Abstract
Many pharmacological treatments for stroke have afforded protection in rodent models but failed to show efficacy in clinical trials. This discrepancy may be due to the lack of long-term functional studies. Previously, delayed administration of the sigma receptor agonist 1,3-di-o-tolylguanidine (DTG) reduced infarct volume after middle cerebral artery occlusion (MCAO) in rats. The present study was conducted to determine whether the protective effects of DTG lead to improvements in behavioral functioning. Rats were subjected to MCAO and administered 7.5, 1.5, or 0.75 mg/kg DTG beginning 24 h post-surgery. Histological outcomes (96 h, 2 weeks, and 5 weeks) were compared with performance on a series of behavioral tests (2 and 4 weeks). Fluoro-Jade staining and immunohistochemistry were used to assess infarct volume and immune cell recruitment. All doses significantly reduced infarct volume and perturbation of striatal white matter tracts at 96 h. These reductions were associated with decreased numbers of CD11b-positive amoeboid microglia/macrophages. Despite short-term efficacy, DTG failed to improve behavioral outcomes or reduce infarct volumes after 96 h. While DTG may prove beneficial as a short-term therapy, these data highlight the importance of long-term functional recovery when evaluating novel therapies to treat stroke.
Keywords: Ischemia, Inflammation, CNS, Therapy, Behavior, Rata
Introduction
Due to the widespread incidence of stroke and the associated costs to society, the need for novel treatments is greater than ever before. The limited therapeutic window of r-tPA therapy has prompted investigations aimed at identifying compounds that can provide neural protection at delayed time points after stroke onset. Despite numerous attempts, pharmacological treatments that have shown efficacy in rodent models have not been proven beneficial in the clinical setting [1–5].
Mounting evidence suggests that the lack of efficacy may be due, at least in part, to the complex immune response to ischemic insult. The initial wave of cell death resulting from ischemia initiates a local [6] and systemic immune response [7–10] that ultimately contributes to neural injury, encompassing both neuronal death and injury to cerebral white matter tracts [11]. Activation of resident microglia and extravasation of peripheral leukocytes into the brain facilitate pro-inflammatory signaling [12, 13]. Despite the purported deleterious role of microglia/macrophages, ablation of these cells increases infarct volume [14] and impairs remyelination following injury [15], indicating that these cells play a complex role in the post-stroke immune response.
Although the precise mechanisms remain unknown, experimental research has provided insight into the progression of ischemic injury. For example, ischemic preconditioning has been shown to reduce neural injury in the middle cerebral artery occlusion (MCAO) model of focal ischemia [16, 17]. Other data showed that splenectomy 2 weeks prior to MCAO resulted in profound neuroprotection that was associated with reduced numbers of activated microglia/macrophages [18]. Because the spleen is a reservoir of immune cells, the splenic response to stroke may involve signaling that recruits these cells to the injured site.
To date, sigma receptors are understudied, yet potentially important, targets to dampen pro-inflammatory signaling that promotes expansion of the core infarction. Administration of the sigma receptor agonist 1,3-di-o-tolylguanidine (DTG) 24 h post-MCAO reduced infarct volume, astrogliosis, and microglia/macrophage recruitment at 96 h [19], suggesting that the delayed inflammatory response is an important mechanism involved in ischemic injury progression. Notably, experiments conducted using primary microglial cultures from rats also showed that DTG blocked several components of microglial activation elicited by both LPS and ATP [20]. These data led to the hypothesis that sigma receptor agonism would provide long-term protection from ischemic injury after stroke. Therefore, the present study was conducted using the rat model of MCAO to determine whether the efficacy of DTG in reducing stroke-induced neural injury leads to functional recovery, as measured by performance on a series of behavioral tests commonly used to assess the efficacy of experimental compounds.
Materials and Methods
Animals
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals with a protocol approved by the Institutional Animal Care and Use Committee at the University of South Florida. Male Sprague–Dawley rats (Harlan, Indian-apolis, IN) weighing 300 to 350 g were housed in a climate-controlled room with water and laboratory chow available ad libitum. A total of 48 animals were used in this study.
Laser Doppler Blood Flow Measurement
Anesthesia was supplied through a nose cone (3% to 4% isoflurane in 100% oxygen, flow rate 2 L/min) and was maintained throughout the procedure. Rats were treated prophylactically with ketoprofen (10 mg/kg s.c.), atropine (0.25 mg/kg s.c.), and Baytril (20 mg/kg i.m.). Ketoprofen injections were continued 3 days post-MCAO to minimize pain and discomfort. Blood perfusion was monitored using the Moor Instruments Ltd. laser Doppler with MoorLAB proprietary Windows-based software (Moor Instruments, Devon, England) on a standard laptop for the duration of the procedure. For Doppler probe insertion, an incision was made just lateral to the midline of the dorsal plates of the skull and a small hole was drilled into the skull at 1 mm posterior and 4 mm lateral to bregma. A hollow, stainless steel guide screw was placed into the hole in the skull and a fiber optic cable (500 μm) was inserted through the screw guide and secured with super glue (Vetbond, 3M). Following MCAO, the screw was removed and the scalp incision closed with surgical sutures. Rats that did not show ≥60% reduction in perfusion during MCAO were excluded from the study.
Permanent Middle Cerebral Artery Occlusion
The methodology employed for MCAO has been previously reported [19]. Briefly, rats were anesthetized, the common carotid artery was separated from the vagus nerve, and blunt dissection was performed to isolate the internal carotid artery (ICA), the external carotid artery (ECA), and the middle cerebral artery (MCA). A 40-mm monofilament was introduced into the ECA, fed distally into the ICA, and advanced approximately 25 mm through the Circle of Willis to the origin of the MCA. The monofilament was then secured into the vessel to achieve permanent occlusion. Reductions in blood flow were recorded using a laser Doppler monitor. Only animals that demonstrated ≥60% reduction in blood flow were included in the study. Following recovery, animals were randomly assigned into treatment groups for each study. Before and during the MCAO induction surgeries, small (100 μl) venous blood samples were obtained and immediately analyzed using the i-STAT handheld clinical analyzers. The physiological parameters that were monitored include hemoglobin, hematocrit, ionized calcium, glucose, sodium, potassium, pH, pCO2, HCO3, TCO2, base excess, pO2, and O2.No differences in these measures were found between the groups of rats used in the treatment studies.
Drug Administration
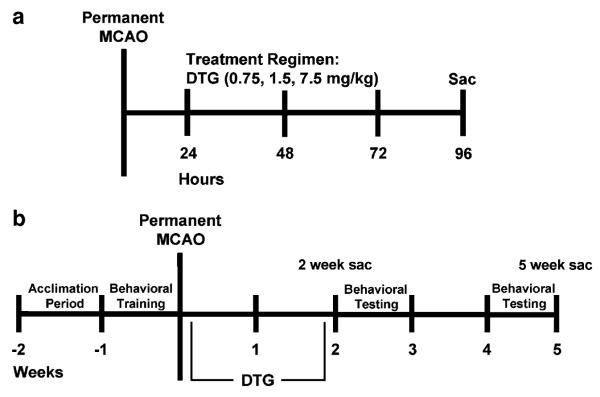
Vehicle or DTG was administered once daily (s.c.) beginning 24 h after MCAO for all experiments. Separate experiments were performed including a dose–response regimen in which animals were killed 96 h post-MCAO and two extended-dose regimens in which animals were killed either 2 or 5 weeks post-MCAO (Fig. 1). For dose–response, animals received a total of three injections. All extended-dose animals received a total of 12 injections. Groups for the dose–response regimen were vehicle (n=4), 7.5 mg/kg DTG (n=4), 1.5 mg/kg DTG (n=4), and 0.75 mg/kg DTG (n=4). For the 2-week extended-dose animals, groups consisted of vehicle (n=4) and 7.5 mg/kg DTG (n=4). For the 5-week extended-dose animals, groups consisted of vehicle (n=9), 7.5 mg/kg DTG (n=7), and 0.75 mg/kg DTG (n=8). Vehicle consisted of DMSO for dose–response rats and 3% lactate diluted in 0.9% sterile saline for extended-dose rats. DTG was prepared fresh each day.
Fig. 1.
Experimental design. For the dose–response experiment (a), animals were subjected to permanent MCAO, administered vehicle or DTG (7.5, 1.5, or 0.75 mg/kg) once daily for 3 days beginning 24 h after MCAO, and killed at 96 h for measurement of infarct volume. For the extended-dose experiments (b), animals subjected to behavioral testing were trained for 1 week prior to MCAO, administered vehicle or DTG (7.5 or 0.75 mg/kg) once daily for 12 days beginning 24 h after MCAO, and tested for performance on the behavioral battery at 2 and 4 weeks post-MCAO (5-week sac). An additional group of animals received the same extended-dose regimen but were killed at 2 weeks post-MCAO for measurement of infarct volume (2-week sac)
Tissue Preparation
Animals were euthanatized at 96 h (dose–response) and 2 or 5 weeks (extended-dose) after MCAO and perfused with 0.9% saline followed by 4% paraformaldehyde in phosphate buffer (pH 7.4). The brains were harvested, post-fixed in paraformaldehyde, and saturated with increasing sucrose concentrations (20%, 30%) in phosphate-buffered saline (PBS, pH 7.4). Brains were frozen and sectioned coronally at 30 μm thickness using a cryostat. Sections were thaw-mounted onto glass slides and stored at −20°C prior to staining. For both immunohistochemistry and Fluoro-Jade staining, six sections were selected from each rat brain at specific intervals (from 1.7 mm anterior to bregma through −3.3 mm posterior to bregma) that included striatal and hippocampal regions of the infarct.
Immunohistochemistry
Immunohistochemistry was performed as previously described [21] to assess microglia/macrophage abundance and morphology at the injured site. Slides were rinsed with PBS, permeabilized and blocked for 60 min (3% Triton-X, 3% 1 M lysine, 10% normal goat serum in PBS), incubated overnight with primary antibody at 4°C, washed three times with PBS, incubated for 60 min with fluorescent-tagged secondary antibody at room temperature, and coverslipped using Vectashield Hard Set mounting media (Vector Labs, Burlingame, CA). The CD11b antigen was detected using mouse anti-OX-42 (Serotec, Raleigh, NC; 1:3,000), myelin basic protein (MBP) was detected using mouse anti-MBP (Abcam, Cambridge, MA; 1:1,000), and neuronal nuclei were detected using mouse anti-NeuN (Chemicon, Temecula, CA; 1:30,000). Secondary antibodies used were goat antimouse Alexa Fluor 488 and 594 (Molecular Probes, Eugene, OR; 1:300).
Fluoro-Jade Staining
Fluoro-Jade (Histochem, Jefferson, AR) staining was performed to label degenerating neurons. This method was adapted from that originally developed by Schmued et al. [22] and has been subsequently detailed [23]. Thaw-mounted sections were placed in 100% ethanol for 3 min followed by 70% ethanol and deionized water for 1 min each. Sections were then oxidized using a 0.06% KMnO4 solution for 15 min followed by three rinses in ddH2O for 1 min each. Sections were then stained in a 0.001% solution of Fluoro-Jade in 0.1% acetic acid for 30 min. Slides were again rinsed, dried at 45°C for 20 min, cleared with xylene, and coverslipped using DPX mounting medium (Electron Microscopy Sciences, Ft. Washington, PA).
Image Analyses and Quantification
For immunohistochemistry, images were acquired using a Zeiss Axioskop2 controlled by Openlab software (Improvision Ltd., Lexington, MA). Photomicrographs were captured with a Zeiss Axiocam Color camera. All images subjected to direct comparisons were captured at the same exposure and digital gain settings to minimize confounds of differential background intensity or false-positive immuno-reactivity across sections.
Fluoro-Jade-stained tissue sections were photographed at ×1.25 magnification with an Olympus IX71 microscope controlled by DP manager software (Olympus America Inc., Melville, NY). Images were then edited with Jasc Paintshop Pro to sharpen and enhance contrast to the same specifications across sections. Total area of neurodegeneration, as indicated by Fluoro-Jade staining, was measured using NIH Image J software. The areas of the contralateral hemispheres were also measured and infarct volumes were determined by dividing the total ipsilateral area occupied by fluorescent stain by the total area of the contralateral hemisphere for each section. Data were then expressed as percent of contralateral.
Behavioral Paradigms
To assess functional recovery, behavioral performance of animals in the 5-week extended-dose group was assessed at baseline (preoperative) and 2 and 4 weeks after MCAO. Spontaneous activity was performed during the light (1-h trial) and dark (12-h trial) phases of the light cycle. All other tests were performed during the light phase between 2 and 4 h after lights on.
Rotorod
Animals were trained on the accelerating Omnirotor rotorod (Omnitech, Rotoscan, Columbus, OH) for three consecutive days prior to baseline data collection. The rotorod was set to accelerate slowly from 0 rpm to a maximum speed of 40 rpm over 180 s. All animals were capable of remaining on the rotorod up to 30 rpm at the conclusion of the training period. During testing, animals were placed on the rotorod and latency to fall was recorded once daily for 3 days. Rats that performed the task up to 40 rpm were assigned a maximum value of 180 s.
Step Test
Animals were held with one forepaw placed on a hard surface and moved in the direction of the placed limb at a steady speed for a distance of 1 m. This was repeated three times for each paw and the bias was calculated as the difference between the number of steps taken with the right and left paw.
Elevated Body Swing Test
Animals were held by the base of the tail and suspended 6 in. above the testing area. The number of lateral elevations to the right or left side was counted over 20 trials. The bias was calculated as the absolute value of the difference between right and left side elevations.
Spontaneous Activity
Spontaneous activity was monitored using the VersaMax System (Accuscan Instruments, Columbus, OH). Animals were placed in a Plexiglas box (35×20×30 cm) and allowed to roam freely during the sample period. Infrared beams mapping the horizontal and vertical planes detected movements reflecting distance, location, time, and count. Data was collected at 5-min intervals for 1 h during the daytime activity test and at 12-h intervals during the nighttime activity test. Data collection began 1 h before lights out during the night test and 2 h after lights on for the daytime test. Thirteen parameters were selected for analysis: horizontal and vertical activity, vertical movement number, vertical time, total distance, center distance, marginal distance, rest time, clockwise and counterclockwise rotations, stereotypy number, stereotypy count, and stereotypy time.
Statistical Analyses
All data were expressed as group mean ± SEM. A value of p<0.05 was considered significant for all analyses. Significance was determined by two-way ANOVA and main effects were subjected to Bonferroni’s post hoc tests.
Results
DTG Reduces Neurodegeneration 96 h after MCAO
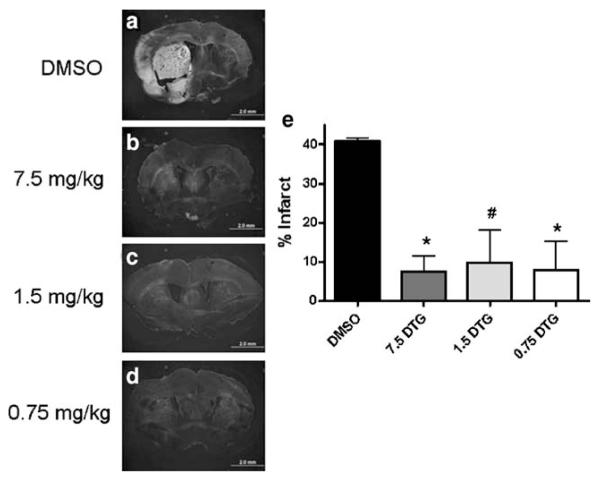
Animals subjected to permanent MCAO were administered 7.5, 1.5, or 0.75 mg/kg DTG once daily for 3 days beginning 24 h after insult and killed at 96 h to determine whether DTG could reduce neurodegenerative damage (Fig. 2). Treatment with vehicle alone resulted in prominent Fluoro-Jade staining throughout the ipsilateral corpus striatum and cortical tissue, while staining in the contralateral hemisphere was virtually absent (Fig. 2a). All doses of DTG tested resulted in greatly reduced Fluoro-Jade staining (Fig. 2b–d). While positive staining was detected at low levels in all treatment groups, the majority of tissues visualized did not appear to show fluorescent labeling above background levels compared with the contralateral hemisphere. Quantification revealed significant reductions in Fluoro-Jade staining at each dose relative to vehicle alone, and each dose was equally efficacious in providing neuroprotection 96 h after stroke (Fig. 2e).
Fig. 2.
DTG dose–response shows efficacy at 96 h. Vehicle-treated animals showed intense Fluoro-Jade staining within the ipsilateral corpus striatum and throughout the cerebral cortex at 96 h, while staining in the contralateral hemisphere showed no fluorescent signal above background levels (a). In sections from animals treated with DTG, Fluoro-Jade staining was predominantly localized to the striatum, occupied smaller regions of tissue, and displayed lower intensity relative to vehicle-treated animals (b–d). Quantification of Fluoro-Jade staining revealed significant reductions at each dose relative to vehicle-treated rats, and all doses demonstrated equal efficacy in reducing neurodegeneration (e). Scale bars=2 mm. Asterisk denotes significance from vehicle at p<0.01. Number sign denotes significance from vehicle at p<0.05
DTG Reduces CD11b-Positive Amoeboid Cells and Preserves White Matter Integrity
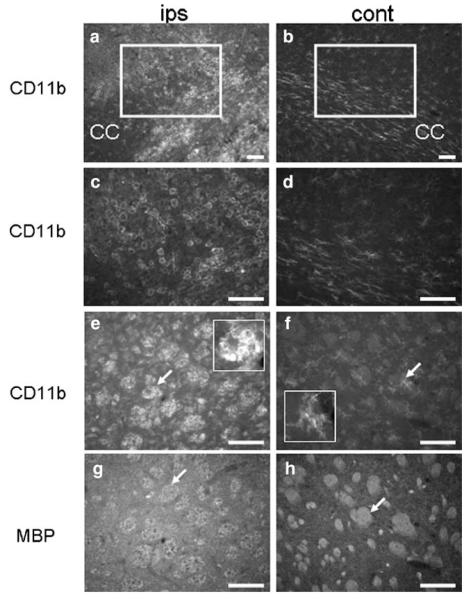
Immunohistochemistry was performed to determine whether the neuroprotective effects of DTG were associated with diminished immune cell recruitment (Fig. 3). CD11b-positive microglia/macrophages were abundant in the ipsilateral hemisphere of animals treated with vehicle alone. These cells displayed the classic amoeboid phenotype associated with injury and localized to the corpus striatum, corpus callosum, and cortical region immediately dorsal to the striatal infarct (Fig. 3a, c). In contrast, immunoreactivity in the contralateral hemisphere was present only in ramified cells, indicative of the resting phenotype, and these cells were far fewer in number compared to the ipsilateral staining profile (Fig. 3b, d). Amoeboid CD11b-positive cells were also seated on the intrafascicular white matter bundles of the ipsilateral corpus striatum (Fig. 3e), while diffusely distributed ramified cells populated the contralateral control hemisphere (Fig. 3f). Importantly, striatal white matter that contained increased numbers of microglia/macrophages also showed greatly reduced MBP immunoreactivity (Fig. 3g) compared to contralateral white matter (Fig. 3h).
Fig. 3.
Microglia/macrophages populate the infarct and are associated with white matter injury at 96 h. CD11b-expressing cells densely populated the ipsilateral corpus striatum, corpus callosum, and cortical regions dorsal to the infarct in tissues from animals treated with vehicle (a). These cells displayed amoeboid morphology consistent with the activated phenotype (c). In the contralateral control hemisphere, CD11b-positive microglia/macrophages were fewer in number (b), were predominantly restricted to the corpus callosum, and displayed ramified or hypertrophic ramified morphology (d). Immunoreactivity for CD11b also localized to cells seated within the intrafascicular white matter bundles of the ipsilateral corpus striatum (e) that showed reduced MBP immunostaining (g), and these effects were not observed in the contralateral control regions (f, h). Arrows in e and f denote the inserts at higher magnification showing amoeboid and ramified microglia, respectively. Arrow in g depicts reduced MBP immunoreactivity in intrafascicular white matter bundles relative to those on the contralateral hemisphere (h, arrow). Scale bars=100 μm (a, b), 50 μm (c–h)
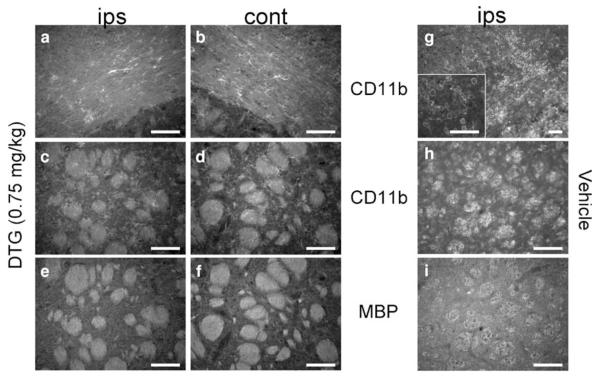
Because all doses of DTG showed equal efficacy in reducing neurodegeneration after MCAO, the lowest dose was selected to determine whether the neuroprotective effects of DTG were associated with a diminished immune cell response (Fig. 4). CD11b-positive cells were present in the ipsilateral hemisphere of animals treated with 0.75 mg/kg DTG. Both hemispheres displayed diffusely distributed, ramified cells. Both the quantity and phenotype present in the ipsilateral corpus callosum (Fig. 4a) and corpus striatum (Fig. 4c) were indistinguishable from those within the respective contralateral regions (Fig. 4b, d). Amoeboidsh-aped cells that were present in tissues from animals treated with vehicle alone (Fig. 4g, h) were not present in DTG-treated sections (4a, c). Consistent with these data, ipsilateral striatal white matter bundles immunostained for MBP (Fig. 4e) were indistinguishable from contralateral staining (Fig. 4f), indicating preserved integrity of white matter after DTG treatment relative to vehicle alone (Fig. 4i).
Fig. 4.
DTG blocks microglia/macrophage recruitment and preserves white matter integrity at 96 h. Tissues from animals administered 0.75 mg/kg DTG showed a similar distribution of ramified CD11b-expressing cells throughout the ipsilateral corpus callosum (a) and corpus striatum (c) relative to contralateral control regions (b, d). Amoeboid-shaped cells were also absent from the ipsilateral hemisphere, in sharp contrast to animals treated with vehicle (g, h). MBP immunoreactivity labeled white matter bundles throughout the corpus striatum of both hemispheres (e, f) and showed greater intensity compared to the ipsilateral staining detected in vehicle-treated animals (i). Scale bars=50 μm, 100 μm (g)
Extended Dosing does not Promote Behavioral Recovery
As DTG treatment reduced neurodegeneration and white matter injury at 96 h, a separate group of animals were subjected to behavioral testing to determine whether this compound also promotes functional recovery weeks after stroke onset. Baselines were recorded prior to MCAO. Rats were then administered 7.5 or 0.75 mg/kg for 12 days beginning 24 h after MCAO and subsequently retested for functional recovery at 2 and 4 weeks (Figs. 5 and 6).
Fig. 5.
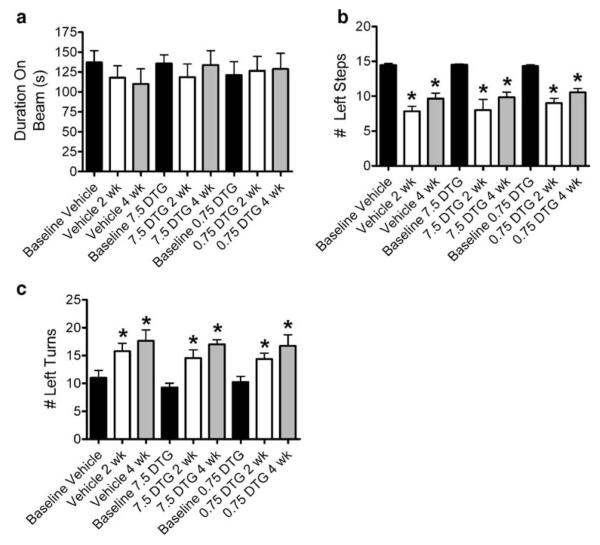
Extended dosing has no effect on rotorod, step test, or elevated body swing test performance. Rats were treated with 7.5 or 0.75 mg/kg for 12 days beginning 24 h after MCAO and behavioral testing was administered at 2 and 4 weeks postsurgery. No differences in rotorod performance were detected relative to baseline across treatment or time point examined (a). Significant reductions in the number of left steps were observed in all treatment groups relative to baseline (b). A trend toward increased left stepping was observed at 4 weeks relative to 2 weeks for each treatment group, yet there were no significant differences across treatment groups over time. Similarly, performance on elevated body swing test demonstrated a trend toward increased left turning at 4 weeks relative to 2 weeks for each treatment group with no significant differences across treatment groups over time. Asterisk denotes significance from baseline at p<0.05
Fig. 6.
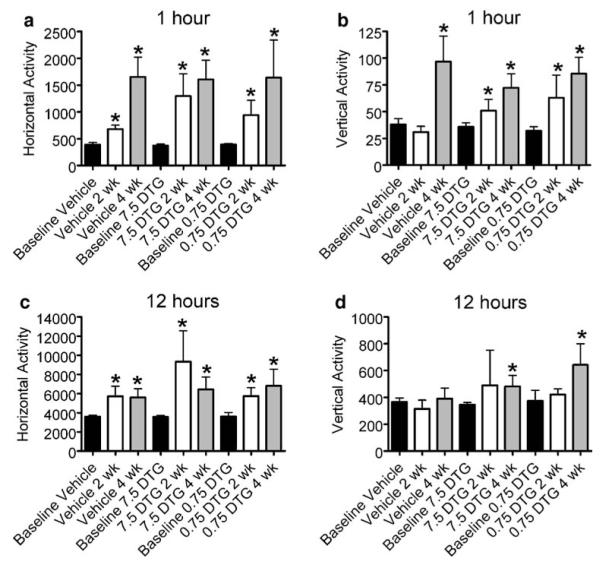
Extended dosing has no effect on open field activity. Rats were treated with 7.5 or 0.75 mg/kg for 12 days beginning 24 h after MCAO and behavioral testing was administered at 2 and 4 weeks postsurgery. Horizontal and vertical open field activity was recorded over two trial periods (1 and 12 h). Horizontal activity was significantly elevated during both trials for all treatment groups relative to baseline but did not differ with respect to treatment or time point examined (a, c). Vertical activity was increased from baseline in vehicle-treated animals only at 4 weeks during the 1-h trial (b). DTG treatment resulted in increased vertical activity relative to baseline in all cases with the exception of the 2-week 12-h trials (d). Asterisk denotes significance from baseline at p<0.05
Although the rotorod test showed no change in performance between any treatment groups at any time point (Fig. 5a), clear deficits were observed with the step test. Animals treated with vehicle alone showed significant reductions in the number of contralateral steps relative to baseline values, yet neither dose of DTG was effective in reducing these deficits (Fig. 5b). Interestingly, all rats showed a bias of contralateral turning that, while significantly increased from baseline, did not differ across treatment groups over time (Fig. 5c). The number of contralateral steps and turns was not statistically significant at 4 weeks compared to 2 weeks, irrespective of treatment.
The overall effects of spontaneous activity demonstrated that treatment with DTG did not differ from vehicle-treated group in hyperactivity. Increased horizontal activity for both the 1- and 12-h testing periods was observed in response to both doses of DTG relative to baseline measures, and activity between treatment groups did not differ between 2 and 4 weeks (Fig. 6a, c). Similarly, vertical activity also increased for both the 1- and 12-h testing periods relative to baseline, and there were no differences across treatment groups over time (Fig. 6b, d).
Extended Dosing does not Reduce Neural Injury at 30 Days
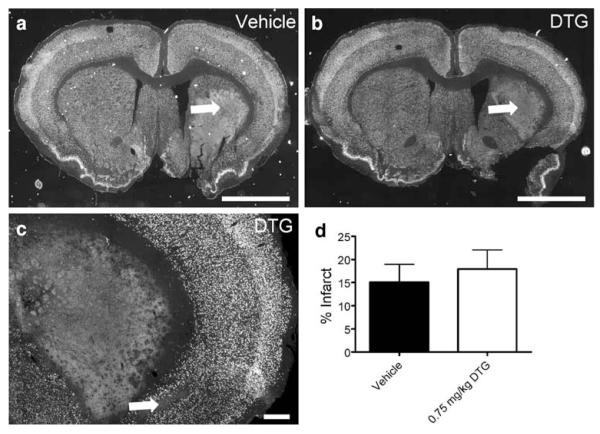
To determine whether the neurohistological outcome was linked to functional recovery at 4 weeks, NeuN immunohistochemistry was performed on tissues from animals in the 5-week extended-dose group (Fig. 7). This neuronal marker was abundant throughout the contralateral hemisphere in the cortical and striatal regions. Prominent ipsilateral striatal lesions were detected in animals from both treatment groups (Fig. 7a, b; arrows). Additional regions of reduced NeuN immunoreactive cells were also evident in cortical tissues (Fig. 7c; arrow). Consistent with the behavioral outcomes, quantification revealed no significant differences in NeuN-positive area measurements between animals treated with vehicle and those administered DTG (Fig. 7d).
Fig. 7.
Extended dosing does not reduce infarct volume at 5 weeks. Tissues from animals in the 5-week extended-dose group were immunohistochemically stained for NeuN and infarct volume was quantified. NeuN immunoreactivity was abundant throughout the contralateral (left) hemispheres of rats treated with vehicle (a) or 0.75 mg/kg DTG (b). Prominent striatal infarcts were detected in animals from both treatment groups (a, b; arrows) and were often accompanied by cortical regions devoid of NeuN-positive cells (c; arrow). Quantification revealed no differences in infarct volume between treatment groups (d). Scale bars=2 mm (a, b), 100 μm (c)
Maintenance of Dosing does not Provide Long-Term Protection
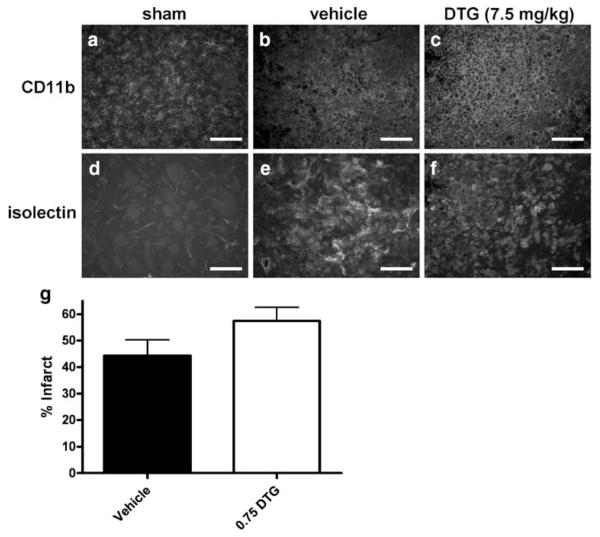
Data showed that repeated dosing for 3 days, beginning 24 h after MCAO, significantly reduced neurodegeneration at 96 h. However, repeated dosing for 12 days showed no longterm efficacy in improving the behavioral or neurohistological outcomes. To further examine this discrepancy, a separate group of animals were subjected to the extended dosing regimen and killed at 2 weeks to determine whether uninterrupted dosing of DTG is necessary to reduce neurodegeneration, or conversely, whether the lack of functional recovery correlates with failure to neuroprotect. Immunohistochemistry was performed on tissues from animals in the 2-week extended-dose group (Fig. 8). CD11b immunoreactivity was detected on amoeboid cells throughout the infarcted regions after treatment with vehicle (Fig. 8b) or 7.5 mg/kg DTG (Fig. 8c). Isolectin also localized to large amoeboid cells that demonstrated morphology similar to phagocytic or “foamy” macrophages (Fig. 8e, f). The CD11b and isolectin staining profiles were indistinguishable between vehicle- and DTG-treated rats. In sham controls, CD11b expression was restricted to cells that exhibited ramified morphology (Fig. 8a) and did not bind isolectin (Fig. 8d), which only labeled a subset of blood vessels within the infarct.
Fig. 8.
Maintaining dosing does not decrease neuroinflammation or infarct volume at 2 weeks. CD11b immunoreactivity localized to amoeboid microglia/macrophages throughout the infarct in tissues from animals treated with vehicle (b) or 7.5 mg/kg DTG (c). Increased isolectin binding was also detected and localized to cells exhibiting morphology consistent with “foamy” macrophages (e, f). This cellular immune response occurred irrespective of treatment and was markedly elevated relative to sham controls, which showed CD11b-expressing cells with ramified morphology (a) and virtually no lectin-binding immune cells (d). Separate tissues were immunohistochemically stained for NeuN and infarct volume was quantified (g). Animals treated with DTG showed no significant differences in infarct volume compared to vehicle-treated controls. Scale bars=50 μm
Infarct volume was quantified using areas devoid of NeuN immunofluorescence (Fig. 8g). Consistent with the 5-week extended-dose animals, quantification of NeuN-positive staining revealed no significant differences in NeuN-positive area measurements across treatment groups at 2 weeks, indicating that DTG was ineffective in reducing long-term neurodegeneration even when there was no withdrawal of the compound prior to histological assessment.
Discussion
Sigma receptor activation has been shown to be beneficial in the treatment of transient [24–27] and permanent [19] MCAO in rodents. Data here show that DTG, a compound that has previously shown promising results in vitro [20] and in vivo [19], demonstrated short-term efficacy by reducing neurodegeneration and white matter injury at 96 h when administered once daily for 3 days beginning 24 h post-MCAO. At the 24-h time point following MCAO, the inflammatory response has begun and is characterized by the presence of activated amoeboid microglia. Sigma receptor activation is known to suppress microglial-mediated inflammation via upregulation of anti-inflammatory cytokines and inhibition of intracellular calcium signaling [20, 28]. Hall et al. has shown that DTG directly blocks microglial activation, inflammation, and migration [20], which is congruent with the protection afforded by DTG in vivo associated with reduced microglia/macrophage recruitment to affected brain regions and a ramified phenotype associated with a quiescent state. These studies verified and confirmed our previous findings showing the protective effects of DTG at 96 h post-MCAO [19].
Surprisingly, this compound showed equal efficacy at all doses tested. While DTG treatment has been associated with the induction of hypothermia, which is known to be protective, the lowest dose tested (0.75 mg/kg) was below the dose necessary for hypothermia to occur [29]. These data suggested not only that DTG exerts actions at multiple targets, but that therapeutic efficacy can be achieved at doses which are below those known to cause side effects in animal models [29].
The next phase of the present study was to determine whether improvement in the neurohistological outcome translates into functional recovery at delayed time points. While most clinical trials use functional recovery at 30 days as a primary endpoint to determine efficacy, to our knowledge, this is the first study to determine therapeutic efficacy of sigma receptor activation beyond 96 h post-MCAO. Despite the potent short-term effects of DTG in reducing Fluoro-Jade staining and preserving MBP-positive white matter tracts, data from the 5-week extended-dose experiment showed that 12 daily injections of DTG failed to promote functional recovery. Importantly, these animals were subjected to behavioral testing 2 and 4 weeks post-MCAO. The fact that animals showed no improvement at 2 weeks or immediately following the completion of dosing, suggested that neither increasing the total dose nor minimizing withdrawal from the compound promoted behavioral recovery. Quantification of NeuN immunoreactivity also confirmed the presence of severe infarctions at 5 weeks, the magnitude of which did not differ significantly across treatment groups.
Although DTG did not provide any long-term protection, the interpretation of these data was complicated by several caveats. The most obvious was the fact that DTG demonstrated potent efficacy in reducing neural injury at 96 h. Thus, it was unknown exactly when the compound failed to combat degenerative processes. Similarly, because the 5-week animals were subjected to behavioral testing at 4 weeks, data was lacking regarding the true association between functional outcome and neurohistological outcome at 2 weeks. In conjunction with conflicting reports regarding the reproducibility and reliability of various behavioral paradigms in the field of stroke research, the final experiment was conducted to determine whether the 12-day dosing regimen reduces infarct volume when animals are killed immediately following treatment. Once again, data showed that the 2-week extended-dose animals displayed substantial cerebral infarctions that occurred irrespective of treatment. The 2-week infarcts were accompanied by increased infiltration of amoeboid-shaped microglia/macrophages that expressed CD11b. These cells also bound isolectin, which occurs in response activation. The fact that DTG effectively reduced infarct volume and immune cell infiltration at 96 h, but not 2 weeks, suggests that infarct expansion is intimately connected with the immune cell response to ischemic injury.
Taken together, these data raise several implications regarding the actions of DTG and the nature of stroke injury in general. The fact that DTG was effective in reducing neurodegeneration and white matter injury in the short-term, despite being administered at a delayed time point, indicates that the delayed immune response is a critical component of infarct expansion. These data also provide evidence that the short-term efficacy of DTG was due, at least in part, to reducing the activation and recruitment of CD11b-expressing immune cells either directly or indirectly. Importantly, this initial protection was insufficient to protect the rat brain from long-term neural injury. This latter point indicates that there are likely other mechanisms or other signaling pathways subserving similar mechanisms, which are involved in promoting progressive stroke injury. However, it is also possible that, after the initial inhibition by DTG, the immune response becomes heightened in a compensatory fashion to a degree where DTG is no longer effective. Thus, although DTG represents a short-term treatment for stroke that may be effective in the clinical setting, a multiple pharmaceutical target approach would be necessary to combat the various elements of stroke-induced injury progression that ultimately impede functional recovery.
Conclusion
Regardless of the precise mechanisms involved, data here clearly show that demonstrating short-term protection is not sufficient to deem a compound promising for the treatment of stroke. As novel therapies continue to fail clinical trials, the field of stroke research would likely benefit from instituting long-term behavioral recovery as the bench mark for efficacy.
Acknowledgements
This work was supported in part by the National Institutes of Health (grant no. 5R21NS060907) and the American Heart Association (grant no. 0715096B).
Contributor Information
Christopher C. Leonardo, Department of Molecular Pharmacology and Physiology, College of Medicine, University of South Florida, MDC Box 9, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612, USA cleonard@health.usf.edu
Aaron A. Hall, Department of Molecular Pharmacology and Physiology, College of Medicine, University of South Florida, MDC Box 9, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612, USA ahall@health.usf.edu
Lisa A. Collier, Department of Molecular Pharmacology and Physiology, College of Medicine, University of South Florida, MDC Box 9, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612, USA lcollier@health.usf.edu
Suzanne M. Green, Department of Neurosurgery, Center of Excellence for Aging and Brain Repair, College of Medicine, University of South Florida, Tampa, FL 33612, USA sgreen1@health.usf.edu
Alison E. Willing, Department of Neurosurgery, Center of Excellence for Aging and Brain Repair, College of Medicine, University of South Florida, Tampa, FL 33612, USA awilling@health.usf.edu
Keith R. Pennypacker, Department of Molecular Pharmacology and Physiology, College of Medicine, University of South Florida, MDC Box 9, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612, USA
References
- 1.Davis SM, Lees KR, Albers GW, Diener HC, Markabi S, Karlsson G, et al. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke. 2000;31(2):347–354. doi: 10.1161/01.str.31.2.347. [DOI] [PubMed] [Google Scholar]
- 2.Elting JW, Sulter GA, Kaste M, Lees KR, Diener HC, Hommel M, et al. AMPA antagonist ZK200775 in patients with acute ischemic stroke: possible glial cell toxicity detected by monitoring of S-100B serum levels. Stroke. 2002;33(12):2813–2818. doi: 10.1161/01.str.0000043823.37955.fb. [DOI] [PubMed] [Google Scholar]
- 3.Limburg M, Hijdra A. Flunarizine in acute ischemic stroke: a pilot study. Eur Neurol. 1990;30(3):121–122. doi: 10.1159/000117326. [DOI] [PubMed] [Google Scholar]
- 4.Lyden P, Jacoby M, Schim J, Albers G, Mazzeo P, Ashwood T, et al. The clomethiazole acute stroke study in tissue-type plasminogen activator-treated stroke (CLASS-T): final results. Neurology. 2001;57(7):1199–1205. doi: 10.1212/wnl.57.7.1199. [DOI] [PubMed] [Google Scholar]
- 5.Diener HC, Cortens M, Ford G, Grotta J, Hacke W, Kaste M, et al. Lubeluzole in acute ischemic stroke treatment: a double-blind study with an 8-hour inclusion window comparing a 10-mg daily dose of lubeluzole with placebo. Stroke. 2000;31(11):2543–2551. doi: 10.1161/01.str.31.11.2543. [DOI] [PubMed] [Google Scholar]
- 6.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 7.Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke. 1992;23(9):1367–1379. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- 8.Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15(1):42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- 9.Jander S, Schroeter M, D’Urso D, Gillen C, Witte OW, Stoll G. Focal ischaemia of the rat brain elicits an unusual inflammatory response: early appearance of CD8+ macrophages/microglia. Eur J NeuroSci. 1998;10(2):680–688. doi: 10.1046/j.1460-9568.1998.00078.x. [DOI] [PubMed] [Google Scholar]
- 10.Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- 11.Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4(2):113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- 12.Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27(10):1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- 13.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19(8):819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacer-bates ischemic injury in the brain. J Neurosci. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35(3):204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- 16.Obrenovitch TP. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev. 2008;88(1):211–247. doi: 10.1152/physrev.00039.2006. [DOI] [PubMed] [Google Scholar]
- 17.Truettner J, Busto R, Zhao W, Ginsberg M, Perez-Pinson M. Effect of ischemic preconditioning on the expression of putative neuroprotective genes in the rat brain. Mol Brain Res. 2002;103:106–115. doi: 10.1016/s0169-328x(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 18.Ajmo CT, Jr, Collier LA, Leonardo CC, Hall AA, Green SM, Womble TA, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajmo C, Jr, Vernon D, Collier L, Pennypacker K, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3(2):89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- 20.Hall AA, Herrera Y, Ajmo CT, Jr, Cuevas J, Pennypacker KR. Sigma receptors suppress multiple aspects of microglial activation. Glia. 2008;57:744–754. doi: 10.1002/glia.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonardo CC, Eakin AK, Ajmo JM, Gottschall PE. Versican and brevican are expressed with distinct pathology in neonatal hypoxic-ischemic injury. J Neurosci Res. 2008;86(5):1106–1114. doi: 10.1002/jnr.21553. [DOI] [PubMed] [Google Scholar]
- 22.Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751(1):37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 23.Duckworth EA, Butler TL, De Mesquita D, Collier SN, Collier L, Pennypacker KR. Temporary focal ischemia in the mouse: technical aspects and patterns of Fluoro-Jade evident neurodegeneration. Brain Res. 2005;1042(1):29–36. doi: 10.1016/j.brainres.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Shen YC, Wang YH, Chou YC, Liou KT, Yen JC, Wang WY, et al. Dimemorfan protects rats against ischemic stroke through activation of sigma-1 receptor-mediated mechanisms by decreasing glutamate accumulation. J Neurochem. 2008;104(2):558–572. doi: 10.1111/j.1471-4159.2007.05058.x. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Kirsch JR, Hashimoto K, London ED, Koehler RC, Traystman RJ. PPBP [4-phenyl-1-(4-phenylbutyl) piperidine] decreases brain injury after transient focal ischemia in rats. Stroke. 1996;27(11):2120–2123. doi: 10.1161/01.str.27.11.2120. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H, Kirsch JR, Hashimoto K, London ED, Koehler RC, Traystman RJ. PPBP [4-phenyl-1-(4-phenylbutyl) piperidine], a potent sigma-receptor ligand, decreases brain injury after transient focal ischemia in cats. Stroke. 1995;26(9):1676–1682. doi: 10.1161/01.str.26.9.1676. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi H, Traystman RJ, Hashimoto K, London ED, Kirsch JR. Postischemic brain injury is affected stereospecifically by pentazocine in rats. Anesth Analg. 1997;85(2):353–357. doi: 10.1097/00000539-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factorbeta1. Int Immunopharmacol. 2006;6(6):1029–1033. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Rawls SM, Baron DA, Geller EB, Adler MW. Sigma sites mediate DTG-evoked hypothermia in rats. Pharmacol Biochem Behav. 2002;73(4):779–786. doi: 10.1016/s0091-3057(02)00903-6. [DOI] [PubMed] [Google Scholar]