Abstract
Nicotine addiction is the leading cause of premature illness and death in the general population. Up to half of all cigarettes are consumed by a minority of the population: persons with schizophrenia and other forms of mental illness. Ironically, despite nicotine dependence being considered a serious and deadly form of addiction in the general population, research on smoking in mental illness is predominantly guided by the idea that smoking has beneficial medication-like treatment effects. This article considers pitfalls of adherence to the self-medication hypothesis as an exclusively held dogma. New evidence from animal modeling work suggests the need to broaden hypothesis-driven research on smoking in mental illness. Adolescent smoking could predispose to mental illness and/or increased nicotine dependence in schizophrenia may represent an involuntary, general addiction vulnerability that has little to do with the ‘helpful’ psychoactive effects of nicotine or other drugs.
Keywords: Nicotine addiction, schizophrenia, dual diagnosis, animal models, neurodevelopmental, self-medication, integrated neurocircuit vulnerability
Nicotine addiction remains the leading cause of premature illness and death in the United States (Mokdad, Marks, Stroup, & Gerberding, 2004). Unfortunately, nicotine addiction is highly concentrated in schizophrenia and other psychiatric populations, who collectively smoke up to 50% of cigarettes consumed (Lasser et al., 2000). These trends contribute to accelerated rates of early illness and death, public heath expenditures, and financial destitution in these vulnerable populations (Brown, Inskip, & Barraclough, 2000; Miller, Paschall, & Svendsen, 2006; Steinberg, Williams, & Ziedonis, 2004). Investigating the causal relationships between mental illness and nicotine addiction is therefore not only a mental health care priority but also a public health priority. As schizophrenia patients have among the highest rates of smoking (70%–90%; Buckley, 1998; Hughes, Hatsukami, Mitchell, & Dahlgren, 1986), this particular form of dual diagnosis has been one of the most intensively studied.
SELF-MEDICATION AND ALTERNATIVE HYPOTHESES
The causal mechanisms responsible for smoking in schizophrenia are undoubtedly complex. However, the bulk of published research and federal funding in support of investigating this phenomenon has been guided by or seeks to support only one hypothesis: nicotine is used by patients in some way as a beneficial agent (i.e., as self-medication). This situation appears ironic, given the attention smoking receives as a deadly, serious addiction in non-mentally ill populations. How is it that our mental health research and clinical communities focus so exclusively on beneficial effects of smoking in populations who suffer the most from it? A rational assumption is that if nicotine addiction is so prevalent and dangerous for a population, they must be getting something positive out of it. And, the self-medication hypothesis does have empirical support. Numerous studies support the view that the decision to smoke as a volitional act of self-treatment is the result of a helpful lock-and-key–like interaction between disease-specific nicotinic acetylcholinergic receptor (nAChR) abnormalities in schizophrenia and nicotine’s unique cognitive or mood-altering properties (Leonard et al., 2001). Some aspects of schizophrenia-based nAChR abnormalities may be partially normalized by smoking, and nicotine can improve cognitive or sensory gating deficits (Breese et al., 2000; Durany et al., 2000; George et al., 2006).
The possibility that research based on the self-medication hypothesis could advance our understanding of schizophrenia remains promising. But, as with many dogmas in science, the dominance of the self-medication hypothesis has been so complete that little attention has been paid to the limitations of its suppositions. For instance, do schizophrenia patients generally become “compulsively compliant” on medications just because they have proven efficacy? No. While few clinicians would argue that nicotine “therapy” as opposed to antipsychotic, antidepressant, or mood-stabilizing medication would be an acceptable treatment, patients are often not compliant with their prescribed medication regimens and/or will forgo small medication co-pays to afford reinforcing behaviors such as smoking or gambling (Chambers & Potenza, 2001; Steinberg, Williams, & Ziedonis, 2004). Do patients with disorders involving cognitive and nAChR deficits similar to those in schizophrenia (which might also improve with nicotine treatment) generally pick up smoking? No. For example, while cross-sectional studies have shown decreased rates of smoking (but schizophrenia-like declines in nAChR densities [Kadir, Darreh-Shori, Almkvist, Langstrom, & Nordberg, 2007]) in patients with Alzheimer’s disease, midlife smoking is actually a disease risk factor, and not a protective agent, for later dementia (Reitz, den Heijer, van Duijn, Hofman, & Breteler, 2007). Do schizophrenia patients generally use reinforcing drugs because of their positive cognitive psychoactive effects? No. Substance disorders involving cocaine, alcohol, and cannabis—drugs widely known to impair cognition and/or generate or exacerbate psychosis—are all increased in schizophrenia at rates above those in the general population and with proportional increases similar to smoking (Dixon, 1999; Regier et al., 1990).
Perhaps the most detrimental aspect of the self-medication hypothesis, as a widely held dogma, has been a lack of interest and effort in exploring alternative hypotheses. For instance, might nicotine addiction in schizophrenia represent a case of a far more general, non–drug specific, and involuntary vulnerability to addiction due to an interaction between both disease processes (Chambers, Krystal, & Self, 2001)? Or might smoking in adolescence (when smoking usually starts) actually predispose to or enhance mental illness trajectories in vulnerable populations (Slotkin, 2008)?
Mounting evidence points to an unmet need for investigating both of these possibilities. With respect to addiction vulnerability, both schizophrenia and addiction to nicotine (and other major addictive drugs) typically begin around adolescence, and common frontal cortical-striatal circuits are involved in the genesis of both diseases (Berg & Chambers, 2008; Chambers et al., 2001; Chambers, Taylor, & Potenza, 2003, p. 639). With respect to nicotine as a causative agent for mental illness, recent preclinical studies have demonstrated that adolescent nicotine exposure produces long-lasting effects on adult brain function. These include declines in cholinergic transmission and its modulation of monoamine transmitters (e.g., serotonin), a prolonged nicotinic withdrawal syndrome involving sustained cognitive deficits, enhancement of anxiety, and altered nicotine receptor levels that resist abstinence-induced renormalization (Slawecki, Thorsell, El Koury, Mathe, & Ehlers, 2005; Slotkin, 2008).
A key feature that distinguishes these alternative theories from the self-medication hypothesis is that they do not assume that there has to be something “good” or “beneficial” underlying the co-morbidity, and in fact, they point to mechanisms or outcomes that are detrimental. With respect to addiction vulnerability, the core idea in our concept and criteria for addictions is that drug taking becomes compulsive despite negative consequences, not because of positive ones (American Psychiatric Association, 2000). Objectively measurable benefits from drug use are neither necessary nor sufficient to provide a rationale or mechanism for the addiction, and subjective claims of drug benefit are routinely couched as pathological rationalizations for continued drug use. Neurobiological changes sculpted by drugs in the addictive process occur not because of any rational thinking or moral judgment about what is “good,” but as an involuntarily occurring pharmacological impact: a “hijacking” of neural systems that govern motivation (Newlin, 2002). Further, these motivational changes occur not in any unique relation to any one of the diverse and often unique psychoactive or neurotransmitter effects of abused drugs, but by virtue of their shared effects within the same neurocircuits that govern motivation (e.g., frontal cortical-striatal systems), involving the same neurotransmitter systems (e.g., dopamine, glutamate, and GABA [gamma–aminobytyric acid]; Di Chiara & Imperato, 1988; Kauer & Malenka, 2007).
Another implication of the distinction between self-medication, betting on good, and the alternative theories, focusing on the detrimental, is that these alternative hypotheses cannot be ethically tested or directly explored in human beings using the necessary level of well-controlled, prospective, interventional studies. Thus, animal modeling will have to serve as a key avenue toward exploring these alternative hypotheses.
ANIMAL MODELING NICOTINE EFFECTS IN SCHIZOPHRENIA
In animal modeling of dual diagnosis, the choice of which animal model to study is an important step. Over 60 different animal models of schizophrenia have been described; they all have their strengths and weaknesses and differential degrees to which they model specific aspects of schizophrenia or schizophrenia as a multifaceted syndrome (Koenig, 2008). This writer and colleagues have utilized the neonatal ventral hippocampal lesion (NVHL) model of schizophrenia in rats to study dual diagnosis, on its strength as being a well-studied model (>100 publications) that captures multiple clinical, neurobiological, and neurodevelopmental features of schizophrenia. For instance, positive symptom traits, associated with extreme behavioral responses to stimuli that provoke dopamine transmission, emerge in periadolescence and are ameliorated with neuroleptics (Lipska, Jaskiw, & Weinberger, 1993; Lipska & Weinberger, 1994). Schizophrenia-like cognitive deficits emerge earlier, in a prodromal fashion (Chambers, Moore, McEvoy, & Levin, 1996), with negative symptoms like grooming and social deficits (Becker, Grecksch, Bernstein, Hollt, & Bogerts, 1999; Flores, Silva-Gomez, Ibanez, Quirion, & Srivastava, 2005). NVHL rats also show abnormalities in anatomy, function, and plasticity of frontal cortical-striatal circuits, many of which have been identified in human schizophrenia (Flores, Alquicer, et al., 2005; Goto & O’Donnell, 2002; Tseng, Lewis, Lipska, & O’Donnell, 2007).
With respect to addiction vulnerability, NVHL rats show accentuated acquisition to cocaine self-administration, unstable binging during maintenance of drug intake, and increased drug seeking after withdrawal and upon drug-induced relapse (Chambers & Self, 2002). Similar increases in drug-seeking effort have been documented in methamphetamine self-administration (Brady, McCallum, Glick, & O’ Donnell, 2008). In behavioral tests measuring impulsive approach behavior to natural reward, NVHL rats show increased impulsivity without drug history that is uniquely increased with cocaine history (Chambers, Jones, Brown, & Taylor, 2005).
With respect to nicotine, we have tested aspects pertaining to both self-medication and integrated addiction vulnerability hypotheses. In 8-arm radial-maze testing of contextual learning and working memory, adult NVHL rats show learning deficits consistent with impaired frontal cortical-hippocampal coordination in schizophrenia (Chambers et al., 1996). To test whether nicotine could ameliorate these deficits to provide self-medication efficacy, we gave acute nicotine injections to both NVHL and control rats after learning the maze to asymptotic performance. Neither NVHLs nor controls showed improvement in working memory function. The performance of control rats was already so high that there was little room for improvement, but the NVHL rats had plenty of room to improve. Although these findings did not support the self-medication hypothesis, they by no means ruled it out, as our test conditions were extremely narrow in scope. In testing the cognitive effects of the nAChR blocker mecamylamine, control rats showed new deficits in working memory comparable to NVHL rats at baseline, while NVHL rats again showed little change. Based on these findings, we concluded that NVHL rats were insensitive to the cognitive effects of nAChR manipulation, perhaps due to the loss of hippocampal-bearing nicotinic receptor fields.
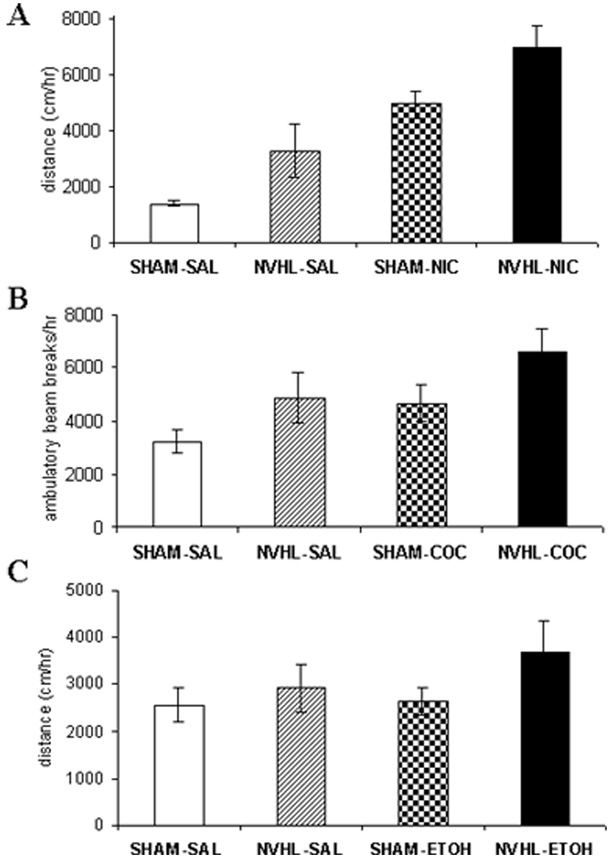
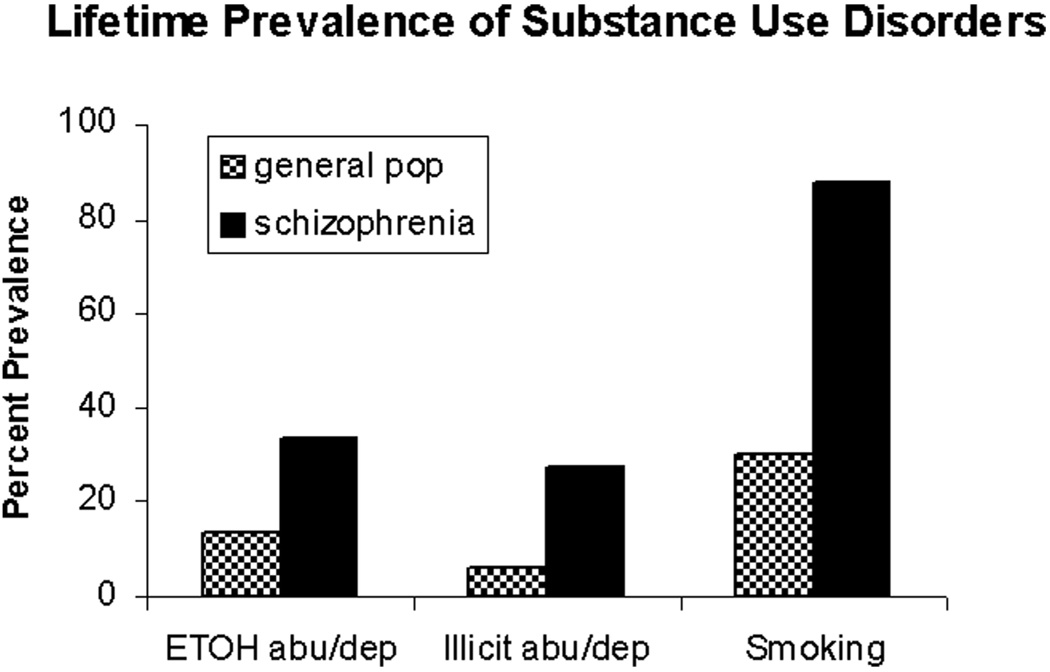
With respect to testing aspects of nicotine addiction vulnerability, a different picture has emerged. Behavioral sensitization is a process by which repeated, unchanging doses of an addictive drug provoke long-lasting growth of specific behaviors (e.g., exploratory locomotion). It is an involuntary effect produced by experimenter-delivered injections of different addictive drugs, involving the same circuits and neurotransmitters implicated in the addiction process. These aspects are closely related to involuntary, drug-induced growth of motivational drives underlying addiction (Robinson & Berridge, 1993). Unlike the situation in the cognitive study, NVHL rats showed accentuated short-term behavioral sensitization to nicotine compared with control rats (Berg & Chambers, 2008). Then, when NVHL and control rats, with and without prior nicotine history, were challenged with a new dose of nicotine, a stepwise progression in behavioral activation emerged: control/saline history rats showed the most normal (lowest) response, NVHL/nicotine history rats showed the most abnormal (largest) response, and the other groups fell intermediately (Figure 1A). These results demonstrated that NVHL rats are not merely insensitive to nicotine (as in the cognitive testing) but in fact are hypersensitive to it in a functional domain that is relevant to involuntary processes underlying addiction. These results are made even more interesting in consideration of prior findings showing that two other addictive drugs, cocaine and alcohol, also produce similar long-term sensitization profiles in NVHL rats (Figure 1B and 1C; Chambers & Taylor, 2004; Conroy, Rodd, & Chambers, 2007). Although nicotine, cocaine, and alcohol are well known to have quite different psychoactive profiles mediated by diverse neurotransmitter effects, they are all addictive via more shared neurotransmitter effects involving common cortical-striatal circuits that govern motivated behavior, addiction, and behavioral sensitization. Given that schizophrenia patients abuse nicotine, alcohol, and illicit drugs like cocaine with rates exceeding those in the general population (Figure 2), our preclinical findings are suggestive of a fundamental neurocircuit-based vulnerability to the addiction process in schizophrenia that is neither voluntary nor drug-specific.
FIGURE 1.
Long-term behavioral sensitization profiles of NVHL versus SHAM-operated control rats (i.e., rats receiving sham surgeries in which no excitotoxin was delivered) as demonstrated by acute drug challenges delivered 2 to 4 weeks after an initial series of drug or saline injections. Long-term sensitization profiles to nicotine (A), cocaine (B), and alcohol (C) show similar overall patterns in which NVHL rats with a drug history show the greatest behavioral activation to new drug injections. In panel A (data from Berg & Chambers, 2008), rats received subcutaneous injections of nicotine (0.5 mg/kg for 15 days over 3 weeks) or saline followed by a single challenge dose of 0.5 mg/kg nicotine 2 weeks later. In panel B (data from Chambers & Taylor, 2004), rats received intraperitoneal cocaine (15 mg/kg) or saline for 7 days, followed by cocaine challenge (15 mg/kg) 4 weeks later. In panel C (data from Conroy et al., 2007), rats received intraperitoneal alcohol (1.0 g/kg for 15 days over 3 weeks) or saline followed by a single challenge dose of 0.25 g/kg of alcohol 2 weeks later. Significant lesion effects were found in all three studies.
FIGURE 2.
Compilation of epidemiological data revealing elevated percent prevalences of substance use disorders in schizophrenia spans addictive drug classes including alcohol (ETOH abu/dep), illicit drugs (e.g., cocaine and cannabis; illicit abu/dep), and nicotine (smoking). Data were compiled from Regier et al., 1990, and Hughes et al., 1986.
Clearly, more work is needed to explore the nature of these findings using different preclinical addiction paradigms, neurobiological assays, and alternative animal models of mental illness. The possibility that self-medication makes some contribution to nicotine dependence in mental illness should continue to be explored. However, it is also time to begin exploring alternative explanations that actually focus on smoking as a deadly, irrational addiction, not only in those who are mentally healthy but especially in the mentally ill.
Acknowledgments
This work was supported by the National Institute on Drug Abuse–supported Career Development Award (K08 DA-019850) and the Indiana Division of Mental Health and Addictions, Family and Social Services Administration.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Becker A, Grecksch G, Bernstein HG, Hollt V, Bogerts B. Social behavior in rats lesioned with ibotenic acid in the hippocampus: Quantitative and qualitative analysis. Psychopharmacology. 1999;144:333–338. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology. 2008;54:1201–1207. doi: 10.1016/j.neuropharm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O’Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berlin) 2008;200:205–215. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Brown S, Inskip H, Barraclough B. Causes of excess mortality of schizophrenia. British Journal of Psychiatry. 2000;177:212–217. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Substance abuse in schizophrenia: A review. Journal of Clinical Psychiatry. 1998;59 Supplement 3:26–30. [PubMed] [Google Scholar]
- Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology. 2005;179:470–478. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JK, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Schizophrenia and pathological gambling (letter) American Journal of Psychiatry. 2001;158:497–498. doi: 10.1176/appi.ajp.158.3.497-a. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: An animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: Sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biological Psychiatry. 2004;56:308–316. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacology Biochemistry & Behavior. 2007;86:386–394. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences, USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. Dual diagnosis of substance abuse in schizophrenia: Prevalence and impact on outcomes. Schizophrenia Research. 1999;35:S93–S100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- Durany N, Zochling R, Bioisl KW, Paulus W, Ransmayr G, Tatchner T, et al. Human post-mortem striatal alpha4-beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson’s syndrome. Neuroscience Letters. 2000;287:109–112. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, et al. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Flores G, Silva-Gomez AB, Ibanez O, Quirion R, Srivastava LK. Comparative behavioral changes in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus and the prefrontal cortex. Synapse. 2005;56:147–153. doi: 10.1002/syn.20140. [DOI] [PubMed] [Google Scholar]
- George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, et al. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: Involvement of nicotine receptor mechanisms. Schizophrenia Research. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. Journal of Neuroscience. 2002;22:9070–9077. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. American Journal of Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Kadir A, Darreh-Shori T, Almkvist O, Langstrom B, Nordberg A. Changes in brain 11C-nicotine binding sites in patients with mild Alzheimer’s disease following rivastigmine treatment as measured by PET. Psychopharmacology. 2007;191:1005–1014. doi: 10.1007/s00213-007-0725-z. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature Reviews Neuroscience. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koenig J. Animal models of schizophrenia. [Retrieved July 1, 2008];Schizophrenia Research Forum. 2008 from http://www.schizophreniaforum.org/res/models/default.asp.
- Lasser K, Boyd JW, Woolhandler S, Heimmerlstein D, McCormick D, Bor D. Smoking in mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adler L, Benhammou K, Berger R, Breese CR, Drebring C, et al. Smoking and mental illness. Pharmacology Biochemistry and Behavior. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology. 1994;10:199–205. doi: 10.1038/npp.1994.22. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Paschall CB, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatric Services. 2006;57:1482–1487. doi: 10.1176/ps.2006.57.10.1482. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup JS, Gerberding JL. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Newlin DB. The self-perceived survival ability and reproductive fitness (SPFit) theory of substance use disorders. Addiction. 2002;97:427–445. doi: 10.1046/j.1360-0443.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drugs of abuse. Journal of the American Medical Association. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Reitz C, den Heijer T, van Duijn C, Hofman A, Breteler MMB. Relation between smoking and risk of dementia and Alzheimer disease: The Rotterdam study. Neurology. 2007;69:998–1005. doi: 10.1212/01.wnl.0000271395.29695.9a. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell AK, El Koury A, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: Relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39:369–377. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescence? Neurotoxicology and Teratology. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Williams JM, Ziedonis DM. Financial implications of cigarette smoking among individuals with schizophrenia. Tobacco Control. 2004;13:206. [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biological Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]