Abstract
The syntheses and cytotoxic activity of coumarin-estrogen conjugates are described. In vitro results indicated that conjugates 10, 11 and 13 show growth inhibitory activities at 5-dose concentration (100, 10, 1, 0.1, 0.01 μM) against the following NCI-7- human breast cancer cell lines: BT-549, HS 578T, MCF 7, MDA-MB-231/ATCC, MDA-MB-435, NCI/ADR-RES, and thus serve as new leads for further development of antibreast cancer agent.
Keywords: coumarin, 17β-estradiol, conjugates, cytotoxicity, antiproliferative activity and breast cancer
Introduction
Breast cancer is the second leading cause of cancer death in American women behind lung cancer [1]. Breast, ovary and gonads produce an abundance of estrogens via the aromatase and sulphatase pathways [2]. Estrogens stimulate the proliferation of normal and malignant cells in these organs through “estrogen receptor (ER)” via the induction of nucleic acid synthesis and activation of growth regulatory genes. Post-menopausal women, whose production of ovarian estrogen has ceased with estrogens originating in extra-glandular tissues, accounted for approximately 80% of all breast cancer cases [3]. It has been reported that about one-third of all postmenopausal breast cancer cases are hormone-dependent, which involve the stimulation of cancer cell proliferation by estrogens [4, 5]. Thus, the new approach for the successful treatment of postmenopausal breast cancers (hormone-dependent) involves the use of therapeutic agents that prevent the biosynthesis or physiological action of estrogen on tumor cells [6].
There is an over-expressed ER in breast tumor cell in the earlier stage of breast cancer and during hormonal treatment [7]. The non-selectivity and acute toxicity of many antitumor agents have been the major deterrent in their usage for treating human cancer [8]. Among the current cancer therapy focusing on the improvement of drug selectivity, conjugation of cytotoxic drug components to a carrier with either selectivity toward the tumors or the tumor tissues has proven to be an effective strategy in the development of efficient antitumor drugs with high therapeutic indices [9-12]. Estradiol (E2) has served as a framework for the attachment of various substituents; e.g. cytotoxic moieties, radioisotopes, dietary antioxidants, affinity and photoaffinity-labeling groups, of which several E2 conjugates have advanced as synthetic ligands for therapeutic applications targeting the ER [13-15]. Studies have shown that conjugated groups provide biomolecules with novel properties, such as catalytic activity, altered hydrophobicity or bioaffinity [16]. During the past decades, the application of bioconjugates (i.e. biomolecules bearing unnatural organic structures) in molecular and cell biology has significantly increased [17]. Furthermore, studies involving the concept of ER-targeting have generated ligands with high affinity for calf-uterine ER and selective toxicity for the ER-positive cell line MCF-7.
Coumarins, naturally occurring benzofurans, exhibit useful and diverse biological activities. In recent years, there has been a growing interest in their synthesis and possible applications for drug discovery. Coumarin derivatives have been found to be useful in photochemotherapy, antitumor and anti-HIV therapy [18, 19], and as stimulants for the central nervous system (CNS) [20], antibacterial [21, 22], anti-inflammatory [23], anti-coagulants [24], and dyes [25]. Among the many pharmacological actions of coumarins, antioxidant and antiproliferative actions are the most extensively examined. The antiproliferative coumarins display both cytostatic and cytotoxic activities [26, 27]. For example, coumarin (1) and its active metabolite, 7-hydroxycoumarin (2), demonstrated growth-inhibitory (cytostatic) in human malignant cell lines, such as A549 (lung), ACHN (renal), H727 (lung), MCF-7 (breast) and HL-60 (leukemia) [28, 29], and have also demonstrated activity against prostate cancer, malignant melanoma, and metastatic renal cell carcinoma in clinical trials [30-32].
In the present study, we hypothesize based on the novel concept of biconjugation that the conjugation of antitumor coumarin to estrogen would result in potential agents with selective antiproliferative effect in the breast tissues. The combination of antitumor coumarin to estrogen, a carrier with strong affinity for the ER, would mediate selective delivery of the coumarin to the cells or tissues bearing a high concentration of ERs. This in turn would result in better penetration and selective cytotoxicity of coumarin towards ER enriched breast tumor cells. Previous studies had shown that coupling of cytotoxic porphyrins with 17β-estradiol resulted in improved antitumor activity in the target tissues as the result of sufficient binding to the ER, allowing selective accumulation of the conjugates in ER-rich cells [33-36].
Results and Discussion
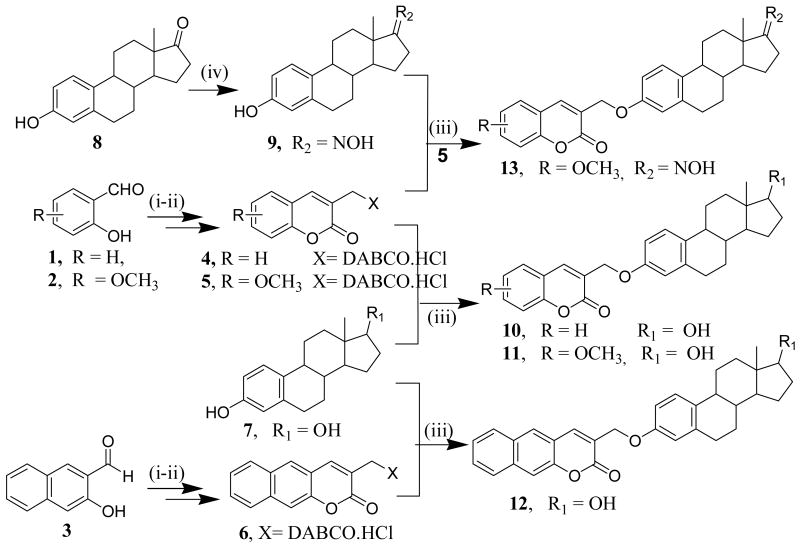
The coumarin quaternary salts (4-6) were prepared following literature methods [37, 38] via the intramolecular Baylis-Hillmann reaction using 1,4-diazabicyclo [2.2.2] octane (DABCO) as a catalyst, which were then coupled to 17β-estradiol (7) and estrone oxime (9) to afford conjugates 10-13 (Scheme 1).
Scheme 1.
Reagents: i) Acryloychloride, NaH, reflux; ii) DABCO, CH2Cl2, rt, iii) K2CO3, CH3CN, reflux, overnight, iv) NH2OH, THF, reflux
The 17β-estradiol (7) was dissolved in acetonitrile and treated with K2CO3. The mixture was stirred for 45 mins; followed by addition of coumarin quaternary salt (4) and then subsequently refluxed overnight to afford product, 10 (60% yield) with a molecular ion at m/z 431.27 (M+ +1) corresponding to the coupled conjugate. The 1H NMR spectrum of conjugate 10 shows a singlet at δ 5.00 ppm corresponding to OCH2- linkage involving the coumarin and the hydroxyl group at the C-3 of the A ring of 17β-estradiol. Other characteristic peaks between δ 6.75- 7.92 ppm correspond to the coumarin five aromatic protons (C-1′ - C-5′) and the C-1, C-2 and C-4 protons of the A ring of 17β-estradiol. None of the conjugates showed any C-17 hydroxy peak when the spectra were recorded in CDCl3 as NMR solvent has indicated in the experimental section. However, the 1H NMR spectrum of conjugate 12 in acetone-d6 showed the presence of the C-17 hydroxyl peak at δ 3.50ppm, which disappeared upon the addition of D2O.
Using the same reaction condition we coupled the 17β-estradiol (7) with coumarin quaternary salts (5 & 6) to yield the corresponding conjugates (11 & 12). Furthermore, conjugate 13 was prepared by coupling coumarin quaternary salt 5 with estrone oxime (9), generated by reacting estrone (8) with hydroxylamine in THF.
These conjugates were purified using preparative plate chromatography and recrystallized from acetone (in overall 65% isolated yields), characterized using 1H NMR, 13C NMR, and elemental and MS analyses and then tested for antiproliferative activities.
Cytotoxicity
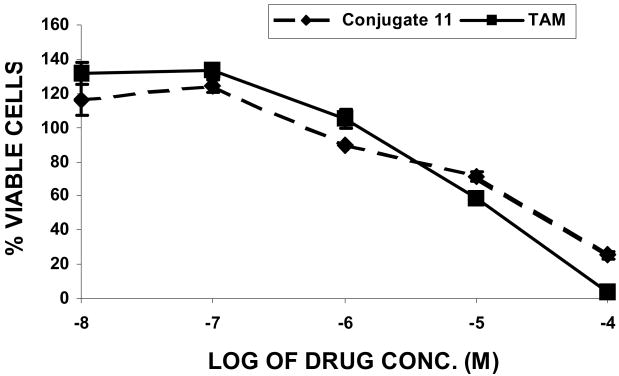
The initial tests conducted by our research group using MTT assay [39,40] at concentration range 100 μm-0.1 nm with conjugate 11 in the presence of 10 nm concentration of estradiol demonstrated significant antiproliferative activity in the human breast cancer MCF-7 cells (GI50 = 9.55 μM), comparable to that of 4-hydroxytamoxifen (GI50 = 9.33 μM) (Figure 1). Estradiol was used to do competitive growth inhibitory studies mimicking physiological estradiol concentration. This initial result intrigued us to conduct further studies against series of cell lines in the National Cancer Institute Developmental Therapeutics (NCIDT) program to determine three dose-dependent parameters (GI50, TGI, and LC50) for conjugates 10-13. GI50 is the concentration of the drug resulting in 50% growth reduction in comparison to the untreated control, LC50 is concentration of the compound leading to the 50% of net cell death following treatment, and TGI is the drug concentration resulting in total growth inhibition.
Figure 1.
In vitro cytotoxicity study in MCF-7 human breast cancer cell line as assessed by MTT assay.
The in-vitro testing results obtained from NCIDT program demonstrated that the conjugates 10-13 possess antiproliferative activities against the following full NCI-7 cell panels: (BT-549, HS 578T, MCF 7, MDA-MB-231/ATCC, MDA-MB-435, NCI/ADR-RES and T-47D) at single dose tested (10 μM). These cell lines differ in term of their pathology, and all are classified as invasive ductal carcinoma cell lines, except NCI/ADR-RES and MDA-MB-231/ATCC, which are adenocarcinoma cell lines. This classification is primarily based on the observed gene expression corresponding to the tumor from which the cell lines were derived. All of the above conjugates, except conjugate 12, were selected for 5-dose testing since they satisfied the pre-determined threshold inhibition criteria (single dose, 10 μM), which were designed to efficiently capture compounds with anti-proliferative activity. The data for the 5-dose testing (100, 10, 1, 0.1, 0.01 μM) is reported in (Table 1).
Table 1.
GI50, TGI and LC50 values (μM) for conjugates 10, 11 and 13 for 5-dose assay in the full NCI-7-cell line panels (Data obtained from NCI's in vitro tumor-cell screen).
| Breast cancer cell (Type) | GI50 (μM) of Conjugate | TGI (μM) of conjugate | LC50 (μM) of conjugate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 11 | 13 | 10 | 11 | 13 | 10 | 11 | 13 | |
| MCF-7 (Invasive ductal carcinoma) | 100 | 3.63 | 12.6 | 100 | 15.0 | 42.5 | 100 | 100 | 100 |
| NCI/ADR-RES (Adenocarcinoma) | 29.5 | 26.2 | 12.4 | 100 | 100 | 100 | 100 | 100 | 100 |
| MDA-MB-231/ATCC (Adenocarcinoma) | 100 | 2.80 | 4.85 | 100 | 14.4 | 18.4 | 80.5 | 80.5 | 48.1 |
| HS 578T (Invasive ductal carcinoma) | 27.6 | 17.7 | 29.4 | 100 | 100 | 87.5 | 100 | 100 | 100 |
| MDA-MB-435 (Invasive ductal carcinoma) | 1.22 | 5.40 | 11.4 | 100 | 100 | 100 | 100 | 100 | 100 |
| BT-549 (Invasive ductal carcinoma) | 4.53 | 27.0 | 12.1 | 100 | 100 | 41.2 | 100 | 100 | 100 |
| T-47D (Invasive ductal carcinoma) | 100 | 32.0 | 8.45 | 100 | 100 | 38.2 | 100 | 100 | 100 |
GI50 = growth inhibition by 50%; TGI = 100% (total) growth inhibition, signifying a cytostatic effect; LC50 = 50% cell kill or lethal concentration, signifying a cytotoxic effect.
Comparisons of the GI50 values among the conjugates 10, 11 and 13 showed that conjugate 10 displayed the highest antiproliferative activity against MDA-MB-435, conjugate 11 against ER-enriched MCF-7, and conjugates 11 and 13 against MDA-MB-231/ATCC breast cancer cell lines. As far as the distinction between noninvasive and invasive breast cancer cell lines, overall conjugates 11 and 13 appear to be active against both cell types while conjugate 10 was surprisingly inactive against MDA-MB-231/ATCC (noninvasive), MCF-7 (Invasive) and T-47D (Invasive) breast cancer cell lines. In general, it was shown that cytotoxicity occurred at around 100 μM for all of the conjugates. Furthermore, conjugate 11 displayed the most cytostatic properties based upon GI50 values being less than LC50 values.
Conclusion
The synthesis and cytotoxic activities of a new group of coumarin-estrogen conjugates as antiproliferative agents have been described. Initial preliminary activity results demonstrated that conjugate 11 possesses significant antiproliferative and growth inhibitory activities in MCF-7 cell at 100 μm-0.1 nm concentration range comparable to that of the active metabolite of the clinically used drug, 4-hydroxytamoxifen. The in vitro results obtained from the NCI have validated this result by showing that conjugates 10, 11 and 13 possess significant growth inhibitory activities against panel of breast cancer cell lines. On the basis of these results, conjugates 11 and 13 could be considered as attractive leads for the further development of anti-breast cancer agents.
Experimental
General
Commercial grade solvents and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Alfa Aesar (Ward Hill, MA, USA) and used without further purification. NMR spectra were recorded on Varian 300 MHz spectrometer. The appropriate deuterated solvents are indicated in the procedure, and line positions recorded in ppm from the reference signal. Infrared (IR) spectra were recorded on Fourier Transformation Infrared (FTIR-8400S) spectrometer (SHIMADZU, Japan). Elemental analyses were carried out by Atlantic Microlab, Inc., Norcross. GA. ESI-TOF Mass Spectrometer was recorded on Agilent 6210 TOF with 1200 HPLC using fast atom bombardment (FAB), (TOF H+). Melting points were determined on a Gallenkamp (UK) apparatus and are uncorrected. The purification of the conjugates was carried out using preparative plate chromatography obtained from Analtech (Newark, DE, USA).
Synthesis of coumarins quaternary ammonium salt (6)
The preparation and characterization of coumarins quaternary ammonium salt (4 & 5) have been reported previously [37, 38] and the same method was utilized in the present experiment to synthesize the compound (6).
To a suspension of NaH (50% dispersion in oil; 0.86 g, 18 mmol) in dry THF (15 ml) under nitrogen was added 3-hydroxynaphthalene-2-carbaldehyde (2.36 g, 13.7 mmol) and the resulting mixture was boiled under reflux for 1h to generate the anion. To the cooled solution of the anion, acryloyl chloride (1.45 ml, 17.9 mmol) in dry THF (15 ml) was added dropwise with stirring, and the reaction mixture was boiled under reflux for 3 h. The reaction was quenched by the addition of water (20 ml) and the resulting mixture extracted with diethyl ether. The organic layer was washed with saturated brine, dried over anhydrous MgSO4, and concentrated in vacuo to afford, as yellow oil, 2-formylnaphthalen-3-yl acrylate (2.04 g, 66%). To a solution of 2-formylnaphthalen-3-yl acrylate (2.48 g, 11 mmol) in dry dichloromethane (23 ml) at −10 °C was added DABCO (1.27 g, 11 mmol), and the stirred solution was allowed to warm to rt over several hours. The resulting precipitate was filtered off, washed with dichloromethane and recrystallized from methanol and dichloromethane to afford, as a pale yellow solid, coumarin quaternary ammonium salt (6)
Compound (6)
(2.01 g, 51%), m.p. < 283.8 °C (dec.); 1H-NMR (300 MHz, CD3OD) 3.18-3.30 [m, 6H, (CH2)3N+], 3.50-3.61 [m, 6H, (CH2)3N+], 4.55 (2H, s, CH2), 7.46 (d, J 9 Hz, 1H), 7.61(t, J 7.5 Hz, 1H), 7.75(t, J 7.4 Hz, 1H), 7.97(d, J 8.1 Hz, 1H), 8.16 (d, J 9.3 Hz, 1H), 8.53 (d, J 8.7 Hz, 1H), 9.26 (s, 1H); 13C-NMR (75MHz, CD3OD) 45.1 (NCH2CH2N+), 52.7 (+NCH2CH2N), 63.1 (CH2N+), 113.1, 114.1, 116.3, 122.1, 126.6, 129.0, 129.4, 130.7, 135.6, 147.4 and 155.0 (Ar-C) and 161.9 (C=O); HR-MS (ESI-MS) (m/z): calcd for C20H21O2N2+, 321.1603: found, 321.1601; analysis (% calcd, % found for C20H21O2N2Cl): C (67.32, 77.42), H (5.93, 6.66).
Synthesis of Coumarin-17β-estradiol conjugates (10-12)
To a mixture of estradiol (7) (0.554 mmol, 150 mg) and K2CO3 (2.215 mmol, 300 mg) in acetonitrile (10 ml) was added the coumarin quaternary ammonium salt (4) (0.665 mmol, 198.8 mg). The mixture was refluxed overnight, allowed to cool to rt and the solid was removed by filtration. The filtrate was collected and acetonitrile removed in vacuo to afford clear yellow oil residue that was purified by preparative plate chromatography [elution with Dichloromethane / Ethyl acetate (5:1)] and then recrystallized using acetone to afford the conjugate 10. The quaternary ammonium salts, 5 and 6, were used to afford conjugates 11 and 12, respectively, following this methodology.
Conjugate 10
(120 mg, 60%) as white solid, m.p. = 234-236 °C. γmax (KBr)/cm-1 1712.7 (C=O), and 2923.9 (CH2); 1H-NMR (300 MHz, CDCl3) 0.80 (s, 3H, 13-CH3), 1.18-2.40 (m, 13H), 2.84-2.88 (m, 2H, 6-CH2-), 3.70-3.76 (t, J 7.8 and 9 Hz, 1H, 17α-CH), 5.00 (s, 2H, OCH2-), 6.75(d, J 2.7 Hz, 1H), 6.80-6.84 (dd, J 2.7 Hz, 1H), 7.22-7.38 (overlapping signals, 3H), 7.50-7.60 (overlapping signal, 2H), and 7.92 (s, 1H); 13C-NMR (75MHz, CDCl3) 11.27 (CH3), 23.36, 26.55, 27.43, 30.00, 30.85, 36.97, 39.05, 64.68 (-CH2), 43.50, 44.21, 50.34, 82.11 (CH-), 112.50, 114.87, 116.83, 119.35, 124.76, 125.40, 126.76, 128.13, 131.51, 133.86, 138.55, 138.68, 153.38, 156.19, 160.47 (Ar-C and C=O); HR-MS (ESI-MS) (m/z): [M+H]+ calcd for C28H31O4+, 431.2222: found, 431.2217; analysis (% calcd, % found for C28H30O4): C (78.11,74.24), H (7.02, 6.76).
Conjugate 11
(158.2 mg, 62%) as white solid, m.p. = 211-212 °C. γmax (KBr)/cm-1 1713.09 (C=O) and 2930.41(CH2); 1H-NMR (300 MHz, CDCl3) 0.78 (s, 3H, 13-CH3), 1.18-2.34 (m, 13H), 2.85-2.88 (m, 2H, 6-CH2-), 3.37 (d, J 5.7 Hz, 1H, 17α-CH), 3.98 (s, 3H, -OCH3), 5.01 (2H, s, OCH2-), 6.75 (d, J 2.4 Hz, 1H), 6.79 (dd, J 2.4 and 3 Hz, 1H), 7.06-7.11 (t, J 7.5 Hz, 2H), 7.20-7.26 (2H), and 7.90 (s, 1H); 13C-NMR (75MHz, CDCl3) 11.6 (-CH3), 56.24 (OCH3), 23.11, 26.29, 27.18, 29.80, 30.58, 36.67, 64.31 (-CH2), 38.76, 42.25, 43.95, 50.0, 81.90 (CH-), 112.23, 113.14, 114.52, 119.30, 119.70, 124.45, 125.31, 126.57, 133.53, 138.32, 138.51, 142.72, 147.13, 155.87, 159.76 (Ar-C and C=O); HR-MS (ESI-MS) (m/z): [M+H]+ calcd for C29H33O5+, 461.2328: found, 461.2325; analysis (% calcd, % found for C29H32O5): C (75.63, 75.11), H (7.00, 7.05).
Conjugate 12
(160 mg, 60%) as white solid, m.p. = 198-200 °C. γmax(KBr)/cm-1 1712.67 (C=O) and 2908.45(CH2); 1H-NMR (300 MHz, CDCl3) 0.78 (s, 3H, 13-CH3), 1.36-2.36 (m, 13H), 2.85-2.91 (m, 2H, 6-CH2-), 3.71-3.76 (t, J 8.1 Hz, 1H, 17α-CH), 5.01 (2H, s, OCH2-), 6.87 (s, 1H), 6.88-6.91 (d, J 4.8 Hz, 1H), 7.26-7.28 (t, J 4.8 and 3.6 Hz 1H), 7.511(d, J 9 Hz, 1H), 7.55-7.60 (t, J 7.5 and 7.8 Hz, 1H), 7.68-7.73 (t, J 6.9 and 8.4 Hz, 1H,), 7.92 (d, J 19.2 Hz, 2H), 7.99 (d, J 9.0 Hz, 2H), 8.33 (d, J 8.4, 1H) and 9.71 (s, 1H,); 13C-NMR (75MHz, CDCl3) 11.06 (-CH3), 23.11, 26.29, 27.19, 29.81, 30.55, 36.67, 64.58 (-CH2), 38.74, 43.95, 49.99, 81.90 (CH-), 112.35, 113.31, 114.66, 116.76, 121.81, 124.09, 126.10, 126.61, 128.16, 128.96, 129.02, 130.37, 132.67, 133.63, 134.38, 138.35, 152.64, 155.93, 160.43 (Ar-C and C=O); HR-MS (ESI-MS) (m/z): [M+H]+ calcd for C32H33O4+, 481.2379: found, 481.2361; analysis (% calcd, % found for C32H32O4): C (79.97, 77.42), H (6.71, 6.66).
Synthesis of Coumarin-17-oximino-estradiol conjugates (Conjugate 13)
A solution of estrone (1.0 mmol), hydroxylamine hydrochloride (0.14 g, 2.0 mmol) and pyridine (0.16 ml, 2.0 mmol) in 10 ml absolute methanol was refluxed for 3.5 h. After cooling, the solvent was removed, following by addition of 10 ml water to afford a white precipitate, 9 that was collected using vacuum filtration (60% yield). To a mixture of 9 (0.554 mmol, 158 mg) and K2CO3 (2.215 mmol, 300 mg) in acetonitrile (10 ml) was added coumarin quaternary ammonium salt 4 (0.665 mmol, 198.8 mg). The mixture was refluxed overnight. Upon cooling the solid was removed by filtration. The filtrate was collected and acetonitrile removed in vacuo to a afford clear yellow oil residue that was purified by preparative plate chromatography [elution with DCM / EtOAc (5:1) and then recrystallization using acetone to afford the conjugate 13 (64% yield).
Conjugate 13
(168 mg, 64%) as white solid, m.p. = 216-217 °C. γmax(KBr)/cm-1 1712.67 (C=O) and 2923.88 (CH2); 1H-NMR (300 MHz, CDCl3) 0.96 (s, 3H, 13-CH3), 1.26-2.58 (m, 13H), 2.88 (m, 2H, 6-CH2-), 3.98 (s, 3H, -OCH3), 5.01 (2H, s, OCH2-), 6.75 (d, J 2.7 Hz, 1H), 6.82 (dd, J 2.4 Hz, 1H), 7.06-7.10 (1H), 7.20-7.26 (overlapping signals, 3H), and 7.88 (s, 1H); 13C-NMR (75MHz, CDCl3) 17.43 (-CH3), 56.52 (OCH3), 23.13, 25.24, 26.42, 27.42, 29.91, 34.31, 38.35, 64.63 (-CH2), 44.26, 44.47, 53.17, (CH-), 112.64, 113.57, 114.83, 119.60, 120.01, 124.62, 125.61, 126.72, 133.48, 138.33, 138.76, 147.45, 156.27, 159.94, 162.0 and 171.59 (Ar-C and C=O); HR-MS (ESI-MS) (m/z): [M+H]+ calcd for C29H32NO5+, 474.2280: found, 474.2238; analysis (% calcd, % found for C29H31NO5): C (73.55, 71.99), H (6.60, 6.49).
Cytotoxicity Studies [41, 42]
MCF-7 (purchased from ATCC) human breast cancer cells were cultured in phenol red-free MEM (500 mL) supplemented with L-glutamine (5 mL), sodium pyruvate (5 mL), MEM non-essential amino acids (5 mL), pen/strep (5 mL), human insulin (1.25 mL) and 10% fetal bovine serum (50 mL). The MCF-7 cells were propagated in 75-cm2 flask under 37 °C air with 5% CO2 with media change every 3-4 days. Once confluent, the media was removed and cells washed with phosphate buffer solution (PBS). The cells were then detached using 2 mL of 0.25% trypsin/EDTA solution (incubation 5–10 minutes) followed by the addition of growth media containing estradiol at the concentration of 10 nM. To each well of 96-well microplate, 100 μl of cell suspension was added at final density of 104 cells per well. After the cells were allowed to attach and grow overnight, the growth media was removed and different drugs treatments (conjugate 11 and 4-hydroxytamoxifen) ranging from 100 nM to 0.1 nM in the growth media were added. Incubation lasted for 4 days at 37 °C air with 5% CO2 and GI50 values determined using MTS assay and GraphPad Prism 3.0
National Cancer Institute (NCI) screening procedures [41-46]
The cytotoxic activity of the conjugates 10, 11 and 13 was evaluated at the National Cancer Institute (NCI, Bethesda, Maryland, USA). The following breast cancer lines: BT-549, HS 578T, MCF 7, MDA-MB-231/ATCC, MDA-MB-435, NCI/ADR-RES and T-47D were used as a pre-screen for the large 7 cell line panel. Cell suspensions were diluted according to particular cell type, follow by addition of the expected target cell density (5000-40,000 cells per well based on cell growth characteristics) into 96-well microtiter plates using a 100 μL pipet. In order to inoculate, a preincubation period of 24 h at 37 °C for stabilization was permitted. The test concentrations were diluted twice in 100 μL aliquots and added to the microtiter plate wells at time zero. The tested conjugates 10, 11 and 13 were evaluated at five concentrations (100, 10, 1, 0.1and 0.01 μM) in the NCI's screening panel. The cells were incubated for 48 h in 5% CO2 atmosphere and 100% humidity and assayed using the sulforhodamine B assay (SRB), a protein-binding dye. The special concentration parameters were determined using plate reader and microcomputer. The cytotoxic effects of each conjugate was expressed as the molar drug concentrations required for 50% growth inhibition (GI50), total growth inhibition (TGI), and 50% cell kill (LC50).
Acknowledgments
Faculty Research Development Funds (NIH/NCRR grant G12 RR0 3020, NIH/NCRR grant 1 C06 RR12512-01 and NIH/DHHS grant 1 S11 ES011182 01) is gratefully acknowledged for financial support and The National Cancer Institute (NCI, Bethesda, Maryland, USA) is acknowledged for the cytotoxicity assays.
References
- 1.Boyle P. Breast cancer control: signs of progress, but more work required. Breast. 2005;14:429–38. doi: 10.1016/j.breast.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Swamy N, James DA, Mohr SC, Hanson RN, Ray R. An estradiol-porphyrin conjugate selectively localizes into estrogen receptor-positive breast cancer cells. Bioorg Med Chem. 2002;10:3237–43. doi: 10.1016/s0968-0896(02)00242-0. [DOI] [PubMed] [Google Scholar]
- 3.Brueggemeier RW, Richards JA, Joomprabutra S, Bhat AS, Whetstone JL. Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J Steroid Biochem Mol Biol. 2001;79:75–84. doi: 10.1016/s0960-0760(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 4.Woo LL, Purohit A, Malini B, Reed MJ, Potter BV. Potent active site-directed inhibition of steroid sulphatase by tricyclic coumarin-based sulphamates. Chem Biol. 2000;7:773–91. doi: 10.1016/s1074-5521(00)00023-5. [DOI] [PubMed] [Google Scholar]
- 5.Henderson IC, Canellos GP. Cancer of the breast: the past decade (first of two parts) N Engl J Med. 1980;302:17–30. doi: 10.1056/NEJM198001033020104. [DOI] [PubMed] [Google Scholar]
- 6.Hamelers IH, Van Schaik RF, Sussenbach JS, Steenbergh PH. 17beta-Estradiol responsiveness of MCF-7 laboratory strains is dependent on an autocrine signal activating the IGF type I receptor. Cancer Cell Int. 2003;3:1–10. doi: 10.1186/1475-2867-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotteland M, May E, May-Levin F, Contesso G, Delarue JC, Mouriesse H. Estrogen receptors (ER) in human breast cancer. The significance of a new prognostic factor based on both ER protein and ER mRNA contents. Cancer. 1994;74:864–71. doi: 10.1002/1097-0142(19940801)74:3<864::aid-cncr2820740312>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Devraj R, Barrett JF, Fernandez JA, Katzenellenbogen JA, Cushman M. Design, synthesis, and biological evaluation of ellipticine-estradiol conjugates. J Med Chem. 1996;39:3367–74. doi: 10.1021/jm9602930. [DOI] [PubMed] [Google Scholar]
- 9.Krohn K, Kulikowski K, Leclercq G. Diethylstilbestrol-linked cytotoxic agents: synthesis and binding affinity for estrogen receptors. J Med Chem. 1989;32:1532–8. doi: 10.1021/jm00127a022. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt BF, Hernandez L, Rouzer C, Czerwinski G, Chmurny G, Michejda CJ. Peptide-linked 1,3-dialkyl-3-acyltriazenes: gastrin receptor directed antineoplastic alkylating agents. J Med Chem. 1994;37:3812–8. doi: 10.1021/jm00048a016. [DOI] [PubMed] [Google Scholar]
- 11.Varga JM, Asato N, Lande S, Lerner AB. Melanotropin-daunomycin conjugate shows receptor-mediated cytotoxicity in cultured murine melanoma cells. Nature. 1977;267:56–8. doi: 10.1038/267056a0. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa-Goto K, Yamada K, Nakamura S, Chen TH, Chiang PC, Bastow KF, Wang SC, Spohn B, Hung MC, Lee FY, Lee FC, Lee KH. Antitumor agents. 258. Syntheses and evaluation of dietary antioxidant--taxoid conjugates as novel cytotoxic agents. Bioorg Med Chem Lett. 2007;17:5204–9. doi: 10.1016/j.bmcl.2007.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodine PV, Harris HA, Lyttle CR, Komm BS. Estrogenic effects of 7alpha-methyl-17alpha-ethynylestradiol: a newly discovered tibolone metabolite. Steroids. 2002;67:681–6. doi: 10.1016/s0039-128x(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 14.el Amouri H, Vessieres A, Vichard D, Top S, Gruselle M, Jaouen G. Syntheses and affinities of novel organometallic-labeled estradiol derivatives: a structure-affinity relationship. J Med Chem. 1992;35:3130–5. doi: 10.1021/jm00095a006. [DOI] [PubMed] [Google Scholar]
- 15.Khan EH, Ali H, Tian H, Rousseau J, Tessier G, Shafiullah, van Lier JE. Synthesis and biological activities of phthalocyanine-estradiol conjugates. Bioorg Med Chem Lett. 2003;13:1287–90. doi: 10.1016/s0960-894x(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 16.Canete M, Villanueva A, Dominguez V, Polo S, Juarranz A, Stockert JC. Meso-tetraphenylporphyrin: photosensitizing properties and cytotoxic effects on cultured tumor cells. Int J Oncol. 1998;13:497–504. doi: 10.3892/ijo.13.3.497. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed N, Dubuc C, Rousseau J, Benard F, van Lier JE. Synthesis, characterization, and estrogen receptor binding affinity of flavone-, indole-, and furan-estradiol conjugates. Bioorg Med Chem Lett. 2007;17:3212–6. doi: 10.1016/j.bmcl.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Nofal ZM, El-Zahar MI, AbdEl-Karim SS. Novel Coumarin Derivatives with expected Biological Activity. Molecules. 2000;5:99–113. [Google Scholar]
- 19.Takeuchi Y, Xie L, Cosentino LM, Lee KH. Anti-AIDS Agents-XXVIII. Synthesis and Anti-HIV Activity of Methoxy Substituted 3′, 4′-di-O-(-)Camphanoyl- (+)-Cis Khellactone (DCK) Analogues. Bioorg Med Chem Lett. 1997;7:2573–2578. doi: 10.1016/s0960-894x(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 20.Moffet RS. Central Nervous System Depressants. VII.1 Pyridyl Coumarins. J Med Chem. 1964;7:446–449. doi: 10.1021/jm00334a010. [DOI] [PubMed] [Google Scholar]
- 21.Al-Haiza MA, Mostafa MS, El-Kady MY. Synthesis and Biological Evaluation of some new coumarin derivatives. Molecules. 2003;8:275–286. [Google Scholar]
- 22.Musiciki B, Periers AM, Laurin P, Ferroud D, Benedetti Y, Lachaud S, Chatreaux F, Haesslein JL, LLtis A, Pierre C, Khider J, Tessol N, Airault M, Demassey J, Dupuis-Hamelin C, Lassaigne P, Bonnefoy A, Vicat P, Klich M. Improved antibacterial activities of coumarin antibiotics bearing 5′,5′-dialkylnoviose: biological activity of RU 79115. Bioorg Med Chem Lett. 2000;10:1695–1699. doi: 10.1016/s0960-894x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- 23.Fylaktakidou KC, Hadipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and Synthetic coumarin derivatives with Anti-inflammatory/antioxidant activities. Current Pharmaceutical Design. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 24.Jung J, Kin J, Park O. Simple and cost effective syntheses of 4-hydroxycoumarin. Synth Commun. 1999;29:3587–3595. [Google Scholar]
- 25.Wang Z, Hara K, Dan-oh Y, Kasada C, Shinpo A, Suga S, Arakawa H, Sugihara H. Photophysical and (Photo) electrochemical Properties of a coumarin Dye. J Phys Chem B. 2005;109:3907–3914. doi: 10.1021/jp044851v. [DOI] [PubMed] [Google Scholar]
- 26.Stanchev S, Momekov G, Jensen F, Manolov I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur J Med Chem. 2008;43:694–706. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Marshall ME, Ryles M, Butler K, Weiss L. Treatment of advanced renal cell carcinima (RCC) with coumarin and cimetidine: long-term follow-up of patients on a phase I trial. J Cancer Res Clin Oncol. 1994;120:535–538. [Google Scholar]
- 28.Stanchev S, Momekov G, Jensen F, Manolov I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur J Med Chem. 2008;43:694–706. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Marshall ME, Mohler JL, Edmonds K, Williams B, Butler K, Ryles M, Weiss L, Urban D, Bueschen A, Markiewicz M. An updated review of the clinical development of coumarin (1,2-benzopyrone) and 7-hydroxycoumarin. J Cancer Res Clin Oncol. 1994;120:S39–42. doi: 10.1007/BF01377124. [DOI] [PubMed] [Google Scholar]
- 30.Thornes RD, Daly L, Lynch G, Breslin B, Browne H, Browne HY, Corrigan T, Daly P, Edwards G, Gaffney E. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J Cancer Res Clin Oncol. 1994;120:S39–4. doi: 10.1007/BF01377122. [DOI] [PubMed] [Google Scholar]
- 31.Marshall ME, Butler K, Fried A. Phase I evaluation of coumarin (1,2-benzopyrone) and cimetidine in patients with advanced malignancies. Mol Biother. 1991;3:170–178. [PubMed] [Google Scholar]
- 32.Mohler JL, Gomella LG, Crawford ED, Glode LM, Zippe CD, Fair WR, Marshall ME. Phase II evaluation of coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma. Prostate. 1992;20:123–131. doi: 10.1002/pros.2990200208. [DOI] [PubMed] [Google Scholar]
- 33.Ali H, Ahmed N, Tessier G, van Lier JE. Synthesis and biological activities of nucleoside-estradiol conjugates. Bioorg Med Chem Lett. 2006;16:317–9. doi: 10.1016/j.bmcl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 34.James DA, Swamy N, Paz N, Hanson RN, Ray R. Synthesis and estrogen receptor binding affinity of a porphyrin-estradiol conjugate for targeted photodynamic therapy of cancer. Bioorg Med Chem Lett. 1999;9:2379–84. doi: 10.1016/s0960-894x(99)00390-x. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Strobl JS, Bane S, Schilling JK, McCracken M, Chatterjee SK, Rahim-Bata R, Kingston DG. Design, synthesis, and bioactivities of steroid-linked taxol analogues as potential targeted drugs for prostate and breast cancer. J Nat Prod. 2004;67:152–9. doi: 10.1021/np030296x. [DOI] [PubMed] [Google Scholar]
- 36.Swamy N, James DA, Mohr SC, Hanson RN, Ray R. An estradiol-porphyrin conjugate selectively localizes into estrogen receptor-positive breast cancer cells. Bioorg Med Chem. 2002;10:3237–43. doi: 10.1016/s0968-0896(02)00242-0. [DOI] [PubMed] [Google Scholar]
- 37.Drewes SE, Njamela OL, Emslie ND, Ramesar N, Field JS. Synth Commun. 1993;23:2807–2815. [Google Scholar]
- 38.Musa MA. PhD thesis. Rhodes University; South Africa: 2003. unpublished result. [Google Scholar]
- 39.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–42. [PubMed] [Google Scholar]
- 40.Ford CH, Richardson VJ, Tsaltas G. Comparison of tetrazolium colorimetric and [3H]-uridine assays for in vitro chemosensitivity testing. Cancer Chemother Pharmacol. 1989;24:295–301. doi: 10.1007/BF00304761. [DOI] [PubMed] [Google Scholar]
- 41.Monks A, Scudiero DA, Skehan P, Shoemaker RH, Paull KD, Vistica DT, Hose C, Langley J, Cronice P, Vaigro-Wolf M, Gray-Goodrich M, Campbell H, Mayo MR. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–66. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 42.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 43.Shoemaker RH, Monks A, Alley MC, Scudiero DA, Fine DL, McLemore TL, Abbott BJ, Paull KD, Mayo JG, Boyd MR. Development of human tumor cell line panels for use in disease-oriented drug screening. Prog Clin Biol Res. 1988;276:265–86. [PubMed] [Google Scholar]
- 44.Stinson SF, Alley MC, Kenny S, Fiebig H, Boyd MR. Morphologic characterization of Human Carcinoma Cell Lines. Proc Am Assoc Cancer Res. 1989;30:613. [Google Scholar]
- 45.Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–8. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 46.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]