Abstract
A healthy taste system is important to the maintenance of nutrition and overall quality of life, and taste disorders are associated with many inflammatory states. We previously determined the immune cells in normal human gustatory tissue; they are predominantly dendritic cells and CD4 T cells with a few macrophages and B lymphocytes present. There are, however, few reports of the subtypes of resident lymphocytes in or near taste tissues. The present study further characterized the distribution and population of the major subtypes of T cells in situ within biopsies of healthy human fungiform papillae (FP). Immunohistochemical analyses indicated that T-helper (Th)1 cells (CCR5+) were more predominant in FP than Th2 T cells (CCR4+). CD45RO+ memory T cells were the principal T cells in gustatory tissue, whereas CD45RA+ naive T cells were uncommon. Regarding subcompartments of the tissue, most intraepithelial lymphocytes of FPs were γ/δ T cells, whereas the major subtype of lymphocytes in the lamina propria were α/β T cells. Regulatory T cells that express CTLA-4 (CD152) and interleukin-2 receptors (IL-2R, CD25) were found at low levels in FP. The T cells stand ready to respond to inflammatory and infectious insults and may play a role in the taste alterations observed during acute and chronic inflammatory states.
Keywords: lymphocyte subset, memory T cells, regulatory T cells, taste tissue, Th1 cells
Introduction
A healthy and functioning taste system is important to the maintenance of ingestion, proper diet, and overall well-being. Taste alterations and taste loss affect the pleasure patients derive from food, their willingness to eat, and their desire to participate in social gatherings related to food, such as eating meals with family and friends (Breslin et al. 1997; Varga et al. 2000). In general, immune regulation and inflammatory status are important to the normal physiology of the taste system. In many inflammatory disorders, taste function is altered. Whereas the mechanisms for these taste alterations are unknown, there are several immune cells and molecules in and near taste tissue that likely play causal roles. For example, several key cells of the immune system reside in or near human gustatory tissue (fungiform papillae, FP; Feng et al. 2009). Functional molecules of the immune signaling pathways reside in taste buds as well (Wang et al. 2007), and these molecules are differentially regulated in taste tissue in clinical studies (Mann 2002) and in experimental paradigms (Phillips and Hill 1996; Cavallin and McCluskey 2005). Patients with chronic inflammatory and autoimmune diseases report taste dysfunctions (Mann 2002). Also, inflammation induced by radiotherapy to the oral cavity for treatment of head and neck cancer is strongly correlated with either hypogeusia or ageusia and the consequent loss of interest in feeding (Conger and Wells 1969; Esses et al. 1988; Nelson 1998; Yamashita et al. 2006). The exogenous use of cytokines, such as interferons (IFNs), is associated with taste disorders, and in experimental models, IFNs cause apoptosis of taste cells (Abdollahi and Radfar 2003; Wang et al. 2007). Rats with reduced nerve responses to sodium caused by the experimental denervation of anterior tongue taste via chorda tympani nerve transection followed by dietary sodium restriction are accompanied by compromised immune function (Cavallin and McCluskey 2005). Furthermore, the activation of certain immune responses with model infectious agents and immune activators, such as lipopolysaccharide from bacteria, may promote more rapid recovery from gustatory neural injury and the return of normal taste function (Cavallin and McCluskey 2005), suggesting a positive regulatory role on taste tissue. This induced recovery of taste function appears to be mediated, in part, by increased activation of macrophages and T cells (Cavallin and McCluskey 2005; Phillips and Hill 1996).
We previously identified the distribution and population of various immune cells in the epithelia and lamina propria of FPs and found that dendritic cells (DC), macrophages, and T lymphocytes were the constitutive guardians of anterior lingual human taste tissue, with DCs and T cells predominating under healthy conditions. Low levels of macrophages and B lymphocytes were detected. T lymphocytes were frequently present throughout the FP with CD4+ T cells more prevalent than CD8+ T cells (Feng et al. 2009). These results suggest that DCs and CD4+ T cells may play important roles in affecting gustatory function in inflammatory states as they defend the tissue. CD4+ T cells act as helper T cells. The functions of T helper cells in the immune system are very important and unique. Although they have no direct cytotoxic or phagocytic activity to pathogens or cancer cells, they are involved in regulating the activities of most other immune cells, such as antigen (Ag) presentation on immune cells, B-cell antibody production, CD8 T-cell cytotoxicity, and bactericidal activity of phagocytes. CD4+ T-helper (Th) cells are highly diverse in function and can be identified by molecular markers on their cell surface and cytokines they produce to delineate them into different subtypes, including Th1, Th2, Th3 cells; regular, naive and memory T cells; resting and activated T cells; and others.
Accumulating evidence suggests that the oral immune system is pivotal in mediating the interactions between host and microbia that shape the oral environment. Oral homeostasis arises from a highly dynamic balance between host protective immunity and regulatory mechanisms. This regulation is achieved by a number of T-cell populations with different roles in immune regulation and their acting through a set of shared regulatory pathways. However, little is known about T-cell subsets in human gustatory tissue. Here, we identify the populations of major T-cell subsets controlling immune responsiveness in anterior lingual gustatory tissue. We used immunohistochemistry to mark the surface antigens associated with T-cell functions and activations. The results show that the subsets of T cells in human taste tissue feature prevalent Th1 cells and memory cells, a high frequency of intraepithelial γ/δ T cells and low level of activated and regulatory T cells (Treg) under healthy conditions. This work provides normative data for the subsequent study of inflammatory processes in patients with gustatory perceptual and behavioral dysfunctions, as well as for the treatment of taste disorders related to immune responses and acute and chronic inflammatory states.
Materials and methods
Human subjects
Twelve healthy adult subjects (six men and six women; age 35.8 ± 9.6 years) were recruited through the Department of Otolaryngology at Thomas Jefferson University and were evaluated by the physicians. The evaluations were performed to ensure that all the subjects had normal taste perception and were free of systemic inflammation, immune-related diseases, and gustatory lingual disease, and were not taking prescription medications. None presented with clinical complaints or abnormal taste perceptions. Taste self- evaluation was conducted via a questionnaire established by the Monell/Jefferson Smell and Taste Clinic at Thomas Jefferson University. The collection of human tissue was conducted after subjects provided informed consent in accordance with the Declaration of Helsinki according to a protocol approved by the Institutional Review Board of Thomas Jefferson University and the Office of Regulatory Affairs University of Pennsylvania.
Tissue sampling
Four human lingual FPs were collected from anterior tongue with microscissors from each of the subjects by a licensed surgeon as described elsewhere (Rossier et al. 2004); in total, 48 biopsies were collected for this study. Biopsies were immediately placed in 4% paraformaldehyde–phosphate-buffered saline (PBS) for 1–2 h at 4°C, processed through a series of 10–30% sucrose solutions dissolved in H2O for cryoprotection, and then embedded in M1 matrix medium (Shandon) and frozen using an acetone–dry ice bath. Frozen sections were cut at a thickness of 10 μm through the entire biopsy, mounted on StarFrost slides (Mercedes Medical) and stored at −20°C until analyzed.
Immunohistochemistry
We used previously described immunohistochemistry techniques (Yee and Rawson 2005) to identify immune cell populations in FPs. Briefly, primary and secondary antibodies were selected for their specificity, sensitivity, and availability. Frozen sections were dried in an oven at 37°C for 30 min and rehydrated 10 min with 0.1 M PBS at pH 7.0. Endogenous peroxidase was blocked by 3% hydrogen peroxide for 10 min. Tissue sections were then washed in PBS for 3 × 5 min, incubated in Superblock (Pierce) for 1 h at room temperature (RT) to block nonspecific-binding sites. Mouse antibodies specifying the following cell surface markers were employed as primary antibodies and incubated with the tissue sections for 2 h at RT: T-cell receptor (TCR)-γ/δ, CD45RA, CD45RO, CCR4, CCR5, CD25, CD152, CD123 (or overnight at 4°C for TCR-α/β; BD Pharmingen). The characteristics and biological functions of the antibodies applied in this research are listed in Table 1. After a 3 × 5 min PBS wash, tissue sections were incubated for 1 h with secondary biotinylated antibody then in prediluted streptavidin–horseradish peroxidase (HRP) complex (Anti-Ig HRP Detection Kits; BD Pharmingen) for 45 min. Immunoreactivity was detected using diaminobenzidine (DAB) as the chromogen. Sections were counterstained with hematoxylin and 0.3% (v/v) diluted ammonia prior to dehydration and coverslipping. Controls for nonspecific binding were performed by substituting primary antibody with an isotypic control immunoglobulin G or excluding primary antibodies. Each antibody was used on 48 sections from the 12 subjects.
Table 1.
Description of antibodies used for immunohistochemistry
| Ag | Antibody Source | Dilution | Ag description |
| CD3 | BD Biosciences | 1:50 | Pan T lymphocytes |
| TCR-α/β | BD Biosciences | 1:10 | TCR-α/β T lymphocytes |
| TCR-γ/δ | eBiosciences | 1:50 | TCR-γ/δT Lymphocytes |
| CD45RA | eBiosciences | 1:50 | Unprimed T lymphocytes |
| CD45RO | eBiosciences | 1:100 | Primed T lymphocytes |
| CCR4 | BD Biosciences | 1:50 | Th2 lymphocytes |
| CCR5 | BD Biosciences | 1:50 | Th1 lymphocytes |
| CD25 | BD Biosciences | 1:50 | Activated/Treg lymphocytes |
| CD152 | BD Biosciences | 1:50 | Treg lymphocytes |
| CD123 | eBiosciences | 1:50 | Plasmacytoid dendritic cells |
Computer-assisted image analysis and cellular quantification
The method of cell quantification has been published previously (Robert et al. 1995; Phipps et al. 2004; Feng et al. 2009). Brightfield images were captured at a 10 × 10 magnification using the Nikon DXM 1200C attached to a Nikon Eclipse 80i microscope. The density of specific immunoreactive cells in both the epithelium and lamina propria of FPs were quantitatively measured using Image-Pro Plus image analysis software 6.0. This software allows analysis of brown- or orange-stained areas of tissue sections (obtained after immunohistochemistry with DAB staining) with light blue counterstaining. On the computer screen, two masks were used. One mask was used to define a threshold to determine the total areas of the epithelium and lamina propria in measured section. A second mask was applied over the captured image based on the color range of DAB-reactive immune cells. The masked areas within the epithelium and lamina propria were measured and summed, and the size for each region was calculated. The cell population was expressed as the ratio of the masked colored area (cells) to the total area of tissue region measured. The accuracy of the computer evaluation of color was checked and validated by human visual appraisal of each section at 10 × 10 magnification. All slides were computer analyzed by the same person. For each subject and antibody, four sections from four different FPs were analyzed and the relevant values were averaged. This protocol was developed while assessing sections with different densities of positive staining with experimenters blind to the antibody used.
Statistical analysis
The two-sample t-test was performed using the PRISM software package (GraphPad) to compare the differences of cells (TCR-α/β, TCR-γ/δ, CD45RA, CD45RO, CCR4, CCR5, CD25, CD152, and CD123) in the compartments of epithelium and lamina propria. The differences in cellular intensities of α/β T cells versus γ/δ T cells, CD45RA+ “naive” T cells versus CD45RO “memory” T cells, and CCR4+ Th2 versus CCR5+ Th1 cells in the compartments of epithelium and lamina propria were also analyzed. P < 0.05 denotes significant difference.
Results
Th1 (CCR5+) and Th2 (CCR4+) cells
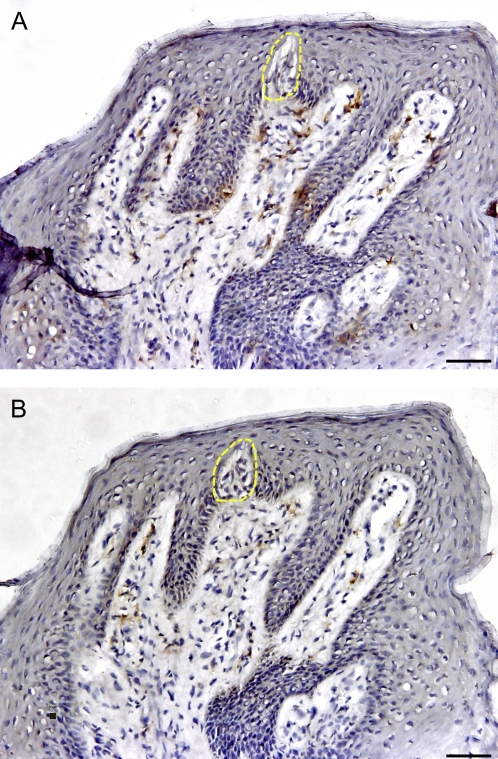
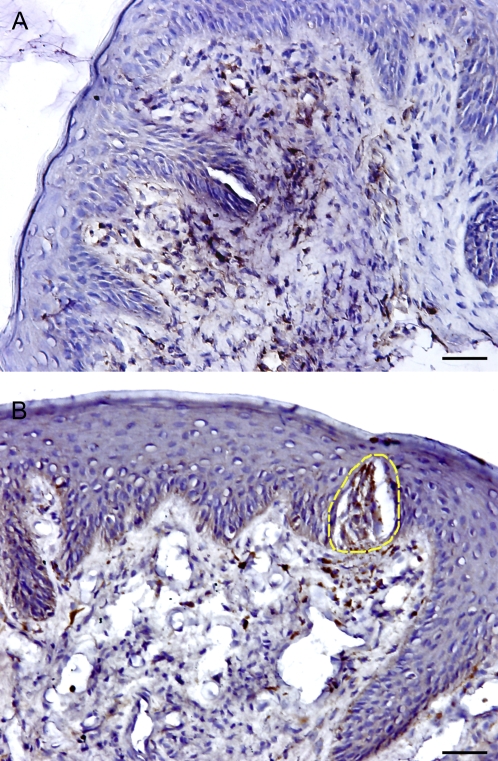
To explore the general presence and distribution of Th1 and Th2 T cells, we identified chemokine receptors CCR5 and CCR4, which are expressed on Th1 and Th2 cells, respectively, in human FP biopsies (Baudouin et al. 2005). Our results show that CCR5-expressing Th1 cells were consistently present in the epithelial and lamina propria compartments of taste tissues and were scattered throughout the sections (Figure 1). Quantification of cellular density using computer-assisted image analysis indicated that the percentage area of CCR5+ staining comprised 1.33% of the epithelium and 4.86% of the lamina propria in virtually all sections. In contrast, CCR4-positive Th2 T cells were only identified in 66.6% (8 out of 12) subjects, indicating that one-third of all FP sections contained no detectable CCR4-expressing cells. The densities of CCR4-expressing cells were also significantly lower than for CCR5 both in the epithelial (0.38% vs. 1.33%) and in the lamina propria (0.61% vs. 4.86%) compartments of FPs. These data clearly demonstrate that CCR5+ Th1 cells are more prevalent than CCR4+ Th2 cells in human lingual taste tissue.
Figure 1.
Presence and distribution of CCR4+ (Th2) and CCR5+ (Th1) cells in the human FP. The brown color in the sections shows the specifically stained cells. (A) CCR5+ expressing Th1 cells. (B) CCR4+ expressing Th2 cells. The yellow dotted line shows taste bud. The scale bar indicates 20 μm.
Regulatory T cells
To study the population and distribution of Treg cells and activated T cells, sections of human FP were labeled with antibodies against CTLA-4 (CD152) and interleukin-2 receptor (IL-2R, CD25). Both these two molecules are expressed on Treg cells (Uhlig and Powrie 2005), and CD25 is also expressed on activated T cells (Marzano et al. 2009). These two biomarkers were both used as they serve as cross-references for Treg in FP. Human FPs express similar low levels of CD25 and CD152 in the epithelial and lamina propria compartments, as shown in Figure 2. There were no significant differences in densities between the cells identified by these two markers either in the epithelium or underlying lamina propria of taste tissues.
Figure 2.
Presence and distribution of CD25+ and CD152+ cells in the human FP. The brown color in the sections shows the specifically stained cells. (A) CD25+ expressing T cells. (B) CD152+ expressing T cells. The scale bar indicates 20 μm.
Memory T cells and naive T cells
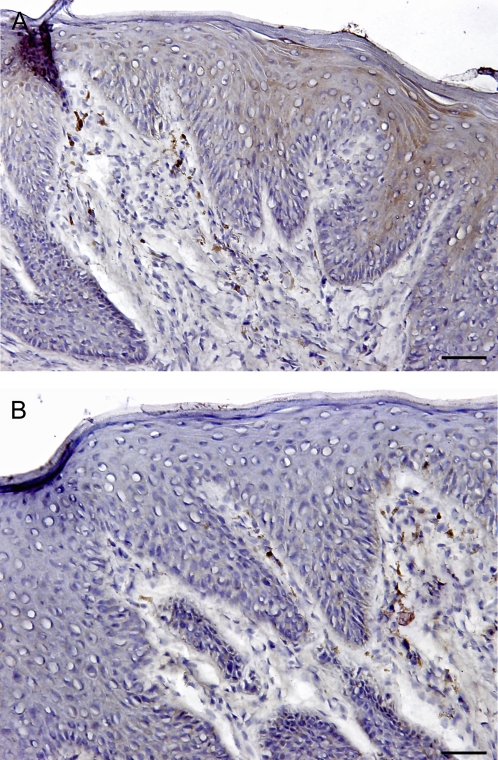
T cells generally consist of a mixture of naive T cells (CD45RA+) and memory T cells (CD45RO+). After antigen stimulation, some naive T cells transition to memory cells and activated cells. CD45RA+ naive and CD45RO+ memory cells were diffusely distributed in the epithelium and lamina propria of most human FPs. In some sections, CD45RO+ cells formed small clusters within epithelium and especially the lamina propria, as shown in Figure 3. Overall, the density of CD45RO+ expressing cells was higher than that of CD45RA+ cells in most FP sections. The percentage of CD45RO+ stained area was 4.08% of the epithelium and 9.9% of the lamina propria, whereas the CD45RA+ stained areas were only 2.32% and 1.45% in epithelium and lamina propria, respectively (Figure 6). These data indicate that CD45RO+ memory cells are more abundant T cells than are CD45RA+ naive T cells in FP.
Figure 3.
Presence and distribution of CD45RA+ (naive T cells) and CD45RO+ (memory T cells) in the human FP. The brown color in the sections shows the specifically stained cells. (A) CD45RA+ expression and distribution. (B) CD45RO+ expression and distribution. The yellow dotted line shows taste bud. The scale bar indicates 40 μm.
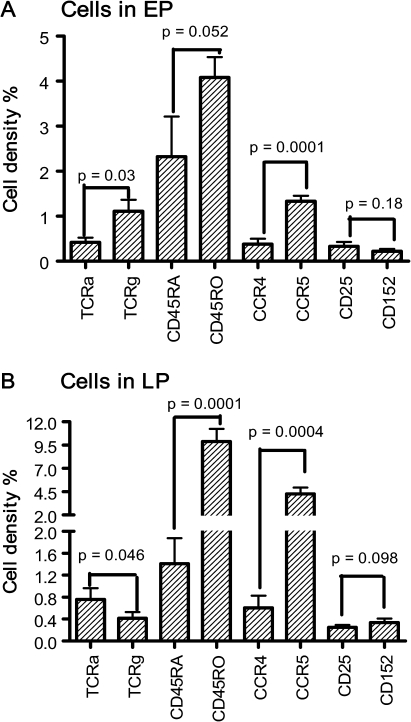
Figure 6.
Cellular populations in epithelium and lamina propria. The cell densities are presented as the percentages of specifically stained area against the total area of the histological section. Data are expressed as mean ± SE, n = 12, which is the number of subjects analyzed. EP, epithelium; LP, lamina propria; TCRa, α/β T cells; TCRg, γ/δ T cells.
α/β T cells and γ/δ T cells
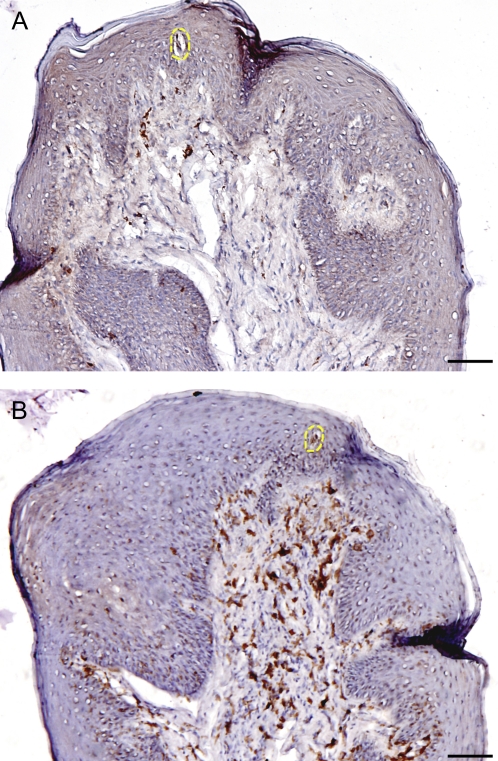
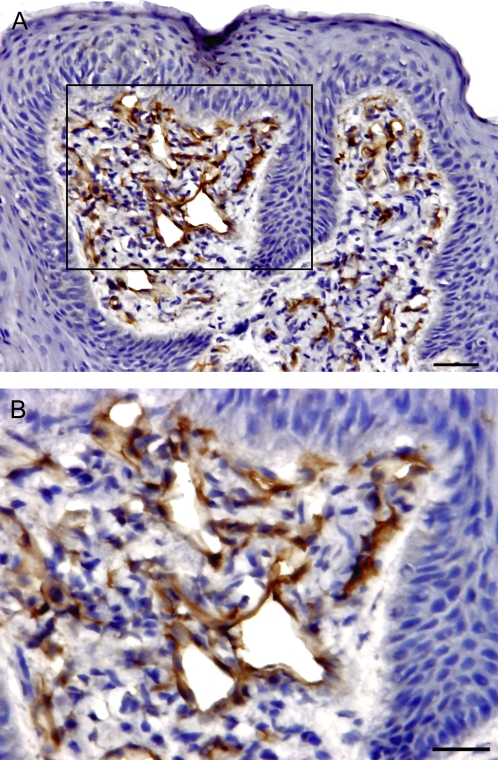
The distribution and population of T cells with α/β TCR and γ/δ TCR were identified in epithelium and lamina propria of human FPs. α/β TCR-expressing cells comprise 90% of T cells, whereas γ/δ TCR-expressing cells comprise only 10% of cells, but are enriched in the mucosal epithelia in general. Both α/β T cells and γ/δ T cells were observed scattered throughout the FPs but with very different distributions within the epithelial and lamina propria compartments. The majority of α/β T cells are localized in the lamina propria but γ/δ T cells are more prevalent in the epithelium (Figures 4 and 6). Quantitative analysis on cellular density of sections found that the ratio of α/β T cells to γ/δ T cells are 1:3 in the epithelium and the approximately inverted ratio 2:1 in the lamina propria of FP tissue. This result clearly demonstrates that most intraepithelial lymphocytes are γ/δ T cells in human peripheral taste tissue.
Figure 4.
Presence and distribution of α/β T cells and γ/δ T cells in the human FP. The brown color in the sections shows the specifically stained cells. (A) α/β T cells. (B) γ/δ T cells. The yellow dotted line shows taste bud. The scale bar indicates 20 μm.
CD123+ plasmacytoid DCs
DC cells, including plasmacytoid (PDCs) and myeloid DCs, have a crucial role in regulation of T cell–mediated immune responses. PDCs tend to create an environment that favors a Th2-biased immune reaction. Relatively high and variable numbers of CD123+ PDCs were identified in the lamina propria of all samples but few were found in the epithelial compartment of the same tissues. In addition to PDCs, which were diffusely located in the connective tissue, endothelial venules in the FP tissues also expressed strong CD123, as shown in Figure 5. In contrast to the distribution feature of the CD1a+ Langerhans cells that are exclusively present in the epithelial compartment of the FP tissues, as we demonstrated previously (Feng et al. 2009), CD123+ PDCs were exclusively present in connective tissue beneath the epithelium (Figure 5).
Figure 5.
Presence and distribution of CD123+ PDCs in the FP. The brown color in the sections shows the specifically stained cells. (A) CD123+ DCs exclusively present in lamina propria, with few present in epithelial compartment. The scale bar indicates 20 μm. (B) High magnification of image showing that endothelial venules in FP tissues also express CD123. The scale bar indicates 10 μm.
Quantification of the cell populations
Quantification of the relative density of each identified lymphocyte subset in the epithelium and the lamina propria is summarized in Figure 6. In the epithelium, the densities of the stained cells are, in descending order: CD45RO, CD45RA, CCR5, γ/δ T cells, α/β T cells, CD25, and CD152. In the lamina propria, the order of the cell populations are CD45RO, CCR5, CD45RA, α/β T cells, CCR4, γ/δ T cells, CD152, and CD25. CD45RO+ cells are clearly the most frequent cell types in both epithelial and lamina propria compartments of the tissue, whereas CD25 and CD152 are the least commonly observed cells. Overall, the densities of most subsets of T cells are higher in the lamina propria than in the epithelium, except γ/δ T cells and CD45RA+ naive T cells. Analysis of relative pairs of subsets of T cells demonstrates that α/β T cells are significantly less common than γ/δ T cells in the epithelium but more numerous in the lamina propria (P < 0.05). The densities of CD45RO+ memory T cells are significantly higher than are CD45RA+ naive T cells in both epithelial and lamina propria compartments (P = 0.05 and P < 0.001, respectively). CCR5+ Th1 cells are also significantly more numerous than CCR4+ Th2 cells in both the epithelium and the lamina propria (P < 0.001). A similar density of CD25+ and CD152+ cells was observed in the epithelium and the lamina propria. The results are presented in Figure 6.
Discussion
We determined the identity and distribution of the major subtypes of T cells in situ in the biopsies of healthy human FP to better understand potential interactions between the immune system and the gustatory system, particularly the possible regulatory roles of T cells on taste activity. All the biopsies of FP were collected from volunteers who had neither signs of systemic inflammation nor clinical signs of significant oral diseases. Therefore, the results represent the immune cell populations in human gustatory tissue under healthy conditions. Our overall results show that the subsets of T cells in human taste tissues are characterized by frequent intraepithelial γ/δ T cells, dominant memory cells T cells and prevalent Th1 cells, with low levels of activated T cells and Treg.
Establishing the functional diversity of helper T lymphocytes, such as the Th1 and Th2 subclasses, as well as Treg, is useful for understanding the mechanisms of immune regulation both in healthy and disease conditions. To analyze the Th1 and Th2 cells in human taste tissue, we focused on cells that express two chemokine receptors, CCR5 and CCR4, which are associated with the Th1 and Th2 cells, respectively (Campbell and HayGlass 2000; Trinh et al. 2008). These differential patterns of chemokine receptor expression have been widely applied to identify Th1- and Th2-type cells in clinical and animal experiments (Baudouin et al. 2005; Elhini et al. 2005; Trinh et al. 2007). Prior to this study, little was known of their presence in the human lingual taste system. A high level of CCR5 (Th1) expression was observed in all subjects. In contrast, a weak expression of CCR4 (Th2) was identified and appeared in only a subset of individuals examined. Clearly, Th1 cells are more prevalent in human peripheral taste tissue than are Th2 cells. This strongly suggests that local immune responses in human taste tissue are Th1-type biased. The Th1 and Th2 balance is a key component of the digestive mucosal immune defense against pathogens and immune tolerance. Whereas Th1 cells secrete the cytokines IFN and tumor necrosis factor, Th2 cells secrete IL-4, IL-5, IL-9, and IL-13. Previous studies demonstrated the presence of Th1 cytokine IFN-mediated signaling pathways in taste bud cells and inflammation-induced taste alteration. Cytokine communication between receptor cells in taste buds and surrounding immune microenvironments related to Th1 and Th2 cells may contribute to the maintenance of normal taste activity and the immunotolerance of nutrients in the oral cavity.
The CCR5 ligands include Macrophage Inflammatory Protein-1α (MIP-1α) and MIP-1β, which recruit both macrophages and neutrophils and were recently identified in taste tissue (Cavallin and McCluskey 2007). CCR4 is the main receptor for macrophage-derived chemokines, as well as thymus and activation-regulated chemokines. The interaction between chemokines and their receptors is an important step in the control of leukocyte migration into sites of inflammation. Recent studies suggest that the dysregulation of Th1–Th2 balance is associated with inflammation-related chemosensory disorders, such as in chronic rhinosinusitis (Elhini et al. 2005) and oral lichen planus (Iijima et al. 2003). These findings of different patterns of chemokine receptor expression demonstrate a possible mechanism of selective induction of migration and activation of Th1- and Th2-type cells during inflammation into peripheral human taste tissue.
We also demonstrated the expression of two important cellular markers for Treg cells, CD25 and CTLA-4 (CD152), in human FPs. Both markers exhibited a similar low level of expression and similar tissue distribution pattern in taste tissue. CD25 is now known to be IL-2Rα, an early activation marker on T cells and also serves as a biomarker of Treg cells and has been widely used with the marker FoxP3 to identify CD3+CD25+ Treg cells (Fontenot et al. 2005). Similar to CD25, CD152 (CTLA-4) is also expressed on activated T cells and some types of Treg cells (Read et al. 2000); however, it downregulates T-cell activation through negative TCR signaling and competitive antagonism of CD28- and B7-mediated costimulation (Carreno et al. 2000). We believe that the Treg cells in human gustatory tissue may play a role in the maintenance of oral homeostasis and interactions between the local immune system and the taste system; however, the exact role of these cells in taste tissue remains unknown.
The human T-cell population can also be divided into functionally distinct and largely reciprocal subsets based on their differential expression of CD45 isoforms (CD45RA, CD45RO). CD45RO+ memory T cells respond maximally to recall Ag and provide help for specific immune responses, such as antibody synthesis. In contrast, the CD45RA+ naive population responds poorly to recall Ag and lacks helper function for specific immune response (Morimoto and Schlossman 1993). Here, we identified memory T-cell and naïve T-cell subsets in taste tissue by examination of CD45RO and CD45RA. We note that CD45RO+ memory cells are the principal T-cell type in human taste tissue. The results agree with previous reports of human gingival (Colasante et al. 1992) and buccal mucosa (Walton et al. 1998). The CD45RO+ T cells in local tissue may reflect conversion of T cells from naive (CD45RA+) to memory (CD45RO+) phenotype at the site of exposure to repeated Ag stimulation (Sanders et al. 1988). This conversion is believed to be unidirectional and irreversible. We believe that the high frequency of cells expressing CD45RO in the gustatory system mirrors the process of immature and naïve CD45RA cell conversions into memory cells after activation by oral antigens from food and oral bacteria. As only a low level of activation marker CD25 was displayed in the same sections of FP, this may indicate that most CD45RO+ memory T cells in human FPs are in a resting state instead of activated state. This scenario is consistent with observations of relatively weak immune responses in the oral cavity during healthy conditions.
T lymphocytes recognize Ag through the TCRs. There are two structurally distinct types of CD3-associated T-cell receptors, namely heterodimers composed of two pairs of glycoprotein subunits: α/β (α/β TCR) or γ/δ (γ/δ TCR). Over 90% of human peripheral T lymphocytes express α/β TCR and less than 10% express γ/δ TCR (Acuto and Reinherz 1985; Groh et al. 1989), but γ/δ T cells are reported to be relatively enriched within mucosal epithelia and seem to play a specific role in mucosal immunity (Goodman and Lefrancois 1988). γ/δ T cells may coordinate innate and acquired immune responses that maintain the integrity of epithelial tissues (Komano et al. 1995; Mak and Ferrick 1998; Hayday and Tigelaar 2003). They are also increased in diseased mucosa (Yeung et al. 2000). Here, we identified the α/β T cells and γ/δ T cells in FP and discovered that they are differentially distributed in the epithelial and lamina propria compartments. The majority of α/β T cells are located in the lamina propria but γ/δ T cells are more prevalent in the epithelium where taste buds reside. The ratio of α/β T cells to γ/δ T cells is 1:3 in epithelium but is 2:1 in lamina propria of FP tissue. This distribution may contribute to the highly controlled immunotolerance in oral mucosa, which is heavily exposed to high levels of environmental antigens such as nutrients and various bacteria.
The population and distribution of CD123+ PDCs were identified in this project to expand our study of the heterogeneity of DCs in taste tissue. PDCs, originally believed to be plasmacytoid T cells in skin (Eckert and Schmid 1989), have been recently identified as a subset of DCs (Liu 2005). Previously, we identified four different subsets of DCs that populate the mucosa of FP; the prototypical DCs that express CD11c, Langerhans cells that express CD1a, immature DCs that express DC-SIGN, and mature DCs that express CD83. These DCs are distributed either in both the epithelia and lamina propria (CD11, CD83, and DC-SIGN) or exclusively in epithelium (CD1a) (Feng et al. 2009). Presently, we found the distribution of CD123+ PDCs to be distinct from the other classes of DCs in papillae in that CD123+ PDCs are exclusively present in the lamina propria of the FP tissues. These differences in distribution likely reflect the specific function of these subsets of DCs in local immune responses. As a unique subset of DCs, PDCs recognize viral, bacterial, and self-nucleic acids. They produce type-I IFN, activate T cells, and play a crucial role in the initiation of immune responses including antiviral and antitumoral immune responses (Marafioti et al. 2008). They are featured by a high level of IL-3 receptor α-chain (CD123) expression, and this expression may be upregulated by T cell–produced IL-3 via autocrine or paracrine stimulation. It is believed that the CD123 expression is associated with Th2-cell immune reactions; note that Th2 cells were less commonly observed in the lamina propria than Th1 cells (Figure 1). The compartmentalization of DCs in the different regions of taste tissue may be important to the immune surveillance of this tissue under the normal, healthy conditions and immune response in some diseases.
In summary, we have described the characteristic subsets of T lymphocytes in the human peripheral taste tissue. As an important part of the immune cell network in the lingual gustatory system, these subtypes of T cells might have regulatory roles in protecting the local tissue. These observations provide basic cellular knowledge for the roles of immune regulation and inflammation on gustatory activity in health and disease.
Funding
This work was supported by National Institutes of Health [DC 02995 and P50 DC 06760 to P.A.S.B].
Acknowledgments
We thank the volunteers and their families for their participations, Linda Wysocki for her technical help in histology, Suzanne Alarcon and Anne Ledyard for their invaluable assistance in tissue collection and Dr Karen K. Yee for her valuable assistance with figure preparation. Conflict of interest: All authors declare that there are no conflicts of interest.
References
- Abdollahi M, Radfar M. A review of drug-induced oral reactions. J Contemp Dent Pract. 2003;4(1):10–31. [PubMed] [Google Scholar]
- Acuto O, Reinherz EL. The human T-cell receptor. Structure and function. N Engl J Med. 1985;312(17):1100–1111. doi: 10.1056/NEJM198504253121706. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Liang H, Bremond-Gignac D, Hamard P, Hreiche R, Creuzot-Garcher C, Warnet JM, Brignole-Baudouin F. CCR 4 and CCR 5 expression in conjunctival specimens as differential markers of T(H)1/ T(H)2 in ocular surface disorders. J Allergy Clin Immunol. 2005;116(3):614–619. doi: 10.1016/j.jaci.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Chapman GB, Mattes RD, Beauchamp GK, Cowart BJ. Quality of life for patients with chemical senses disorders. Chem Senses. 1997;22:650. [Google Scholar]
- Campbell JD, HayGlass KT. T cell chemokine receptor expression in human Th1- and Th2-associated diseases. Arch Immunol Ther Exp (Warsz) 2000;48(6):451–456. [PubMed] [Google Scholar]
- Carreno BM, Bennett F, Chau TA, Ling V, Luxenberg D, Jussif J, Baroja ML, Madrenas J. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165(3):1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Lipopolysaccharide-induced up-regulation of activated macrophages in the degenerating taste system. J Neurosci Res. 2005;80(1):75–84. doi: 10.1002/jnr.20438. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Upregulation of the chemokine monocyte chemoattractant protein-1 following unilateral nerve injury in the peripheral taste system. Neurosci Lett. 2007;413(3):187–190. doi: 10.1016/j.neulet.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Colasante A, Rosini S, Piattelli A, Artese L, Aiello FB, Musiani P. Distribution and phenotype of immune cells in normal human gingiva: active immune response versus unresponsiveness. J Oral Pathol Med. 1992;21(1):12–16. doi: 10.1111/j.1600-0714.1992.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Conger AD, Wells MA. Radiation and aging effect on taste structure and function. Radiat Res. 1969;37(1):31–49. [PubMed] [Google Scholar]
- Eckert F, Schmid U. Identification of plasmacytoid T cells in lymphoid hyperplasia of the skin. Arch Dermatol. 1989;125(11):1518–1524. [PubMed] [Google Scholar]
- Elhini A, Abdelwahab S, Ikeda K. Th1 and Th2 cell population in chronic ethmoidal rhinosinusitis: a chemokine receptor assay. Laryngoscope. 2005;115(7):1272–1277. doi: 10.1097/01.MLG.0000165380.64445.EE. [DOI] [PubMed] [Google Scholar]
- Esses BA, Jafek BW, Hommel DJ, Eller PM. Histological and ultrastructural changes of the murine taste bud following ionizing irradiation. Ear Nose Throat J. 1988;67(7):478–484. 487–478, 493. [PubMed] [Google Scholar]
- Feng P, Yee KK, Rawson NE, Feldman LM, Feldman RS, Breslin PA. Immune cells of the human peripheral taste system: dominant dendritic cells and CD4 T cells. Brain Behav Immun. 2009;23(6):760–766. doi: 10.1016/j.bbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Goodman T, Lefrancois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AK, Strominger JL, Brenner MB. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169(4):1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3(3):233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- Iijima W, Ohtani H, Nakayama T, Sugawara Y, Sato E, Nagura H, Yoshie O, Sasano T. Infiltrating CD8+ T cells in oral lichen planus predominantly express CCR5 and CXCR3 and carry respective chemokine ligands RANTES/CCL5 and IP-10/CXCL10 in their cytolytic granules: a potential self-recruiting mechanism. Am J Pathol. 2003;163(1):261–268. doi: 10.1016/S0002-9440(10)63649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, Mombaerts P, Tonegawa S, Yamamoto H, Itohara S. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 1995;92(13):6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4(7):764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- Mann NM. Management of smell and taste problems. Cleve Clin J Med. 2002;69(4):329–336. doi: 10.3949/ccjm.69.4.329. [DOI] [PubMed] [Google Scholar]
- Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, Dictor M, Hansmann ML, Pileri SA, Dyer MJ, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111(7):3778–3792. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- Marzano AV, Vezzoli P, Fanoni D, Venegoni L, Berti E. Primary cutaneous T-cell lymphoma expressing FOXP3: a case report supporting the existence of malignancies of regulatory T cells. J Am Acad Dermatol. 2009;61(2):348–355. doi: 10.1016/j.jaad.2008.11.894. [DOI] [PubMed] [Google Scholar]
- Morimoto C, Schlossman SF. P. Rambotti Lecture. Human naive and memory T cells revisited: new markers (CD31 and CD27) that help define CD4+ T cell subsets. Clin Exp Rheumatol. 1993;11(3):241–247. [PubMed] [Google Scholar]
- Nelson GM. Biology of taste buds and the clinical problem of taste loss. Anat Rec. 1998;253(3):70–78. doi: 10.1002/(SICI)1097-0185(199806)253:3<70::AID-AR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Phillips LM, Hill DL. Novel regulation of peripheral gustatory function by the immune system. Am J Physiol. 1996;271(4 Pt 2):R857–R862. doi: 10.1152/ajpregu.1996.271.4.R857. [DOI] [PubMed] [Google Scholar]
- Phipps S, Habib FK, McNeill A. Quantitative morphometric analysis of individual resected prostatic tissue specimens, using immunohistochemical staining and colour-image analysis. BJU Int. 2004;94(6):919–921. doi: 10.1111/j.1464-410X.2004.05060.x. [DOI] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M, Costa P, Bressolle F, Mottet N, Navratil H. Percentage area density of epithelial and mesenchymal components in benign prostatic hyperplasia: comparison of results between single biopsy, multiple biopsies and multiple tissue specimens. Br J Urol. 1995;75(3):317–324. doi: 10.1111/j.1464-410x.1995.tb07342.x. [DOI] [PubMed] [Google Scholar]
- Rossier O, Cao J, Huque T, Spielman AI, Feldman RS, Medrano JF, Brand JG, le Coutre J. Analysis of a human fungiform papillae cDNA library and identification of taste-related genes. Chem Senses. 2004;29(1):13–23. doi: 10.1093/chemse/bjh002. [DOI] [PubMed] [Google Scholar]
- Sanders ME, Makgoba MW, Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988;9(7–8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Trinh L, Brignole-Baudouin F, Pauly A, Liang H, Houssier M, Baudouin C. Th1- and Th2-related chemokine and chemokine receptor expression on the ocular surface in endotoxin-induced uveitis. Mol Vis. 2008;14:2428–2434. [PMC free article] [PubMed] [Google Scholar]
- Trinh L, Brignole-Baudouin F, Raphael M, Dupont-Monod S, Cassoux N, Lehoang P, Baudouin C. Th1 and Th2 responses on the ocular surface in uveitis identified by CCR4 and CCR5 conjunctival expression. Am J Ophthalmol. 2007;144(4):580–585. doi: 10.1016/j.ajo.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Uhlig HH, Powrie F. The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol. 2005;27(2):167–180. doi: 10.1007/s00281-005-0206-6. [DOI] [PubMed] [Google Scholar]
- Varga EK, Breslin PA, Cowart BJ. The impact of chemosensory dysfunction on quality of life. Chem Senses. 2000;25:654. [Google Scholar]
- Walton LJ, Macey MG, Thornhill MH, Farthing PM. Intra-epithelial subpopulations of T lymphocytes and Langerhans cells in oral lichen planus. J Oral Pathol Med. 1998;27(3):116–123. doi: 10.1111/j.1600-0714.1998.tb01926.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. Inflammation activates the interferon signaling pathways in taste bud cells. J Neurosci. 2007;27(40):10703–10713. doi: 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Nakagawa K, Tago M, Nakamura N, Shiraishi K, Eda M, Nakata H, Nagamatsu N, Yokoyama R, Onimura M, et al. Taste dysfunction in patients receiving radiotherapy. Head Neck. 2006;28(6):508–516. doi: 10.1002/hed.20347. [DOI] [PubMed] [Google Scholar]
- Yee KK, Rawson NE. Immunolocalization of retinoic acid receptors in the mammalian olfactory system and the effects of olfactory denervation on receptor distribution. Neuroscience. 2005;131(3):733–743. doi: 10.1016/j.neuroscience.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarstrom S, Hammarstrom ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47(2):215–227. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]