Abstract
IncP-9 plasmids are important vehicles for degradation and resistance genes that contribute to the adaptability of Pseudomonas species in a variety of natural habitats. The three completely sequenced IncP-9 plasmids, pWW0, pDTG1 and NAH7, show extensive homology in replication, partitioning and transfer loci (an ∼25 kb region) and to a lesser extent in the remaining backbone segments. We used PCR, DNA sequencing, hybridization and phylogenetic analyses to investigate the genetic diversity of 30 IncP-9 plasmids as well as the possibility of recombination between plasmids belonging to this family. Phylogenetic analysis of rep and oriV sequences revealed nine plasmid subgroups with 7–35 % divergence between them. Only one phenotypic character was normally associated with each subgroup, except for the IncP-9β cluster, which included naphthalene- and toluene-degradation plasmids. The PCR and hybridization analysis using pWW0- and pDTG1-specific primers and probes targeting selected backbone loci showed that members of different IncP-9 subgroups have considerable similarity in their overall organization, supporting the existence of a conserved ancestral IncP-9 sequence. The results suggested that some IncP-9 plasmids are the product of recombination between plasmids of different IncP-9 subgroups but demonstrated clearly that insertion of degradative transposons has occurred on multiple occasions, indicating that association of this phenotype with these plasmids is not simply the result of divergent evolution from a single successful ancestral degradative plasmid.
INTRODUCTION
As agents of horizontal gene transfer (HGT), plasmids contribute considerably to bacterial evolution and adaptation (Thomas, 2000a). Plasmids are extremely diverse and distributed universally in a range of hosts and ecological niches (Turner et al., 2002). Until recently the available molecular tools, such as primers and probes, for identification and classification of plasmids from Gram-negative bacteria originated largely from clinical studies (Couturier et al., 1988; Gotz et al., 1996). The limited usefulness of these in broad ecological studies prompted projects to catalogue and characterize diverse replicons from various environmental contexts (Dahlberg et al., 1997; Kobayashi & Bailey, 1994; Sobecky et al., 1997). Plasmid sequencing also provides valuable insights into their evolution, confirming their mosaic nature based on genetic modules of different origins, and indicating the importance of recombination, cointegration and mobile element insertions [insertion sequence (IS) elements, transposons and integrons] in plasmid diversity (Boyd et al., 1996; Osborn et al., 2000; Tan, 1999; Thomas, 2000b; Toussaint & Merlin, 2002; Tsuda et al., 1999).
Species of the Pseudomonas genus are found in most soil and aquatic environments and some are implicated in diseases of humans, animals and plants (Wackett, 2003). Natural isolates of Pseudomonas often harbour plasmids, which are currently classified into 14 incompatibility groups (Boronin, 1992; Thomas & Haines, 2004). Plasmid-encoded traits contribute to the genetic plasticity and adaptability of pseudomonads in various ecological niches. The IncP-9 plasmid family includes large self-transmissible plasmids associated with degradation and antibiotic- and toxic metal-resistance markers (Korfhagen et al., 1978). The best-known IncP-9 plasmids are pWW0 (Williams & Murray, 1974) and NAH7 (Dunn & Gunsalus, 1973), the paradigms of prokaryotic degradation of mono- and polyaromatic compounds (toluene/xylenes and naphthalene) (Eaton, 1994; Harayama & Rekik, 1989; Kasai & Harayama, 2004; Ramos et al., 1997; Yen & Gunsalus, 1982), and these have been frequently used in natural and genetically modified biodegraders for bioremediation of polluted sites (Lee et al., 1995; Nüsslein et al., 1992; Panke et al., 1998; Parales et al., 2002; Ramos & Timmis, 1987; Ramos et al., 1987; Top et al., 2002).
Hybridization studies with IncP-9 plasmids R2, pMG18, NAH7, pWW0 and SAL (Bayley et al., 1979; Heinaru et al., 1978; Lehrbach et al., 1983) have indicated that diverse IncP-9 replicons share about 6 kb of DNA sequence that must encode functions essential for plasmid survival (‘core sequence’). When sequences of IncP-9 plasmids pMT2, pWW0, pDTG1 and NAH7 became available the relationships between them were estimated by sequence comparison and phylogenetic analysis (Dennis & Zylstra, 2004; Dennis, 2005; Greated & Thomas, 1999; Greated et al., 2002; Sota et al., 2006). PCR primers that target the basic replicon functions of IncP-9 have facilitated studies on the diversity and prevalence of naturally occurring IncP-9 plasmids (Greated & Thomas, 1999; Krasowiak et al., 2002). Thus, the genetic diversity of a limited number of phenotypically different IncP-9 plasmids (Krasowiak et al., 2002) and large collections of IncP-9 Nah replicons (Izmalkova et al., 2006; Leuchuk et al., 2006) have been evaluated by RFLP analysis of replication/maintenance loci, selected nah determinants or complete plasmid genomes. Environmental studies indicate a wide distribution of IncP-9-like replicons in manure, soils and coastal waters and their involvement in natural HGT of the naphthalene-degradation trait (Herrick et al., 1997; Krasowiak et al., 2002; Smalla et al., 2000).
Collaboration with groups in Russia, Belarus and Germany made available a large collection of IncP-9 plasmids, while complete plasmid sequences and tools for analysis and detection of IncP-9 replicons allow analysis of genetic diversity among these plasmids using PCR, sequencing and hybridization. This study presents a subclassification of IncP-9 plasmids based on sequence analysis of the replication loci, oriV and rep. Subsequent analysis of selected backbone loci indicated that phylogenetically distant IncP-9 plasmids must retain many common genes, while modules encoding similar phenotypes have been inserted at different sites in the conserved plasmid backbone, indicating multiple independent acquisitions of these functions.
METHODS
Bacterial strains, plasmids and growth conditions.
Pseudomonas strains carrying IncP-9 plasmids used in this study are listed in Table 1. Additional strains used were Pseudomonas putida BS 394 (cys− Rifr Strr) (Russian Academy of Sciences collection) and Escherichia coli DH5α (F− φ80dlacZΔM15 endA thi-1 recA1 gyrA96 relA1 hsdR17 supE44 deoR Δ(lacZYA-argF) U169; Gibco-BRL). Bacteria were propagated in Luria broth or M9 minimal medium, supplemented with 1.5 % agar when required (Sambrook & Russell, 2001). The cultivation temperature was 30 °C for Pseudomonas spp. and 37 °C for E. coli. To select for catabolic plasmids, bacteria were maintained on M9 lacking glucose but supplemented with an appropriate source of carbon and energy such as naphthalene [0.05–0.1 g naphthalene (Sigma) placed on the lid of an inverted Petri dish], caprolactam and toluene (0.1–0.15 % and 5 mM added to molten agar before pouring, respectively). Cysteine and proline (40 μg ml−1) were added for growth of auxotrophic strains. Antibiotic-resistance plasmids were retained by supplementing medium with antibiotics as follows: ampicillin, 100 μg ml−1; kanamycin, 50 μg ml−1; tetracycline, 10–25 μg ml−1; streptomycin, 50 μg ml−1 and gentamicin, 10 μg ml−1. PCR products were cloned into a T-overhang vector (pGEM-T Easy; Promega).
Table 1.
Strains used in this study
Cb, Gm, Km, Sm, Su, Tc and Hg, resistance to antibiotics carbenicillin, gentamicin, kanamycin, streptomycin, sulphonamide, tetracycline and to mercury ions, respectively; Uv, ultraviolet light protection; Ant, Cap, Nah, Phn, Sal, Tol and Xyl, ability to degrade anthracene, ε-caprolactam, naphthalene, phenanthrene, salicylic acid, toluene and (m- and p-) xylenes, respectively.
| Strain | Plasmid | Size (k)b | Plasmid phenotype | Source of isolate | Reference or provider* |
|---|---|---|---|---|---|
| P. putida PpG7 | NAH7 | 83 | Nah Sal | Coal-tar-contaminated soil, CA, USA | Dunn & Gunsalus (1973) |
| P. putida BS 202 | NPL-1 | 100 | Nah Sal | Coal-tar-contaminated soil near coal tar mine, Makeevka, Ukraine | Izmalkova et al. (2006); RAS |
| Pseudomonas spp. 8C | p8C | 110 | Nah Sal Phn | Oil-contaminated soil, Tumen region, Western Siberia, Russia | Izmalkova et al. (2006); RAS |
| Pseudomonas spp. 15C | p15C | 110 | Nah Sal Phn | Oil-contaminated soil, Tumen region, Western Siberia, Russia | Izmalkova et al. (2006); RAS |
| P. putida BS 238 | pBS2 | 130 | Nah Sal | Soil from territory of metallurgical plant, Nizhniy Tagil, Russia | Izmalkova et al. (2006); RAS |
| P. putida BS 3710 (cys−) | pBS216 | 83 | Nah Sal Phn | Soil from territory of metallurgical plant, Magnitogorsk, Russia | Izmalkova et al. (2006); RAS |
| P. putida BS 639 (cys−) | pBS240 | 160 | Nah | Coke chemical plant, Kemerovo, Russia | RAS |
| P. putida BS 638 (cys−) | pBS243 | 160 | Nah | Soil from territory of metallurgical plant, Magnitogorsk, Russia | RAS |
| P. putida BS 394 (cys−) | pBS265 | 130 | Cap | Chemical plant sewage, Severodonetsk, Ukraine | Krasowiak et al. (2002); RAS |
| P. putida BS 394 (cys−) | pBS267 | 130 | Cap | Chemical plant sewage, Severodonetsk, Ukraine | Krasowiak et al. (2002); RAS |
| P. putida BS 394 (cys−) | pBS268 | 85 | Cap | Chemical plant sewage, Kemerovo, Russia | Mavrodi et al. (2003); RAS |
| P. putida BS 3701 | pBS1141, pBS1142 | 100, 60 | Nah Sal Phn Ant cryptic | Coke chemical plant, Vidnoe, Moscow region, Russia | Izmalkova et al. (2006); RAS |
| P. putida BS 3750 | pBS1181 | 120 | Nah Sal Phn | Oil-contaminated soil, Tumen region, Western Siberia, Russia | Izmalkova et al. (2006); RAS |
| P. putida BS 3790 | pBS1191, pBS1192 | 100, 60 | Nah Sal Phn Ant cryptic | Oil-contaminated soil, Tumen region, Western Siberia, Russia | Izmalkova et al. (2006); RAS |
| P. putida NCIB 9816-4 | pDTG1 | 83 | Nah | Coal-tar-contaminated site, Bangor, Wales, UK | Dennis & Zylstra (2004); Evans et al. (1965) |
| Unknown† | pFKY1 | 200 | Nah Sal | Oil-contaminated site, Japan | M. Tsuda, unpublished data |
| P. putida M | pM3 | 75 | Sm Tc Uv | Sewage and soil from different industrial and agricultural locations in Belarus and Azerbaijan | Greated et al. (2000); Titok et al. (1991) |
| P. putida M (pro−) | pM77 | 75 | Sm Tc | Soil from the area of sewage treatment plant, Minsk, Belarus | Krasowiak et al. (2002); BSU |
| P. putida M (pro−) | pM80 | 75 | Sm Tc | Soil from the area of sewage treatment plant, Minsk, Belarus | Krasowiak et al. (2002); BSU |
| P. putida AC34 | pMG18 | 100 | Cb Gm Km Sm Su Hg | Japan | Jacoby & Matthew (1979) |
| P. putida 10a | pNL4 | 75 | Nah Sal | Soil from a distillery area, Minsk, Belarus | Leuchuk et al. (2006); BSU |
| E. coli C600‡ | pNL15‡ | 75 | Sm | Soil from a petrol station area, Minsk, Belarus | Leuchuk et al. (2006); BSU |
| Pseudomonas fluorescens 41a | pNL22 | 100 | Nah Sal | Soil from a petrol station area, Minsk, Belarus | Leuchuk et al. (2006); BSU |
| P. putida 21a | pNL25 | 75 | Nah Sal | Soil from a railway station area, Minsk, Belarus | Leuchuk et al. (2006); BSU |
| Pseudomonas spp. 58 | pNL29 | nd§ | Nah Sal | Soil from a petrol station area, Minsk, Belarus | Leuchuk et al. (2006); BSU |
| Pseudomonas aeruginosa 56 | pNL31 | nd§ | Nah Sal | Soil from the roadside, Minsk, Belarus | Leuchuk et al. (2006); BSU |
| P. fluorescens 18d | pNL60 | 120 | Nah Sal | Soil from the foundry area, Homel, Belarus | Leuchuk et al. (2006); BSU |
| Pseudomonas aureofaciens OV17 | pOV17 | 85 | Nah | Oat rhizosphere from oil-contaminated soil, Western Siberia, Russia | RAS |
| P. putida SN11 | pSN11 | 83 | Nah Sal | Salt-contaminated soil from chemical plant, Berezniki, Ural, Russia | Izmalkova et al. (2006); RAS |
| P. putida SVS15 | pSVS15 | 90 | Tol Xyl | Piece of rubber from used-car-tyre storage, Minsk, Belarus | Sentchilo et al. (2000) |
| P. putida mt-2 (PaW1) | pWW0 | 116.58 | Tol Xyl | USA | Greated et al. (2002); Williams & Murray (1974) |
| P. aeruginosa ML 4262 | R2 | 73 | Cb Sm Su Uv | Japan | Kawakami et al. (1972) |
*RAS and BSU, bacterial strain collections obtained from Russian Academy of Sciences and Belarus State University, respectively.
†Plasmid obtained by exogenous isolation.
‡Plasmid was labelled with mini-Tn5 (Km) and transferred by conjugation into E. coli C600 (selection for Km Nah− phenotype) from the natural host P. fluorescens 42 (Leuchuk et al., 2006).
§Not determined.
DNA sequences.
Published plasmid sequences used in this study were: pMT2 (accession no. AF078924), pWW0 (AJ344068), pDTG1 (AF491307), NAH7 (AB237655), pBBR1 (X66730) and pBI709 (partial sequence, AY299015). Newly determined sequences generated in this work were deposited in GenBank Database under the accession numbers EU499619–EU499641 for oriV and EU499644–EU499666 for rep.
DNA isolation and manipulation.
Plasmid DNA was extracted for screening purposes using the alkaline lysis method (Birnboim & Doly, 1979) or with a Wizard Plus SV Miniprep kit (Promega) for sequencing. Large plasmids were isolated with a Qiagen Midiprep kit and total genomic DNA was extracted using a GenElute Bacterial Genomic DNA kit (Sigma). Restriction endonuclease digestion and agarose gel electrophoresis were carried out using established techniques (Sambrook & Russell, 2001). Restriction enzymes were from New England Biolabs, MBI Fermentas, Invitrogen and Boehringer Mannheim (Roche). PCR products were purified from agarose gel or reaction mixtures using a High Pure PCR Product Purification kit (Roche) or GeneClean Spin kit (Bio 101). Bacteria were transformed using a standard CaCl2 transformation protocol (Sambrook & Russell, 2001).
PCR amplification of rep, oriV and selected backbone loci.
PCR primers used are listed in Supplementary Table S1 available with the online version of this paper. Putative rep and oriV were amplified from IncP-9 plasmids with repF and repR (Greated & Thomas, 1999) and orisF and orisR primer pairs. PCR was performed using Bio-X-Act DNA Polymerase (Bioline); total genomic DNA or crude cell lysate was used as a DNA template. The reaction included 3 mM MgCl2, 0.8 mM dNTPs, 0.2 μM of each primer and 2–4 U polymerase in 1× PCR buffer provided with the polymerase. The thermal cycling profile used was: 5 min denaturation at 94 °C; 30 cycles of denaturation at 94 °C for 1 min, annealing at a specific annealing temperature (Ta) (Supplementary Table S1) for 1 min and elongation at 68 °C for 1 min; final extension at 68 °C for 10 min. Selected backbone loci were amplified from IncP-9 plasmids (total genomic DNA or crude cell lysate) using Taq polymerase (Invitrogen) according to the manufacturer's instructions. The cycling profile was as follows: 5 min denaturation at 94 °C; 30 cycles of denaturation at 94 °C for 1 min, annealing at Ta (Supplementary Table S1) for 1 min, extension at 72 °C for 1 min (or 2.5 min for the last two primer pairs in Supplementary Table S1 due to the increased size of the product) and final extension at 72 °C for 10 min.
DNA sequencing.
Automated sequencing was carried out using the Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer Applied Biosystems) on an ABI Prism 3700 DNA automatic sequencer (Functional Genomics Laboratory, University of Birmingham) according to the manufacturer's instructions. Putative IncP-9 rep PCR products (∼500 bp) were cloned in the pGEM-T Easy vector and sequenced on both strands with pUC18/M13 primers. Purified oriV PCR products (540–600 bp) were sequenced directly using 30–50 ng template and 6 pmol orisF or orisR primer.
Southern blotting.
Selected PCR products or SalI- or AvaI-digested total genomic DNA (300–500 ng) from strains with IncP-9 plasmids were fractionated by 1.5 % agarose gel electrophoresis in Tris-acetate/EDTA buffer and blotted to Hybond-N+ membranes (Amersham) by neutral capillary transfer using 20× SSC buffer [0.3 M sodium citrate, 3 M NaCl, pH 7.0 (Sambrook & Russell, 2001)] for 16 h. DNA was fixed by UV-induced cross-linking at 70 mJ cm−2. Hybridization probes were generated from relevant PCR products (Supplementary Table S1) by random priming labelling with a DIG DNA Labeling and Detection kit (Roche) following the manufacturer's instructions. Hybridizations were performed overnight in hybridization buffer (5×SSC, 0.1 % N-lauroylsarcosine, 0.02 % SDS, 1 % blocking reagent) at 50 °C, followed by two washes in 2×SSC, 0.1 % SDS at room temperature and two washes in 0.1×SSC, 0.1 % SDS at 55 °C. The blots were developed using nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (NBT/BCIP) colour substrate solution, and the image was acquired on a GS-710 densitometer (Functional Genomics Laboratory, University of Birmingham).
Computer analysis.
DNA sequence alignments were generated using the pileup programme from the UWGCG package (Devereux et al., 1984) and edited manually. Phylogenetic analysis was performed using the neighbour-joining (Saitou & Nei, 1987) and maximum-parsimony methods as implemented by clustal_x (v1.83) (Thompson et al., 1997), phylip (Felsenstein, 1989) and mega v3.1 (Tamura et al., 2007) program packages. Phylogenetic trees were displayed with TreeView (v1.6.6) (Page, 1996). Alignments of complete plasmid sequences were obtained using the ACT program from the Artemis package (Rutherford et al., 2000). Protein identity values were determined using blast (Altschul et al., 1997).
RESULTS
Phylogeny of the IncP-9 plasmid family
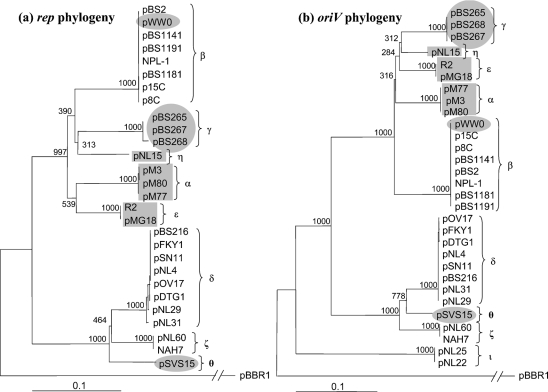
The IncP-9 plasmid phylogeny was inferred using sequences of both rep and oriV, in order to increase accuracy of the analysis. These regions were amplified from each IncP-9 plasmid (Table 1) using rep- and oriV-specific primers (Supplementary Table S1) that should yield products of 398 and 512–537 bp respectively. PCR products for rep and oriV were obtained with 23 and 25 IncP-9 plasmids, respectively (Table 1); only pBS240 and pBS243 gave neither rep nor oriV products. The PCR products were sequenced (oriV and rep sequences of pFKY1 were provided by M. Tsuda, Tohoku University) and multiple sequence alignments were created using UWGCG and clustal_x software (excluding the primer sequences). The rep–oriV sequence from Bordetella bronchiseptica plasmid pBBR1 (co-ordinates 1850–2635), which is distantly related to IncP-9 plasmids but compatible with pMT2 (Greated et al., 2000), was used as an outgroup. The resulting neighbour-joining rep and oriV IncP-9 phylogenies (Fig. 1) revealed significant polymorphism within the family, with 30 plasmids placed in nine subgroups.
Fig. 1.
Neighbour-joining rooted phylogenies of the IncP-9 plasmid family based on sequence analysis of rep (a) and oriV (b) loci. Plasmid subgroups are bracketed and named with letters of the Greek alphabet from α to ι; grey background shapes define plasmid phenotypes: rectangle, multiple antibiotic resistance; oval, toluene/xylene degradation; circle, caprolactam degradation; no shape (clear background), naphthalene degradation. Bootstrap values (out of 1000 replicates) are shown adjacent to branch nodes. pBBR1 sequences were used to root the trees (pBBR1 branches are shortened for convenience). The lengths of horizontal branches correspond to evolutionary distances and the scale bars show the number of substitutions per site.
The subgroups were named with Greek letters (α, β, γ, δ, ε, ŋ, ζ, θ and ι subgroups; Fig. 1), in accordance with previous studies of IncP-9 plasmid diversity (Izmalkova et al., 2006; Krasowiak et al., 2002; Krasowiak, 2003; Leuchuk et al., 2006). IncP-9 plasmids from different phylogenetic clusters showed 74–92 % (rep) and 64–93 % (oriV) DNA sequence identity, while the plasmids from the same subgroup shared 98–100 % identity for these determinants. Although subgroups tend to be associated with a single phenotypic profile, there are exceptions (e.g. the β cluster includes both naphthalene and toluene degradation). Apart from the additional ι cluster in the oriV tree (see below), the rep and oriV phylogenies have similar clusters, thus providing no evidence for incidents of recombination in the replication region. The results were not affected by the specific type of analysis used, since maximum-parsimony analysis also showed identical and well-supported phylogenetic clusters (data not shown), differing only slightly in their branching patterns.
Two Nah plasmids, pNL22 and pNL25, were allocated to a separate subgroup (ι) based on their oriV sequences, which showed only 64–67 % identity with other IncP-9 oriV sequences (Fig. 1). These plasmids gave no PCR products with other available IncP-9 replicon-specific primers (including those targeting rep), regardless of whether the DNA template used was total genomic DNA or purified plasmid DNA. Genomic DNA extracted from bacteria harbouring pNL25 yielded a very faint hybridization signal with the pMT2-derived rep probe (α), while the pNL25-derived oriV probe did not hybridize with plasmids from the α, β and δ subgroups under the stringency conditions used (data not shown). This suggests that the plasmids of the ι subgroup are highly divergent. In classical incompatibility tests with pNL22 and pNL25 plasmid-bearing bacteria, the naphthalene-degradation phenotype was lost with 100 % frequency upon the transfer of pM3 and R2 (Leuchuk et al., 2006). If they possess a second, unrelated, replication and partitioning system they should not be displaced in this way, so pNL22 and pNL25 depend on a functionally related IncP-9 replication and/or partitioning system.
Analysis of diversity in the common backbone of phylogenetically distant IncP-9 plasmids
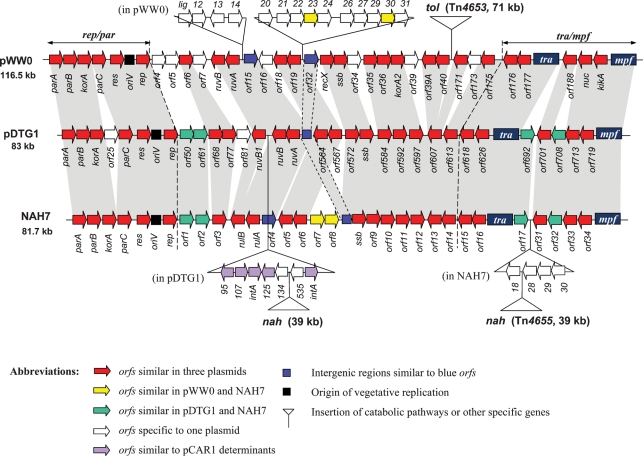
Genome-wide comparison of sequenced IncP-9 plasmids pWW0 (Greated et al., 2002) and pDTG1 (Dennis & Zylstra, 2004) using the Artemis Comparison Tool (ACT) (Fig. 2) provided information about (i) the structure of the common IncP-9 plasmid backbone, (ii) the existence of possible unique regions in each plasmid, (iii) the levels of sequence identity for homologous segments and (iv) insertions of relevant degradation genes; this informationwas used to design tools for comparison of unsequenced plasmid genomes. Subsequent publication of the NAH7 sequence (Sota et al., 2006) confirmed the identification of the ∼35 kb IncP-9 core (34.7, 35.0 and 34.1 kb in pWW0, pDTG1 and NAH7, respectively) including plasmid replication (oriV-rep) and stable maintenance (par and res) functions (4.4–4.9 kb), tra–mpf clusters encoding conjugative transfer apparatus (∼20 kb) and an additional 10 kb segment (variable region; Dennis & Zylstra, 2004). The list of the plasmid backbone determinants together with pair-wise DNA and protein sequence identities is given in Supplementary Tables S2 and S3. To extend our understanding of the sequence divergence in the variable region of the common IncP-9 backbone as well as of the possibility of recombination between related plasmids, PCR primers targeting selected conserved loci in pWW0 and pDTG1 were designed and corresponding pWW0-specific hybridization probes were produced (Fig. 2, Supplementary Table S1). Five primer pairs targeted homologous ORFs in pWW0 and pDTG1 (orf6–7, ruvB–ruvA, orf18–19, orf39A–40 and orf175 in pWW0, and their homologues orf68–77, ruvB–ruvA, orf564–567 and orf607–613 in pDTG1), while two were specific to pWW0 ORFs (orf31–32 and orf176–177). Overall, 13 ORFs from pWW0 and eight from pDTG1 (primarily from the variable backbone segment) were covered by these primer pairs.
Fig. 2.
Schematic representation of alignment of pWW0, pDTG1 and NAH7 plasmid sequences generated using Artemis software. Grey areas connect similar orfs. The tra and mpf blocks included traD–C and mpfJ–R genes showing perfect synteny in three plasmids. Not to scale.
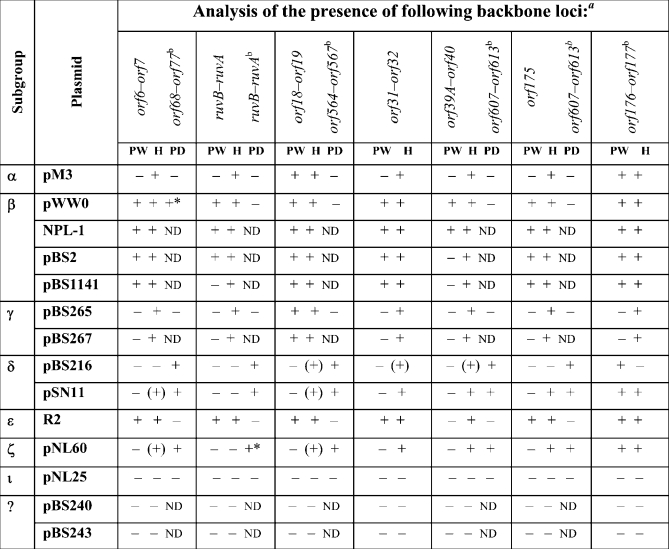
Twelve IncP-9 plasmids representing seven phylogenetic subgroups and the two unclassified plasmids pBS240 and pBS243 (Table 2) were analysed by PCR with these primer pairs and by Southern blotting of digested genomic DNA with hybridization probes. A PCR product of the expected size (∼500 bp) or a visible hybridization signal were considered to be positive results. The results of the PCR and hybridization experiments are summarized in Table 2 and representative hybridization blots are shown in Fig. 3. The results showed that all backbone loci studied were detected in nearly all IncP-9 plasmids tested by either PCR or hybridization analysis or both. Where only one method gave a positive result this might indicate local variations in the level of sequence conservation. The results indicate that the proposed backbone is indeed present in the majority of IncP-9 plasmids. Three plasmids, pNL25 (ι), pBS240 and pBS243 [classified as IncP-9 plasmids on the basis of incompatibility testing (I. A. Kosheleva, unpublished data)], did not yield PCR products or hybridization signals with any primers or probes targeting the backbone determinants. This suggests that these plasmids have a higher level of sequence divergence in the backbone functions. As expected, PCR with pWW0- or pDTG1-specific primers yielded products primarily with plasmids from their cognate subgroups. Plasmid R2 (ε) gave PCR products with all but one pWW0-specific primer, while pM3 (α) and pBS265/267 (γ) gave PCR products with only two and one primer pairs, respectively, while none of the plasmids from pWW0 branch (β, α, ε, γ subgroups) worked with pDTG1-specific primers. However, they all yielded hybridization signals with pWW0-derived probes, consistent with them being more closely related to pWW0 than to pDTG1 (Fig. 1). Plasmids from δ (pBS216 and pSN11) and ζ (pNL60) subgroups gave PCR products with all or almost all of the pDTG1-specific primers. In contrast, they worked with only one pWW0-specific primer pair (orf176–177) and often yielded weak signals in hybridizations with pWW0 probes (Fig. 3). Peculiarly, orf6–7 and ruvB–ruvA were not detected in the δ and ζ plasmids by hybridization with pWW0-specific probes, while PCR with equivalent pDTG1-specific primers gave products of the expected sizes (orf68–77 and ruvA–ruvB pairs in pDTG1). This could be due to high sequence divergence in these loci between pWW0 and the plasmids from the δ and ζ subgroups. The DNA sequence identity for pWW0 orf6–7 and ruvB–ruvA and their homologues in pDTG1 (orf68–7 and ruvA–ruvB, respectively) ranged from 51.3 to 68.7 %, while it was 69.4–78.2 % for other backbone loci analysed (Supplementary Table S2).
Table 2.
PCR- and hybridization-based detection of the selected backbone loci in plasmids representing seven of the nine IncP-9 subgroups using pWW0- and pDTG1-specific tools
a, +, (+) and − correspond to strong, weak and no PCR or hybridization signal, respectively. PW, PCR with pWW0-specific primers; H, hybridization with pWW0-specific probes; PD, PCR with pDTG1-specific primers. An asterisk indicates a PCR product larger than the expected size. nd, Not determined.
b, Pairs of homologous backbone loci from pWW0 and pDTG1 are indicated in this order; if only one orf pair is indicated, it belongs to pWW0.
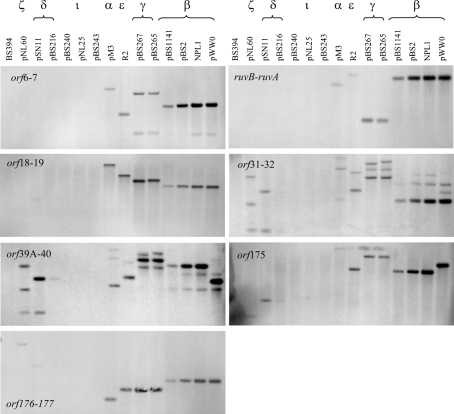
Fig. 3.
Hybridization of selected IncP-9 plasmids with pWW0-derived DIG-labelled probes specific to particular backbone loci. For hybridization, genomic DNA isolated from plasmid-containing bacteria was used; genomic DNA from plasmid-free P. putida BS 394 was used as a negative control (lane ‘BS394’). The DNA was digested with AvaI (orf6–7 and ruvB–ruvA loci) or SalI (others) before loading of the agarose gels, and was hybridized with pWW0-derived probes as described in Methods. In several hybridizations, multiple bands appeared in pWW0 and some test plasmids (orf6–orf7, orf31–orf32, orf39A–orf40). This might result from (i) incomplete digestion of the genomic DNA, (ii) non-specific hybridization or (iii) the presence of another region of homology elsewhere on the plasmid. The last could account for the appearance of multiple bands in the hybridizations of pWW0 and closely related β plasmids with orf31–32 and orf39A–40 probes, since pWW0orf32 and pWW0orf40 show 92.7 and 78.7 % nucleotide sequence identity to pWW0orf15 and pWW0orf171, respectively (Fig. 2).
The data were also scanned for abnormal patterns of hybridization that were not typical of the plasmid classification by ori–rep phylogeny. The PCR results (Table 2) showed that for only one plasmid from the pWW0 branch, R2 (ε), were products amplified with the pWW0-specific primers as well as they were with the β plasmid pBS2 (both plasmids only failed to yield products with orf39A–40 primers). This indicates that R2 might share significant sequence similarity with the backbone of both pWW0 and the β plasmids, although their rep and oriV sequences are as dissimilar (12–13 % divergence) as, for instance, those of pWW0 and α or γ plasmids (12–15 %). Plasmid pSN11 (δ) gave a very strong hybridization signal with the pWW0 orf39A–40 probe, which also may suggest recombination in this region. These cases should be investigated further by additional PCR/hybridization and sequencing analysis to test whether these results do indeed arise from mosaics created by recombination between conserved IncP-9 backbone regions in diverse members of the family.
Analysis of possible insertions of accessory DNA in diverse IncP-9 plasmids
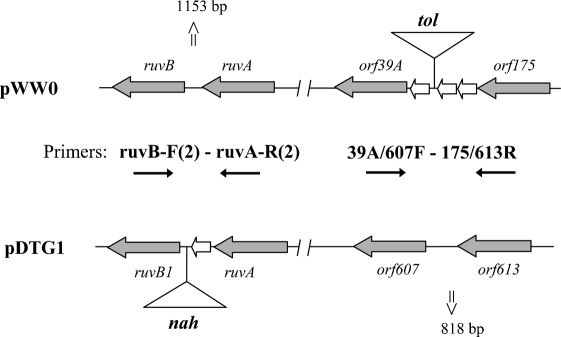
PCR and hybridization analyses were used to determine whether diverse IncP-9 plasmids have insertions of accessory DNA (degradation and resistance genes) in the same backbone sites as in those of pWW0 and pDTG1. To target these sites, the PCR primers 39A/607F–175/613R and ruvB-F(2)–ruvA-R(2) were designed on the basis of the conserved sequences in the determinants surrounding the catabolic gene cassette in one plasmid and in their homologues in other plasmids (Figs 2 and 4). Thus, orf39A and orf175 flank Tn4653 carrying the toluene-degradation pathway in pWW0, while the equivalent ORFs in pDTG1 were orf607 and orf613. Similarly, another primer pair was designed in the ruvB–ruvA (ruvB1–ruvA in pDTG1) region, since in pDTG1 the nah gene insertion occurred in ruvB. As expected pWW0 and pDTG1 gave PCR products with only one primer pair, whose sizes should be 1153 and 818 bp, respectively. PCR products obtained from different IncP-9 plasmids were analysed on agarose gels and hybridized with probes derived from pWW0 (for the ruvB-F(2)–ruvA-R(2) amplicons) or from pBS216 (similar to pDTG1) (for the 39A/607F–175/613R amplicons), as described in Methods. The results are summarized in Table 3 and Fig. 5.
Fig. 4.
Schematic representation of the pWW0 versus pDTG1 plasmid alignment to show the location of the conserved primers specific to determinants flanking the insertions of corresponding catabolic pathways. The conserved primer pairs are shown by black arrows; the genetic determinants used for the primer design are shown by grey arrows; white triangles (tol and nah) indicate insertions of toluene and naphthalene catabolic pathways, respectively. The sizes of the expected PCR products are indicated.
Table 3.
Analysis of possible insertions in two sites of the IncP-9 backbone by PCR and hybridization with plasmids representing the eight best-characterized subgroups
| Subgroup | Plasmid phenotype* | Plasmid | ruvB/ruvB1–ruvA | orf39A/607–orf175/613 | ||
|---|---|---|---|---|---|---|
| Hybridization signal | Presence of insertion† | Hybridization signal | Presence of insertion† | |||
| α | R | pM3 | + | − | − | + |
| β | Tol | pWW0 | + | (−) | − | (+) |
| Nah | NPL-1, pBS2, pBS1141, p8C, p15C, pBS1181 | + | − | + | − | |
| Nah | pBS1191 | + | − | − | + | |
| γ | Cap | pBS265, pBS267 | + | − | − | + |
| δ | Nah | pBS216, pSN11 | − | (+) | + | (−) |
| ε | R | R2 | + | − | − | + |
| R | pMG18 | − | + | − | + | |
| θ | Tol | pSVS15 | + | − | + | − |
| ŋ | R | pNL15 | + | − | − | + |
| ζ | Nah | pNL60 | + | (−) | + | (−) |
*Tol, Nah and Cap, degradation of toluene/xylenes, naphthalene and caprolactam, respectively; R, antibiotic resistance.
†+ or − indicates predicted presence or absence of insertions in uncharacterized plasmids. (+) or (−) indicates known presence or absence of insertions in characterized plasmids (pWW0, δ and ζ plasmids).
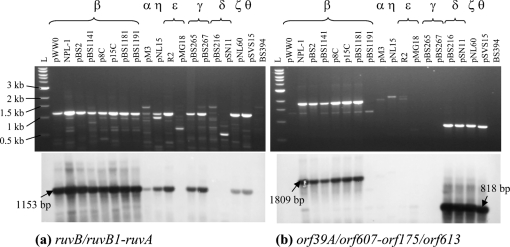
Fig. 5.
PCR and hybridization analyses of selected IncP-9 plasmids with the primers and hybridization probes targeting the sites of accessory DNA insertions in the common plasmid backbone. (a) PCR products obtained from IncP-9 plasmids with the ruvB-F(2)–ruvA-R(2) primers and subsequently hybridized with the pWW0-derived ruvB/ruvB1-ruvA DIG-labelled hybridization probe. (b) PCR products obtained with the 39A/607F–175/613R primers and hybridized with the pBS216-derived hybridization probe orf39A/orf607–orf175/orf613. PCR with genomic DNA from the plasmid-free P. putida BS 394 was used as a negative control (lane ‘BS394’).
Results with primers and probes specific to the ruvB–ruvA locus (Fig. 5a) indicated that this region was intact and of the same length (1.2 kb) in all of the plasmids used, apart from the δ plasmids (pBS216, pSN11) and pMG18 (ε). Therefore, only plasmids from the δ subgroup and possibly pMG18 might carry insertions in ruvB. The PCR and hybridization analysis of the orf39A/607–orf175/613 locus (Fig. 5b, Table 3) indicated that it was not intact in the following plasmids: pWW0 (β, contains transposon insertion in this region), pBS1191 (β), pM3 (α), pNL15 (ŋ), pBS265 and pBS267 (γ), and R2 and pMG18 (ε). Interestingly, the majority of Nah plasmids of the β subgroup (NPL-1, pBS2, p8C, p15C, pBS1141 and pBS1181) yielded a PCR product of ∼1.8 kb. This is very close to the size of an intact orf39A–orf175 region in pWW0 (1809 bp) if it lacked the insertion of Tn4653. These results indicate that Nah plasmids from the β subgroup may be very similar to pWW0 and may carry their accessory DNA in the same position (i.e. between orf39A and orf175; plasmid pBS1191) as well as different backbone sites, but not in ruvB as in pDTG1 (plasmids NPL-1, pBS2, p8C, p15C, pBS1141 and pBS1181). Plasmids from the α, ŋ, γ and ε clusters may also contain insertions in the same region as pWW0, while in pMG18 (ε) both backbone sites analysed might be disrupted by insertions. The pDTG1-related plasmids (δ, ζ and θ) yielded an approximately 800 bp PCR product, indicating the integrity of the orf39A/607–orf175/613 backbone site. Sequencing of NAH7 (ζ subgroup) confirmed that in this plasmid the nah cluster was inserted in a different, third, backbone location, between the tra and mpf genes (Sota et al., 2006), consistent with the results obtained for our ζ plasmid (pNL60).
DISCUSSION
From 30 IncP-9 plasmids this study distinguished nine IncP-9 subgroups (α to ι) based on divergence in rep and oriV sequences. The results are consistent with the existing molecular classifications of IncP-9 plasmids (Izmalkova et al., 2006; Krasowiak et al., 2002; Krasowiak, 2003; Leuchuk et al., 2006) but provide much more reliable distances between the different subgroups as well as confirming the existence of the two main clusters of subgroups. Previous RFLP and sequence analysis of PCR products amplified from replication/maintenance loci in a small selection of IncP-9 plasmids has identified four plasmid subgroups, α, β, γ and ε (Krasowiak et al., 2002; Krasowiak, 2003). The δ subgroup identified by Izmalkova et al. (2006) included plasmids that were positive only in PCR with the IncP-9 rep primers (Greated & Thomas, 1999) but not with other available IncP-9-specific primers (Krasowiak et al., 2002). Finally, several Nah plasmids were allocated to and ζ subgroups by RFLP analysis of their rep products, and to the IncP-9-like replicons (ι subgroup) by incompatibility testing (Leuchuk et al., 2006).
The oriV and rep phylogenies were rooted using replication region sequences from the pBBR1 plasmid (Antoine & Locht, 1992). This showed two main divergent lineages in the evolution of IncP-9 plasmid family: the ‘pWW0 branch’, including clades α, β, γ, ε and ŋ, and the ‘pDTG1 branch’, comprising the δ, ζ and θ subgroups (Fig. 1). Nucleotide sequence divergence between plasmids from these branches was highest (22–26 % for rep and 24–28 % for oriV), while that between subgroups of each branch ranged from 7 to 19 %. Consistent with this, only plasmids from the pWW0-branch (α, β, γ, ε and clusters) but not those from the pDTG1-branch (δ, ζ and θ cluster) yielded PCR products with primers based on replication and maintenance loci of pMT2 (α) (Izmalkova et al., 2006; Krasowiak et al., 2002; Y. R. Sevastsyanovich and others, unpublished results). Our PCR and hybridization analyses and the sequence analysis of NAH7 (Sota et al., 2006) also support the division of the IncP-9 plasmid family into two major clusters (pWW0 and pDTG1 branches).
Three subgroups (α, ε and ŋ) included multiple antibiotic-resistance plasmids isolated from clinical and environmental samples. Diverse plasmids encoding degradative functions (for naphthalene, toluene/xylenes and caprolactam) formed the six remaining subgroups, each including plasmids of the same phenotype, with the exception of the β branch, which included Tol and Nah plasmids. Several IncP-9 plasmids that did not give PCR products with the IncP-9 replicon-specific primers used may create additional subgroups, characterized by higher sequence divergence in the replication region.
The high sequence variability within the replication region of IncP-9 plasmids contrasts, for example, with that exhibited by the IncP-4 (IncQ) and IncP-1 plasmid families. The IncP-4 family is currently divided into three subgroups on the basis of diversity in the replication initiator RepC (Rawlings & Tietze, 2001; Rawlings, 2005). The IncP-1 group includes five subgroups, as demonstrated by the phylogenetic analysis of the determinants involved in replication, maintenance, global gene regulation and conjugal transfer (Bahl et al., 2007; Haines et al., 2006; Harada et al., 2006; Heuer et al., 2004; Vedler et al., 2004). However, the DNA sequence divergence in the replication initiator gene from the plasmids allocated to different IncP-1 subgroups was 22–29 %, with up to 16 % divergence in the β branch alone. On this basis, some of the IncP-9 subgroups might be considered as a single group, since the divergence between them was only 7 %. On the other hand, the variation among the IncP-1β plasmids was relatively continuous, while the IncP-9 plasmids fall more tightly into the groups we have defined, suggesting more distinct clades.
Comparison of the complete sequences of pWW0, pDTG1 and NAH7 identified a 35 kb core composed of a 5 kb replication/stability gene cluster, a 20 kb conjugal transfer/mating pair formation cluster and an additional 10 kb variable region, which probably does not encode functions essential for plasmid survival (Dennis & Zylstra, 2004; Sota et al., 2006). Apart from the atypical IncP-9 replicons (ι subgroup and pBS240 and pBS243), the diverse plasmids analysed here possess a variable backbone segment (10 kb), similar to either the pWW0 or the pDTG1 type, suggesting conservation throughout the IncP-9 family. A blast search of GenBank identified pBI709 (partial sequence accession no. AY299015) from Pseudomonas as an additional family member based on extensive sequence similarity with the IncP-9 backbone (65–72 % overall DNA sequence identity and 52–81 % protein sequence identity between pBI709 and sequenced IncP-9 plasmids; Supplementary Table S3).
Recent studies have suggested that recombination between related plasmids might be more common than previously assumed for incompatible replicons. For example, F-related plasmids of the E. coli reference collection (ECOR) carry recombinant repA, finO and traD genes, as detected by hybridization analysis (Boyd et al., 1996). Sequencing of plasmid pB10 has revealed that its stable inheritance module (klcAB–korC and kleAEF) is closely related to that from pB4, while the rest of the backbone is more similar to that of R751, pTSA and pADP-1 (all plasmids belong to the IncP-1β subgroup; Schluter et al., 2003). The hybridization experiments presented in this study indicated that some plasmids might be the product of recombination between diverse IncP-9 replicons. For example, R2 has an ε plasmid replication region but shares extensive similarity with β (pWW0) plasmids in the variable backbone segment. In addition, R2 may carry its accessory DNA in the same backbone site as in pWW0. The plasmids that showed unexpectedly strong or weak hybridization with the pWW0 sequences [e.g. pSN11, pBS216 (δ) and pBS1141 (β)] may be recombinants of the corresponding backbone loci. More extensive hybridization and sequence analysis involving other parts of the backbone would be worthwhile in order to address the possibility of recombination in this plasmid group.
In the sequenced IncP-9 plasmids three different sites for integration of accessory DNA were identified: at the junction between the two modules, tra and mpf (NAH7), as in the IncP-1 plasmids; in pDTG1ruvB; and between pWW0orf40 and pWW0orf171. The results of this study indicated that diverse IncP-9 replicons carry the insertions of the genes encoding adaptive traits in these, as well as additional, backbone sites. This is consistent with the notion that similar or identical degradative markers have been acquired multiple times by IncP-9 plasmids, raising the question of whether IncP-9 plasmids possess specific features that make them particularly suited to carry such determinants through inherent plasmid properties such as host range, copy number or even effects on host structure or physiology.
Acknowledgments
We are grateful to Professor George A. Jacoby (Lahey Clinic, Burlington, USA) and Professor Jan Roelof Van der Meer (Department of Fundamental Microbiology, University of Lausanne, Switzerland) for providing pMG18 and pSVS15 plasmids, respectively. We are also grateful to Professor Masataka Tsuda, Ryo Miyazaki and Dr Masahiro Sota (Department of Environmental Life Sciences, Tohoku University, Japan) for providing rep and oriV sequences from NAH7 (before the complete plasmid sequence was released) and pFKY1. Y. R. S. and R. K. were recipients of scholarships from the Darwin Trust of Edinburgh. L. E. H. B. was supported by Wellcome Trust grant 067526/Z and A. S. H. was supported by Wellcome Trust grant 06083. This work was supported by INTAS 99-01487 and 01-2389.
Abbreviations
HGT, horizontal gene transfer
Footnotes
The GenBank/EMBL/DDBJ accession numbers for the newly determined oriV and rep sequences of Pseudomonas strains examined in this study are EU499619–EU499641 and EU499644–EU499666, respectively.
Three supplementary tables showing primers used in this work, homologous loci in pWW0, pDTG1 and NAH7 and pair-wise identities for corresponding genes and putative gene products, and identity levels between gene products of pM3 and pBI709 plasmids and their homologues in pWW0, pDTG1 and NAH7 are available with the online version of this paper.
References
- Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J. (1997). Gapped blast and psi-blast: a new generation of protein database search programs. Nucleic Acids Res 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine, R. & Locht, C. (1992). Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from Gram-positive organisms. Mol Microbiol 6, 1785–1799. [DOI] [PubMed] [Google Scholar]
- Bahl, M. I., Hansen, L. H., Goesmann, A. & Sorensen, S. J. (2007). The multiple antibiotic resistance IncP-1 plasmid pKJK5 isolated from a soil environment is phylogenetically divergent from members of the previously established α, β and δ sub-groups. Plasmid 58, 31–43. [DOI] [PubMed] [Google Scholar]
- Bayley, S. A., Morris, D. W. & Broda, P. (1979). The relationship of degradative and resistance plasmids of Pseudomonas belonging to the same incompatibility group. Nature 280, 338–339. [DOI] [PubMed] [Google Scholar]
- Birnboim, H. C. & Doly, J. (1979). A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronin, A. M. (1992). Diversity of Pseudomonas plasmids: to what extent? FEMS Microbiol Lett 79, 461–466. [DOI] [PubMed] [Google Scholar]
- Boyd, E. F., Hill, C. W., Rich, S. M. & Hartl, D. L. (1996). Mosaic structure of plasmids from natural populations of Escherichia coli. Genetics 143, 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier, M., Bex, F., Bergquist, P. L. & Maas, W. K. (1988). Identification and classification of bacterial plasmids. Microbiol Rev 52, 375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg, C., Linberg, C., Torsvik, V. L. & Hermansson, M. (1997). Conjugative plasmids isolated from bacteria in marine environments show various degrees of homology to each other and are not closely related to well-characterized plasmids. Appl Environ Microbiol 63, 4692–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, J. J. (2005). The evolution of IncP catabolic plasmids. Curr Opin Biotechnol 16, 291–298. [DOI] [PubMed] [Google Scholar]
- Dennis, J. J. & Zylstra, G. J. (2004). Complete sequence and genetic organization of pDTG1, the 83 kilobase naphthalene degradation plasmid from Pseudomonas putida strain NCIB 9816-4. J Mol Biol 341, 753–768. [DOI] [PubMed] [Google Scholar]
- Devereux, J., Haeberli, P. & Smithies, O. (1984). A comprehensive set of sequence-analysis programs for the VAX. Nucleic Acids Res 12, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, N. W. & Gunsalus, I. C. (1973). Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol 114, 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, R. W. (1994). Organization and evolution of naphthalene catabolic pathways: sequence of the DNA encoding 2-hydroxychromene-2-carboxylate isomerase and trans-o-hydroxybenzylidenepyruvate hydratase-aldolase from the NAH7 plasmid. J Bacteriol 176, 7757–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, W. C., Fernley, H. N. & Griffiths, E. (1965). Oxidative metabolism of phenanthrene and anthracene by soil Pseudomonas. Biochem J 95, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. (1989). phylip – phylogeny inference package (version 3.2). Cladistics 5, 164–166. [Google Scholar]
- Gotz, A., Pukall, R., Smit, E., Tietze, E., Prager, R., Tschape, H., vanElsas, J. D. & Smalla, K. (1996). Detection and characterization of broad-host-range plasmids in environmental bacteria by PCR. Appl Environ Microbiol 62, 2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greated, A. & Thomas, C. M. (1999). A pair of PCR primers for IncP-9 plasmids. Microbiology 145, 3003–3004. [DOI] [PubMed] [Google Scholar]
- Greated, A., Titok, M., Krasowiak, R., Fairclough, R. J. & Thomas, C. M. (2000). The replication and stable inheritance functions of IncP-9 plasmid pM3. Microbiology 146, 2249–2258. [DOI] [PubMed] [Google Scholar]
- Greated, A., Lambertsen, L., Williams, P. A. & Thomas, C. M. (2002). Complete sequence of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ Microbiol 4, 856–871. [DOI] [PubMed] [Google Scholar]
- Haines, A. S., Akhtar, P., Jones, K., Thomas, C. M., Perkins, C. D., Williams, J. R., Day, M. J. & Fry, J. C. (2006). Plasmids from freshwater environments capable of IncQ retrotransfer are diverse and include pQKH54, a new IncP-1 subgroup archetype. Microbiology 152, 2689–2701. [DOI] [PubMed] [Google Scholar]
- Harada, K. M., Aso, Y., Hashimoto, W., Mikami, B. & Murata, K. (2006). Sequence and analysis of the 46.6-kb plasmid pA1 from Sphingomonas sp. A1 that corresponds to the typical IncP-1β plasmid backbone without any accessory gene. Plasmid 56, 11–23. [DOI] [PubMed] [Google Scholar]
- Harayama, S. & Rekik, M. (1989). Bacterial aromatic ring cleavage enzymes are classified into two different gene families. J Biol Chem 264, 15328–15333. [PubMed] [Google Scholar]
- Heinaru, A. L., Duggleby, C. J. & Broda, P. (1978). Molecular relationships of degradative plasmids determined by in situ hybridisation of their endonuclease-generated fragments. Mol Gen Genet 160, 347–351. [DOI] [PubMed] [Google Scholar]
- Herrick, J. B., Stuart-Keil, K. G., Ghiorse, W. C. & Madsen, E. L. (1997). Natural horizontal transfer of a naphthalene dioxygenase gene between bacteria native to a coal tar-contaminated field site. Appl Environ Microbiol 63, 2330–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer, H., Szczepanowski, R., Schneiker, S., Puhler, A., Top, E. M. & Schluter, A. (2004). The complete sequences of plasmids pB2 and pB3 provide evidence for a recent ancestor of the IncP-1β group without any accessory genes. Microbiology 150, 3591–3599. [DOI] [PubMed] [Google Scholar]
- Izmalkova, T. Y., Mavrodi, D. V., Sokolov, S. L., Kosheleva, I. A., Smalla, K., Thomas, C. M. & Boronin, A. M. (2006). Molecular classification of IncP-9 naphthalene degradation plasmids. Plasmid 56, 1–10. [DOI] [PubMed] [Google Scholar]
- Jacoby, G. A. & Matthew, M. (1979). The distribution of β-lactamase genes on plasmids found in Pseudomonas. Plasmid 2, 41–47. [DOI] [PubMed] [Google Scholar]
- Kasai, Y. & Harayama, S. (2004). Catabolism of PAHs. In Pseudomonas, pp. 463–490. Edited by J.-L. Ramos. New York: Kluwer Academic/Plenum Publishers.
- Kawakami, Y., Mikoshiba, F., Nagasaki, S., Matsumoto, H. & Tazaki, T. (1972). Prevalence of Pseudomonas aeruginosa strains possessing R factor in hospital. J Antibiot (Tokyo) 25, 607–609. [DOI] [PubMed] [Google Scholar]
- Kobayashi, N. & Bailey, M. J. (1994). Plasmids isolated from the sugar-beet phyllosphere show little or no homology to molecular probes currently available for plasmid typing. Microbiology 140, 289–296. [DOI] [PubMed] [Google Scholar]
- Korfhagen, T. R., Sutton, L. & Jacoby, G. A. (1978). Classification and physical properties of Pseudomonas plasmids. In Microbiology-1978, pp. 221–224. Edited by D. Schlessinger. Washington, DC: American Society for Microbiology.
- Krasowiak, R. (2003). Analysis of elements involved in replication of pMT2 and its application for environmental screening of IncP-9 Pseudomonas plasmids. PhD thesis, University of Birmingham, UK.
- Krasowiak, R., Smalla, K., Sokolov, S., Kosheleva, I., Sevastyanovich, Y., Titok, M. & Thomas, C. M. (2002). PCR primers for detection and characterisation of IncP-9 plasmids. FEMS Microbiol Ecol 42, 217–225. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y., Jung, K. H., Choi, S. H. & Kim, H. S. (1995). Combination of the tod and the tol pathways in redesigning a metabolic route of Pseudomonas putida for the mineralization of a benzene, toluene, and p-xylene mixture. Appl Environ Microbiol 61, 2211–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach, P. R., McGregor, I., Ward, J. M. & Broda, P. (1983). Molecular relationships between Pseudomonas IncP-9 degradative plasmids TOL, NAH, and SAL. Plasmid 10, 164–174. [DOI] [PubMed] [Google Scholar]
- Leuchuk, A. A., Bulyha, I. M., Izmalkova, T. Y., Sevastsyanovich, Y. R., Kosheleva, I. A., Thomas, C. M. & Titok, M. A. (2006). Characterisation of Nah-plasmids of IncP-9 group from natural strains of Pseudomonas. Mol Biol 40, 750–757. [PubMed] [Google Scholar]
- Mavrodi, D. V., Kovalenko, N. P., Sokolov, S. L., Parfeniuk, V. G., Kosheleva, I. A. & Boronin, A. M. (2003). Identification of the key genes of naphthalene catabolism in soil DNA. Mikrobiologiia 72, 672–680. [PubMed] [Google Scholar]
- Nüsslein, K., Maris, D., Timmis, K. & Dwyer, D. F. (1992). Expression and transfer of engineered catabolic pathways harbored by Pseudomonas spp. introduced into activated sludge microcosms. Appl Environ Microbiol 58, 3380–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn, A. M., Tatley, F. M. D., Steyn, L. M., Pickup, R. W. & Saunders, J. R. (2000). Mosaic plasmids and mosaic replicons: evolutionary lessons from the analysis of genetic diversity in IncFII-related replicons. Microbiology 146, 2267–2275. [DOI] [PubMed] [Google Scholar]
- Page, R. D. M. (1996). TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12, 357–358. [DOI] [PubMed] [Google Scholar]
- Panke, S., Sanchez-Romero, J. M. & de Lorenzo, V. (1998). Engineering of quasi-natural Pseudomonas putida strains for toluene metabolism through an ortho-cleavage degradation pathway. Appl Environ Microbiol 64, 748–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parales, R. E., Bruce, N. C., Schmid, A. & Wackett, L. P. (2002). Biodegradation, biotransformation, and biocatalysis (B3). Appl Environ Microbiol 68, 4699–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, J. L. & Timmis, K. N. (1987). Experimental evolution of catabolic pathways of bacteria. Microbiol Sci 4, 228–237. [PubMed] [Google Scholar]
- Ramos, J. L., Wasserfallen, A., Rose, K. & Timmis, K. N. (1987). Redesigning metabolic routes: manipulation of TOL plasmid pathway for catabolism of alkylbenzoates. Science 235, 593–596. [DOI] [PubMed] [Google Scholar]
- Ramos, J. L., Marques, S. & Timmis, K. N. (1997). Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through interplay of host factors and plasmid-encoded regulators. Annu Rev Microbiol 51, 341–373. [DOI] [PubMed] [Google Scholar]
- Rawlings, D. E. (2005). The evolution of pTF-FC2 and pTC-F14, two related plasmids of the IncQ-family. Plasmid 53, 137–147. [DOI] [PubMed] [Google Scholar]
- Rawlings, D. E. & Tietze, E. (2001). Comparative biology of IncQ and IncQ-like plasmids. Microbiol Mol Biol Rev 65, 481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, K., Parkhill, J., Crook, J., Horsnell, T., Rice, P., Rajandream, M. A. & Barrel, B. (2000). Artemis: sequence visualization and annotation. Bioinformatics 16, 944–945. [DOI] [PubMed] [Google Scholar]
- Saitou, N. & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. & Russell, D. W. (2001). Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Schluter, A., Heuer, H., Szczepanowski, R., Forney, L. J., Thomas, C. M., Puhler, A. & Top, E. M. (2003). The 64 508 bp IncP-1β antibiotic multiresistance plasmid pB10 isolated from a waste-water treatment plant provides evidence for recombination between members of different branches of the IncP-1β group. Microbiology 149, 3139–3153. [DOI] [PubMed] [Google Scholar]
- Sentchilo, V. S., Perebituk, A. N., Zehnder, A. J. B. & van der Meer, J. R. (2000). Molecular diversity of plasmids bearing genes that encode toluene and xylene metabolism in Pseudomonas strains isolated from different contaminated sites in Belarus. Appl Environ Microbiol 66, 2842–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalla, K., Krogerrecklenfort, E., Heuer, H., Dejonghe, W., Top, E., Osborn, M., Niewint, J., Tebbe, C., Barr, M. & other authors (2000). PCR-based detection of mobile genetic elements in total community DNA. Microbiology 146, 1256–1257. [DOI] [PubMed] [Google Scholar]
- Sobecky, P. A., Mincer, T. J., Chang, M. C. & Helinski, D. R. (1997). Plasmids isolated from marine sediment microbial communities contain replication and incompatibility regions unrelated to those of known plasmid groups. Appl Environ Microbiol 63, 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sota, M., Yano, H., Ono, A., Miyazaki, R., Ishii, H., Genka, H., Top, E. M. & Tsuda, M. (2006). Genomic and functional analysis of the IncP-9 naphthalene-catabolic plasmid NAH7 and its transposon Tn4655 suggests catabolic gene spread by a tyrosine recombinase. J Bacteriol 188, 4057–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K., Dudley, J., Nei, M. & Kumar, S. (2007). MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Tan, H. M. (1999). Bacterial catabolic transposons. Appl Microbiol Biotechnol 51, 1–12. [DOI] [PubMed] [Google Scholar]
- Thomas, C. M. (2000a). The Horizontal Gene Pool. Amsterdam: Harwood Academic Publishers.
- Thomas, C. M. (2000b). Paradigms of plasmid organization. Mol Microbiol 37, 485–491. [DOI] [PubMed] [Google Scholar]
- Thomas, C. M. & Haines, A. S. (2004). Plasmids of the genus Pseudomonas. In Pseudomonas, pp. 197–231. Edited by J.-L. Ramos. London: Kluwer/Plenum.
- Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. & Higgins, D. G. (1997). The clustal_x windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titok, M. A., Maksimova, N. P. & Fomichev, Y. K. (1991). Characteristics of the broad host range IncP-9 plasmid pM3. Mol Genet Microbiol Virol 8, 18–23. [PubMed] [Google Scholar]
- Top, E. M., Springael, D. & Boon, N. (2002). Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol Ecol 42, 199–208. [DOI] [PubMed] [Google Scholar]
- Toussaint, A. & Merlin, C. (2002). Mobile elements as a combination of functional modules. Plasmid 47, 26–35. [DOI] [PubMed] [Google Scholar]
- Tsuda, M., Tan, H. M., Nishi, A. & Furukawa, K. (1999). Mobile catabolic genes in bacteria. J Biosci Bioeng 87, 401–410. [DOI] [PubMed] [Google Scholar]
- Turner, S. L., Bailey, M. J., Lilley, A. K. & Thomas, C. M. (2002). Ecological and molecular maintenance strategies of mobile genetic elements. FEMS Microbiol Ecol 42, 177–185. [DOI] [PubMed] [Google Scholar]
- Vedler, E., Vahter, M. & Heinaru, A. (2004). The completely sequenced plasmid pEST4011 contains a novel IncP1 backbone and a catabolic transposon harboring tfd genes for 2,4-dichlorophenoxyacetic acid degradation. J Bacteriol 186, 7161–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett, L. P. (2003). Pseudomonas putida – a versatile biocatalyst. Nat Biotechnol 21, 136–138. [DOI] [PubMed] [Google Scholar]
- Williams, P. A. & Murray, K. (1974). Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence of the existence of TOL plasmid. J Bacteriol 120, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, K. M. & Gunsalus, I. C. (1982). Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci U S A 79, 874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]