Abstract
Melanosomes are the specialized intracellular organelles of pigment cells devoted to the synthesis, storage and transport of melanin pigments, which are responsible for most visible pigmentation in mammals and other vertebrates. As a direct consequence, any genetic mutation resulting in alteration of melanosomal function, either because affecting pigment cell survival, migration and differentiation, or because interfering with melanosome biogenesis, transport and transfer to keratinocytes, is immediately translated into color variations of skin, fur, hair or eyes. Thus, over one hundred genes and proteins have been identified as pigmentary determinants in mammals, providing us with a deep understanding of this biological system, which functions by using mechanisms and processes that have parallels in other tissues and organs. In particular, many genes implicated in melanosome biogenesis have been characterized, so that melanosomes represent an incredible source of information and a model for organelles belonging to the secretory pathway. Furthermore, the function of melanosomes can be associated with common physiological phenotypes, such as variation of pigmentation among individuals, and with rare pathological conditions, such as albinism, characterized by severe visual defects. Among the most relevant mechanisms operating in melanosome biogenesis are the signal transduction pathways mediated by two peculiar G protein-coupled receptors: the melanocortin-1 receptor (MC1R), involved in the fair skin/red hair phenotype and skin cancer; and OA1 (GPR143), whose loss-of-function results in X-linked ocular albinism. This review will focus on the most recent novelties regarding the functioning of these two receptors, by highlighting emerging signaling mechanisms and general implications for cell biology and pathology.
Keywords: Pigmentation, Albinism, G protein-coupled receptor, Melanosome, Lysosome, Organelle biogenesis
1. INTRODUCTION
1.1 Melanocyte biology and melanosome biogenesis
The color of the skin, hair and eyes of mammals (and to a large extent of other vertebrates) results from the presence and distribution of melanin pigments. These are large biopolymers derived from the progressive oxidation of the amino acid tyrosine in the absence or in the presence of sulfhydryl groups from cysteine, giving rise to black-brown (eumelanin) or yellow-red (pheomelanin) compounds, respectively (Prota et al., 1998). Melanins are exclusively synthesized by a small population of pigment cells, including melanocytes and retinal pigment epithelium (RPE) (King et al., 1995). The former derive embryologically from the neural crest and then migrate in several organs and tissues, including the basal layer of the epidermis, where they play a critical role in photo-protection and camouflage, the eye (choroid and iris stroma), the inner hear, and the leptomeninges. In contrast, the RPE derives from the neuroectoderm, similarly to the neurosensory retina, and is situated behind the photoreceptor layer in the posterior segment of the eye, but also extends anteriorly to form the innermost part of the iris, becoming the iris pigment epithelium (IPE). The RPE is implicated in photo-absorption, provides structural and functional support to photoreceptors, and - last but not least - plays a fundamental role in the development of the retina and visual pathways (Graw, 2003; King et al., 1995).
Despite embryological and functional differences, melanocytes and RPE share the common ability to synthesize melanin pigments within specialized subcellular organelles, termed melanosomes. Melanosomes are part of the secretory/endocytic pathway and, based on their characteristics, are commonly defined as lysosome-related organelles, like dense bodies and α granules in platelets, major histocompatibility complex (MHC) class II compartments in antigen presenting cells, lytic granules in cytotoxic T lymphocytes, and other cell-type specific organelles in granulocytes, osteoclasts, endothelial and lung epithelial cells. Indeed, melanosomes display an acidic luminal pH and contain lysosomal hydrolases, lysosomal-associated membrane proteins (LAMPs) and specific integral membrane proteins, whose transport is regulated by targeting machineries typical of lysosomes, including sorting signals and adaptors. Moreover, melanosomes are abnormal in monogenic disorders affecting lysosomes and multiple lysosome-related organelles, namely the Chediak-Higashi and Hermansky-Pudlak syndromes, indicating a close structural and/or functional relationship between these organelles (Dell’Angelica et al., 2000; Marks and Seabra, 2001). Nevertheless, melanosomes are also different from lysosomes, being responsible for specific functions unrelated to degradation, and in pigment cells they co-exist with conventional lysosomes (Figure 1)(Futter, 2006; Raposo and Marks, 2007).
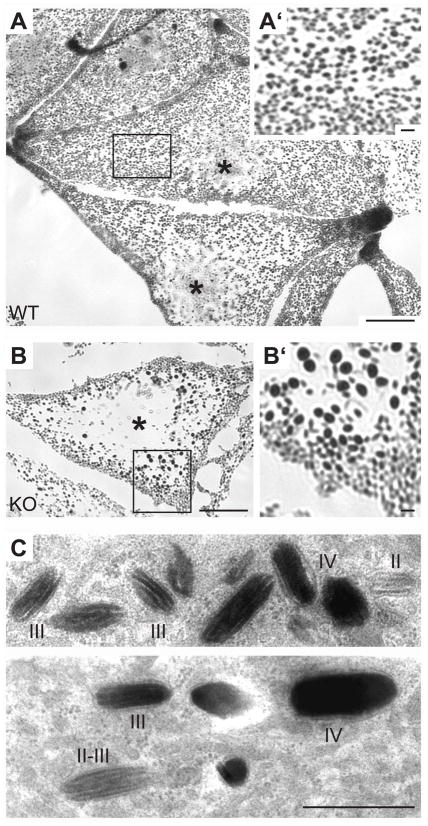
Figure 1.
Optical and ultrastructural features of melanosomes. (A) Representative picture of wild-type mouse melanocytes in culture as observed by bright-field optical microscopy. In this condition, melanosomes represent the only visible dark objects, thanks to the melanin pigment, and show an even distribution and size throughout the cell. (B) Bright-field picture of Oa1-KO mouse melanocytes, showing reduction in melanosome number, presence of macromelanosomes and displacement of the organelles towards the cell periphery. A′ and B′ represent a 3x magnification of delimited areas in A and B, respectively. Asterisks indicate the position of the cell nuclei. Scale bars 10 μm (1 μm for 3x magnifications). (C, D) Conventional electron microscopy of wild-type mouse melanocytes, showing melanosomes at various maturation stages, as indicated. Stages II and III are characterized by longitudinal striatures, without or with progressive melanin deposition (black), respectively, while stage IV melanosomes show no recognizable internal structures and a completely electron dense melanized lumen. Scale bar, 1 μm.
Melanosome biogenesis has been characterized in detail for eumelanin-carrying organelles only. Melanosomes originate from endosomal precursors (Raposo et al., 2001) and subsequently undergo a series of maturation stages, each characterized by unique ultrastructural morphology and melanin content: stage II and III melanosomes correspond to non-pigmented and partially-pigmented immature organelles, respectively, and possess a proteinaceus internal matrix forming regular longitudinal striatures; stage IV melanosomes correspond to fully-melanized mature organelles, whose internal structure becomes completely masked by the eumelanin pigment (Marks and Seabra, 2001; Seiji et al., 1963). A series of pigment cell-specific melanosomal proteins are responsible for this process, including the melanogenic enzymes Tyrosinase and Tyrosinase-related proteins TYRP1 and 2 (the latter being also known as dopachrome tautomerase, DCT) (Yamaguchi et al., 2007); the structural protein Pmel17, representing the main constituent of the internal matrix of the organelles (Berson et al., 2003); the membrane transporters P, MATP/SLC45A2 and SLC24A5, implicated in the control of melanosome pH, osmolarity and calcium content, respectively (Ancans et al., 2001; Lamason et al., 2005; Newton et al., 2001; Puri et al., 2000); and finally the melanosomal G-protein-coupled receptor (GPCR) OA1 (also known as GPR143) (Schiaffino and Tacchetti, 2005).
Mature melanosomes display a considerable motility both along microtubules (MTs), by means of kinesin and dynein motors, and along actin filaments (AFs), by means of a tripartite complex comprising the monomeric GTPase Rab27a, its effector melanophilin (MyRIP in RPE), and the actin-based motor myosin Va (myosin VIIa in RPE) (for review see Barral and Seabra, 2004). In a probably simplified view, based on the effects of reagents that depolymerize actin or tubulin filaments, or disrupt the function of dynein or Myosin Va, these cytoskeletal systems appear to regulate the distribution of melanosomes by generating opposite and competitive forces, as in a “tug of war” in which MTs promote the perinuclear accumulation and AFs support the peripheral dispersal of the organelles (Gross et al., 2002; Wu et al., 1998). In fish or frog melanophores (the melanocyte equivalent in these species), which exploit melanosome motility for rapid color adaptation, the switch between the two transport systems is believed to depend on signaling events, including cAMP and protein kinases, which direct the coordinated dispersion or aggregation of melanosomes in response to extracellular stimuli (for review see Aspengren et al., 2007). In contrast, in mammals melanosome transport is associated with melanogenesis and is thought to proceed in a perinuclear to centrifugal route, leading to the accumulation of mature melanosomes at the cell periphery, where in the case of skin melanocytes the organelles are subsequently transferred via dendritic processes to neighboring keratinocytes (Marks and Seabra, 2001).
Basal pigmentation of both melanocytes and RPE is mostly genetically determined. However, skin melanocytes also display the ability to adapt their melanogenic abilities to a number of extracellular stimuli, either generated by the organism as paracrine and endocrine factors, or by the external environment, like ultraviolet radiation (UVR). Therefore, in the skin the overall process leading to melanosome formation, transport and transfer can be stimulated by signaling pathways rising from the melanocyte plasma membrane, the most relevant of which is mediated by the melanocortin-1 receptor (MC1R), and by its downstream target, the microphthalmia transcription factor (MITF) (Lin and Fisher, 2007).
1.2 Physiological and pathological phenotypes associated to melanosomes
Physiological differences in skin, hair and eye color between individuals, within and among ethnic groups, do not depend on the number of melanocytes, but on the number and size of melanosomes, on the quantity and quality of melanin they contain, and on the efficiency and characteristics of melanosome transfer to keratinocytes, where melanosomes can be more or less effectively distributed around the cell nuclei to screen them from UVR (Alaluf et al., 2002; Thong et al., 2003). The molecular bases for such differences reside in the polymorphic variation of genes implicated in melanosome biogenesis (Sturm, 2009). For instance, a single nucleotide polymorphism (SNP), leading to the substitution of a conserved amino acid within the gene for SLC24A5, accounts by itself for 25–38% of skin color difference between Europeans and Africans (Lamason et al., 2005). On the other hand, within the European population the most polymorphic pigmentation-related gene is certainly MC1R, for which over 60 non-conservative SNPs have been reported, often altering the receptor’s activity, resulting in lighter yellow/red skin and hair color phenotypes, and predisposing to the development of skin cancer (Garcia-Borron et al., 2005; Rees, 2003; Sturm et al., 2003).
When melanosome number, structure and/or function are severely compromised not only in melanocytes, but also in the RPE, a clearly pathological condition is generated, namely albinism. This term comprises a heterogeneous group of diseases, characterized by variable hypopigmentation of the skin (evidently affected in oculocutaneus albinism, not or only mildly affected in ocular albinism) and severe developmental defects of the eyes, including foveal hypoplasia and misrouting of the optic tracts at the chiasm, which are secondary to RPE hypopigmentation (King et al., 1995). Albinism can be associated to abnormalities affecting other cell types in addition to pigment cells, as in the Hermansky-Pudlak and Chediak-Higashi syndromes, which derive from mutation of genes involved in the biogenesis of multiple lysosome-related organelles (Dell’Angelica et al., 2000). In contrast, primary albinism is characterized by exclusive involvement of melanosomes in the pigment cells of the skin and eyes and has been associated so far to five genes, which encode integral membrane proteins localized to melanosomes and essential for their proper biogenesis, namely Tyrosinase, TYRP1, P, MATP/SLC45A2 and OA1.
While partial or complete loss-of-function of the first four genes can lead to oculocutaneus or ocular albinism, or even physiological skin/eye color phenotypes, depending on the residual protein activity and on the resulting melanin synthesis, OA1 mutations are only associated to ocular albinism, with minor involvement of the skin even in the absence of any OA1 activity. Moreover, at variance with the genes for other melanosomal proteins and MC1R, no coding polymorphisms have been associated so far to OA1, underlining the critical role that this receptor plays in the development of the retina, where instead MC1R is not expressed. Therefore, both MC1R and OA1 play critical and unique roles in the physiology and pathology of pigmentation and melanosome biogenesis. In addition, they have recently provided the GPCR and more generally the signaling fields with novel and intriguing insights, which will represent the main focus of this review (Figures 2-3).
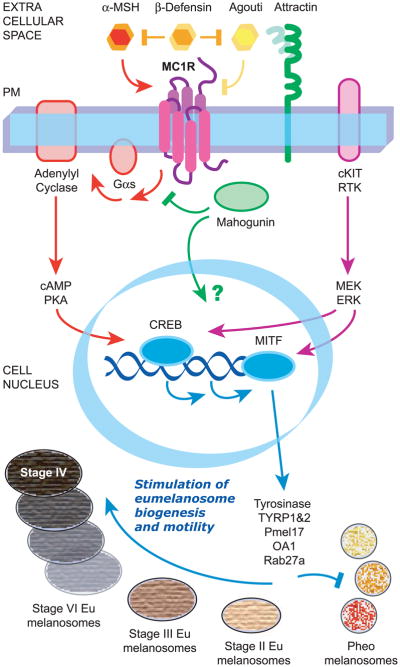
Figure 2.
Schematical overview of the signaling pathway mediated by MC1R. MC1R resides at the plasma membrane of melanocytes and is activated by melanocortins, in particular α-MSH, and inactivated by Agouti protein. To be fully effective, Agouti also needs Attractin, which helps Agouti binding to the receptor, and Mahogunin, which acts at the cytosolic side of the plasma membrane, most likely by competing with Gs for receptor binding. In addition, MC1R has a neutral agonist, β-Defensin, which interferes with both agonist and antagonist binding, thereby highlighting the high degree of constitutive activity of the receptor. The canonical pathway activated by MC1R leads to cAMP increase via Gs and adenylyl cyclase, and proceeds with CREB phosphorylation by PKA and transcription of downstream targets, in particular MITF. Increased MITF activity is further reinforced and regulated by cAMP-independent ERK activation, probably resulting from cross-talk between MC1R and the cKIT receptor. MITF binds to promoters and stimulates transcription of genes coding for melanosomal proteins implicated in the eumelanogenesis pathway, thus inducing a switch from pheomelanin to eumelain synthesis and increasing melanosome number, size and transport. MC1R signaling also reduces the generation of reactive oxygen species and enhances DNA repair mechanisms by still poorly understood mechanisms (not shown). PM, plasma membrane.
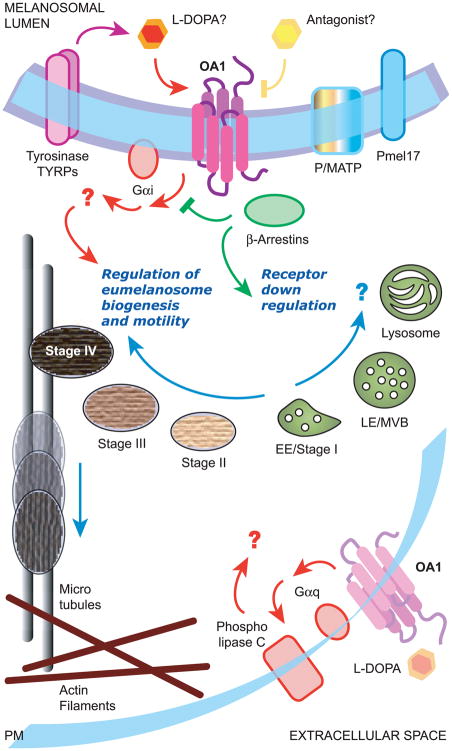
Figure 3.
Schematical overview of the signaling pathway mediated by OA1. Endogenous OA1 resides on the membrane of late endosomes/lysosomes and melanosomes, where it could function as a “sensor” of melanosome maturation and be activated by molecules belonging to the melanin biosynthetic pathway, possibly L-DOPA. It appears coupled to Gi proteins, through which it regulates proper biogenesis of melanosomes and restrains their transport toward the cell periphery by still unknown effectors. While a role in melanosome biogenesis and transport is supported by numerous lines of evidence, it is unclear whether the lysosomal localization of endogenous OA1 in melanocytes has a functional or downregulation role. In addition, a minor fraction of endogenous OA1 might travel to the cell surface of RPE cells, where it could become activated by L-DOPA and in turn trigger a Gq-mediated signaling pathway eventually leading to the secretion of neurotrophic factors. In contrast, exogenous OA1 overexpressed in non-melanocytic cells can be substantially missorted to the plasma membrane, downregulated by arrestins and coupled to Gq proteins, leading to phospholipase C activation and inositol phosphate or intracellular calcium increase (not shown). PM, plasma membrane; EE, early endosomes; LE, late endosomes; MVB, multivesicular bodies.
2. MC1R AND SKIN CANCER
2.1 Overview of MC1R structure, function and associated pathology
Human MC1R is a 317 amino acid seven transmembrane receptor, belonging to Class A of the GPCR superfamily, like rhodopsin and adrenergic receptors. In mammals, it appears strictly related in sequence and ligand-binding features to four additional melanocortin receptors (MC2R to MC5R), characterized by different patterns of expression and physiological roles (Chhajlani and Wikberg, 1992; Cone et al., 1993; Mountjoy et al., 1992). MC1R is mostly expressed in melanocytes, although it has been detected in several additional cell types, including cells of the human immune system, suggesting that it could be implicated in the anti-inflammatory effects elicited by α-melanocyte stimulating hormone (α-MSH, see below; Brzoska et al., 2008). Based on bioluminescence resonance energy transfer as well as biochemical studies, MC1R appears to undergo constitutive dimerization as part of its quaternary structure (Mandrika et al., 2005; Sanchez-Laorden et al., 2006). Since both the wild-type receptor and its allelic variants can homo or hereterodimerize, this structural feature has a series of functional consequences, including dominant-negative effects, on agonist binding and coupling efficiency (Garcia-Borron et al., 2005).
MC1R is activated by melanocortins, a class of secreted peptides derived from a precursor hormone named proopiomelanocortin (POMC) (Eipper and Mains, 1980) and expressed in the pituitary gland and other locations including skin melanocytes and keratinocytes (Slominski et al., 1993). From this precursor several bioactive peptides are generated, including α-MSH and Adrenocorticotrophin (ACTH) of 13 and 39 amino acids, respectively, either of which is able to function as MC1R agonist, in physiological conditions most likely in a paracrine and autocrine fashion, rather than as circulating hormones. In addition, functional and genetic studies have shown that MC1R displays a high level of constitutive activity (Bennett and Lamoreux, 2003; Sanchez-Mas et al., 2004), which can be suppressed by Agouti signal protein (ASP or ASIP, in mice and humans, respectively). Consistently, gain-of-function mutations at the Agouti locus determine completely yellow mice, similarly to loss-of-function mutations of MC1R. In the mouse, Agouti is a paracrine signaling polypeptide of 131 amino acids that is normally produced by the dermis at the base of hair follicles within a specific time frame during hair growth, leading to a typical subapical band of reddish/yellow pigment in between black tip and base. In contrast, the role of Agouti in human pigmentation has been less extensively investigated. Agouti appears to function as an endogenous inverse agonist of MC1R, both by competing with and inhibiting the effect of melanocortins, and by stabilizing the inactive conformation of the receptor, thus decreasing both agonist-dependent and agonist-independent signaling (Garcia-Borron et al., 2005).
MC1R mediates a classical GPCR signal transduction pathway at the melanocyte membrane. Indeed, it is coupled to Gαs and in turn stimulates adenylyl cyclase, leading to cyclic AMP (cAMP) increase, protein kinase A (PKA) activation and consequent phosphorylation of cAMP-responsive element-binding protein (CREB). The latter enhances transcription from responsive promoters, including that of MITF, the master transcription factor regulating melanocyte differentiation, proliferation and survival (Goding, 2000; Steingrimsson et al., 2004). Activation of MC1R in melanocytes stimulates melanogenesis, and in particular eumelanogenesis, by promoting a general transcriptional upregulation of the overall machinery necessary for melanosome biogenesis and transport, including the melanosomal proteins Tyrosinase, TYRP1, TYRP2, Pmel17, OA1 and Rab27a (Cheli et al., 2009). This in turn determines a switch from the default pigment biosynthetic pathway, characterized by pheomelanin production, to the accumulation of eumelanin. In addition, upon MC1R signaling melanocytes produce and transfer to surrounding keratinocytes brown/black melanosomes that are more numerous, bigger and elongated compared to pheomelanin-containing organelles, which are smaller, spherical and do not show evidence of internal structure (Barsh, 2006).
While basal MC1R signaling is maintained by the constitutive activity of the receptor and/or autocrine or paracrine agonist secretion, UVR represents the main environmental stimulus leading to intense MC1R activation in melanocytes, primarily driven by α-MSH secretion from keratinocytes (D’Orazio et al., 2006; Lin and Fisher, 2007). As a result, MC1R increases the eumelanin content in the skin and produces tanning, which works as a mild sunscreen, protecting the skin from photodamage and cancer. It also reduces generation of reactive oxygen species and enhances DNA repair mechanisms in melanocytes, thereby maintaining their genomic stability and preventing malignant transformation to melanoma (Bohm et al., 2005; Kadekaro et al., 2005; Smith et al., 2008). By contrast, reduced MC1R activity leads to prevalent synthesis of pheomelanin, which is less effective in UVR absorption and rather acts as a photosensitizer, generating reactive oxygen species that can cause further DNA damage (Lin and Fisher, 2007). Consistently, extensive genetic and functional studies in humans and mice have revealed that MC1R is a major determinant not only of constitutive pigmentation in mammals, but also of skin phototype, acquired pigmentation (as tanning) and UV sensitivity in humans.
Natural variants of MC1R, typically resulting from non-conservative SNPs, are very frequent in the European population and often modify the receptor’s activity to variable extent, by interfering with cell surface expression, ligand binding, or adenylyl cyclase activation (see Garcia-Borron et al., 2005, for a comprehensive review). The frequency of these variants, and their independent evolution in Neanderthals, suggests that they might have provided human populations migrating out of Africa with a selective advantage during the colonization of European countries (Lalueza-Fox et al., 2007). Indeed, partial or complete MC1R loss-of-function typically leads to lighter complexions in Europeans, which might have facilitated the irradiation of the skin and consequent vitamin D synthesis at northern latitudes. On the other hand, polymorphisms leading to complete loss-of-function of MC1R are also responsible for the red-hair/fair-skin pigmentation phenotype (Valverde et al., 1995), which is characterized by tendency to burn and inability to tan, and has been significantly linked to the development of UV-induced skin cancer, in particular melanoma. So, despite their postulated selective advantage during evolution, MC1R variants have now acquired medical attention in an era in which sun or indoor tanning behaviors - especially in the young generations - have generated both commercial interests and severe public health concerns (Tran et al., 2008).
While MC1R is mostly implicated in pigmentation, other members of the melanocortin receptor family are implicated in critical metabolic processes including regulation of adrenal cortex function, immunoregulation and cytoprotection, homeostatic control of energy balance and body weight in the brain. For aspects related to non-pigmentary roles of the melanocortin system, the reader is referred to excellent reviews on the topic (Barsh, 2006; Brzoska et al., 2008; Kaelin et al., 2008; and references therein).
2.2 Complexity of partners and mechanisms in MC1R signaling: from melanocortins and Agouti, to β-Defensin, Attractin and Mahogunin
Despite the vast amount of information on MC1R, largely coming from the study of natural variants and coat color mutants in humans and mice, complete understanding of the MC1R activation mechanism and downstream signaling is far from complete. As other members of its subfamily, MC1R is quite unique among GPCRs because of the presence of both endogenous peptide agonists and antagonists, such as α-MSH and Agouti protein. Furthermore, recent studies revealed not only a previously unsuspected complexity in MC1R signaling mediated by melanocortins or Agouti, but also the presence of additional players involved in MC1R activation. The latter were identified as the genes responsible for peculiar coat color phenotypes, characterized by a black fur that was not dependent on MC1R, melanocortins or Agouti, and that was insensitive to Agouti gain-of-function mutations: the dominant black (K locus) phenotype in dogs, caused by hypermorphic mutation of the β-defensin CBD103 gene, and the mahogany and mahoganoid phenotypes in mice, caused by loss-of-function mutations of the attractin and mahogunin genes, respectively.
Beta-Defensin 103 is a secreted molecule structurally similar to Agouti, highly expressed in the skin and able to modulate the activity of MC1R, by acting as high-affinity neutral agonist. It competitively antagonizes the binding of both melanocortins and Agouti, without determining per se any effect on the receptor’s activity, as measured by cAMP accumulation, although activation of other signaling pathways cannot be excluded (Candille et al., 2007). The β-defensins (and defensins in general) were previously known for their role in immunity, as an heterogeneous class of secreted peptides, acting as chemokines and endogenous antibiotics, and participating in innate as well as adaptive immune responses (Yang et al., 1999). These novel findings uncover a new class of molecules that play both pigmentary and immunological roles and, considering the known immunomodulatory function of melanocortins, provide further confirmation of the tight link and cross-talk between the melanocortin and immune systems (Brzoska et al., 2008; Kaelin et al., 2008).
To some extent, the same concept applies to Attractin (ATRN), a large type I transmembrane protein of 1428 amino acids, which in the extracellular domain displays several motifs typical of molecules implicated in cell adhesion and axon guidance (Gunn et al., 1999; Nagle et al., 1999). Its name derives from the human homolog (Duke-Cohan et al., 1998), previously isolated as a secreted glycoprotein expressed by activated T lymphocytes. Secreted and transmembrane forms of attractin result from alternative splicing (Tang et al., 2000), and are implicated both in cell guidance and amplification during the immune response and in neurodegenerative processes (Barsh, 2006). In melanocytes, attractin is expressed as a transmembrane form and functions at the cell surface as a low-affinity yet essential co-receptor for Agouti protein, which interacts with attractin with its N-terminus, while the C-terminus is engaged in MC1R binding (He et al., 2001). The transmembrane nature of the protein in melanocytes has also led to the hypothesis that, in addition to stabilizing the Agouti-MC1R interaction, Attractin might be involved in other autonomous signal transduction processes (He et al., 2003).
A possible intracellular effector of attractin might actually be represented by Mahogunin (MGRN1), which like Attractin is evolutionary more ancient than melanocortins and MC1R, being found not only in vertebrates, but in all metazoans. Moreover, similarly to attractin, it is ubiquitously expressed, playing a role both in pigmentation and neurodegeneration (Barsh, 2006). Mahogunin has been identified as an additional accessory protein required for efficacious inhibition of MC1R signaling by Agouti. In fact, its mutation suppresses pheomelanin synthesis even in the presence of hypermorphic Agouti variants, while has not effects in the presence of MC1R loss-of-function (He et al., 2003). However, Mahogunin does not function at an extracellular level, being an intracellular C3HC4 RING containing protein showing E3 ubiquitin ligase activity in vitro, although the physiological substrate remains to be identified (He et al., 2003; Phan et al., 2002). Recent studies by Pérez-Oliva and colleagues (2009) revealed that human melanoma cells express four Mahogunin isoforms, two of which contain nuclear localization signals. All Mahogunin isoforms decrease cAMP accumulation mediated by agonist-stimulated MC1R, but not β2-adrenergic receptor, by a mechanism independent from receptor downregulation and ubiquitylation, and most likely involving competition with Gαs for receptor binding.
Interestingly, when expressed in non-melanocytic cells all Mahogunin isoforms remain in the cytosol in the absence of MC1R expression; however, those containing nuclear localization signals accumulate in the nucleus when co-expressed with the receptor (Pérez-Oliva et al., 2009). These findings point to a mechanism somehow reminiscent of that operated by the well-established multifunctional adaptors of GPCR, namely β-arrestins, whose role has surprisingly not been precisely investigated in relation to MC1R. Arrestins uncouple activated GPCRs from G proteins and, upon appropriate stimuli, β-arrestin 1 is also capable to translocate to the nucleus and regulate CREB-dependent transcription (Beaulieu and Caron, 2005). Although the physiological role of mahogunin in the nucleus remains to be investigated, its coupling with Agouti signaling suggests that it might be involved in the upregulation of the pheomelanin biosynthetic pathway, or that it might contribute to suppression of MITF expression, for instance by means of its ubiquitin ligase activity. Furthermore, given the ubiquitous expression of mahogunin and the pleiotropic effects of its loss-of function, one wonders whether this protein in its various isoforms might represent a novel arrestin-like molecule for specific members of the GPCR superfamily.
New developments in the field have also revealed a previously unappreciated complexity in the MC1R downstream pathway. Indeed, it has long been thought that most, if not all, actions of α-MSH and MC1R are mediated by an increase of intracellular cAMP. However, this paradigm does not seem to apply to activation of mitogen-activated protein kinases ERK1 and 2. In fact, natural variants of MC1R, associated to melanoma and unable to produce cAMP accumulation, are nevertheless able to efficiently activate ERK signaling as the wild-type receptor, possibly via cross-talk with receptor tyrosine kinases (RTKs) like cKIT (Herraiz et al., 2009). Similarly, although somehow reversely, pigmentary and biological effects of Agouti do not appear mediated by cAMP downregulation (Hida et al., 2009). Indeed, melanocyte lines treated with Agouti showed increased pheomelanin synthesis, morphological changes consistent with dedifferentiation (melanoblast-like), and reduced proliferation. However, a C-terminal fragment of Agouti, able to reduce basal and melanocortin-stimulated cAMP levels as the full-length, was instead ineffective with regard to melanin synthesis, dedifferentiation, and proliferation (Hida et al., 2009), suggesting that different portions of Agouti might impact diverse signaling pathways emanating from MC1R, either cAMP-dependent or independent.
Overall, although understanding of the underlying molecular mechanism will require further studies, it is becoming increasingly clear that MC1R and its agonists and antagonists act via multiple signaling pathways and downstream effectors, consistent with the typical behavior of receptors belonging to the GPCR superfamily. Along this line, a recent microarray analysis comparing α-MSH versus Agouti treated melanocytes has initiated to fill the numerous gaps in our understanding of the pheomelanin biosynthetic pathway and the role of Agouti in melanocytes (Le Pape et al., 2009). In addition to confirming the antagonistic and opposite effects of Agouti with respect to melanocortins on the expression of melanogenesis genes, this analysis revealed a number of wider effects. Indeed, Agouti upregulated the expression of genes involved in oncogenesis and dedifferentiation of melanocytes, and downregulated genes involved in DNA repair and antioxidant pathways, thus emerging as a crucial player in the regulation of melanoma susceptibility (Abdel-Malek, 2009; Le Pape et al., 2009).
3. OA1 AND ALBINISM
3.1 Overview of OA1 structure, function and associated pathology
The existence of MC1R was predicted before its actual identification based on the effects of melanocortins on melanocytes. By contrast, OA1 was originally identified as the 404 amino acid protein product of the gene responsible for ocular albinism type 1, isolated by a classical positional cloning strategy from the distal short arm of the X chromosome (Bassi et al., 1995), and was recognized as a GPCR only later on (Schiaffino et al., 1999). The expression of both the human OA1 and mouse Oa1 genes was found to be restricted to the pigment cells of the skin and eyes (melanocytes and RPE) (Bassi et al., 1996; Bassi et al., 1995; Newton et al., 1996), and to be regulated similarly to that of melanogenic enzymes, showing comparable temporal and spatial patterns during development, upregulation by α-MSH and MITF, and downregulation by Agouti (Samaraweera et al., 2001; Surace et al., 2000; Vetrini et al., 2004). In addition, and again similarly to melanosomal proteins and the melanocortin-MC1R system, OA1 is highly conserved in vertebrates, while no ortholog nor homolog has been identified in invertebrate species (Schiaffino and Tacchetti, 2005).
The human and mouse OA1 proteins (also known as GPR143) are integral membrane glycoproteins of the secretory pathway (d’Addio et al., 2000; Samaraweera et al., 2001; Schiaffino et al., 1996; Shen and Orlow, 2001) that share significant structural and functional similarities with G protein-coupled receptors (GPCRs), although based on sequence identities the protein cannot be assigned to any specific GPCR subfamily (Schiaffino et al., 1999). Nevertheless, OA1 was found to bind heterotrimeric Gi, Go and Gq proteins by co-immunoprecipitation and pull-down assays using human melanocyte extracts (Schiaffino et al., 1999), to interact with a yeast/mammalian G protein chimera in a yeast-based signaling assay (Staleva and Orlow, 2006), and to activate heterotrimeric Gq proteins in a mammalian heterologous expression system (Innamorati et al., 2006). In the latter study, in which OA1 was purposely mislocalized to the plasma membrane by overexpression in COS cells and coupled to the promiscuous G protein Gα15, the receptor showed a considerable degree of spontaneous (“constitutive”) activity, a behavior already observed in similar conditions with other GPCRs (Milligan, 2003). It also showed the ability to functionally associate with and be downregulated by arrestins, which typically bind conformationally active GPCRs (Innamorati et al., 2006). Altogether, these findings indicate that OA1 functionally behaves as a canonical GPCR.
However, unlike canonical GPCRs, OA1 is not localized to the cell surface, but in normal human melanocytes and mouse melanocyte lines it is exclusively detectable, either morphologically or biochemically, on the membrane of intracellular organelles, namely late-endosomes/lysosomes and melanosomes (Samaraweera et al., 2001; Schiaffino et al., 1996; Schiaffino et al., 1999). Moreover, similarly to other melanosomal proteins, when expressed in non-melanocytic cells OA1 is localized to late endosomes/lysosomes (Schiaffino et al., 1999; Shen et al., 2001). Finally, when expressed in yeast, OA1 is delivered to the prevacuolar compartment, the functional equivalent of the mammalian late endosome (Staleva and Orlow, 2006). The sorting mechanism driving OA1 to its compartment does not depend on glycosylation (d’Addio et al., 2000; Shen et al., 2001), but rather on specific sorting determinants in the cytosolic domains of the protein (Piccirillo et al., 2006). In fact, OA1 contains two separate sorting signals that are both necessary and sufficient for intracellular retention, as well as lysosomal and melanosomal localization. These are an unconventional dileucine motif within the third cytosolic loop and a novel motif, characterized by a tryptophan-glutamic acid doublet, within the C-terminal tail, both of which must be mutated to promote the plasma membrane localization of OA1 (Piccirillo et al., 2006). Based on these evidences, OA1 can be considered a resident GPCR of lysosomes and melanosomes.
Some initial controversy existed on the precise subcellular localization OA1 in melanocytes, whether predominantly on late-endosomes/lysosomes or melanosomes (Schiaffino and Tacchetti, 2005). However, more recent quantitative studies performed by immunofluorescence and immunoelectron microscopy in MNT1 cells (a pigmented human melanoma line) revealed that OA1 is equally distributed both along the lysosomal and melanosomal pathways (Giordano et al., 2009; Piccirillo et al., 2006). Moreover, OA1 was found associated to all stages of melanosome maturation, although it was particularly enriched in compartments labeled by Pmel17, corresponding to immature melanosomes (Giordano et al., 2009; Piccirillo et al., 2006). Consistently, proteomic analysis of a purified early melanosome fraction obtained from MNT1 cells identified OA1 as a constituent of these organelles (Basrur et al., 2003). Based on sequence homologies and on experimental evidences (Schiaffino and Tacchetti, 2005; Sone and Orlow, 2007), the topological orientation of OA1 on the organelle membrane appears equivalent to canonical seven transmembrane receptors, with the N-terminus toward the lumen and with the C-terminus toward the cytoplasm, and therefore, by comparison with receptors at the plasma membrane, a putative ligand should bind OA1 on the lumenal side of the organelles.
The specific intracellular localization of OA1 suggests that it might regulate signaling processes related to melanosome function. The downstream pathway triggered by OA1 remains mysterious though, and at present can only be hypothesized based on the consequences of its loss-of-function in humans and mice. Ocular albinism type 1 (Nettleship-Falls type; MIM 300500) is an X-linked disorder representing the most common form of ocular albinism and characterized by all typical visual anomalies associated with albinism: retina and iris hypopigmentation, foveal hypoplasia and optic misrouting, resulting in severe reduction of visual acuity, nystagmus, strabismus, photophobia, and loss of stereoscopic vision (King et al., 1995). By contrast, cutaneous changes are usually mild or absent. Several types of mutations were identified within the OA1 gene in patients with ocular albinism, including deletions, frameshifts and stop codons, and a number of missense mutations, the majority of which results in protein misfolding and ER-mediated degradation (d’Addio et al., 2000; Shen et al., 2001), indicating that the disease is due to a loss-of-function mechanism.
The histological hallmark of ocular albinism type 1 is the presence of giant pigmented melanosomes, named macromelanosomes, in skin melanocytes and RPE of patients and in a mouse model of the disorder, suggesting a defect in melanosome biogenesis (Garner and Jay, 1980; Incerti et al., 2000; O’Donnell et al., 1976; Wong et al., 1983). In addition, at variance with the skin, the RPE appears hypopigmented, in part because melanin concentrates in few macromelanosomes instead of being evenly dispersed, but most importantly because the overall number of melanosomes is significantly reduced (by 50% or more) (Cortese et al., 2005; Garner and Jay, 1980; Wong et al., 1983). Therefore, OA1 appears to control both size and number of mature melanosomes, while at variance with other forms of albinism it does not directly affect the activity of Tyrosinase (Cortese et al., 2005). How these defects are generated is not known; however, ultrastructural examination of RPE obtained from Oa1-KO mice, and from Oa1-Tyr-null and Oa1-Matp-null double-mutant mice, revealed that the giant melanosomes only develop in the presence of fully pigmented stage IV organelles, most likely by abnormal overgrowth of apparently normal mature melanosomes (Cortese et al., 2005; Incerti et al., 2000). By contrast, the reduction in melanosome number appears dependent on a reduced rate of melanosome biogenesis. In fact, while macromelanosomes did not develop in the absence of melanin in Oa1-Tyr-null RPE, their number was reduced similarly to Oa1-KO RPE and no signs of increased organelle degradation were observed (Cortese et al., 2005).
The role of OA1 in the development of the visual system is poorly understood as well. The misrouting of the optic tracts, at least in the mouse, develops earlier in embryonic life (between E12.5 and E16) than the reduction in melanosome number and the appearance of macromelanosomes, clearly detectable around and after birth, respectively (Cortese et al., 2005; Incerti et al., 2000; Palmisano et al., 2008). Thus, these defects might represent only an epiphenomenon of the disease and cannot be the direct cause of the visual anomalies. Moreover, in contrast to typical oculocutaneous forms of albinism, melanin is not dramatically reduced in ocular albinism, which is characterized by the unusual coexistence of the typical albino visual defects and the presence of a substantial amount of melanin in the eyes and skin, similarly to autosomal recessive ocular albinism (O’Donnell et al., 1976; O’Donnell et al., 1978).
3.2 Multiplicity of pathways and processes regulated by OA1 signaling: from melanosome biogenesis and motility, to retinal development
Despite that the gene for ocular albinism has been cloned 15 years ago, many gaps remain to be filled in the understanding of its function. This is in part due to the peculiarity of OA1 as an intracellular GPCR, since with the only exception of the KDEL receptor in the Golgi apparatus (Pulvirenti et al., 2008), no other GPCRs that are primarily localized to internal membranes have been described to date. As a melanosomal GPCR, OA1 might transduce information from the organelle lumen to the cytosol, by triggering a signal transduction cascade that regulates proper melanosome biogenesis in an organelle-autonomous fashion. Indeed, similar mechanisms have been previously hypothesized to operate widely in the endomembrane system (Nurnberg and Ahnert-Hilger, 1996). Based on biochemical and morphological analyses in normal human melanocytes, a potential G protein partner for OA1 on melanosomes is Gαi, which coprecipitates and colocalizes with OA1 on the melanosomal membrane (Schiaffino et al., 1999). This possibility has been recently corroborated by the analysis of Gαi3-null mice (Young et al., 2008). Compared to wild-type animals carrying the same genetic background, the RPE of Gαi3-null mice was found to contain fewer and larger melanosomes. In addition, the analysis of the optic pathways revealed a significant reduction of ipsilateral retinofugal projections (Young et al., 2008), similarly to Oa1-KO animals. Thus, although other Gαi subunits are expressed and might be involved in retinal development, Gαi3 plays a clear role in melanosome biogenesis, suggesting that it could participate in OA1 signaling.
The macromelanosomal phenotype suggests a defect in melanosome biogenesis, however it also displays variable expressivity in vivo (Garner and Jay, 1980; O’Donnell et al., 1976; Schnur et al., 1998; Wong et al., 1983) and in vitro, since giant melanosomes were observed in melanocytes obtained from Oa1-KO mice, but not human patients (Palmisano et al., 2008; Schiaffino et al., 2002). It is possible that in different pathological conditions the deficiency of OA1 manifests with alternative phenotypes, which could nevertheless provide insights into the function of the receptor. For instance, primary cultures of human melanocytes from patients with ocular albinism showed no macromelanosomes, but a striking prevalence of mature over immature melanosomes, compared to wild-type or OA1-transduced cells (Schiaffino et al., 2002). Similarly, transient OA1 downregulation in MNT1 cells did not result in the formation of classical macromelanosomes (Giordano et al., 2009).
Nevertheless, detailed ultrastructural studies revealed, together with a significant reduction in melanosome number, the appearance of enlarged mixed organelles, possibly representing the precursors of macromelanosomes. The aberrant melanosomes contained disorganized fibrillar structures positive for Pmel17, variably associated with an electron dense core of melanin and proteins of mature melanosomes, namely TYRP1 and LAMP1, indicating anomalous protein segregation between immature and mature melanosomal stages (Giordano et al., 2009). Thus, although the main detectable abnormalities in vivo are in the number and size of mature melanosomes, OA1 might actually play a major role at earlier melanosomal stages, by regulating the traffic of late-stage melanosomal markers to developing organelles (Giordano et al., 2009). Together, these findings suggest that the receptor could act as a negative regulator of melanosome maturation, by delaying the enrichment in melanogenic enzymes until melanosomes reach the appropriate maturation step. Lack of this control could lead to melanosome overgrowth, accelerated maturation or mixed organelles in different experimental or cellular conditions.
Melanosome biogenesis is thought to proceed by a concomitant and progressive transport of mature organelles toward the cell periphery, implying the presence of signaling mechanisms, coordinating membrane and protein traffic responsible for organelle maturation with the activity of molecular motors responsible for organelle transport. Similar principles operate in other professional secretory cells, to avoid premature release of immature cargo; however how maturation of a lumenal cargo is coordinated with protein sorting and organelle motility on the cytosolic side of an organelle membrane is not known (Raposo et al., 2007). Given its features, OA1 might play a role in this coordination, by acting as a “sensor” of melanosome maturation. Indeed, the characterization of Oa1-KO mouse melanocytes revealed that these cells show not only a reduced number of melanosomes and the presence of macromelanosomes, but also an abnormal distribution of the organelles that appear displaced toward the cell periphery (Palmisano et al., 2008). Interestingly, an equivalent displacement toward the apical membrane was observed in the RPE of Oa1-KO mice, at early embryonic stages relevant for the development of the visual pathways, when melanosome size and number are not altered yet (Palmisano et al., 2008).
Time-lapse video-microscopy and organelle tracking analysis revealed that in the absence of OA1 function, melanosomes move significantly less frequently along microtubules; however, this phenotype was only observed in the presence of an intact actin cytoskeleton. In fact, upon disruption of actin based transport, melanosomes were able to aggregate in the perinuclear area as wild-type organelles and displayed a comparable motility along microtubules (Palmisano et al., 2008). On the other hand, both wild-type and Oa1-KO melanosomes showed a similar ability to recruit a GFP-tagged Myosin Va construct (Palmisano et al., 2008), indicating that increased actin-mediated capture of the latter does not depend on gross abnormalities in motor binding due to biogenetic defects. Although at present additional hypotheses cannot be ruled out, these finding suggest that the cytoskeleton represents a downstream effector of OA1 and that the receptor plays a regulatory role in melanosome motility, by favoring MT-based versus AF-based transport. In this way OA1 might retain immature melanosomes in the perinuclear area and avoid their premature transport to the cell periphery.
Intriguingly, proper delivery of TYRP1 to maturing melanosomes has been recently shown to require peripheral positioning of melanocyte-specific recycling endosomal compartments by the kinesin motor KIF13A (Delevoye et al., 2009). It is tempting to speculate that, in the absence of OA1, premature centrifugal transport of immature organelles would determine their abnormal fusion with TYRP1-containing transport intermediates, leading to abnormal melanosome biogenesis. Overall, although the mechanisms implicated remain to be identified, OA1 appears to act by restraining both melanosome maturation and peripheral transport. Given its localization to melanosomes, its ligand could belong to the melanin biosynthetic pathway and act either as an agonist, which activates OA1 on the right organelles, or as an inhibitor, which switches off the receptor when melanosomes have reached the correct maturation stage. Consistently, L-DOPA was identified as a ligand for OA1 by using an heterologous expression system based on OA1 overexpression in CHO cells. In fact, in these conditions L-DOPA was able to induce an increase in intracellular calcium and to bind the receptor although with low affinity, while untransfected cells and related compounds, such as tyrosine and dopamine, showed no measurable second messenger activity (Lopez et al., 2008).
Despite being considered as a by-product of the initial steps in melanin biosynthesis, L-DOPA has long been known as a critical molecule in retinal development, in particular for its ability to regulate the rate and timing of cell proliferation and cell cycle exit, which are altered in albinism (Ilia and Jeffery, 1999; Lavado et al., 2006; Tibber et al., 2006). Theoretically, L-DOPA could act directly on the neurosensory retina, being secreted from the RPE, where it is physiologically generated by Tyrosinase, or indirectly, by inducing the RPE to provide trophic or mitogenic factors to neighbouring neurons. Lopes et al. (2008) propose that L-DOPA might act in the latter way, as an autocrine molecule that becomes secreted from the RPE and in turn stimulates OA1 activation on the plasma membrane of the same cells, thereby inducing secretion of neurotrophic factors such as pigment epithelium-derived factor (PEDF). Thus, in addition to its role in melanosome biogenesis and transport via Gαi, OA1 would control the proliferation of the neurosensory retina by a Gαq-dependent downstream pathway. This is an attractive hypothesis that would explain why melanin is not dramatically reduced in the absence of OA1 function, yet the retinal defect is the same as in the complete absence of melanin in other types of albinism. It would also provide a common effector pathway, with OA1 representing the bottleneck mechanisms responsible for the surprisingly homogeneous visual abnormalities found in albinism, despite the number of different genes and mutations involved.
Nevertheless, loss-of-function of OA1 has also an obvious effect on number, size and distribution of melanosomes, which could per se be responsible for the disease, as in autosomal recessive ocular albinism, which is clinically comparable to ocular albinism type 1 and is characterized by residual Tyrosinase or P activity allowing some degree of melanin synthesis (Hutton and Spritz, 2008; O’Donnell et al., 1978). Moreover, although small amounts of endogenous OA1 were detected at the cell surface of RPE cells in situ and in low-tyrosine culture conditions (means of 3.5% and 4.5%, respectively; Lopez et al., 2008), the functional significance of this finding remains unclear, since the bulk of the protein is located intracellularly and in particular on melanosomes, where it is not degraded, but clearly plays a critical function (Schiaffino and Tacchetti, 2005). Finally, the involvement of endogenous OA1 in an autocrine loop resulting in secretion of neurotrophic factors from the RPE awaits confirmation, since the effects of L-DOPA on calcium increase and PEDF secretion were not assessed in RPE cells lacking OA1 (Lopez et al., 2008). Therefore, further studies will be required to unambiguously determine the role of L-DOPA in OA1 signaling and vice versa whether the effects of L-DOPA in retinal development are mediated by OA1.
4. CONCLUSIONS AND FUTURE DIRECTIONS
Melanocytes represent a unique biological model to address general issues relevant both to cell biology and molecular medicine, since many genes and pathways used by the pigmentary system are representative of other physiological processes. In particular, melanosome biogenesis and transport have parallels to the generation and release of secretory organelles in other cell types, including secretory lysosomes in hematopoietic cells and secretory granules in neuroendocrine cells. In addition, studies on melanosomes are facilitated (i) by their natural black color, which allows prompt visualization in bright field microscopy without the need for specific markers, and (ii) by the number of genes controlling their biogenesis and transport that have already been identified and characterized, thanks to molecular genetics approaches. Indeed, mutations at loci relevant for melanosome function are typically non-lethal, yet give clear pigmentation phenotypes, resulting in obvious skin/fur color changes, in humans, mice, and other vertebrates.
In this scenario, the MC1R and OA1-mediated pathways provide extremely innovative and fertile examples of signal transduction systems, playing fundamental roles in the biology and pathology of pigmentation and able to provide crucial insights into the regulation of melanosome biogenesis and transport, but also into the development of cancer and albinism. Additional signaling pathways are beginning to emerge in these processes, including the involvement of melanosome associated protein kinases, such as PKA, PKC and MAPK (Deacon et al., 2005; Park et al., 2004; Park et al., 2007; Semenova et al., 2009), of phosphoinositide turnover (Chow et al., 2007; Marks, 2008), and of the molecular machinery implicated in autophagosome biosynthesis (Ganesan et al., 2008; Marks, 2009). Future studies will hopefully provide us with a more complete understanding of this colorful, fascinating and instructive field.
Acknowledgments
I wish to thank present and past lab members for their invaluable contribution to several studies described here. I also thank Drs. T. Daniele and I. Palmisano for critical reading of the manuscript and Dr. A. Palmigiano for providing the pictures of melanocytes displayed in Figure 1. I apologize to colleagues whose relevant work could not be cited due to space limitations. Work in my laboratory was supported by the National Institutes of Health/National Eye Institute (grant N. 5R01EY014540), Telethon-Italy (grant N. GGP08156), and the Vision of Children Foundation, San Diego-CA.
ABBREVIATIONS
- ACTH
adrenocorticotrophin
- AF
actin filaments
- a-MSH
a-melanocyte stimulating hormone
- ASP/ASIP
mouse/human Agouti signal protein
- cAMP
cyclic AMP
- CREB
cAMP-responsive element-binding protein
- GPCR
G protein-coupled receptor
- LAMP
lysosomal-associated membrane protein
- MC1R
melanocortin 1 receptor (human and mouse)
- MITF
microphthalmia transcription factor
- MT
microtubules
- OA1
ocular albinism type 1 receptor (human and mouse)
- PEDF
pigment epithelium-derived factor
- POMC
proopiomelanocortin
- RPE
retinal pigment epithelium
- RTK
receptor tyrosine kinase
- SNP
single nucleotide polymorphism
- TYR
Tyrosinase
- TYRP1
Tyrosinase-related protein 1
- TYRP2/DCT
Tyrosinase-related protein 2/dopachrome tautomerase
- UVR
ultraviolet radiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Malek ZA. Agouti signal protein: crossing the ‘yellow’ signal and arriving to pathways that affect tumorgenesis. Pigment Cell Melanoma Res. 2009;22:253–4. doi: 10.1111/j.1755-148X.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002;15:112–8. doi: 10.1034/j.1600-0749.2002.1o071.x. [DOI] [PubMed] [Google Scholar]
- Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res. 2001;268:26–35. doi: 10.1006/excr.2001.5251. [DOI] [PubMed] [Google Scholar]
- Aspengren S, Hedberg D, Wallin M. Melanophores: A model system for neuronal transport and exocytosis? J Neurosci Res. 2007;85:2591–600. doi: 10.1002/jnr.21132. [DOI] [PubMed] [Google Scholar]
- Barral DC, Seabra MC. The melanosome as a model to study organelle motility in mammals. Pigment Cell Res. 2004;17:111–8. doi: 10.1111/j.1600-0749.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- Barsh GS. Regulation of pigment type switching by agouti, melanocortin signaling, attractin, and mahoganoid. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System. Oxford: Blackwell Publishing Ltd; 2006. pp. 395–409. [Google Scholar]
- Basrur V, Yang F, Kushimoto T, Higashimoto Y, Yasumoto K, Valencia J, et al. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J Proteome Res. 2003;2:69–79. doi: 10.1021/pr025562r. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Incerti B, Easty DJ, Sviderskaya EV, Ballabio A. Cloning of the murine homologue of the Ocular Albinism type 1 (OA1) gene: sequence, genomic structure and expression analysis in pigment cells. Genome Research. 1996;6:880–5. doi: 10.1101/gr.6.9.880. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Schiaffino MV, Renieri A, De Nigris F, Galli L, Bruttini M, et al. Cloning of the gene for ocular albinism type 1 from the distal short arm of the X chromosome. Nat Genet. 1995;10:13–9. doi: 10.1038/ng0595-13. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Caron MG. Beta-arrestin goes nuclear. Cell. 2005;123:755–7. doi: 10.1016/j.cell.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Lamoreux ML. The color loci of mice--a genetic century. Pigment Cell Res. 2003;16:333–44. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Berson JF, Theos AC, Harper DC, Tenza D, Raposo G, Marks MS. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol. 2003;161:521–33. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, et al. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280:5795–802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29:581–602. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, et al. A beta-defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–23. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli Y, Ohanna M, Ballotti R, Bertolotto C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2009;23:27–40. doi: 10.1111/j.1755-148X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- Chhajlani V, Wikberg JE. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309:417–20. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD, Mountjoy KG, Robbins LS, Nadeau JH, Johnson KR, Roselli-Rehfuss L, et al. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann N Y Acad Sci. 1993;680:342–63. doi: 10.1111/j.1749-6632.1993.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Cortese K, Giordano F, Surace EM, Venturi C, Ballabio A, Tacchetti C, et al. The ocular albinism type 1 (OA1) gene controls melanosome maturation and size. Invest Ophthalmol Vis Sci. 2005;46:4358–64. doi: 10.1167/iovs.05-0834. [DOI] [PubMed] [Google Scholar]
- d’Addio M, Pizzigoni A, Bassi MT, Baschirotto C, Valetti C, Incerti B, et al. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum Mol Genet. 2000;9:3011–8. doi: 10.1093/hmg/9.20.3011. [DOI] [PubMed] [Google Scholar]
- D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–4. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Deacon SW, Nascimento A, Serpinskaya AS, Gelfand VI. Regulation of bidirectional melanosome transport by organelle bound MAP kinase. Curr Biol. 2005;15:459–63. doi: 10.1016/j.cub.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H, et al. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol. 2009;187:247–64. doi: 10.1083/jcb.200907122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. Faseb J. 2000;14:1265–78. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- Duke-Cohan JS, Gu J, McLaughlin DF, Xu Y, Freeman GJ, Schlossman SF. Attractin (DPPT-L), a member of the CUB family of cell adhesion and guidance proteins, is secreted by activated human T lymphocytes and modulates immune cell interactions. Proc Natl Acad Sci U S A. 1998;95:11336–41. doi: 10.1073/pnas.95.19.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper BA, Mains RE. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980;1:1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Futter CE. The molecular regulation of organelle transport in mammalian retinal pigment epithelial cells. Pigment Cell Res. 2006;19:104–11. doi: 10.1111/j.1600-0749.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- Ganesan AK, Ho H, Bodemann B, Petersen S, Aruri J, Koshy S, et al. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Garner A, Jay BS. Macromelanosomes in X-linked ocular albinism. Histopathology. 1980;4:243–54. doi: 10.1111/j.1365-2559.1980.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Giordano F, Bonetti C, Surace EM, Marigo V, Raposo G. The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum Mol Genet. 2009;18:4530–45. doi: 10.1093/hmg/ddp415. [DOI] [PubMed] [Google Scholar]
- Goding CR. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000;14:1712–28. [PubMed] [Google Scholar]
- Graw J. The genetic and molecular basis of congenital eye defects. Nat Rev Genet. 2003;4:876–88. doi: 10.1038/nrg1202. [DOI] [PubMed] [Google Scholar]
- Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–65. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn TM, Miller KA, He L, Hyman RW, Davis RW, Azarani A, et al. The mouse mahogany locus encodes a transmembrane form of human attractin. Nature. 1999;398:152–6. doi: 10.1038/18217. [DOI] [PubMed] [Google Scholar]
- He L, Eldridge AG, Jackson PK, Gunn TM, Barsh GS. Accessory proteins for melanocortin signaling: attractin and mahogunin. Ann N Y Acad Sci. 2003;994:288–98. doi: 10.1111/j.1749-6632.2003.tb03192.x. [DOI] [PubMed] [Google Scholar]
- He L, Gunn TM, Bouley DM, Lu XY, Watson SJ, Schlossman SF, et al. A biochemical function for attractin in agouti-induced pigmentation and obesity. Nat Genet. 2001;27:40–7. doi: 10.1038/83741. [DOI] [PubMed] [Google Scholar]
- Herraiz C, Jimenez-Cervantes C, Zanna P, Garcia-Borron JC. Melanocortin 1 receptor mutations impact differentially on signalling to the cAMP and the ERK mitogen-activated protein kinase pathways. FEBS Lett. 2009;583:3269–74. doi: 10.1016/j.febslet.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Hida T, Wakamatsu K, Sviderskaya EV, Donkin AJ, Montoliu L, Lynn Lamoreux M, et al. Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: a cAMP-independent pathway. Pigment Cell Melanoma Res. 2009;22:623–34. doi: 10.1111/j.1755-148X.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SM, Spritz RA. A comprehensive genetic study of autosomal recessive ocular albinism in Caucasian patients. Invest Ophthalmol Vis Sci. 2008;49:868–72. doi: 10.1167/iovs.07-0791. [DOI] [PubMed] [Google Scholar]
- Ilia M, Jeffery G. Retinal mitosis is regulated by dopa, a melanin precursor that may influence the time at which cells exit the cell cycle: analysis of patterns of cell production in pigmented and albino retinae. J Comp Neurol. 1999;405:394–405. doi: 10.1002/(sici)1096-9861(19990315)405:3<394::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Incerti B, Cortese K, Pizzigoni A, Surace EM, Varani S, Coppola M, et al. Oa1 knock-out: new insights on the pathogenesis of ocular albinism type 1. Hum Mol Genet. 2000;9:2781–8. doi: 10.1093/hmg/9.19.2781. [DOI] [PubMed] [Google Scholar]
- Innamorati G, Piccirillo R, Bagnato P, Palmisano I, Schiaffino MV. The melanosomal/lysosomal protein OA1 has properties of a G protein-coupled receptor. Pigment Cell Res. 2006;19:125–35. doi: 10.1111/j.1600-0749.2006.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–9. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Kaelin CB, Candille SI, Yu B, Jackson P, Thompson DA, Nix MA, et al. New ligands for melanocortin receptors. Int J Obes (Lond) 2008;32 (Suppl 7):S19–27. doi: 10.1038/ijo.2008.234. [DOI] [PubMed] [Google Scholar]
- King RA, Hearing VJ, Creel DJ, Oetting WS. Albinism. In: Scriver CR MDCM, Beaudet AL MD, Sly WS MD, Valle D MD, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill, Inc; 1995. pp. 4353–92. [Google Scholar]
- Lalueza-Fox C, Rompler H, Caramelli D, Staubert C, Catalano G, Hughes D, et al. A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science. 2007;318:1453–5. doi: 10.1126/science.1147417. [DOI] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–6. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- Lavado A, Jeffery G, Tovar V, de la Villa P, Montoliu L. Ectopic expression of tyrosine hydroxylase in the pigmented epithelium rescues the retinal abnormalities and visual function common in albinos in the absence of melanin. J Neurochem. 2006;96:1201–11. doi: 10.1111/j.1471-4159.2006.03657.x. [DOI] [PubMed] [Google Scholar]
- Le Pape E, Passeron T, Giubellino A, Valencia JC, Wolber R, Hearing VJ. Microarray analysis sheds light on the dedifferentiating role of agouti signal protein in murine melanocytes via the Mc1r. Proc Natl Acad Sci U S A. 2009;106:1802–7. doi: 10.1073/pnas.0806753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6:e236. doi: 10.1371/journal.pbio.0060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrika I, Petrovska R, Wikberg J. Melanocortin receptors form constitutive homo- and heterodimers. Biochem Biophys Res Commun. 2005;326:349–54. doi: 10.1016/j.bbrc.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Marks MS. FIG4, Charcot-Marie-Tooth disease, and hypopigmentation: a role for phosphoinositides in melanosome biogenesis? Pigment Cell Melanoma Res. 2008;21:11–4. doi: 10.1111/j.1755-148X.2007.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS. Eating thyself toward the dark side? Pigment Cell Melanoma Res. 2009;22:251–2. doi: 10.1111/j.1755-148X.2009.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2:738–48. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- Milligan G. Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol Pharmacol. 2003;64:1271–6. doi: 10.1124/mol.64.6.1271. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–51. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Nagle DL, McGrail SH, Vitale J, Woolf EA, Dussault BJ, Jr, DiRocco L, et al. The mahogany protein is a receptor involved in suppression of obesity. Nature. 1999;398:148–52. doi: 10.1038/18210. [DOI] [PubMed] [Google Scholar]
- Newton JM, Cohen-Barak O, Hagiwara N, Gardner JM, Davisson MT, King RA, et al. Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am J Hum Genet. 2001;69:981–8. doi: 10.1086/324340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton JM, Orlow SJ, Barsh GS. Isolation and characterization of a mouse homolog of the X-linked ocular albinism (OA1) gene. Genomics. 1996;37:219–25. doi: 10.1006/geno.1996.0545. [DOI] [PubMed] [Google Scholar]
- Nurnberg B, Ahnert-Hilger G. Potential roles of heterotrimeric G proteins of the endomembrane system. FEBS Lett. 1996;389:61–5. doi: 10.1016/0014-5793(96)00584-4. [DOI] [PubMed] [Google Scholar]
- O’Donnell FE, Jr, Hambrick GW, Jr, Green WR, Iliff WJ, Stone DL. X-linked ocular albinism. An oculocutaneous macromelanosomal disorder. Arch Ophthalmol. 1976;94:1883–92. doi: 10.1001/archopht.1976.03910040593001. [DOI] [PubMed] [Google Scholar]
- O’Donnell FE, King RA, Green WR, Witkop CJ., Jr Autosomal recessively inherited ocular albinism. A new form of ocular albinism affecting females as severely as males. Arch Ophthalmol. 1978;96:1621–5. doi: 10.1001/archopht.1978.03910060255013. [DOI] [PubMed] [Google Scholar]
- Palmisano I, Bagnato P, Palmigiano A, Innamorati G, Rotondo G, Altimare D, et al. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum Mol Genet. 2008;17:3487–501. doi: 10.1093/hmg/ddn241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Wu H, Killoran CE, Gilchrest BA. The receptor for activated C-kinase-I (RACK-I) anchors activated PKC-beta on melanosomes. J Cell Sci. 2004;117:3659–68. doi: 10.1242/jcs.01219. [DOI] [PubMed] [Google Scholar]
- Park M, Serpinskaya AS, Papalopulu N, Gelfand VI. Rab32 regulates melanosome transport in Xenopus melanophores by protein kinase a recruitment. Curr Biol. 2007;17:2030–4. doi: 10.1016/j.cub.2007.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Oliva AB, Olivares C, Jimenez-Cervantes C, Garcia-Borron JC. Mahogunin ring finger-1 (MGRN1) E3 ubiquitin ligase inhibits signaling from melanocortin receptor by competition with Galphas. J Biol Chem. 2009;284:31714–25. doi: 10.1074/jbc.M109.028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan LK, Lin F, LeDuc CA, Chung WK, Leibel RL. The mouse mahoganoid coat color mutation disrupts a novel C3HC4 RING domain protein. J Clin Invest. 2002;110:1449–59. doi: 10.1172/JCI16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo R, Palmisano I, Innamorati G, Bagnato P, Altimare D, Schiaffino MV. An unconventional dileucine-based motif and a novel cytosolic motif are required for the lysosomal and melanosomal targeting of OA1. J Cell Sci. 2006;119:2003–14. doi: 10.1242/jcs.02930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prota G, D’Ischia M, Napolitano A. The chemistry of melanins and related metabolites. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne J-P, editors. The Pigmentary System. New York: Oxford University Press, Inc; 1998. pp. 307–32. [Google Scholar]
- Pulvirenti T, Giannotta M, Capestrano M, Capitani M, Pisanu A, Polishchuk RS, et al. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat Cell Biol. 2008;10:912–22. doi: 10.1038/ncb1751. [DOI] [PubMed] [Google Scholar]
- Puri N, Gardner JM, Brilliant MH. Aberrant pH of melanosomes in pink-eyed dilution (p) mutant melanocytes. J Invest Dermatol. 2000;115:607–13. doi: 10.1046/j.1523-1747.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Raposo G, Marks MS. Melanosomes - dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol. 2007;8:786–97. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol. 2001;152:809–24. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JL. Genetics of hair and skin color. Annu Rev Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- Samaraweera P, Shen B, Newton JM, Barsh GS, Orlow SJ. The mouse ocular albinism 1 gene product is an endolysosomal protein. Exp Eye Res. 2001;72:319–29. doi: 10.1006/exer.2000.0962. [DOI] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Sanchez-Mas J, Martinez-Alonso E, Martinez-Menarguez JA, Garcia-Borron JC, Jimenez-Cervantes C. Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol. 2006;126:172–81. doi: 10.1038/sj.jid.5700036. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mas J, Hahmann C, Gerritsen I, Garcia-Borron JC, Jimenez-Cervantes C. Agonist-independent, high constitutive activity of the human melanocortin 1 receptor. Pigment Cell Res. 2004;17:386–95. doi: 10.1111/j.1600-0749.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Baschirotto C, Pellegrini G, Montalti S, Tacchetti C, De Luca M, et al. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc Natl Acad Sci U S A. 1996;93:9055–60. doi: 10.1073/pnas.93.17.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino MV, d’Addio M, Alloni A, Baschirotto C, Valetti C, Cortese K, et al. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nat Genet. 1999;23:108–12. doi: 10.1038/12715. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Dellambra E, Cortese K, Baschirotto C, Bondanza S, Clementi M, et al. Effective retroviral-mediated gene transfer in normal and mutant human melanocytes. Hum Gene Ther. 2002;13:947–57. doi: 10.1089/10430340252939050. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Tacchetti C. The ocular albinism type 1 (OA1) protein and the evidence for an intracellular signal transduction system involved in melanosome biogenesis. Pigment Cell Res. 2005;18:227–33. doi: 10.1111/j.1600-0749.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Schnur RE, Gao M, Wick PA, Keller M, Benke PJ, Edwards MJ, et al. OA1 mutations and deletions in X-linked ocular albinism. Am J Hum Genet. 1998;62:800–9. doi: 10.1086/301776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiji M, Shimao K, Birbeck MS, Fitzpatrick TB. Subcellular localization of melanin biosynthesis. Ann N Y Acad Sci. 1963;100:497–533. [PubMed] [Google Scholar]
- Semenova I, Ikeda K, Ivanov P, Rodionov V. The protein kinase A-anchoring protein moesin is bound to pigment granules in melanophores. Traffic. 2009;10:153–60. doi: 10.1111/j.1600-0854.2008.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Orlow SJ. The ocular albinism type 1 gene product is an N-glycoprotein but glycosylation is not required for its subcellular distribution. Pigment Cell Res. 2001;14:485–90. doi: 10.1034/j.1600-0749.2001.140609.x. [DOI] [PubMed] [Google Scholar]
- Shen B, Rosenberg B, Orlow SJ. Intracellular distribution and late endosomal effects of the ocular albinism type 1 gene product: consequences of disease-causing mutations and implications for melanosome biogenesis. Traffic. 2001;2:202–11. doi: 10.1034/j.1600-0854.2001.020306.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Wortsman J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol Cell Endocrinol. 1993;93:C1–6. doi: 10.1016/0303-7207(93)90131-3. [DOI] [PubMed] [Google Scholar]
- Smith AG, Luk N, Newton RA, Roberts DW, Sturm RA, Muscat GE. Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. J Biol Chem. 2008;283:12564–70. doi: 10.1074/jbc.M800480200. [DOI] [PubMed] [Google Scholar]
- Sone M, Orlow SJ. The ocular albinism type 1 gene product, OA1, spans intracellular membranes 7 times. Exp Eye Res. 2007;85:806–16. doi: 10.1016/j.exer.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staleva L, Orlow SJ. Ocular albinism 1 protein: trafficking and function when expressed in Saccharomyces cerevisiae. Exp Eye Res. 2006;82:311–8. doi: 10.1016/j.exer.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18:R9–17. doi: 10.1093/hmg/ddp003. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Box NF, Newton RA, Shepherd AG, Chen W, et al. Genetic association and cellular function of MC1R variant alleles in human pigmentation. Ann N Y Acad Sci. 2003;994:348–58. doi: 10.1111/j.1749-6632.2003.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Surace EM, Angeletti B, Ballabio A, Marigo V. Expression pattern of the ocular albinism type 1 (Oa1) gene in the murine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2000;41:4333–7. [PubMed] [Google Scholar]
- Tang W, Gunn TM, McLaughlin DF, Barsh GS, Schlossman SF, Duke-Cohan JS. Secreted and membrane attractin result from alternative splicing of the human ATRN gene. Proc Natl Acad Sci U S A. 2000;97:6025–30. doi: 10.1073/pnas.110139897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong HY, Jee SH, Sun CC, Boissy RE. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br J Dermatol. 2003;149:498–505. doi: 10.1046/j.1365-2133.2003.05473.x. [DOI] [PubMed] [Google Scholar]
- Tibber MS, Whitmore AV, Jeffery G. Cell division and cleavage orientation in the developing retina are regulated by L-DOPA. J Comp Neurol. 2006;496:369–81. doi: 10.1002/cne.20920. [DOI] [PubMed] [Google Scholar]
- Tran TT, Schulman J, Fisher DE. UV and pigmentation: molecular mechanisms and social controversies. Pigment Cell Melanoma Res. 2008;21:509–16. doi: 10.1111/j.1755-148X.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–30. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Vetrini F, Auricchio A, Du J, Angeletti B, Fisher DE, Ballabio A, et al. The microphthalmia transcription factor (Mitf) controls expression of the ocular albinism type 1 gene: link between melanin synthesis and melanosome biogenesis. Mol Cell Biol. 2004;24:6550–9. doi: 10.1128/MCB.24.15.6550-6559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L, O’Donnell FE, Jr, Green WR. Giant pigment granules in the retinal pigment epithelium of a fetus with X-linked ocular albinism. Ophthalmic Paediatrics and Genetics. 1983;2:47–65. [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JAr. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143:1899–918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–61. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Young A, Powelson EB, Whitney IE, Raven MA, Nusinowitz S, Jiang M, et al. Involvement of OA1, an intracellular GPCR, and G alpha i3, its binding protein, in melanosomal biogenesis and optic pathway formation. Invest Ophthalmol Vis Sci. 2008;49:3245–52. doi: 10.1167/iovs.08-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]