31–35), suggesting that altering the functional phenotype of donor-reactive T cells would be an ancillary effect of CXCR3 inhibition. These current findings support our previous studies testing a CXCR3 small-molecule inhibitor that did not affect alloantigen-primed T cell infiltration into cardiac allografts but did decrease the number of donor-reactive CD8 T cells producing IFN-γ and the level of IFN-γ mRNA in the graft tissue (36). The current report extends and confirms these findings by implicating novel roles for CXCL9 and CXCL10 in the development of donor antigen reactive T cells to IFN-γ-producing cells.
To date, the extrachemotactic activities of the CXCR3-binding chemokines in antigen-specific T cell priming have been relatively unknown. Studies from other laboratories have shown that neutralization of CXCL9 can impair the anti-donor IFN-γ response by reactive CD4 T cells and that recombinant CXCL9 can stimulate T cell proliferation in vitro suggesting that CXCL9 may function as a costimulatory molecule during the priming of T cells to various inflammatory stimuli (21, 37). The current report demonstrates that the absence of CXCL9 significantly decreases the frequency of donor-reactive, IFN-γ-producing CD8 and CD4 T cells (Figure 1A and B), and this corresponds to decreased IFN-γ production in the cardiac allograft. We were unable to demonstrate an effect of CXCL9 on CD4 or CD8 T cell proliferation in vivo (Figure 8), indicating that CXCL9 influences the polarization of expanding effector T cells rather than proliferation in vivo. Additionally, we demonstrate that both donor- and recipient-derived CXCL9 can influence T cell priming. In agreement with the paradigm that interstitial dendritic cells migrate out of the graft to the recipient spleen (4), we show that CXCL9 derived from donor cells of hematopoietic origin are responsible for early CXCL9 production in the recipient spleen (Figure 2 and 3C). Furthermore, the dramatic increase in CXCL9 production by day 5–6 post-transplant in allograft recipient spleens is due, in part, to B cell production of CXCL9 (Figure 3B).
The role of NK cells in allograft rejection has yet to be fully elucidated. Several studies indicate an important role for NK cells at the interface between innate and adaptive immune responses, demonstrating that NK cells assist in CD4 T cell priming in response to inflammatory stimuli (38, 39). Others have employed semi-allogeneic graft combinations or have used costimulation blockade to impair T cell responses in order to uncover a role for NK cells in allograft rejection (40, 41). Interestingly, we have shown that in the spleens of recipients of full MHC-mismatched cardiac allografts, NK cells produce IFN-γ early post-transplant, and this induces the production of CXCL9 that influences the polarization of donor-reactive CD8 T cells (Figure 5). It is important to note that NK cells produce IFN-γ during the first 24–48 hours post transplantation of either syngeneic or allogeneic cardiac grafts, indicating that NK cell activation occurs as a result of the innate, non-specific systemic inflammatory response to cardiac transplantation. While it is unclear from our studies how NK cells are activated to produce IFN-γ post transplant, increased levels of pro-inflammatory cytokines like TNF-α are likely to play a role since blockade of TNF-α depresses early IFN-γ in the spleen (data not shown). Alternatively, NK cells may be activated by so called alarmins or danger signals like HMGB-1, which are released by activated endothelium following ischemia-reperfusion injury of syngeneic and allogeneic grafts (42–44).
Mounting evidence from several groups has focused on the mechanisms underlying allograft rejection in the absence of IFN-γ. Although donor-specific production of IFN-γ remains a hallmark of clinical and experimental allograft rejection, uncovering alternative effector cytokines becomes important when anti-rejection therapy is specifically targeted to limiting IFN-γ-induced rejection. In allograft recipients genetically deficient in IFN-γ, CD8-mediated graft rejection is resistant to costimulation blockade and occurs independently of IL-4 (45). These findings suggested that in the absence of donor-reactive T cells capable of producing IFN-γ, other effector mechanisms are activated to mediate allograft rejection. Recent studies using Th1 deficient T-bet−/− recipients demonstrated that cardiac allografts were rejected in an IL-17 dependent manner, and that the rejected grafts had histological findings consistent with the development of allograft vasculopathy (46, 47). Since CXCL9−/− allograft combinations rejected with the same pace as wild-type grafts, we investigated whether other effector functions were over-expressed in these groups. IL-17A production by CD8 T cells was significantly increased in CXCL9-deficient allograft recipients, and this correlated with increased levels of IL-17A mRNA in the graft tissue. In contrast to recent reports indicating a role for IL-17-mediated accelerated graft loss due to vasculopathy in an IFN-γ-deficient environment, we did not observe an overt vasculopathy in grafts from CXCL9−/− combinations (data not shown).
Given that CXCL9 and CXCL10 both bind CXCR3, we sought to determine whether CXCL10 also played a role in the polarization of donor-reactive CD8 T cells. Surprisingly, the absence of CXCL10 in allograft combinations resulted in a significant increase in the number of IFN-γ-producing CD8 T cells as compared to wild-type controls (Figure 7A). Taken in context with subsequent in vitro experiments (Figure 7B), these data indicate that CXCL9 and CXCL10 antagonize each other in the allograft recipient spleen to promote effector CD8 T cell polarization. When CXCL9 is neutralized, the effect of unopposed CXCL9 in CXCL10-deficient allograft recipients is abrogated (Figure 7C). We hypothesize that CD8 T cells use the CXCR3 axis as a costimulatory pathway where binding of CXCL9 promotes differentiation to an IFN-γ-producing phenotype and binding of CXCL10 may either promote an IL-17-producing phenotype or may simply inhibit generation of an IFN-γ-producing phenotype with development to an IL-17-producing phenotype being the default pathway. Current studies are aimed at determining the mechanism that provides a differential signaling cascade through CXCR3 depending on which ligand binds. Previous reports demonstrating that CXCL9 and CXCL10 bind to CXCR3 in different manners and at least induce different downstream signals to mediate chemotaxis suggest that different signaling mechanisms may influence the costimulatory nature of the receptor (48, 49).
Although our data suggest that CXCL9 and CXCL10 act as antagonistic costimulation molecules, an alternative mechanism underlying our findings is possible. Elegant work from the Germain laboratory has demonstrated that in models of infection and inflammation, chemokines produced by individual cells within the lymph node create a microchemotactic environment and that CD4 T cell interaction with DCs induces these DCs to produce MIP-1α that recruits naïve CCR5+ CD8 T cells to the DC (50, 51). The chemokines may either promote CD8 T cell migration to a cluster of APC-CD4 T cell complexes (52), or chemokines may be produced by competent APCs to recruit naïve CD8 T cells following `licensing' by the CD4 T cells (53). Taken in this context, our results raise the possibility that the CXCR3 axis plays a role in the priming of naïve T cells by acting as a chemotactic factor. This may provide a rationale as to why CXCL9 and CXCL10 production in the allograft recipient spleen have such variant effects on differentiation of T cells. An APC that has been licensed to prime a CD8 T cell to an IFN-γ-producing phenotype may produce high levels of CXCL9. In contrast, CXCL10 production may mark an APC that expresses costimulatory ligands that polarize T cells to an IL-17-producing phenotype. Reconciling our disparate results with CXCL9−/− and CXCL10−/− allograft combinations using the microchemotaxis model would require two separate populations of DCs migrating from the allograft, one producing CXCL9 and one producing CXCL10. Although we have no direct evidence to support either model at this point, we believe that the alternate signaling hypothesis outlined above is more likely, and we have focused our current efforts to test this as a mechanism.
Recent evidence has shown that CXCR3 expression on CD4+ Tregs is regulated by T-bet induction following exposure to IFN-γ during a Th1-type immune response (54). Conceivably, this mechanism may serve to bring regulatory cells to the site of inflammation in order to dampen aberrant immune responses. Could expression of CXCR3 on Tregs and the differential signaling via binding of CXCL9 or CXCL10 contribute to the results observed in this study? First, there is little evidence that Tregs function effectively in the face of a complete MHC-mismatched alloimmune response, making this possibility unlikely. Second, in the absence of CXCL9, there appears to be no effect on anti-donor CD8 T cell proliferation (Figure 8A), again implicating that Tregs are not effective at dampening this immune response. Finally, we demonstrate a measurable difference in CD8 T cell differentiation in the presence and absence of CXCL9 in vitro, indicating that the effect of CXCL9 and CXCL10 on CD8 T cell priming occurs at the level of the CD8 Tcell; although the possibility of Tregs being involved in this process in a limited MHC-mismatch or a minor antigen-mismatched graft combination in vivo cannot be ruled out.
Collectively, this study provides novel evidence that CXCR3-binding chemokines function as costimulation molecules for CD8 T cells during priming to alloantigen in that CXCL9 and CXCL10 can skew the balance of effector phenotypes. In addition to these findings providing novel insights into T cell development following alloantigen exposure, they present important implications and targets for the pharmacological prolongation of graft survival in clinical transplantation.
Figure 10.
ACKNOWLEDGEMENTS
The authors thank the Cleveland Clinic Biological Resources Unit and their staff for the exceptional care and maintenance of the experimental animals used. Adrian Morelli (University of Pittsburgh) generously provided the peptide used in this work.
JMR was supported in part by the Case Western Reserve University MSTP (NIH T32 GM07250) and an individual NRSA (NIH F30 HL940052). This work was supported by NIH RO1 AI51620 and AI40459 (RLF).
Footnotes
The authors have no conflicting financial interests.
REFERENCES
- 1.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3:525–533. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 2.Hall BM, Dorsch SE. Cells mediating allograft rejection. Immunol Rev. 1984;77:31–59. doi: 10.1111/j.1600-065x.1984.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo LG, Halloran PF. Role of IFN-gamma in allograft rejection. Crit Rev Immunol. 2002;22:317–349. [PubMed] [Google Scholar]
- 4.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 6.Gorbachev AV, Fairchild RL. CD40 engagement enhances antigen-presenting langerhans cell priming of IFN-gamma-producing CD4+ and CD8+ T cells independently of IL-12. J Immunol. 2004;173:2443–2452. doi: 10.4049/jimmunol.173.4.2443. [DOI] [PubMed] [Google Scholar]
- 7.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffner U, Lu B, Hildebrandt GC, Teshima T, Williams DL, Reddy P, Ordemann R, Clouthier SG, Lowler K, Liu C, Gerard C, Cooke KR, Ferrara JL. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol. 2003;31:897–902. doi: 10.1016/s0301-472x(03)00198-x. [DOI] [PubMed] [Google Scholar]
- 9.el-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Curr Opin Immunol. 2002;14:562–568. doi: 10.1016/s0952-7915(02)00382-5. [DOI] [PubMed] [Google Scholar]
- 10.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, Feng J, Novick AC, Fairchild RL. Chemokine and receptor-gene expression during early and late acute rejection episodes in human cardiac allografts. Transplantation. 2003;75:2044–2047. doi: 10.1097/01.TP.0000069601.73079.94. [DOI] [PubMed] [Google Scholar]
- 12.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, Feng J, Novick AC, Fairchild RL. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75:72–78. doi: 10.1097/00007890-200301150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Aizenstein BD, Puchalski A, Burmania JA, Hamawy MM, Knechtle SJ. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am J Transplant. 2004;4:432–437. doi: 10.1111/j.1600-6143.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 14.Miura M, Morita K, Kobayashi H, Hamilton TA, Burdick MD, Strieter RM, Fairchild RL. Monokine induced by IFN-gamma is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J Immunol. 2001;167:3494–3504. doi: 10.4049/jimmunol.167.6.3494. [DOI] [PubMed] [Google Scholar]
- 15.Yun JJ, Fischbein MP, Whiting D, Irie Y, Fishbein MC, Burdick MD, Belperio J, Strieter RM, Laks H, Berliner JA, Ardehali A. The role of MIG/CXCL9 in cardiac allograft vasculopathy. Am J Pathol. 2002;161:1307–1313. doi: 10.1016/S0002-9440(10)64407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi H, Koga S, Novick AC, Toma H, Fairchild RL. T-cell mediated induction of allogeneic endothelial cell chemokine expression. Transplantation. 2003;75:529–536. doi: 10.1097/01.TP.0000048377.59350.E4. [DOI] [PubMed] [Google Scholar]
- 18.Park MK, Amichay D, Love P, Wick E, Liao F, Grinberg A, Rabin RL, Zhang HH, Gebeyehu S, Wright TM, Iwasaki A, Weng Y, DeMartino JA, Elkins KL, Farber JM. The CXC chemokine murine monokine induced by IFN-gamma (CXC chemokine ligand 9) is made by APCs, targets lymphocytes including activated B cells, and supports antibody responses to a bacterial pathogen in vivo. J Immunol. 2002;169:1433–1443. doi: 10.4049/jimmunol.169.3.1433. [DOI] [PubMed] [Google Scholar]
- 19.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 21.Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, Belperio J, Strieter RM, Bonavida B, Ardehali A. Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol. 2004;172:7417–7424. doi: 10.4049/jimmunol.172.12.7417. [DOI] [PubMed] [Google Scholar]
- 22.Campbell JD, Gangur V, Simons FE, HayGlass KT. Allergic humans are hyporesponsive to a CXCR3 ligand-mediated Th1 immunity-promoting loop. FASEB J. 2004;18:329–331. doi: 10.1096/fj.02-0908fje. [DOI] [PubMed] [Google Scholar]
- 23.Gangur V, Simons FE, Hayglass KT. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. FASEB J. 1998;12:705–713. doi: 10.1096/fasebj.12.9.705. [DOI] [PubMed] [Google Scholar]
- 24.Manicone AM, Burkhart KM, Lu B, Clark JG. CXCR3 ligands contribute to Th1-induced inflammation but not to homing of Th1 cells into the lung. Exp Lung Res. 2008;34:391–407. doi: 10.1080/01902140802221987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Cui YZ, Hisha H, Yang GX, Fan TX, Jin T, Li Q, Lian Z, Ikehara S. Optimal protocol for total body irradiation for allogeneic bone marrow transplantation in mice. Bone Marrow Transplant. 2002;30:843–849. doi: 10.1038/sj.bmt.1703766. [DOI] [PubMed] [Google Scholar]
- 27.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, Rose NR. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol. 2004;164:807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farber JM. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A. 1990;87:5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amichay D, Gazzinelli RT, Karupiah G, Moench TR, Sher A, Farber JM. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157:4511–4520. [PubMed] [Google Scholar]
- 30.Ebnet K, Simon MM, Shaw S. Regulation of chemokine gene expression in human endothelial cells by proinflammatory cytokines and Borrelia burgdorferi. Ann N Y Acad Sci. 1996;797:107–117. doi: 10.1111/j.1749-6632.1996.tb52953.x. [DOI] [PubMed] [Google Scholar]
- 31.Schenk AD, Rosenblum JM, Fairchild RL. Chemokinedirected strategies to attenuate allograft rejection. Clin Lab Med. 2008;28:441–454. vii. doi: 10.1016/j.cll.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akashi S, Sho M, Kashizuka H, Hamada K, Ikeda N, Kuzumoto Y, Tsurui Y, Nomi T, Mizuno T, Kanehiro H, Hisanaga M, Ko S, Nakajima Y. A novel small-molecule compound targeting CCR5 and CXCR3 prevents acute and chronic allograft rejection. Transplantation. 2005;80:378–384. doi: 10.1097/01.tp.0000166338.99933.e1. [DOI] [PubMed] [Google Scholar]
- 33.Schnickel GT, Bastani S, Hsieh GR, Shefizadeh A, Bhatia R, Fishbein MC, Belperio J, Ardehali A. Combined CXCR3/CCR5 blockade attenuates acute and chronic rejection. J Immunol. 2008;180:4714–4721. doi: 10.4049/jimmunol.180.7.4714. [DOI] [PubMed] [Google Scholar]
- 34.Turner JE, Steinmetz OM, Stahl RA, Panzer U. Targeting of Th1-associated chemokine receptors CXCR3 and CCR5 as therapeutic strategy for inflammatory diseases. Mini Rev Med Chem. 2007;7:1089–1096. doi: 10.2174/138955707782331768. [DOI] [PubMed] [Google Scholar]
- 35.Wijtmans M, Verzijl D, Leurs R, de Esch IJ, Smit MJ. Towards small-molecule CXCR3 ligands with clinical potential. ChemMedChem. 2008;3:861–872. doi: 10.1002/cmdc.200700365. [DOI] [PubMed] [Google Scholar]
- 36.Rosenblum JM, Zhang QW, Siu G, Collins TL, Sullivan T, Dairaghi DJ, Medina JC, Fairchild RL. CXCR3 antagonism impairs the development of donor-reactive, IFN-gamma-producing effectors and prolongs allograft survival. Transplantation. 2009;87:360–369. doi: 10.1097/TP.0b013e31819574e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 39.Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNerney ME, Lee KM, Zhou P, Molinero L, Mashayekhi M, Guzior D, Sattar H, Kuppireddi S, Wang CR, Kumar V, Alegre ML. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 41.Maier S, Tertilt C, Chambron N, Gerauer K, Huser N, Heidecke CD, Pfeffer K. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7:557–562. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 42.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemiareperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y, Yin H, Han J, Huang B, Xu J, Zheng F, Tan Z, Fang M, Rui L, Chen D, Wang S, Zheng X, Wang CY, Gong F. Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am J Transplant. 2007;7:799–808. doi: 10.1111/j.1600-6143.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 44.Rao DA, Pober JS. Endothelial injury, alarmins, and allograft rejection. Crit Rev Immunol. 2008;28:229–248. doi: 10.1615/critrevimmunol.v28.i3.40. [DOI] [PubMed] [Google Scholar]
- 45.Bishop DK, Wood S. Chan, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166:3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 46.Burrell BE, Csencsits K, Lu G, Grabauskiene S, Bishop DK. CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J Immunol. 2008;181:3906–3914. doi: 10.4049/jimmunol.181.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colvin RA, Campanella GS, Manice LA, Luster AD. CXCR3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Mol Cell Biol. 2006;26:5838–5849. doi: 10.1128/MCB.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 50.Germain RN, Bajenoff M, Castellino F, Chieppa M, Egen JG, Huang AY, Ishii M, Koo LY, Qi H. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunol Rev. 2008;221:163–181. doi: 10.1111/j.1600-065X.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 51.Bajenoff M, Egen JG, Qi H, Huang AY, Castellino F, Germain RN. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28:346–352. doi: 10.1016/j.it.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 53.Beuneu H, Garcia Z, Bousso P. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J Immunol. 2006;177:1406–1410. doi: 10.4049/jimmunol.177.3.1406. [DOI] [PubMed] [Google Scholar]
- 54.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]