Abstract
The possibility that, following early auditory deprivation, the remaining senses such as vision are enhanced has been met with much excitement. However, deaf individuals exhibit both better and worse visual skills than hearing controls. We show that, when deafness is considered to the exclusion of other confounds, enhancements in visual cognition are noted. The changes are not, however, widespread but are selective, limited, as we propose, to those aspects of vision that are attentionally demanding and would normally benefit from auditory-visual convergence. The behavioral changes are accompanied by a reorganization of multisensory areas, ranging from higherorder cortex to early cortical areas, highlighting cross-modal interactions as a fundamental feature of brain organization and cognitive processing.
Introduction
Compensatory plasticity holds that the lack of auditory stimulation experienced by deaf individuals is met by enhancements in visual cognition. However, reports in the educational and cochlear implant literature document deficient visual cognition in deaf individuals. This discrepancy is probably due to the complex etiology of deafness. When free from various confounding factors, deafness per se is seen to shift the spatial distribution of attention such that attention to the peripheral visual field, but not the central visual field, is heightened. Associated neural bases reveal a widespread reorganization from higher association cortices to early sensory cortices. A common feature found across reorganized areas is their fundamental multimodal organization, reinforcing recent views on the role of multimodal integration at all stages of cognitive processing [1].
The complex etiology of deafness
The bulk of the literature on deafness reports either no change in or worse performance by deaf individuals on a variety of tasks as compared to hearing [2,3]. Amid this large literature describing deficiencies in deaf individuals, some recent evidence documents enhancement of a few perceptual and cognitive skills following congenital deafness [4,5]. Discrepancies in the literature might be largely explained by the fact that most studies reporting deficient functions typically include deaf subjects with heterogeneous backgrounds, whereas the studies documenting enhanced functions focus exclusively on a very small subsample of the deaf population, known as Deaf native signers. These individuals are born deaf to deaf parents; they are profoundly deaf; and they have no associated central nervous system damage. In addition, they achieve their language development milestones at the same rate and time as hearing individuals by virtue of being born within a signing community [6]. Study of this population, representing only about 5% of the total deaf population, enables the effect of auditory deprivation to be evaluated, with minimal confounds from other factors such as language deprivation, abnormal cognitive development due to communication disruption, or comorbidity associated with deafness [7] (Box 1). The studies described below focus on Deaf native signers (henceforth shortened to Deaf).
Box 1. Etiology of deafness
In the USA, over 20 million people have been diagnosed with hearing loss, representing a prevalence rate of 9% [52]. This group is extremely heterogeneous in terms of degree, onset, time course and etiology of hearing loss. Of interest here is severe to profound hearing loss (71 + dB loss in the better ear), which is primarily due to sensorineural deficits in the auditory (VIII) nerve, representing approximately a quarter of those with hearing loss [52].The etiology of hearing loss can be hereditary (~50%) or acquired by several mechanisms such as prenatal or perinatal infections (cytomegalovirus, rubella and herpes simplex), postnatal infections (meningitis), premature birth, anoxia, trauma, or as a result of ototoxic drugs (e.g. aminoglycosides) administered during pregnancy. Many of these causes have been associated with other, sometimes severe, neurological sequelae that affect behavioral, cognitive and psychosocial functioning [53,54].
Hereditary deafness is associated with over 350 genetic conditions [55]. Seventy to eighty percent of those are autosomal recessive, 15–20% are autosomal dominant, and 2–3% are X-linked or mitochondrial in origin [56,57]. About a third of these genetic conditions are associated with syndromes [58]. There is a high variability in the chromosome loci for non-syndromic recessive forms, with more than 53 of the loci reported including a mutation of the GJB2 gene [59]. Within the dominant patterns of transmission, 38 genes, consecutively numbered DFNA1–38, have been mapped to 15 different chromosomes, whose functions relate to hair cell formation, potassium recycling into the endolymph, and gap junction proteins [57].
Although not all hereditary cases of deafness are non-syndromic, hereditary-deafened subjects used in the behavioral and imaging studies discussed here are born to deaf parents and have unremarkable neurological and psychiatric histories. In the USA, many individuals who have severe to profound hearing loss before the age of 3 use American Sign Language (ASL) as their first language. This group relies on visual routes to learning and language access, and has similar values, beliefs and behaviors that reflect Deaf culture. The community of ASL users is often referred to in the literature as a linguistic minority community because of the similarities it has with other minority communities in terms of language and culture [60,61].
Selective effects of deafness on visual cognition
Changes in visual cognition following congenital deafness are highly specific. Not all aspects of vision are modified. Visual sensory thresholds are comparable in Deaf and hearing individuals, be it for brightness discrimination, visual flicker, different aspects of contrast sensitivity, or direction and velocity of motion [8–11]. Enhanced performance has been reported in some areas, such as processing of the visual periphery or motion processing, but only under conditions of attention. For example, Neville and Lawson [12] showed that Deaf individuals performed faster and better than hearing controls when asked to detect the direction of motion of a peripheral target at an attended location. Deaf and hearing individuals showed comparable performance for central targets. A selective enhancement in Deaf individuals for stimuli that are peripheral or in motion and require attentional selection has been demonstrated using a variety of paradigms [13–19] (Table 1). As independent anatomical evidence indicates that both motion processing and peripheral vision are predominantly mediated through the dorsal visual pathway [20], these results have led to the proposal that magnocellular visual functions – known to dominate the dorsal visual pathway – might be more easily shaped by experience [21,22]. The dorsal pathway hypothesis holds that, although there is overlap and interconnectivity between dorsal and ventral streams, the two show different functional biases and different susceptibility to altered experience. Several studies support the view that dorsal visual functions might be especially susceptible to the effects of deafness [17,18,21–23]. However, the accumulated results also indicate highly specific plastic changes within dorsal visual pathway functions. For example, sensory thresholds for motion direction and velocity are not altered by early deafness, even when tested in the visual periphery [9–11]. Similarly, recruitment of MT/MST, a brain area highly specialized for visual motion processing and one of the main targets of the dorsal visual pathway, is comparable in Deaf and hearing participants who are viewing moving stimuli passively [23,24]. Thus, Deaf and hearing individuals do not differ on motion tasks that are purely perceptual. When, then, do they differ on motion tasks? The study of MT/MST provides a telling story: comparable recruitment is noted when viewing stimuli passively, but enhanced MT/MST recruitment is observed in Deaf participants when motion stimuli have to be attended to peripherally while central motion is ignored [23,24]. This pattern echoes a general trend in the literature, whereby the greatest population differences have been reported for motion stimuli in the visual periphery under conditions that engage selective attention, such as when the location or time of arrival of the stimulus is unknown or when the stimulus has to be selected from among distractors. Accordingly, Deaf individuals outperform hearing controls when asked to detect the presence of moving light points at unpredictable locations in the periphery [22]. Deaf individuals are faster and more accurate than hearing controls in detecting the direction of motion of a small square at an attended location while ignoring squares flashing at unattended locations [12]. Electrophysiological recordings indicate an increased N1 component – associated with a modulation of visual attention – when Deaf subjects perform this task. Similar increases in N1 amplitude have been noted when Deaf individuals are presented with abrupt onset squares flashed at three possible locations randomly [13] or when monitoring drifting low-spatial frequency gratings for a rare target [18]. As predicted by enhanced peripheral vision, the N1 enhancement in Deaf individuals is more pronounced for peripheral than for central stimuli. Taken together, these results suggest that changes in visual, and possibly tactile, processing after auditory deprivation are best revealed under conditions of attention (Table 1).
Table 1.
Deaf and hearing individuals differ on attention measures, especially in the visual periphery, but not on sensory measures
| Task | Findingsa | Refs |
|---|---|---|
| Sensory measures | ||
| Visual | ||
| Brightness discrimination | D = H | [70] |
| Visual temporal discrimination | D = H | [71–73] |
| Contrast sensitivity | D = H | [8,22] |
| Motion direction | D = H | [9,11,19] |
| Motion velocity | D = H | [10] |
| Tactile | ||
| Frequency discrimination | D = H | [5] |
| Attention measures | ||
| Visual – central field | ||
| Stimulus onset – static | D = H | [14] |
| Visual search | D = H | [26,27] |
| Sustained attention and alerting | D < H | [3] |
| Orienting | D = H | [15,25] |
| Processing of central distractors | D < H | [16] |
| Visual – peripheral field | ||
| Stimulus onset – static | D > H | [14] |
| Motion processing | D > H | [12,21] |
| Orienting and reorienting | D > H | [15,19] |
| Processing of peripheral distractors | D > H | [16,19,28 |
| Tactile | ||
| Frequency change detection | D > H | [5] |
D = H, no population difference; D > H, deaf Ss demonstrated enhanced attention compared with hearing Ss; D < H = deaf Ss demonstrated worse attention compared with hearing Ss.
Deafness alters the spatial distribution of visual attention
Differences between Deaf and hearing individuals are noted mostly under conditions of attention, as when processing in the face of uncertainty and/or selecting a target from among distractors. Could Deaf/hearing differences be better characterized as a generalized attentional difference? This proposal needs qualification as few population differences have been documented on standard attentional paradigms. Studies of attentional orienting, using the Posner-cueing paradigm, report no robust changes, except in the presence of a competing central load [15,19,25]. Although one study reported a tendency for more effective visual search in Deaf than in hearing individuals [26], other reports have failed to replicate the effect [19,27]. The only population effect observed was that Deaf adults terminated target-absent trials faster than hearing adults; this result might reflect differences in decision criteria rather than attention between the two populations. Finally, studies of executive attention suggest comparable abilities across the populations, with a change only in the relative saliency of central and peripheral distractors [16,28]. Thus, attentional differences seem to have been documented between Deaf and hearing individuals mostly when central and peripheral processing are pitted against each other. The changes documented after early deafness are therefore best captured in terms of a change in the spatial distribution of visual spatial attention, whereby Deaf individuals exhibit enhanced peripheral attention compared to hearing individuals.
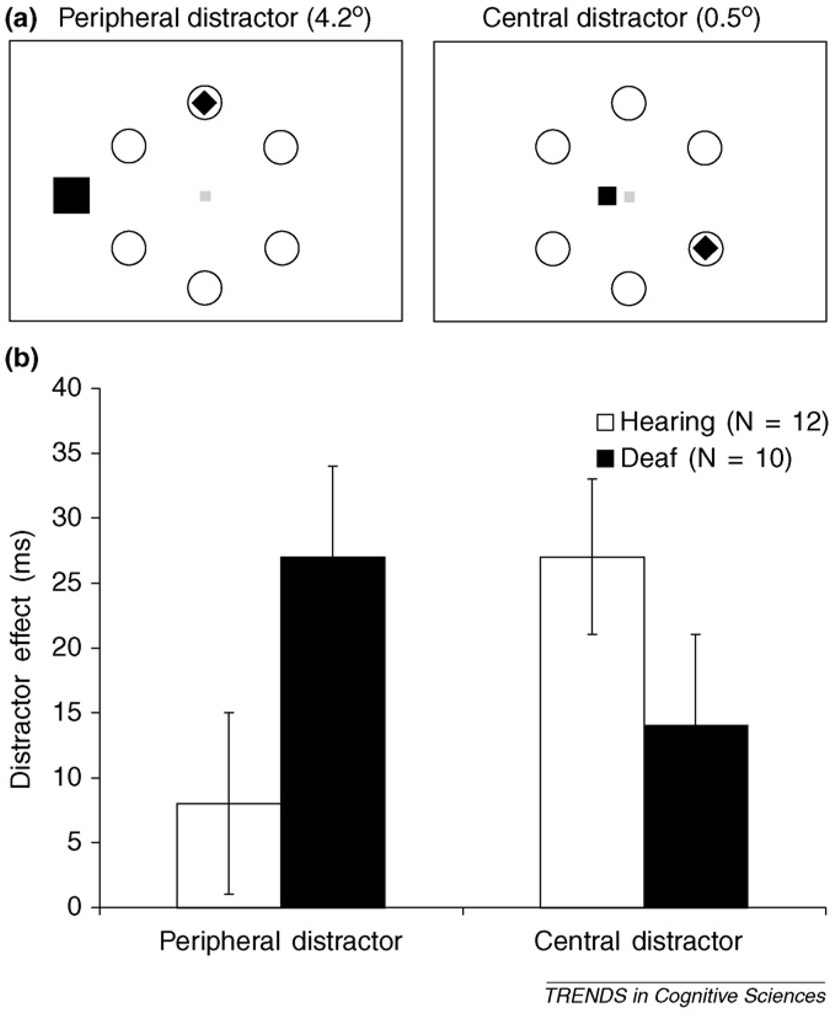
The most direct evidence for the enhanced peripheral attention of Deaf individuals probably comes from a series of experiments in which the effect of an irrelevant distractor on the performance of Deaf and hearing individuals was compared, as a function of whether the distractor was presented centrally or peripherally. Subjects were presented with a display containing six target rings in a circular pattern around fixation [16] (Figure 1a). Subjects were warned that either a square or a diamond would appear in one of the six rings, and they were to decide as fast and as accurately as possible which shape was presented. Also appearing on every trial, outside of the target rings, was a distractor shape. This shape could be the same shape as either the target or the alternative target. By comparing reaction times in trials where the distractor was the same to those where it was different, the effect of the distractors can be measured, allowing for an assessment of attentional resources available at the distractor location. Indeed, the currently accepted basis for this effect is that the size of the distractor effect reflects the amount of processing the distractor receives [29]. The proposal that Deaf individuals have greater attentional resources in the periphery therefore predicts that peripheral distractors will be more distracting for Deaf than for hearing individuals. This was contrasted with a condition where the distractor was presented centrally.
Figure 1.
The proposal that Deaf individuals have greater attentional resources in the visual periphery predicts that peripheral distractors should be more distracting to Deaf than to hearing individuals. (a) The spatial distribution of attention as a function of eccentricity was measured by comparing the extent to which peripheral and central distractors interfere with target performance in Deaf and hearing individuals. (b) Hearing individuals exhibit greater distractability from central than from peripheral distractors, in line with the view of heightened central attention in the hearing population. By contrast, Deaf individuals exhibit greater distractability from peripheral distractors, supporting the view that Deaf individuals have enhanced peripheral attention.
Results confirmed the known finding that, in hearing individuals, central distractors are more distracting than peripheral distractors. By contrast, Deaf individuals were more distracted by peripheral distractors than were hearing individuals, and less so by central distractors (Figure 1b). These findings establish that, whereas in hearing individuals attention is at its peak in the center of the visual field, Deaf individuals show greater attention at peripheral locations. This work carries practical significance, as a recurrent point of view in deaf education is that deaf individuals are easily distracted, cannot stay on task, and lack the ability to focus attention. This conclusion is drawn mainly from the finding that deaf children under-perform compared with hearing children or deaf children with cochlear implants on tasks that require a central focus of attention [2]. Our findings call for a reinterpretation of this greater distractability of deaf individuals. Our work confirms the view that Deaf individuals are more distractible than hearing individuals when the task is central and distraction peripheral. However, our studies show that this is only the case under these conditions. When subjects are asked to perform a peripheral task and ignore central distraction, hearing individuals are more distracted than Deaf individuals. Thus this distraction effect is not a sign of deficient visual attention in the Deaf population, but rather emerges from a different distribution of attentional resources over the visual field in Deaf and hearing populations. This view is confirmed by brain imaging studies that exploit the well-documented layout of the motion pathway in humans to ask how central versus peripheral attention differentially recruits neural circuits in Deaf and hearing individuals. These studies report that, as expected, the main brain areas implicated in motion processing – MT/MST – show greater recruitment under central than under peripheral attention in hearing individuals. By contrast, Deaf individuals display the opposite pattern, with greater recruitment under peripheral than under central attention, again reflecting greater sensitivity to peripheral events in the Deaf population [23] (Figure 2a and 2b).
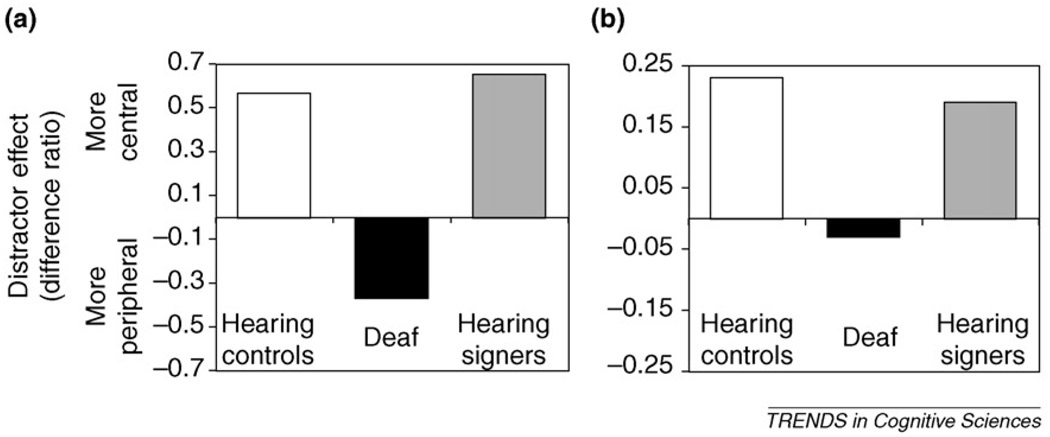
Figure 2.
(a) The extent to which central versus peripheral distractors interfere with the target task can be computed as a difference ratio: (central — peripheral)/(central + peripheral). Both hearing non-signers and hearing signers demonstrate positive ratios, indicating more interference from central than peripheral distractors. Deaf subjects, however, demonstrate negative ratios, indicating more interference from peripheral distractors. This suggests that deafness, not sign language use, is the driving factor behind enhanced distribution of attention to the periphery in adults with early deafness. (b) The same ratio can be calculated using MT/MST activation data from an fMRI task. Again, positive values are observed for hearing non-signers and hearing signers, indicating greater activation when attention is directed towards the center of the visual field. A negative value for Deaf subjects indicates the reverse pattern, reflecting the greater sensitivity to peripheral events in the Deaf population at the level of neural organization.
Overall, these results suggest a core compensatory mechanism of cross-modal plasticity through enhanced modulation of spatial attention in the remaining modalities. Such compensatory changes are best revealed under attentionally demanding conditions arguably because spatial attention resources are limited. In the absence of the auditory modality, the limited attentional resources might be enhanced over the remaining visual modality, as compared to when attentional resources have to be distributed across all modalities [30]. In addition, the compensatory changes seem to be particularly notable for those visual functions, such as peripheral processing, that would normally have benefited from convergence with the now missing auditory input.
Neural correlates of cross-modal plasticity
The enhanced peripheral vision noted in Deaf individuals could be mediated by several distinct brain mechanisms. First, the sensory representation of the peripheral field in early visual cortex might be expanded in genetically deaf individuals. In hearing individuals, it is known that a larger part of the visual cortex is dedicated to processing central vision than peripheral vision. A greater amount of visual cortex might be dedicated to the processing of the visual periphery in Deaf individuals. Although possible, the existing data provide little support for this hypothesis, with the most recent work by Fine et al. [24] reporting similar central/peripheral organization in early visual areas in Deaf and hearing individuals. Second, recent work in the macaque describes projections from auditory areas to the parts of V1 and V2, which represent the far peripheral visual field [31,32]. In the absence of auditory inputs, these visual areas might become more susceptible to intramodal modulations, such as the attentional ones observed behaviorally in deaf individuals. This remains an open question as no study has yet investigated attentional modulation in higher visual cortex at such large eccentricities. Third, multisensory associative cortical areas might reorganize in the face of missing auditory input by displaying a greater sensitivity to the remaining modalities, such as vision. Support for this view comes from studies reporting changes in Deaf individuals in the posterior parietal cortex, one of the main centers for visual attention but also an area that integrates information from different senses [23]. This result is similar to that documented in the animal literature and is well accounted for by a competitive, Hebbian-like mechanism whereby, in the absence of competition from auditory inputs, the remaining modalities exert a greater influence over multimodal cortices [21,33]. Fourth, the auditory cortex might reorganize to mediate other functions such as vision. This view is supported by the findings that auditory areas in the superior temporal sulcus, just caudal to the primary auditory cortex, show greater recruitment in Deaf than in hearing individuals when processing visual, tactile or signed stimuli [23,24,34–37] (Box 2 for the effects of signing on brain and behaviour). The mechanism at work here might be very similar to the Hebbian learning seen in cross-modal areas; indeed, although part of the auditory system at large, there is mounting evidence that neurons in the caudal part of the auditory cortex integrate information from a variety of domains, including somatosensory information, biological motion, face processing and visual information [38–40]. In accordance with a competitive Hebbian mechanism during development, the extent of cross-modal plasticity observed in auditory areas increases with greater dB loss [41] and earlier onset of dB loss [42].
Box 2. Separating the effects of deafness from those of the use of a visual-manual language
Plastic changes observed in Deaf individuals might be due to either congenital deafness or to the early acquisition of a visual-manual language such as American Sign Language (ASL). Studies comparing Deaf and hearing signers – typically hearing children of Deaf individuals – indicate that these factors have different and separable effects. The enhanced peripheral processing reported in Deaf individuals is not observed in hearing signers, suggesting that deafness is a major driving force of that change [12,16,23,24,62]. For example, as shown in Figure 2a, hearing signers are more distracted by central than by peripheral distractors and thus pattern like hearing non-signers and unlike Deaf individuals on this task. Similarly, brain markers of enhanced peripheral processing, such as enhanced N1 or greater recruitment of MT/MST when monitoring peripheral stimuli, are observed in Deaf individuals but not in hearing signers (Figure 2b).
This is not to say that signing does not lead to profound changes in visual skills. First, the use of ASL induces a left hemisphere lateralization for motion processing in signers, Deaf or hearing individuals [9,11,23,62]. As initially proposed by Neville and Lawson [62], the reliance of ASL on motion processing seems to enhance motion processes in the left, language-dominant hemisphere. Second, there is ample research indicating that use of a sign language also modifies higher cognitive skills such as mental rotation abilities, image generation, some aspects of face processing, and short-term memory capacity [63–69]. Deafness and the acquisition of ASL therefore have different and separable effects on the organization of visual and cognitive functions.
Even cortices that were once considered unimodal could receive direct afferents from other modalities, possibly allowing them to exhibit cross-modal plasticity [1]. This mechanism could explain the large reorganization seen in the primary visual cortex of congenitally blind individuals [43], as new evidence accumulates for direct auditory and somatosensory inputs to the primary visual cortex [31,32,44,45]. The case of the primary auditory cortex in the Deaf remains puzzling. The primary auditory cortex sustains its size in Deaf individuals – and thus remains available for functional recruitment [46,47]. Several studies indicate a peak of reorganization in the area caudal to the primary auditory cortex, with activation from that peak spreading, in some studies, to the posterior part of the primary auditory cortex [24,37,41,48]. However, the few studies in congenitally deaf cats and deaf humans in which the primary auditory cortex was delineated on a subject by subject basis, rather than through brain averaging, indicates little functional change beyond the area caudal to the primary auditory cortex [23,49,50].It might be that adequate stimulation has not yet been used – the primary auditory cortex in the Deaf might become recruited only for visual stimuli in the far periphery or for some form of tactile stimulation – or it might be that the connectivity that would allow the primary auditory cortex to reorganize and be taken over by the remaining modalities is lacking. Indeed, research on old world primates documents inputs from other modalities one synapse away from the primary auditory cortex, but not within the primary auditory cortex proper [40,51].
Overall, in the absence of auditory input, cortices that receive multimodal inputs are seen to reorganize (Figure 3). Although most often found in higher cortical areas that are fundamentally multimodal, reorganization is also noted in earlier sensory areas, supporting the view that multisensory convergence is a common feature of cortical organization.
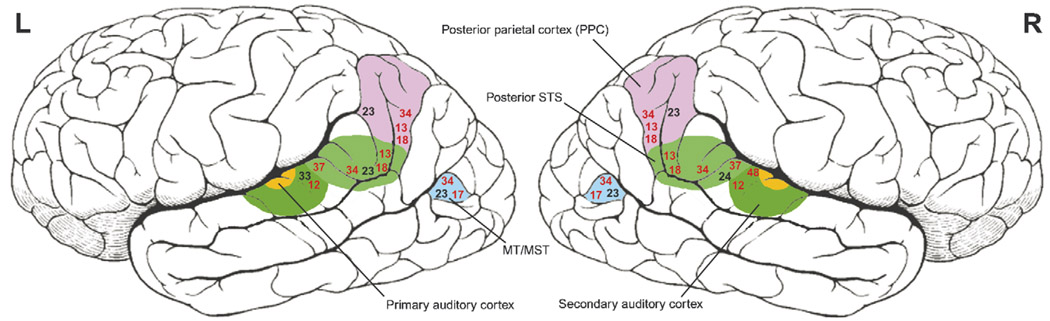
Figure 3.
Brain imaging studies indicate cortical changes associated with cross-modal plasticity in Deaf individuals in three main multisensory areas – the secondary auditory cortex, the posterior superior temporal sulcus (STS) and the posterior parietal cortex (PPC) – as well as in MT/MST when using moving stimuli under conditions of attention. Numbers correspond to cited references; those references reporting Tailarach coordinates are represented with black numbers, whereas those providing only approximate brain locations (as well as ERP and MEG studies) are represented by red numbers.
Summary
The study of plastic changes in Deaf individuals, born profoundly deaf within the Deaf community, highlights the impact of auditory deprivation on cognition without the confounds often associated with deafness. Auditory deprivation leads to enhanced peripheral visual attention, an enhancement particularly notable when contrasted with central attention. Such behavioral change could put deaf individuals at risk in academic or clinical settings that typically rely on the use of centrally presented tasks in often distracting environments. The present results call for a re-evaluation of the best approaches to promote cognitive excellence in the deaf population (Box 3).
Box 3. Questions for future research
Although Deaf adults show enhanced peripheral vision and a reorganization of multimodal areas, little is known about the developmental time-course of these effects. Do young deaf children already show similar enhancement or do these changes appear only slowly during development? Could there be a price to pay during development for these compensatory changes observed in adulthood?
Among Deaf individuals, enhanced behavioral skills co-occur with a reorganization of multimodal areas as seen in the auditory cortex or in the parietal cortex. Although suggestive, the link between cortical reorganization and behavior is only correlational. In future research, it will be important to establish more directly the causal relationship between the reorganization of multimodal areas and behavioral changes.
The behavioral skills modified after deafness seem to be specific, limited to attentionally demanding tasks that would have benefited from sensory convergence. Is this a common feature of cross-modal plasticity? To which extent can results from the literature on blindness also be understood in this framework?
How should the normal cognitive reorganization that occurs in Deaf individuals be appropriately considered when conducting psychological evaluations in the deaf population? How generalizable is the reorganization observed in Deaf signers to the remaining 95% of the deaf community who are born to hearing parents and are not raised with access to fluent users of ASL during infancy and early childhood? How can teachers and educational administrators for the deaf take into consideration the unique strengths of Deaf individuals when developing teaching strategies and curricula?
Deafness and fluency in a signed language have different and separable effects on perception and cognition. However, it remains possible that the effects observed in Deaf signers discussed here stem from the co-occurrence of deafness and signing. Future research focusing on oral deaf subjects who have little exposure to a sign language, but who have hereditary deafness and an absence of comorbidity, are needed to clarify whether deafness is necessary or sufficient to bring about the changes described in Deaf individuals.
Other aspects of visual cognition show few changes, revealing a high degree of specificity in plastic changes after auditory deprivation. At the neural level, the shift in the distribution of attention is accompanied by a greater recruitment of multimodal brain areas, not only in higher association cortices but also in earlier, sensory cortices. This reinforces the view that multisensory convergence is an ubiquitous feature of brain and cognitive behavior.
Acknowledgements
We thank Helen Neville, Elissa Newport and Courtney Stevens for their helpful feedback and stimulating discussions. D.B. acknowledges support from NIH-DC04418, the Charles A. Dana Foundation and the James S. McDonnell Foundation.
References
- 1.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn. Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Quittner AL, et al. The impact of cochlear implants on young deaf children: new methods to assess cognitive and behavioral development. Arch. Otolaryngol. Head Neck Surg. 2004;130:547–554. doi: 10.1001/archotol.130.5.547. [DOI] [PubMed] [Google Scholar]
- 3.Parasnis I, et al. Deaf adults without attention deficit hyperactivity disorder display reduced perceptual sensitivity and elevated impulsivity on the Test of Variables of Attention (T.O.V.A.) J. Speech Lang. Hear. Res. 2003;46:1166–1183. doi: 10.1044/1092-4388(2003/091). [DOI] [PubMed] [Google Scholar]
- 4.Neville HJ. Intermodal competition and compensation in development. In: Diamond A, editor. The Development and Neural Bases of Higher Cognitive Function. New York Academy of Sciences Press; 1990. pp. 71–91. [DOI] [PubMed] [Google Scholar]
- 5.Levanen S, Hamdorf D. Feeling vibrations: enhanced tactile sensitivity in congenitally deaf humans. Neurosci. Lett. 2001;301:75–77. doi: 10.1016/s0304-3940(01)01597-x. [DOI] [PubMed] [Google Scholar]
- 6.Newport E, Meier R. The acquisition of American Sign Language. In: Slobin D, editor. The Crosslinguistic Study of Language Acquisition. Erlbaum; 1985. pp. 881–938. [Google Scholar]
- 7.Mitchell RE, Karchmer MA. Chasing the mythical ten percent: parental hearing status of deaf and hard of hearing students in the United States. Sign Language Stud. 2002;4:128–163. [Google Scholar]
- 8.Finney EM, Dobkins KR. Visual contrast sensitivity in deaf versus hearing populations: exploring the perceptual consequences of auditory deprivation and experience with a visual language. Brain Res. Cogn. Brain Res. 2001;11:171–183. doi: 10.1016/s0926-6410(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 9.Bosworth RG, Dobkins KR. Left hemisphere dominance for motion processing in deaf signers. Psychol. Sci. 1999;10:256–262. [Google Scholar]
- 10.Brozinsky CJ, Bavelier D. Motion velocity thresholds in deaf signers: changes in lateralization but not in overall sensitivity. Brain Res. Cogn. Brain Res. 2004;21:1–10. doi: 10.1016/j.cogbrainres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Bosworth RG, Dobkins KR. Visual field asymmetries for motion processing in deaf and hearing signers. Brain Cogn. 2002;49:170–181. doi: 10.1006/brcg.2001.1498. [DOI] [PubMed] [Google Scholar]
- 12.Neville HJ, Lawson DS. Attention to central and peripheral visual space in a movement detection task: an event related potential and behavioral study. II. Congenitally deaf adults. Brain Res. 1987;405:268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- 13.Neville HJ, et al. Altered visual-evoked potentials in congenitally deaf adults. Brain Res. 1983;266:127–132. doi: 10.1016/0006-8993(83)91314-8. [DOI] [PubMed] [Google Scholar]
- 14.Loke WH, Song S. Central and peripheral visual processing in hearing and nonhearing individuals. Bull. Psychon. Soc. 1991;29:437–440. [Google Scholar]
- 15.Parasnis I, Samar VJ. Parafoveal attention in congenitally deaf and hearing young adults. Brain Cogn. 1985;4:313–327. doi: 10.1016/0278-2626(85)90024-7. [DOI] [PubMed] [Google Scholar]
- 16.Proksch J, Bavelier D. Changes in the spatial distribution of visual attention after early deafness. J. Cogn. Neurosci. 2002;14:687–701. doi: 10.1162/08989290260138591. [DOI] [PubMed] [Google Scholar]
- 17.Bavelier D, et al. Visual attention to the periphery is enhanced in congenitally deaf individuals. J. Neurosci. 2000;20:RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong B, et al. Auditory deprivation affects processing of motion, but not color. Brain Res. Cogn. Brain Res. 2002;14:422–434. doi: 10.1016/s0926-6410(02)00211-2. [DOI] [PubMed] [Google Scholar]
- 19.Bosworth RG, Dobkins KR. The effects of spatial attention on motion processing in deaf signers, hearing signers, and hearing nonsigners. Brain Cogn. 2002;49:152–169. doi: 10.1006/brcg.2001.1497. [DOI] [PubMed] [Google Scholar]
- 20.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 21.Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat. Rev. Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 22.Stevens C, Neville H. Neuroplasticity as a double-edged sword: deaf enhancements and dyslexic deficits in motion processing. J. Cogn. Neurosci. 2006;18:701–714. doi: 10.1162/jocn.2006.18.5.701. [DOI] [PubMed] [Google Scholar]
- 23.Bavelier D, et al. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. J. Neurosci. 2001;21:8931–8942. doi: 10.1523/JNEUROSCI.21-22-08931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine I, et al. Comparing the effects of auditory deprivation and sign language within the auditory and visual cortex. J. Cogn. Neurosci. 2005;17:1621–1637. doi: 10.1162/089892905774597173. [DOI] [PubMed] [Google Scholar]
- 25.Parasnis I. Allocation of attention, reading skills, and deafness. Brain Lang. 1992;43:583–596. doi: 10.1016/0093-934x(92)90084-r. [DOI] [PubMed] [Google Scholar]
- 26.Stivalet P, et al. Differences in visual search tasks between congenitally deaf and normally hearing adults. Brain Res. Cogn. Brain Res. 1998;6:227–232. doi: 10.1016/s0926-6410(97)00026-8. [DOI] [PubMed] [Google Scholar]
- 27.Rettenbach R, et al. Do deaf people see better? Texture segmentation and visual search compensate in adult but not in juvenile subjects. J. Cogn. Neurosci. 1999;11:560–583. doi: 10.1162/089892999563616. [DOI] [PubMed] [Google Scholar]
- 28.Sladen DP, et al. Visual attention in deaf and normal hearing adults: effects of stimulus compatibility. J. Speech Lang. Hear. Res. 2005;48:1529–1537. doi: 10.1044/1092-4388(2005/106). [DOI] [PubMed] [Google Scholar]
- 29.Lavie N. Distracted and confused? Selective attention under load. Trends Cogn. Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Eimer M, et al. Cross-modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J. Cogn. Neurosci. 2002;14:254–271. doi: 10.1162/089892902317236885. [DOI] [PubMed] [Google Scholar]
- 31.Falchier A, et al. Anatomical evidence of multimodal integration in primate striate cortex. J. Neurosci. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. Int. J. Psychophysiol. 2003;50:19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 33.Sadato N, et al. Cross-modal integration and plastic changes revealed by lip movement, random-dot motion and sign languages in the hearing and deaf. Cereb. Cortex. 2005;15:1113–1122. doi: 10.1093/cercor/bhh210. [DOI] [PubMed] [Google Scholar]
- 34.Finney EM, et al. Visual stimuli activate auditory cortex in deaf subjects: evidence from MEG. Neuroreport. 2003;14:1425–1427. doi: 10.1097/00001756-200308060-00004. [DOI] [PubMed] [Google Scholar]
- 35.Petitto LA, et al. Speech-like cerebral activity in profoundly deaf people processing signed languages: implications for the neural basis of human language. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13961–13966. doi: 10.1073/pnas.97.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neville H, et al. Cerebral organization for language in deaf and hearing subjects: biological constraints and effects of experience. Proc. Natl. Acad. Sci. U. S. A. 1998;3:922–929. doi: 10.1073/pnas.95.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levanen S, et al. Vibration-induced auditory-cortex activation in a congenitally deaf adult. Curr. Biol. 1998;8:869–872. doi: 10.1016/s0960-9822(07)00348-x. [DOI] [PubMed] [Google Scholar]
- 38.Allison T, et al. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 39.Brosch M, et al. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J. Neurosci. 2005;25:6797–6806. doi: 10.1523/JNEUROSCI.1571-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeder CE, et al. Anatomical mechanisms and functional implications of multisensory convergence in early cortical processing. Int. J. Psychophysiol. 2003;50:5–17. doi: 10.1016/s0167-8760(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 41.Lambertz N, et al. Cross-modal plasticity in deaf subjects dependent on the extent of hearing loss. Brain Res. Cogn. Brain Res. 2005;25:884–890. doi: 10.1016/j.cogbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Sadato N, et al. Age-dependent plasticity in the superior temporal sulcus in deaf humans: a functional MRI study. BMC Neurosci. 2004;5:56. doi: 10.1186/1471-2202-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theoret H, et al. Behavioral and neuroplastic changes in the blind: evidence for functionally relevant cross-modal interactions. J. Physiol. (Paris) 2004;98:221–233. doi: 10.1016/j.jphysparis.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Laemle L, et al. Cross-modal innervation of primary visual cortex by auditory fibers in congenitally anophthalmic mice. Neurosci. Lett. 2006;396:108–112. doi: 10.1016/j.neulet.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Cappe C, Barone P. Heteromodal connections supporting multi-sensory integration at low levels of cortical processing in the monkey. Eur. J. Neurosci. 2005;22:2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- 46.Penhune V, et al. The morphometry of auditory cortex in the congenitally deaf measured using MRI. Neuroimage. 2003;20:1215–1225. doi: 10.1016/S1053-8119(03)00373-2. [DOI] [PubMed] [Google Scholar]
- 47.Emmorey K, et al. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10049–10054. doi: 10.1073/pnas.1730169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finney EM, et al. Visual stimuli activate auditory cortex in the deaf. Nat. Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- 49.Kral A, et al. Delayed maturation and sensitive periods in the auditory cortex. Audiol. Neurootol. 2001;6:346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura H, et al. Sign language ‘heard’ in the auditory cortex. Nature. 1999;397:116. doi: 10.1038/16376. [DOI] [PubMed] [Google Scholar]
- 51.Schroeder C, et al. Somatosensory input to auditory association cortex in the macaque monkey. J. Neurophysiol. 2001;85:1322–1327. doi: 10.1152/jn.2001.85.3.1322. [DOI] [PubMed] [Google Scholar]
- 52.Ries PW. Prevalence and characteristics of persons with hearing trouble: United States, 1990–91. Vital Health Stat. 1994;10188:1–75. [PubMed] [Google Scholar]
- 53.Hauser PC, et al. Hard of hearing, deafness, and being deaf. In: Farmer JE, et al., editors. Treating Neurodevelopmental Disabilities: Clinical Research and Practice. Guilford Press; 2006. pp. 119–131. [Google Scholar]
- 54.King BH, et al. Psychiatric aspects of blindness and severe visual impairment, and deafness and severe hearing loss in children. In: Coffey CE, Brumback RA, editors. Textbook of Pediatric Neuropsychiatry. American Psychiatric Association; 2006. pp. 397–423. [Google Scholar]
- 55.Martini A, et al. An introduction to genetics of normal and defective hearing. Ann. N. Y. Acad. Sci. 1997;830:361–374. doi: 10.1111/j.1749-6632.1997.tb51908.x. [DOI] [PubMed] [Google Scholar]
- 56.Bussoli TJ, Steel KP. The molecular genetics of inherited deafness – current and future applications. J. Laryngol. Otol. 1998;112:523–530. doi: 10.1017/s0022215100141003. [DOI] [PubMed] [Google Scholar]
- 57.Petersen MB. Non-syndromic autosomal-dominant deafness. Clin. Genet. 2002;62:1–13. doi: 10.1034/j.1399-0004.2002.620101.x. [DOI] [PubMed] [Google Scholar]
- 58.Petit C. Genes responsible for human hereditary deafness: symphony of a thousand. Nat. Genet. 1996;14:385–391. doi: 10.1038/ng1296-385. [DOI] [PubMed] [Google Scholar]
- 59.McGuirt WT, Smith RJH. Connexin 26 as a cause of hereditary hearing loss. Am. J. Audiol. 1999;8:93–100. doi: 10.1044/1059-0889(1999/016). [DOI] [PubMed] [Google Scholar]
- 60.Ladd NP. In Search of Deafhood. Multilingual Matters; 2003. [Google Scholar]
- 61.Padden C, Humphries T. Inside Deaf Culture. Harvard University Press; 2005. [Google Scholar]
- 62.Neville HJ, Lawson DS. Attention to central and peripheral visual space in a movement decision task. III. Separate effects of auditory deprivation and acquisition of a visual language. Brain Res. 1987;405:284–294. doi: 10.1016/0006-8993(87)90297-6. [DOI] [PubMed] [Google Scholar]
- 63.Boutla M, et al. Short-term memory span: insights from sign language. Nat. Neurosci. 2004;7:997–1002. doi: 10.1038/nn1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emmorey K, Bellugi U. Visual imagery and visual-spatial language: enhanced imagery abilities in deaf and hearing ASL signers. Cognition. 1993;46:139–181. doi: 10.1016/0010-0277(93)90017-p. [DOI] [PubMed] [Google Scholar]
- 65.Emmorey K, McCullough S. Mental rotation within linguistic and non-linguistic domains in users of American sign language. Cognition. 1998;68:221–246. doi: 10.1016/s0010-0277(98)00054-7. [DOI] [PubMed] [Google Scholar]
- 66.Bettger J, et al. Enhanced Facial Discrimination: Effects of Experience with American Sign Language. University of California: Institute for Neural Computation; 1997. [DOI] [PubMed] [Google Scholar]
- 67.Wilson M, et al. Modality of language shapes working memory: evidence from digit span and spatial span in ASL signers. J. Deaf Stud. Deaf Educ. 1997;2:150–160. doi: 10.1093/oxfordjournals.deafed.a014321. [DOI] [PubMed] [Google Scholar]
- 68.Capirci O, et al. Teaching sign language to hearing children as a possible factor in cognitive enhancement. J. Deaf Stud. Deaf Educ. 1998;3:135–142. doi: 10.1093/oxfordjournals.deafed.a014343. [DOI] [PubMed] [Google Scholar]
- 69.Arnold P, Murray C. Memory for faces and objects by deaf and hearing signers and hearing nonsigners. J. Psycholinguist. Res. 1998;27:481–497. doi: 10.1023/a:1023277220438. [DOI] [PubMed] [Google Scholar]
- 70.Bross M. Residual sensory capacities of the deaf: a signal detection analysis of a visual discrimination task. Percept. Mot. Skills. 1979;48:187–194. doi: 10.2466/pms.1979.48.1.187. [DOI] [PubMed] [Google Scholar]
- 71.Bross M, Sauerwein H. Signal detection analysis of visual flicker in deaf and hearing individuals. Percept. Mot. Skills. 1980;51:839–843. doi: 10.2466/pms.1980.51.3.839. [DOI] [PubMed] [Google Scholar]
- 72.Mills C. Perception of visual temporal patterns by deaf and hearing adults. Bulletin of the Psychonomic Society. 1985;23:483–486. [Google Scholar]
- 73.Poizner H, Tallal P. Temporal processing in deaf signers. Brain and Language. 1987;30:52–62. doi: 10.1016/0093-934x(87)90027-7. [DOI] [PubMed] [Google Scholar]