Abstract
Epidemiological evidence suggests that a diet abundant in fruits and vegetables may protect against colon cancer. Bioactive compounds, including flavonoids and limonoids, have been shown to possess anti-proliferative and anti-tumorigenic effects in various cancer models. This experiment investigated the effects of four citrus flavonoids and one limonoid mixture at the promotion stage of chemically induced colon cancer in rats. Male Sprague Dawley rats (n = 10 rats/group) were randomly allocated to one of six diets formulated to contain 0.1% apigenin, 0.02% naringenin, 0.1% hesperidin, 0.01% nobiletin, 0.035% limonin glucoside/obacunone glucoside mixture, or a control diet (0% flavonoid/limonoid). Rats received experimental diets for 10 wk and were injected with azoxymethane (15 mg/kg) at wk 3 and 4. Excised colons were evaluated for aberrant crypt foci (ACF) formation, colonocyte proliferation (PCNA assay), apoptosis (TUNEL assay), and expression of iNOS and COX-2 (immunoblotting). When compared to the control diet, apigenin lowered the number of high multiplicity ACF (HMACF > 4 AC/focus) by 57% (P < 0.05), while naringenin lowered both the number of HMACF by 51% (P < 0.05) and the proliferative index by 32% (P < 0.05). Both apigenin and naringenin increased apoptosis of luminal surface colonocytes (78% and 97%, respectively; P < 0.05) when compared to the control diet. Hesperidin, nobiletin, and the limonin glucoside/obacunone glucoside mixture did not affect these variables. The colonic mucosal protein levels of iNOS or COX-2 were not different among the six diet groups. The ability of dietary apigenin and naringenin to reduce HMACF, lower proliferation (naringenin only), and increase apoptosis may contribute toward colon cancer prevention. However, these effects were not due to mitigation of iNOS and COX-2 protein levels at the ACF stage of colon cancer.
Keywords: Citrus, limonoids, flavonoids, colon cancer
Introduction
Aging of the ever-increasing US population is estimated to cause a 45% increase in overall cancer incidence rates, which includes a 52% increase in the incidence of colon cancer by 2030 (1). Despite the prominence of colon cancer mortality rates, colon cancer may be preventable when risk factors such as diet and lifestyle are modified (2), as evidenced by studies of individuals migrating from low- to high-risk areas (3,4). The revised WCRF/AICR (5) report on cancer reiterated the relative chemoprotection derived from diets containing larger proportions of plant-derived foods. However, not all the evidence supports these conclusions, as some studies have found little or no relationship between consumption of fruits and vegetables and colon cancer (6–7).
One of the studies indicating protection by diets with elevated fruit and vegetable servings is a prospective cohort study of Swedish women, in which the risk of colon cancer was greatest for those with the lowest intake of fruits and vegetables. Fruit consumption had a greater association with lowered risk (4). Similarly, a population study by Risch et al. (8) suggested that fruits, especially citrus fruits, contain biologically active compounds besides vitamin C that may play a role in preventing many chronic diseases including cancer. For example, orange juice (9) or a 15% orange-pulp diet (10) was able to suppress chemically induced colon tumors in rats. Among the constituents in citrus that may provide protection are the bioactive flavonoids and limonoids (11–12).
Au et al. (13) found that ornithine decarboxylase (ODC) activity and ACF numbers were reduced by 0.1% apigenin, but it only modestly reduced tumor formation in AOM injected rats and failed to reduce tumors in Min-mice. Aberrant crypt foci in rats also are reduced by diets containing 20 mg/kg hesperetin (the aglycone of hesperidin) (14). A mixture of 20 mg apigenin and 20 mg epigallocathechin-gallate suppressed colon neoplasia recurrence rates in human subjects with resected colon cancers (15). Similarly, we have shown that grapefruit pulp or isolated limonin and naringin suppress high multiplicity aberrant crypt foci (HMACF) because of lower levels of proliferation and enhanced apoptosis (16). These effects occurred in parallel with lower levels of iNOS and COX-2 in response to limonin in the diet and a lower level of iNOS in response to naringin in the diet, suggesting changes in nitric oxide and/or prostaglandin synthesis may be mediating the benefits derived from these dietary interventions. Kohno et al. (17) found that 0.01% nobiletin decreased PGE2 production in rats.
Based on this evidence, we hypothesized that citrus flavonoids (hesperidin, nobiletin, apigenin, naringenin) and limonoids (a limonin glucoside/obacunone glucoside mixture) would act as chemopreventive agents at the promotion stage of colon carcinogenesis. The potential mechanisms were theorized to involve regulation of proliferation and apoptosis caused by lower levels of expression of the pro-inflammatory mediators iNOS and COX-2.
Materials & Methods
Animals and Study Design
The animal use protocol for this work was approved by the University Laboratory Animal Care Committee of Texas A&M University and conformed to the National Institute of Health guidelines. Sixty male weanling (21-d) Sprague-Dawley rats (Harlan, Houston, TX) were individually housed in a temperature- and humidity-controlled animal facility with a 12-h light-dark photoperiod. Food and water were freely available at all times. After acclimation to the facility, the rats were stratified by body weight to one of six diets (10 rats/diet), which they consumed for a total of 10 wk. All rats were injected with the colon carcinogen azoxymethane (AOM, Midwest Research Institute, Kansas City, MO; 15 mg/kg) on day 21 and 28 of the experiment. Body weights and intake were recorded on day 7, 21, 56, and 77. Rats were terminated 6-wk after the last AOM injection and colon tissues collected.
Diets
The six diets (control, 0.1% hesperidin, 0.01% nobiletin, 0.02% naringenin, 0.1% apigenin, 0.035% limonin glucoside/obacunone glucoside mixture) contained levels of experimental compounds previously shown to suppress either colon or oral cancer (17–19). We have also demonstrated protection against colon cancer by 0.02% naringin (16), and in order to compare those results with the current work, we used the same concentration for naringenin (the aglycone of naringin).
The experimental compounds were added to the diets at the expense of dextrose. Hesperidin (97% pure), naringenin (96% pure), and apigenin (97% pure) were isolated from methanol extracts of powdered dried peel from citrus reticulata, citrus junos, and citrus aurantium L., respectively (20). Nobiletin (97% pure) was crystallized from hexane extracts of dried citrus peel (21) and the limonin glucoside/obacunone glucoside mixture (76% pure) was purified from ethanol extracts of citrus seeds (22). Diets were mixed and stored in a −20°C freezer until use. The diets were analyzed by high performance liquid chromatography (HPLC) as previously described (23) to verify the final diet concentrations of the experimental compounds.
Collection of Tissue Samples
Six weeks after the second AOM injection, each rat was euthanized, the colon excised, and two 1-cm sections of the distal colon were fixed in ethanol (70% EtOH) or paraformaldehyde (4% PFA). After flushing with ice-cold PBS, the remaining colon was split open and cut in half longitudinally. One half was fixed in 70% EtOH for aberrant crypt foci analysis and the other half was scraped to prepare protein isolates. The mucosal scrapings were homogenized in a protein buffer (50 mM Tris-HCl pH 7.2, 250 mM sucrose, 2 mM EDTA, 1 mM EGTA, 50 μM NaF, 1% Triton X-100, 100 μM sodium orthovanadate, 4% Sigma Protease Inhibitor Cocktail, and 10 mM beta-mercaptoethanol), lysed and centrifuged at 15,000 g for 20 min at 4°C. The supernatant was stored at −80°C until use.
Aberrant Crypt Foci
This assay was performed using our standard procedures (16), in which the fixed colon tissue was stained with 0.5% methylene blue and examined at 40X to quantify aberrant crypt numbers. Aberrant crypt foci containing more than four aberrant crypts were categorized as HMACF, as they are more likely to correlate with subsequent formation of adenomas and/or adenocarcinomas (24–25).
Colonocyte Proliferation
Proliferation was measured with the proliferating cell nuclear antigen (PCNA) assay using reported procedures (16). Ethanol-fixed colon tissue sections were incubated with a murine anti-PC-10 monoclonal antibody (dilution 1:50; Signet Lab, Dedham, MA) followed by incubation with biotinylated anti-mouse IgG from the Vectastain ABC Elite kit (Vector Lab, Burlingame, CA). Negative control tissues were prepared by incubating with PBS instead of anti-PC-10 antibody. Tissue sections were then stained with diaminobenzidine tetrahydrochloride (DAB; Sigma Chemical, St Louis, MO) and counterstained with hematoxylin (Sigma Chemical, St Louis, MO). PCNA-containing nuclei were counted in 25 crypt columns per rat, with the total number of proliferating cells/crypt column, the proportion of proliferating cells and the position of the highest proliferating cell (proliferative zone) recorded.
Colonocyte Apoptosis
The incidence of apoptosis in the colon epithelia was measured by TUNEL assay according to the method of Hong et al. (26). Samples (4% PFA-fixed) from the distal colon were pretreated with proteinase K (Ambion, Austin, TX) to remove crosslinking caused by PFA. A working solution containing terminal deoxynucleotide transferase (TdT)-reaction buffer was incubated with tissue sections in a pre-warmed humidified chamber, with the subsequent addition of anti-digoxigenin peroxidase. Visualization of the antibody-antigen complex was accomplished by staining with DAB. Positive control tissues were prepared by nicking DNA with deoxyribonuclease I (Ambion) and negative control tissues were prepared by substituting PBS for TdT in the working solution. Apoptotic cells were quantified both within the crypt and on the luminal surface for 50 crypt columns per rat. For each rat, the proportion of apoptotic cells within each crypt column and corresponding mucosal surface cells were recorded.
Immunoblot Analysis of iNOS and COX-2
Protein concentrations of the supernatants extracted from the colonic mucosal homogenates were determined by a BCA Protein Assay kit using the manufacturer’s instructions (Pierce, Rockford, IL). Samples (30 μg protein) were separated by Novex® 4–12% Tris-Glycine gels for 3.5 h at 15 mA and 150 v and electrophoretically transferred to Invitrolon PVDF membranes (Invitrogen, Carlsbad, CA) for 2 h at 300 mA and 25 v; both at 4°C (16). TriChromRanger prestained molecular weight marker mix (Pierce) was loaded into one of the wells of each gel for identification of the relative positions of the desired protein bands. After the transfer procedure, membranes were thoroughly rinsed in Millipore™ water, dipped into 100% methanol and air dried for 15 min. The membranes were then cut horizontally into three pieces, using the bands of the color marker as a guide, so that each piece of the membrane contained one protein band of interest. The three membrane pieces were individually rehydrated with methanol and processed separately. After blocking with 2% bovine serum albumin for 1 h at room temperature (Fisher, Pittsburgh, PA), the membranes were incubated overnight at 4°C with goat polyclonal anti-COX-2 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-iNOS antibody (1:375; Cayman Chemicals, Ann Arbor, MI), or goat polyclonal anti-β-actin (1:10,000; Santa Cruz Biotechnology). The membranes were then incubated for 1 h at room temperature with a bovine anti-goat (COX-2 1:60,000;β-actin 1:300,000; Santa Cruz Biotechnology) or a goat anti-rabbit IgG-HRP (horseradish peroxidase) conjugate (iNOS 1:60,000; Cayman Chemicals). The antibody-antigen complexes were developed with West Femto Maximum Sensitivity Substrate (Pierce), and the band intensities were scanned and quantified with a Bio-Rad Fluor-Imager (Bio-Rad Lab, Hercules, CA), and Quantity One software (Bio-Rad Lab). The loading control was β-actin.
Statistical Analysis
Animal weight and diet intake data were analyzed by one-way ANOVA or a repeated measures model using the GLM or Mixed Procedures in SAS (SAS Institute Inc., Cary, NC). Evaluation of ACF, proliferation and apoptotic indices, and iNOS and COX-2 protein expression were analyzed by one-way ANOVA using the Proc Mixed method in SAS. Least squares means were separated using a Fisher’s protected LSD for multiple comparisons. Pearson’s correlation analyses in SAS were used for evaluation of the relationships between ACF formation and proliferation or apoptosis, and between proliferation or apoptosis and iNOS or COX-2.
Results
Diet Analysis
In order to verify the concentrations of experimental compounds in the diets, aliquots collected throughout the experiment were analyzed by HPLC using the concentrations and retention times of known standards. Quantification of the limonin glucoside/obacunone glucoside mixture in the final diet was not possible, as no standards existed. However, using available limonoid glucoside standards and HPLC methods we identified that the mixture primarily contained limonin glucoside and obacunone glucoside (22). The measured concentrations of experimental compounds were 0.096% for hesperidin, 0.007% for nobiletin, 0.037% for naringenin, and 0.115% for apigenin. Except for naringenin, the concentrations were similar to the intended concentrations (0.1% for hesperidin, 0.01% for nobiletin, 0.02% for naringenin, and 0.1% for apigenin).
Food Intake and Weight Gain
Intake and weight gain were recorded throughout the experiment in order to determine if the experimental compounds influenced these variables. Rats tolerated the diets well as there were no differences in food intake among any of the diet groups overall or at any of the time points measured. However, the body weight of rats in the apigenin group was less than the body weight of rats from the hesperidin, nobiletin, naringenin, and limonin glucoside/obacunone glucoside mixture groups on day 56, and from all diet groups on day 77 (Table 1). When weight gain over the experimental period was considered, apigenin rats gained less than rats consuming the limonin glucoside/obacunone glucoside mixture diet (Table 1).
Table 1.
Mean body weights and body weight gain a
| Diet Group | Experiment Day | Weight Gain | ||||
|---|---|---|---|---|---|---|
| 0 | 7 | 21 | 56 | 77 | ||
| g | ||||||
| Control | 66.7a | 124.6a | 194.0a | 332.4a,b | 381.0a | 313.8a,b |
| Hesperidin | 66.9a | 126.5a | 199.4a | 336.0a | 381.3a | 313.9a,b |
| Nobiletin | 67.1a | 126.9a | 198.3a | 336.5a | 383.5a | 315.8a,b |
| Naringenin | 67.1a | 126.6a | 197.5a | 338.5a | 384.4a | 316.8a,b |
| Apigenin | 66.7a | 125.0a | 194.5a | 320.9a | 365.3a | 298.0a |
| LG/OG mixturec | 67.4a | 123.8a | 196.5a | 335.7a | 386.5a | 318.6a |
| SEM | 4.3 | 7.8 | 7.8 | 7.8 | 7.8 | 9.5 |
Values given are LS means ± SEM, n = 10 rats/group. Means in a column without a common letter differ, P < 0.05.
Limonin glucoside/obacunone glucoside mixture.
Incidence of Aberrant Crypt Foci
Development of preneoplastic lesions (aberrant crypts; AC) is a hallmark of colon carcinogenesis in both humans and animal models of the disease (27), with HMACF being predictive of eventual tumor formation (28). In rats provided with the experimental compounds, the total number of AC and the number of aberrant crypts per centimeter of colon were not different from those in rats provided with the control diet. In rats provided with the control diet, AOM induced an average of 3.94 HMACF per rat (Fig. 1), with naringenin and apigenin yielding fewer (51 and 57%, respectively, P < 0.05) HMACF than in the control rats. The number of HMACF in rats provided with hesperidin, nobiletin, and limonin glucoside/obacunone glucoside mixture were not different from the rats provided with the control diet.
Figure 1.
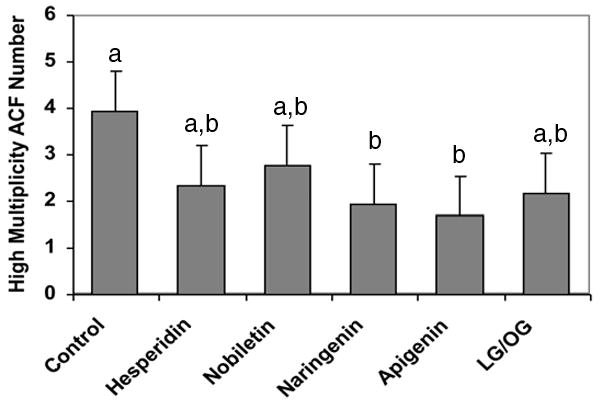
Effects of experimental diets on incidence of high multiplicity aberrant crypt foci (HMACF > 4 aberrant crypts/focus) per colon. Values are LS means ± SEM, n = 10 rats/diet. a,b Bars with different letters are significantly different, P < 0.05.
Colonocyte Proliferation
Loss of control over proliferation is a well-recognized transformation occurring during carcinogenesis. Therefore, we documented changes in the number, position and proportion of proliferating cells within the colon crypts. Naringenin lowered (P < 0.05) both the number of proliferating colonocytes and the proliferative index, compared to the control diet (Table 2). In addition, rats provided with naringenin had a smaller (P < 0.05) proliferative zone (18% lower) when compared with rats in the control group (Table 2). There were no differences in the proliferative index or proliferative zone in rats consuming diets containing hesperidin, nobiletin, or apigenin, compared to rats provided the control diet. However, rats provided with the limonin glucoside/obacunone glucoside mixture did have a lower position of the highest labeled cell, compared to the control diet (Table 2). Colonic crypt heights were not different among the diets.
Table 2.
Effect of experimental diets on number of proliferating cells, location of highest proliferating cell, and crypt height in colon of Sprague-Dawley ratsa
| Diet Group | Proliferating cell number | Number of cells/crypt column | Proliferative index (%) | Location of highest proliferating cell (proliferative zone) |
|---|---|---|---|---|
| Control | 6.64a | 32.05a | 20.74a | 14.98a |
| Hesperidin | 5.77a,b | 32.16a | 18.01ab | 13.76a,b |
| Nobiletin | 4.94a,b | 31.21a | 15.85ab | 12.58a,b |
| Naringenin | 4.47b | 31.98a | 14.03b | 12.12b |
| Apigenin | 4.77a,b | 32.11a | 14.98ab | 12.78a,b |
| LG/OG mixturec | 4.76a,b | 31.96a | 15.16ab | 12.37b |
| SEM | 0.85 | 0.42 | 2.69 | 1.10 |
Values are LS means ± SEM, n = 10 rats/group. Means in a column without a common letter differ, P < 0.05.
Limonin glucoside/obacunone glucoside mixture.
Apoptosis
Induction of apoptosis among transformed cells is one mechanism whereby dietary compounds may protect against colon carcinogenesis. In order to determine if the experimental compounds were affecting colonocyte apoptosis, we documented the number and proportion of apoptotic cells within the crypt columns and along the luminal surface adjacent to the selected crypt columns. The number of apoptotic cells in the colonic crypts of all rats was only different between the naringenin and limonin glucoside/obacunone glucoside mixture diets (Table 3). Because extensive staining of apoptotic cells was observed on the luminal surface, the luminal surface colon cells were evaluated for the extent of apoptosis as well. The luminal surface apoptotic index for rats provided with dietary naringenin was 97% and apigenin was 78% higher (P < 0.05) than rats provided with the control diet (Table 3). The extent of apoptosis in luminal surface cells in rats consuming hesperidin, nobiletin, or the limonin glucoside/obacunone glucoside mixture were not different from control rats.
Table 3.
Effect of experimental diets on apoptosis within colonic crypt columnsa
| Diet Group | Number of apoptotic cells/crypt column | Luminal surface apoptotic index (%) |
|---|---|---|
| Control | 0.20a,b | 20.71a |
| Hesperidin | 0.19a,b | 27.88a,b |
| Nobiletin | 0.22a,b | 26.67a,b |
| Naringenin | 0.32a | 40.81c |
| Apigenin | 0.18a,b | 36.93b,c |
| LG/OG mixtured | 0.14b | 24.50a,b |
| SEM | 0.06 | 3.81 |
Values given are LS means ± SEM, n = 10 rats/group. Means in a column without a common letter differ, P < 0.05.
Limonin glucoside/obacunone glucoside mixture.
Expression of iNOS and COX-2
Based on the literature describing the potential anti-inflammatory effects of compounds related to those used in this study, we sought to document the expression of iNOS and COX-2. There were no differences detected in the expression of iNOS and COX-2 enzymes in the colonic mucosa of rats among the diet groups because of the extensive variability in these measurements (Supplemental Figure). Interestingly, when the data for rats in all diet groups were combined, there was a positive correlation between the levels of iNOS and COX-2 enzyme expression (r = 0.504, P < 0.05).
Correlation Analysis
The proliferative zone was negatively correlated with surface apoptotic index (r=−0.279, P < 0.05) when data for all diet groups were combined. In addition, there was a tendency for a negative relationship between proliferative index and surface cell apoptosis for the pooled data (r=−0.237, P = 0.068). In rats provided the naringenin diets, COX-2 level was positively correlated with proliferative index (r=0.896, P < 0.05).
Discussion
The aim of this study was to investigate the chemopreventive ability of four citrus flavonoids (hesperidin, nobiletin, naringenin, and apigenin) and one citrus limonoid mixture (containing limonin glucoside and obacunone glucoside) at the promotion stage of AOM-induced colon carcinogenesis in Sprague-Dawley rats. Dietary naringenin (0.037%) and apigenin (0.115%) reduced (P < 0.05) the incidence of HMACF in the rat colon by 51% and 57%, respectively, relative to the control diet. Although apigenin has been shown to inhibit AOM-induced ACF in CF-1 mice (29), to our knowledge, this is the first study to demonstrate the ability of naringenin, a major flavonoid found in grapefruit, to reduce AOM-induced HMACF in Sprague-Dawley rats. Since HMACF are likely to correlate with future tumor formation (24), these results suggest that dietary naringenin and apigenin may protect against colon carcinogenesis, similar to their effects in other tumor models (30–31).
The concentrations of hesperidin, nobiletin, and limonin glucoside/obacunone glucoside mixture used in this study did not significantly suppress HMACF formation compared to the control diet. These observations contrast two studies in which dietary hesperidin (0.1%) and nobiletin (0.01%) inhibited ACF in F344 rats (17–18). Contrasting results may be due to strain differences between Sprague-Dawley and F344 rats or the time points at which the observations were made. Taylor et al. (32) showed febrile differences between the two rat strains after lipopolysaccharide injections and Tennekes et al. (33) found Sprague-Dawley rats were more predisposed to certain tumors than F344 rats. A pioneer of the ACF model reported that ACF appear within 2 wk after carcinogen injection, with numbers peaking at 8 wk (34–35). Rats in the studies by Tanaka et al. (18) and Kohno et al. (17) were terminated 2 wk after the last carcinogen injection, thus there may not have been sufficient time for the ACF to reach their peak development. Since our study sacrificed the rats 6 wk after the last AOM injection, the higher numbers of ACF observed in the hesperidin and nobiletin diet groups may be more reflective of the peak stage of aberrant crypt formation.
Some studies have found that some citrus limonoids have chemopreventive properties, which was not observed with the 0.035% limonin glucoside/obacunone glucoside mixture used in this study. Tanaka et al. (12) found the aglycone form of limonin or obacunone (each at 0.02% of the diet) inhibited ACF formation in F344 rats. Because we were unable to confirm the final concentration of these compounds in our mixed diet, we do not know if the disparity in responses were due to differences in the dose delivered. However, a more probable reason for the difference in responses between our study and those of Tanaka et al. (12) are due to the glycosidic form of limonin and obacunone not generating the same protection against AOM-induced ACF as the aglycones.
Increased proliferation and suppressed apoptosis are typical in tumorigenesis (36). In the current study, rats provided with naringenin had a reduced proportion of proliferating colon cells and smaller expansion of the proliferative zone than the control diet. The anti-proliferative effects of naringenin also have been demonstrated in HT29 colon cancer cells (11). Even though hesperidin, nobiletin, apigenin, and the limonin glucoside/obacunone glucoside mixture yielded reductions in proliferation ranging between 13.2 and 27.8%, these differences were not significant. Cell culture experiments have reported anti-proliferative effects for hesperetin, the aglycone form of hesperidin (30), nobiletin (37), apigenin (38), and a limonoid glucoside mixture (39). The apparent discordance between in vivo and in vitro studies raises the question of whether the metabolism of these compounds alters their effectiveness and/or functionality in an in vivo system. For example, Hanske et al. (40) recently demonstrated that apigenin-7-glucoside is metabolized to not only the aglycone form of apigenin, but also to low levels of naringenin (and other compounds) in vivo. Thus, although apigenin may be effective in reducing proliferation in vitro, its capacity to effect this change in vivo may have been reduced by its metabolism within the intestinal tract.
The extent of apoptosis within the colonic crypt columns was limited, and there were no differences in the apoptotic index among the diet groups, except between the naringenin and limonin glucoside/obacunone glucoside mixture diets. Tumors often develop when epithelial cells do not undergo apoptosis and instead accumulate on the luminal surface of the colon (41), thus apoptosis was measured in surface cells in this study. When compared to the control diet, surface cell apoptosis was enhanced by 97% and 78% in rats provided with naringenin and apigenin, respectively. Because changes in apoptosis may be more important in predicting colon tumorigenesis than cell proliferation alone (42), the up-regulation of apoptosis observed with naringenin and apigenin may provide a possible explanation for their ability to inhibit HMACF. The results from this study extend existing in vitro observations and demonstrate that naringenin and apigenin are able to induce apoptosis in vivo. Pro-apoptotic effects were not seen in rats from hesperidin, nobiletin, and limonin glucoside/obacunone glucoside mixture diet groups.
While there were no significant correlations between HMACF and either cell proliferation or apoptosis in this study, there was a slight negative correlation between the extent of the proliferative zone and surface cell apoptosis when data for all six diets were pooled. There was also a tendency for a negative relationship between proliferative index within crypt column and surface cell apoptosis for the pooled data. These results suggest that cell kinetics such as cell proliferation and apoptosis may have been modulated but the individual effects of each diet were not strong enough to achieve significance. In any case, the negative relationship between cell proliferation and apoptosis is in agreement with evidence from the literature that increased cell proliferation together with decreased apoptosis may serve as a permissive environment for cancer development (36).
Another contributor to cancer development may be the induction of pro-inflammatory processes (43–44). Increased expression of two pro-inflammatory enzymes, COX-2 and iNOS, has been reported in human colorectal adenomas and adenocarcinomas (45–46). Apigenin and naringenin suppressed COX-2 or iNOS expression as well as NO and PGE2 levels in LPS-stimulated macrophages (47). Yet, in the current study, no differences in the levels of COX-2 or iNOS proteins among the six diet groups were observed. The data suggest that the protective effects of these bioactive compounds do not result from changes in the level of COX-2 or iNOS proteins in this in vivo colon cancer model. One possible explanation for the discrepancies between the in vitro and in vivo studies may be that most cell culture assays use transformed cancer cells so the products of tumor promoting genes (e.g., iNOS or COX-2) are highly expressed. Samples from animal models, as occurs in humans, include cells that are not all transformed or cancerous. As a result, any protective effects in such scenarios would more likely be harder to detect. Because the current study only measured the protein levels but not activities of iNOS and COX-2 it is not possible to know if the levels of NO and PGE2 were affected by the compounds. Interestingly, there was a positive correlation between COX-2 level and proliferative zone in rats provided with naringenin, which was expected based on the literature linking PGE2 and cell proliferation (48). There was also a positive correlation between the enzyme expression levels of iNOS and COX-2 in the current study, when the data for rats in all diet groups were combined. Several cell culture studies and animal inflammatory models have shown the co-induction or co-regulation of iNOS and COX-2 enzymes (49).
Together, the data from this study indicate that naringenin and apigenin provide promise as naturally occurring chemopreventive agents against colon carcinogenesis. Although hesperidin, nobiletin, and the limonin glucoside/obacunone glucoside mixture did not exhibit protective effects, they should not be discounted from future consideration since they have demonstrated effectiveness in other disease models. Furthermore, the differences in effect or lack of effect between different rat strains provided with the same compounds highlights the fact that higher biological systems are heterogeneous, suggesting that individual compounds at a certain dosage may be beneficial for some individuals but not for others. Therefore, more research in this area is needed to determine the optimum nutritional profile if we wish to pursue the goal of individualized medicine and nutritional recommendations.
Supplementary Material
Acknowledgments
The experimental compounds were provided by Texas A&M University-Kingsville Citrus Center. We wish to thank Dr. Kil Sun Yoo of the Texas A&M University Vegetable and Fruit Improvement Center for performing the analyses to determine the level of experimental compounds in the diets.
Footnotes
Author contributions: All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; TL, JV, ST, LD, and NDT conducted the experiments, MEM, NW, and RJC performed statistical analyses, BP provided the experimental compounds, TL, JV, RSC, JRL and NDT materially participated in data interpretation and manuscript preparation.
Supported by funds from the USDA/IFAFS (2001-52102-11257), the Texas Higher Education Coordinating Board (ATP 003658-0359c-2001 and ATP 010366-0194-2001), USDA/CSREES (2003-34402-13647, 2004-34402-14768), NIH (R37-CA05730), and NIEHS P30-ES09106.
LITERATURE CITED
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Van Duijnhoven FJB, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland A, Olsen A, Overvad K, Thorlacius-Ussing O, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Kaaks R, Linseisen J, Boein H, Nöthlings U, Trichopoulou A, Trichopoulos D, Misirli G, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Peeters PHM, van Gils CH, Ocké MC, Lund E, Engeset D, Skeie G, Suárez LR, González CA, Sánchez M-J, Dorronsoro M, Navarro C, Barricarte A, Berglund G, Manjer J, Hallmans G, Palmqvist R, Bingham SA, Khaw K-T, Key TJ, Allen NE, Boffetta P, Slimani N, Rinaldi S, Gallo V, Norat T, Riboli E. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 3.Potter JD. Colorectal cancer: Molecules and populations. J Natl Cancer Inst. 1999;91:916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 4.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 5.WCRF/AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 6.Hung H-C, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 7.Deneo-Pellegrini H, Boffetta P, De Stefani E, Ronco A, Brennan P, Mendilaharsu M. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev. 2002;11:369–375. doi: 10.1097/00008469-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Risch HA, Jain M, Choi NW, Fodor JG, Pfeiffer CJ, Howe GR, Harrison LW, Craib KJ, Miller AB. Dietary factors and the incidence of cancer of the stomach. Am J Epidemiol. 1985;122:947–959. doi: 10.1093/oxfordjournals.aje.a114199. [DOI] [PubMed] [Google Scholar]
- 9.Miyagi Y, Om AS, Chee KM, Bennink MR. Inhibition of azoxymethane-induced colon cancer by orange juice. Nutr Cancer. 2000;36:224–229. doi: 10.1207/S15327914NC3602_12. [DOI] [PubMed] [Google Scholar]
- 10.Kossoy G, Ben-Hur H, Stark A, Zusman I, Madar Z. Effects of a 15% orange-pulp diet on tumorigenesis and immune response in rats with colon tumors. Oncol Rep. 2001;8:1387–1391. doi: 10.3892/or.8.6.1387. [DOI] [PubMed] [Google Scholar]
- 11.Frydoonfar HR, McGrath DR, Spigelman AD. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid naringenin. Colorectal Dis. 2003;5:149–152. doi: 10.1046/j.1463-1318.2003.00444.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Maeda M, Kohno H, Murakami M, Kagami S, Miyake M, Wada K. Inhibition of azoxymethane-induced colon carcinogenesis in male F344 rats by the citrus limonoids obacunone and limonin. Carcinogenesis. 2001;22:193–198. doi: 10.1093/carcin/22.1.193. [DOI] [PubMed] [Google Scholar]
- 13.Au A, Li B, Wang W, Roy H, Koehler K, Birt D. Effect of dietary apigenin on colonic ornithine decarboxylase activity, aberrant crypt foci formation, and tumorigenesis in different experimental models. Nutr Cancer. 2006;54:243–251. doi: 10.1207/s15327914nc5402_11. [DOI] [PubMed] [Google Scholar]
- 14.Aranganathan S, Selvam JP, Nalini N. Effect of hesperetin, a citrus flavonoid, on bacterial enzymes and carcinogen-induced aberrant crypt foci in colon cancer rats: a dose dependent study. J Pharm Pharmacol. 2008;60:1385–1392. doi: 10.1211/jpp/60.10.0015. [DOI] [PubMed] [Google Scholar]
- 15.Hoensch H, Groh B, Edler L, Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J Gastroenterol. 2008;14:2187–2193. doi: 10.3748/wjg.14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27:1257–1265. doi: 10.1093/carcin/bgi318. [DOI] [PubMed] [Google Scholar]
- 17.Kohno H, Yoshitani S, Tsukio Y, Murakami A, Koshimizu K, Yano M, Tokuda H, Nishino H, Ohigashi H, Tanaka T. Dietary administration of citrus nobiletin inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Life Sci. 2001;69:901–913. doi: 10.1016/s0024-3205(01)01169-9. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T, Makita H, Kawabata K, Mori H, Kakumoto M, Satoh K, Hara A, Sumida T, Tanaka T, Ogawa H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. 1997;18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 19.Miller EG, Gonzales-Sanders AP, Couvillon AM, Wright JM, Hasegawa S, Lam LK. Inhibition of hamster buccal pouch carcinogenesis by limonin 17-β-D-glucopyranoside. Nutr Cancer. 1992;17:1–7. doi: 10.1080/01635589209514167. [DOI] [PubMed] [Google Scholar]
- 20.Raman G, Jayaprakasha GK, Cho M, Brodbelt JS, Patil BS. Isolation of structurally similar citrus flavonoids by flash chromatography. Anal Lett. 2004;37:3005–3016. [Google Scholar]
- 21.Raman G, Jayaprakasha GK, Cho M, Brodbelt J, Patil BS. Rapid adsorptive separation of citrus polymethoxylated flavones in non-aqueous conditions. Sep Purif Technol. 2005;45:147–152. [Google Scholar]
- 22.Poulose SM, Harris ED, Patil BS. Citrus limonoids induce apoptosis in human neuroblastoma cells and have radical scavenging activity. J Nutr. 2005;135:870–877. doi: 10.1093/jn/135.4.870. [DOI] [PubMed] [Google Scholar]
- 23.Patil BS, Vanamala J, Hallman G. Irradiation and storage influence on bioactive components and quality of early and late season ‘Rio Red’ grapefruit (Citrus paradisi Macf) Postharvest Biol Technol. 2004;34:53–64. [Google Scholar]
- 24.Cheng L, Lai M-D. Aberrant crypt foci as microscopic precursors of colorectal cancers. World J Gastroenterol. 2003;9:2642–2649. doi: 10.3748/wjg.v9.i12.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roncucci L, Pedroni M, Vaccina F, Benatti P, Marzona L, De Pol A. Aberrant crypt foci in colorectal carcinogenesis. Cell and crypt dynamics. Cell Prolif. 2000;33:1–18. doi: 10.1046/j.1365-2184.2000.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong MY, Chapkin RS, Wild CP, Morris JS, Wang N, Carroll RJ, Turner ND, Lupton JR. Relationship between DNA adduct levels, repair enzyme, and apoptosis as a function of DNA methylation by azoxymethane. Cell Growth Differ. 1999;10:749–758. [PubMed] [Google Scholar]
- 27.Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr. 2007;137:2576S–2579S. doi: 10.1093/jn/137.11.2576S. [DOI] [PubMed] [Google Scholar]
- 28.Pretlow TP, O’Riordan MA, Somich GA, Amini SB, Pretlow TG. Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis. 1992;13:1509–1512. doi: 10.1093/carcin/13.9.1509. [DOI] [PubMed] [Google Scholar]
- 29.Au A, Li B, Wang W, Roy H, Birt DF. Apigenin inhibits colonic ornithine decarboxylase activity in cultured cells and mouse colon and aberrant crypt foci formation in CF-1 mice. FASEB J. 1999;13:A582. [Google Scholar]
- 30.So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer. 1996;26:167–181. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- 31.Tatsuta A, Iishi H, Baba M, Yano H, Murata K, Mukai M, Akedo H. Suppression by apigenin of peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin Exp Metastasis. 2001;18:657–662. doi: 10.1023/a:1013133803806. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AN, Tio DL, Romeo HE. The febrile response to intraperitoneal lipopolysaccharide: Strain and gender differences in rats. J Neuroimmunol. 2005;158:86–93. doi: 10.1016/j.jneuroim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Tennekes H, Kaufmann W, Dammann M, van Ravenzwaay B. The stability of historical control data for common neoplasms in laboratory rats and the implications for carcinogenic risk assessment. Regul Toxicol Pharmacol. 2004;40:293–304. doi: 10.1016/j.yrtph.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93:55–71. doi: 10.1016/0304-3835(95)03788-X. [DOI] [PubMed] [Google Scholar]
- 35.McLellan EA, Medline A, Bird RP. Sequential analyses of the growth and morphological characteristics of aberrant crypt foci: Putative preneoplastic lesions. Cancer Res. 1991;51:5270–5274. [PubMed] [Google Scholar]
- 36.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 37.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Antiproliferative activity of flavonoids on several cancer lines. Biosci Biotechnol Biochem. 1999;63:896–899. doi: 10.1271/bbb.63.896. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–110. [PubMed] [Google Scholar]
- 39.Tian Q, Miller EG, Ahmad H, Tang L, Patil BS. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr Cancer. 2001;40:180–184. doi: 10.1207/S15327914NC402_15. [DOI] [PubMed] [Google Scholar]
- 40.Hanske L, Loh G, Sczesny S, Blaut M, Braune A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J Nutr. 2009;139:1095–1102. doi: 10.3945/jn.108.102814. [DOI] [PubMed] [Google Scholar]
- 41.Hong MY, Chang WL, Chapkin RS, Lupton JR. Relationship among colonocyte proliferation, differentiation, and apoptosis as a function of diet and carcinogen. Nutr Cancer. 1997;28:20–29. doi: 10.1080/01635589709514548. [DOI] [PubMed] [Google Scholar]
- 42.Chang WL, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 43.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 44.Philpott M, Ferguson LR. Immunonutrition and cancer. Mutat Res. 2004;551:29–42. doi: 10.1016/j.mrfmmm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: Implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 46.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 47.Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–931. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 48.Wendum D, Masliah J, Trugnan G, Flejou J-F. Cyclooxygenase-2 and its role in colorectal cancer development. Virchows Arch. 2004;445:327–333. doi: 10.1007/s00428-004-1105-2. [DOI] [PubMed] [Google Scholar]
- 49.Surh Y-J, Chun K-S, Cha H-H, Han SS, Keum Y-S, Park K-K, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


