Abstract
1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine (KS119) is a prodrug of the 1,2-bis(sulfonyl)hydrazine class of antineoplastic agents designed to exploit the oxygen-deficient regions of cancerous tissue. Thus, under reductive conditions in hypoxic cells this agent decomposes to produce the reactive intermediate 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine (90CE), which in turn generates products that alkylate the O6-position of guanine in DNA. Comparison of the cytotoxicity of KS119 in cultured cells lacking O6-alkylguanine-DNA alkyltransferase (AGT) to an agent such as Onrigin™, which through base catalyzed activation produces the same critical DNA G-C cross-link lesions by the generation of 90CE, indicates that KS119 is substantially more potent than Onrigin™ under conditions of oxygen deficiency, despite being incompletely activated. In cell lines expressing relatively large amounts of AGT, the design of the prodrug KS119, which requires intracellular activation by reductase enzymes to produce a cytotoxic effect, results in an ability to overcome resistance derived from the expression of AGT. This appears to derive from the ability of a small portion of the chloroethylating species produced by the activation of KS119 to slip through the cellular protection afforded by AGT to generate the few DNA G-C cross-links that are required for tumor cell lethality. The findings also demonstrate that activation of KS119 under oxygen-deficient conditions is ubiquitous, occurring in all of the cell lines tested thus far, suggesting that the enzymes required for reductive activation of this agent are widely distributed in many different tumor types.
Keywords: Oxygen-deficient cells; O6-Alkylguanine-DNA alkyltransferase; 1,2-Bis(sulfonyl)hydrazines; KS119; Onrigin™
1. Introduction
It has been well documented that vascular anomalies in solid tumors can result in the formation of hypoxic/anoxic areas in which oxygen is severely limited or in some cases completely absent [1-4]. The presence of oxygen-deficient regions within tumors has significant implications for tumor growth, development, and invasiveness [5]. However, these oxygen-deficient regions possess properties which make them preferential targets for hypoxia directed prodrug chemotherapy. Thus, although oxygen-deficient regions in neoplastic tissue may be the furnace that drives tumor growth and lethal metastatic spread [6,7], these regions also may be considered to be sites of vulnerability through their capacity to actively convert certain prodrugs by reductive enzymatic activation into forms preferentially lethal for tumors relative to normal tissue, thereby producing specificity for neoplastic tissues.
Hif-1 is known to orchestrate the cellular response to oxygen deprivation [8,9]. This signaling protein must interface with the cellular oxygen sensing system and with angiogenesis inducers to modulate a complicated molecular program that carefully controls the cellular and tissue response to limited supplies of oxygen [10-12]. There is evidence that neoplastic cells can and must usurp or manipulate this process in order to spread throughout the body [6,7,12]. Thus, preferential targeting of tumors through selective prodrug activation by reductive enzymes in oxygen-deficient regions within malignant masses may not only permit therapeutic eradication of primary tumor masses, but perhaps more importantly, disrupt the processes which permit tumor spread and metastasis.
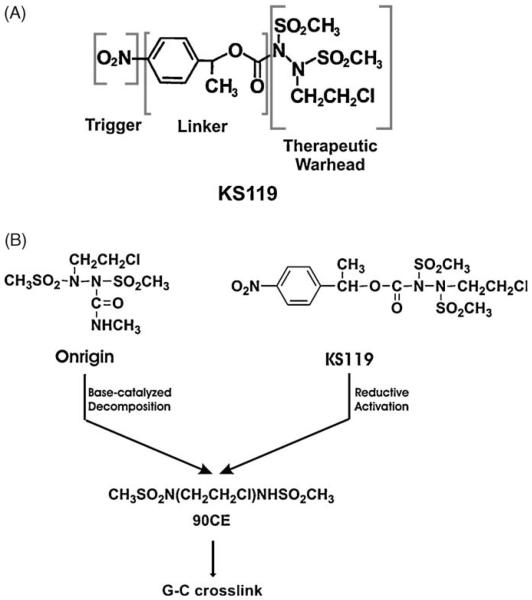
We have been devising ways to optimize the effectiveness of the 1,2-bis(sulfonyl)hydrazine prodrug Onrigin™ (cloretazine, laromustine, VNP40101M, 101M) and its active primary decomposition product, 90CE, against neoplastic tumors. This agent, which has completed phase II clinical trials for the treatment of acute myelogenous leukemia (AML), where it has clearly shown efficacy in de novo poor risk elderly AML patients, has also undergone initial evaluation against glioblastoma and small cell lung carcinoma [13-16]. Since Onrigin™ is activated by base catalyzed fragmentation to yield the activated product 90CE in both extracellular and intracellular compartments, we have attempted to increase the intracellular localization of 90CE in neoplastic cells, through the development of 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine (KS119, Fig. 1A), a second generation DNA O6-guanine targeting agent, which, after intracellular reductive activation in oxygen-deficient neoplastic cells, produces the primary cytotoxic product 90CE. Thus, KS119 was specifically designed to exploit oxygen-deficient areas of malignant tissue [17-19] by serving as a prodrug activated by reductive enzymes in tumors to release the active therapeutic component (Fig. 1B). The reductase enzymes in oxygen-deficient cells preferentially activate KS119 to generate 90CE by converting the 4-nitro substituent to the 4-amino or 4-hydroxylamino product which then spontaneously fragments within hypoxic regions of tumors; whereas, under aerobic conditions back oxidation of the one electron radical product occurs rapidly to regenerate the parental prodrug. The redox cycling that occurs under aerobiosis inherently directs the activation of KS119 to only occur within oxygen-deficient tumor cells, making it improbable that net activation will occur spontaneously in aerated tumor cells or by decomposition in extracellular areas.
Fig. 1.
Schematic diagrams depicting the structures of KS119, Onrigin and 90CE. (Panel A) Schematic diagram of the contributing portions of 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine (KS119). (Panel B) Diagram indicating the metabolic activation pathways of KS119, Onrigin, and 90CE.
In the studies reported herein, KS119 cytotoxicity was compared under oxygenated and enzymatically generated oxygen-deficient [20] conditions to agents such as 90CE and Onrigin™, which produce identical DNA lesions, but are not preferentially activated under oxygen-deficient conditions and, therefore, the activation of these agents is not localized to oxygen-deficient cells. Our results suggest that the tumor specificity attained by KS119 in oxygen-deficient cells can overcome the cellular resistance to O6-DNA guanine chloroethylating agents mediated by O6-alkylguanine-DNA alkyltransferase (AGT), even in cell lines with extremely high levels of expression of this repair protein. AGT is a DNA repair protein that catalyzes the transfer of alkyl groups from the O-6 position of guanine to cysteine-145 of the AGT protein, restoring the O-6 position of guanine to its native state [21]. There is no known acceptor that removes the alkyl group from cysteine-145 of AGT, so one AGT protein is required to repair each lesion; once alkylated, the AGT protein is degraded by the proteasome [21].
2. Materials and methods
2.1. Cell culture
All cell lines were maintained under 5% CO2 in α-Minimal Essential Medium (α-MEM) or Dulbecco's Minimal Essential Medium (DMEM) supplemented with 10% FBS. All cell culture reagents were purchased from Invitrogen (Carlsbad, CA).
2.2. Cell lines
The O6-alkylguanine-DNA alkyltransferase (AGT) expressing cell lines were generated as previously described [22]. Briefly, CHO/AA8 Chinese hamster, EMT6 mammary carcinoma, and U251 glioblastoma cells which are deficient in AGT (containing below the level of detection of less than 600 AGT molecules/cell) were transfected using lipofectamine (Invitrogen, USA) using the manufacturer's recommended conditions, with a plasmid vector containing a neomycin resistance gene and a CMV promoter driven cDNA of the human AGT gene [22]. Cells were screened for G418 resistance and prospective transfectants were single cell cloned. Clones expressing AGT were identified by Western blot analysis with a monoclonal antibody specific for AGT (Neomarkers; Freemont, California). AGT levels were quantified using an [3H]-O6-benzylguanine binding assay [23].
2.3. Chemical syntheses
The 1,2-bis(sulfonyl)hydrazine prodrugs were synthesized by previously published procedures [18,24]. All other drugs and chemicals were purchased from the Sigma Chemical Co. (St. Louis, MO).
2.4. Toxicity studies
Clonogenic survival assays were performed essentially as described previously [20]. Cells were seeded into 25 cm2 plastic tissue culture flasks at 5–8 × 105 cells per flask. When confluent, cells were treated with antineoplastic agents dissolved in DMSO in a total volume of 10 ml of medium for 24 h at 37 °C. For oxygen-deficient conditions, cells were incubated with 1,2-bis(sulfonyl)-hydrazines in the presence of 2 Units(U)/ml of glucose oxidase (Sigma G6641), 120 U/ml of catalase (Sigma, C1345) in high glucose DMEM (Invitrogen). Flasks were flushed with nitrogen for 10 s and the caps screwed on tightly. This facilitates oxygen depletion of the medium by glucose oxidase through removal of residual oxygen containing air and denial of the entry of additional air. After treatment, monolayers were rinsed, and cells were detached by trypsinization, suspended in culture medium, counted and sequential cell dilutions were plated in duplicate into 6-well plates at a density of 1 × 102, 1 × 103, or 1 × 104 cells per well. Seven to 10 days later, colonies were fixed, stained with crystal violet (0.25%) in 80% methanol and quantified. For studies involving O6-benzylguanine (O6-BG), cells were pretreated for 4 h in the presence of O6-BG prior to the addition of the cytotoxic agent. All analyses were corrected for plating efficiency in the presence of vehicle (DMSO) at concentrations equivalent to those used for exposure to the test 1,2-bis(sulfonyl)hydrazine. DMSO concentrations were ≤0.05%, and non-toxic. Cells under aerobic conditions were treated under similar conditions and cytotoxic agent concentrations, but in unsealed flasks without glucose oxidase and catalase. Cells were then washed, harvested by trypsinization, and assayed for survival using a clonogenic assay described previously [22,25,26]. In the absence of cells, no measurable direct metabolism of KS119 was detected in the presence of the glucose oxidase and catalase enzyme components of our oxygen deficiency generating system [20].
2.5. HPLC determination of the disappearance of KS119 in cell cultures under conditions of oxygenation and oxygen deficiency
For these experiments, 107 cells/ml, were incubated for various times with KS119 under oxygenation or enzyme generated oxygen deficiency described above. For oxygenated studies, cells were incubated in 25 cm2 flasks in shallow 5 ml layers of growth medium containing 10% FBS (α-MEM for DU145 cells, or DMEM for EMT6 cells) with shaking at 37 °C. Experiments under oxygen-deficient conditions were performed in sealed 1.5 ml tubes in the presence of 2 U/ml of glucose oxidase (Sigma G6641), 120 U/ml of catalase (Sigma C1345) and 10 mM added glucose in 1 ml of growth medium. Cell supernatant samples containing KS119 were mixed with an equal volume of acetonitrile and allowed to stand at room temperature for 15 min to allow precipitation of most of the protein, then centrifuged at 10,000 × g for 5 min. The supernatant was then analyzed by HPLC using a 5 μm 220 mm × 4.6 mm C-18 reverse-phase column (RP-18, Applied Biosystems); elution was accomplished with 34.5% acetonitrile in buffer (0.03 M KH2PO4/1.0 mM NaN3, pH 5.4) for 5 min, followed by a 34.5–75.0% acetonitrile linear gradient in buffer, at a flow rate of 0.6 ml/min. Absorbance was monitored at 280 nm using a 168 UV/Vis detector (Beckman Coulter, Fullerton, CA). KS119 eluted at approximately 35 min.
2.6. AGT assays
AGT assays were performed essentially as described by Ishiguro et al. [23]. Determination of AGT activity relied upon stoichiometric covalent transfer of radioactive benzyl residues from [benzene-3H]O6-benzylguanine to AGT, and the numbers of AGT molecules/cell were calculated based upon radioactivity in a 70% methanol precipitable fraction, the specific activity of [benzene-3H]O6-benzylguanine and Avogadro's number as described [23].
3. Results
3.1. Toxicity studies in cells lacking AGT
KS119 was designed to be preferentially activated by bioreduction within cells under conditions of oxygen deficiency. The distribution and nature of the cellular reductive enzymes required for this activation are unknown, but in enzymatic systems, NADPH: cytochrome P450 reductase and xanthine/xanthine oxidase are capable of reducing KS119 in a one electron reaction (unpublished observations). Studies in three different cell lines have demonstrated that the activation of KS119 is prolonged and that only a portion of this agent (<40%) is converted to 90CE after exposure to cell monolayers for 24 h under conditions of oxygen deficiency [20].
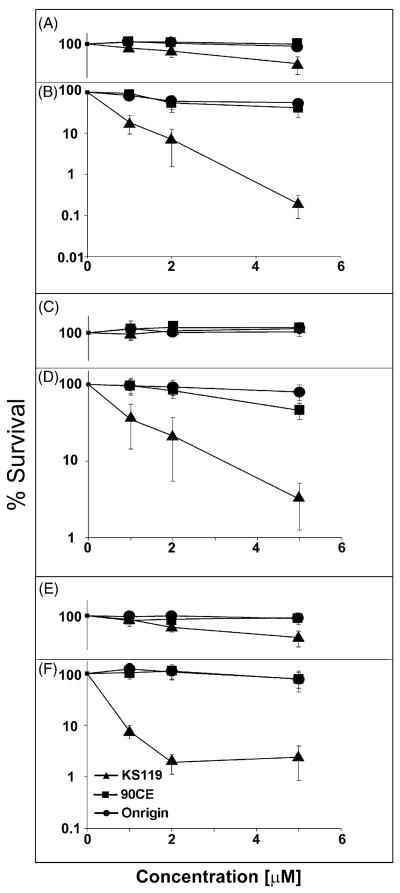
We have compared the toxicity of KS119 to Onrigin™ and 90CE (Fig. 1B), all of which generate the same chloroethylating species that ultimately produce the G-C cross-linking DNA lesion, in three parental cell lines each lacking the expression of AGT. To accomplish this comparison, CHO/AA8 Chinese hamster ovary cells, U251 human glioblastoma cells, and EMT6 murine mammary carcinoma cells were treated with KS119, Onrigin™, or 90CE for 24 h under oxygenated conditions or those of enzyme generated oxygen deficiency and the resulting cell survival was measured by clonogenic assays. As shown in Fig. 2(A–F), KS119 was considerably more efficacious than either Onrigin™ or 90CE at equivalent concentrations under conditions of oxygen deficiency. This is particularly impressive since the total amount of KS119 being activated is very low under these conditions, requiring 24 h to reach <40%, compared to 90CE (half life = 30 s) and Onrigin™ (half life = 1 h), which are relatively rapidly activated independent of enzymatic action or the degree of oxygenation. These findings imply that KS119 can be activated by oxygen-deficient cells of solid tumors, thereby providing a selective advantage in generating tumor cell toxicity under conditions of oxygen deficiency, which is rare in normal tissue, but is known to be present in poorly vascularized and disorganized tumor tissue. Furthermore, intracellular activation can increase the effective delivery of the toxic agent, since the active cytotoxic metabolite is produced directly inside the oxygen-deficient neoplastic tissue where reductive activation occurs and is not produced in extracellular environments (i.e., the blood stream and normal tissue) where distribution throughout the host is occurring. Although, as expected, increased concentrations of Onrigin™ and 90CE have been shown to result in significant cell toxicity, as shown in Table 1, the 50% lethal concentration (LC50value) for KS119 is from 6- to 28-fold lower than that of Onrigin™ and 90CE under conditions of oxygen deficiency. The studies conducted imply that the reductase enzymes required for activation of KS119 to 90CE are widespread, being expressed in various cell types.
Fig. 2.
Clonogenic experiments using CHO/AA8, U251 and EMT6 cell lines treated with various agents under conditions of oxygenation or oxygen deficiency. CHO/AA8 (oxygenated, panel A; oxygen-deficient, panel B), U251 cells (oxygenated, panel C; oxygen-deficient, panel D), and EMT6 cells (oxygenated, panel E; oxygen-deficient, panel F) were treated with varying concentrations of KS119 (▲), 90CE (■), or Onrigin (●). The vertical axis indicates the percent survival over untreated controls; the horizontal axis indicates the concentration of the agent employed in μM. Oxygen depleted samples were treated for 24 h in the presence of 2 U/ml of glucose oxidase, 120 U/ml of catalase and 25 mM glucose, before harvesting and plating of cells to measure cloning efficiency. All points are the result of at least three independent experimental determinations ±SEM.
Table 1.
LC50 values in μM for cell lines deficient in AGT under oxygen deficiency (OD) or oxygenation (O).
| Agent | U251 |
EMT6 |
CHO/AA8 |
|||
|---|---|---|---|---|---|---|
| OD | O | OD | O | OD | O | |
| KS119 | 0.8a (6.8)b | >5.0 (<1.5) | 0.5 (28) | 3.2 (6.9) | 0.6 (11.8) | 3.4 (2.8) |
| Onrigin | 5.3 (1.0) | 6.9 (1.1) | 10 (1.4) | 18 (1.2) | 15 (0.47) | 7.8 (1.2) |
| 90CE | 5.4 (1.0) | 7.3 (1.0) | 14 (1.0) | 22 (1.0) | 7.1 (1.0) | 9.4 (1.0) |
Values are the result of at least three independent determinations.
The fold difference in cytotoxicity between KS119 or onrigin and 90CE was determined by dividing the measured LC50 value of 90CE by that of onrigin or KS119. These values are in agreement with the predicted maximum values expected by calculations using the 90CE flux in cells in model systems.
3.2. Toxicity studies in cells expressing AGT
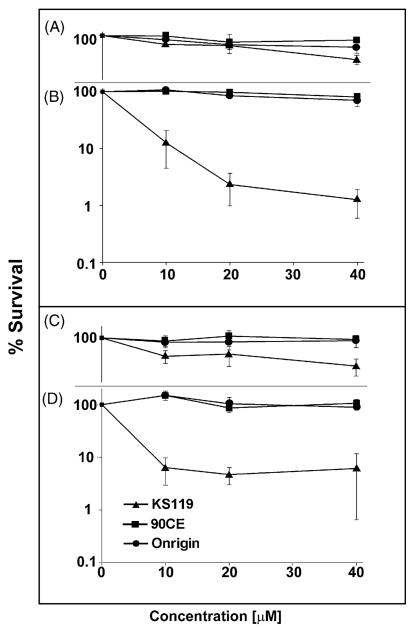
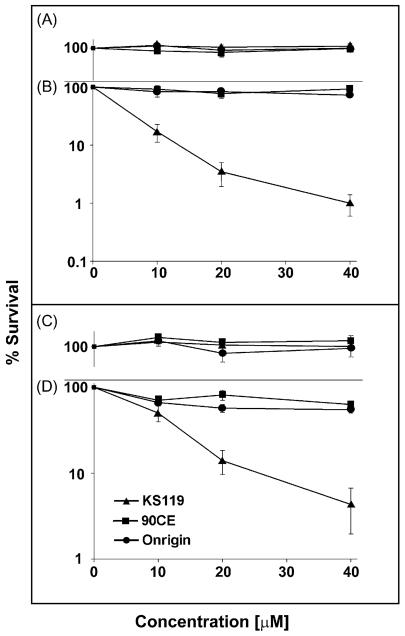
To ascertain the ability of the prodrug KS119 to overcome AGT mediated resistance to DNA O6-guanine alkylation, a series of AGT transfected cell lines, CHO/hAGT7, EMT6/hAGT18, CHO/hAGT135, and U251/hAGT325, which contain a range of human AGT levels (7000 to 325,000 molecules/cell), as well as a human prostate carcinoma cell line DU145, which naturally expresses a moderately high level of 42,000 AGT molecules/cell (Table 2), were evaluated for sensitivity to KS119. AGT levels were quantified in these cell lines by measuring the degree of [3H]-O6-benzylguanine binding to cysteine-145 of the AGT protein, an assay developed in this laboratory [23]. The two transfected cell lines, which stably expressed the lowest levels of AGT (CHO/hAGT7 and EMT6/hAGT18), exhibited sensitivity to KS119; while, under conditions of oxygen deficiency, relatively little or no inhibition was produced by this agent in the presence of aeration (Fig. 3A–D). Transfected cell lines with exceedingly high levels of human AGT expression, CHO/hAGT135 and U251/hAGT325 (135,000 and 325,000 molecules of AGT/cell, respectively) were also sensitive to this 1,2-bis(sulfonyl)hydrazine prodrug under conditions of oxygen deficiency (Fig. 4B and D). In addition, the DU145 human prostate carcinoma cell line which displays one of the highest levels of natural (untransfected) AGT expression among the cell lines surveyed in this laboratory [23] was found to be inhibited by this agent in clonogenic assays under oxygen-deficient conditions (Fig. 5). In contrast, the non-directed antitumor agents employed, Onrigin™ and 90CE, were not effective at similar concentrations, the maximum concentration of these 1,2-bis(sulfonyl)hydrazines employed in these experiments being determined by the aqueous solubility limit of KS119, which is approximately 40 μM.
Table 2.
AGT concentration as determined by the [3H]O6-benzylguanine binding method.
| Cell linea | AGT molecules/cellb |
|---|---|
| U251 | <600 |
| U251/hAGT325 | 325,000 |
| EMT6 | <600 |
| EMT6/hAGT18 | 18,000 |
| CHOAA8 | <600 |
| CHO/hAGT7 | 7000 |
| CHO/hAGT135 | 135,000 |
| DU145 | 42,000 |
AGT levels were determined using the [3H]O6-benzylguanine binding method of Ishiguro et al. [22].
AGT amounts are given in molecules per cell. A value of <600 AGT molecules/cell is below the limits of detection of the measurement technique employed and therefore taken as indicative of an AGT non-expressing cell line.
Fig. 3.
Survival of cells expressing relatively low to moderate levels of AGT treated with various agents under conditions of oxygenation or oxygen deficiency. CHO/hAGT7 (oxygenated, panel A; oxygen-deficient, panel B), and EMT6/hAGT18 cells (oxygenated, panel C; oxygen-deficient, panel D) were treated with varying concentrations of KS119 (▲), 90CE (■), or Onrigin (●). The vertical axis indicates the percent survival over untreated controls; the horizontal axis indicates the concentration of the agent employed in μM. Oxygen depleted samples were treated for 24 h in the presence of 2 U/ml of glucose oxidase, 120 U/ml of catalase and 25 mM glucose, before harvesting and plating of cells to measure cloning efficiency. All points are the result of at least three independent experimental determinations ±SEM.
Fig. 4.
Survival of cells expressing relatively high levels of AGT after treatment with various agents under conditions of oxygenation or oxygen deficiency. CHO/hAGT135 (oxygenated, panel A; oxygen-deficient, panel B), and U251/hAGT325 cells (oxygenated, panel C; oxygen-deficient, panel D), were treated with varying concentrations of KS119 (▲), 90CE (■), or Onrigin (●). The vertical axis indicates the percent survival over untreated controls; the horizontal axis indicates the concentration of the agent employed in μM. Oxygen-deficient samples were treated for 24 h in the presence of 2 U/ml of glucose oxidase, 120 U/ml of catalase and 25 mM glucose, before harvesting and plating of cells to measure cloning efficiency. All points are the result of at least three independent experimental determinations ±SEM.
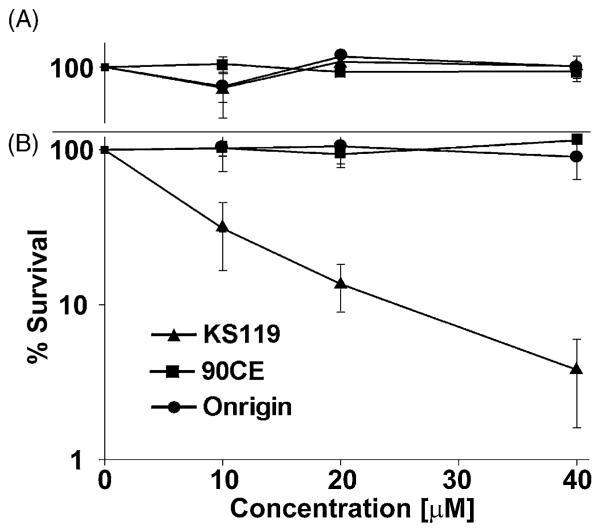
Fig. 5.
Clonogenic experiments showing the survival of DU145 cells, which naturally express relatively high levels of AGT, after treatment with various agents under conditions of oxygenation or oxygen deficiency. DU145 cells (oxygenated, panel A; oxygen-deficient, panel B), were treated with varying concentrations of KS119 (▲), 90CE (■), or Onrigin (●). The vertical axis indicates the percent survival over untreated controls; the horizontal axis indicates the concentration of the agent employed in μM. Oxygen depleted samples were treated for 24 h in the presence of 2 U/ml of glucose oxidase, 120 U/ml of catalase and 25 mM glucose, before harvesting and plating of cells to measure cloning efficiency. All points are the result of at least three independent experimental determinations ±SEM.
3.3. Metabolism studies
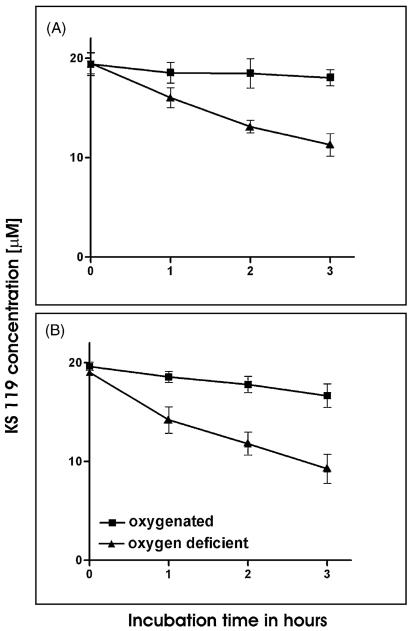
Previous studies have indicated that KS119 was metabolized relatively slowly to an active product by cells in monolayers, with only 20–40% being activated over a 24 h period of exposure under conditions of oxygen deficiency [20]. Since high cell densities may possibly be a more accurate model of solid tumor masses than experiments conducted with single cell monolayers, we measured the extent of metabolic activation of KS119 using two different cell lines, the EMT6 mouse mammary carcinoma and the DU145 human prostate carcinoma (42% and 51% metabolized in a 3 h period, respectively), under conditions of enzymatically generated oxygen deficiency at 107 cells/ml (Fig. 6). Under the aerobic conditions employed in these studies much less metabolism of KS119 was detected over 3 h time periods with EMT6 (23% metabolism) and DU145 cells (5% metabolism). These studies demonstrate, in contrast to experiments with single cell monolayers, that under the appropriate conditions of high cellular density and oxygen deficiency deemed to be more comparable to that occurring in a tumor mass, KS119 can be relatively rapidly metabolized by neoplastic cells. These experiments measured the loss of KS119 and, although we assume that the disappearance of prodrug represents activation to 90CE, it is conceivable that metabolites might be formed that do not result in DNA cross-linking. Therefore, the relative loss of parental KS119 prodrug under both conditions of oxygenation and oxygen deficiency does not necessarily reflect the relative magnitude of DNA cross-linking that might be occurring.
Fig. 6.
Metabolic activation of KS119 using high densities of DU145 and EMT6 cells. For these experiments, 107 cells/ml were incubated with KS119 (DU145 cells, panel A; EMT6 cells, panel B) under oxygenation (■) or oxygen deficiency (▲) for various periods of time. Cells suspension samples were withdrawn, mixed with an equal volume of acetonitrile to lyse cells, quench all reactions, extract drug, and precipitate proteins. These mixtures were then allowed to stand for 15 min at room temperature, then centrifuged at 10,000 × g for 5 min and the supernatants were analyzed by HPLC. The Y-axis indicates drug concentrations as measured by absorbance at 280 nm. All points are the result of at least three independent experimental determinations ±SD.
3.4. Calculated possible levels of 90CE generated from KS119 and Onrigin™ under conditions of oxygenation and oxygen deficiency
We have estimated the possible levels of 90CE generated from KS119 and Onrigin™ under conditions of oxygenation and enzymatically generated oxygen deficiency compared to the flux of 90CE in these cell lines deficient in AGT (Table 1). Whereas 90CE would only be produced from KS119 inside cells under conditions of oxygen deficiency, Onrigin™ is capable of generating 90CE both intracellularly and extracellularly. Using the HPLC results from Fig. 6 for KS119 and previously published findings on Onrigin™ decomposition [27], we calculate that the cellular flux of 90CE generated by KS119 activation under conditions of oxygen deficiency is approximately 30-fold greater at maximal levels than that of Onrigin™ under similar conditions. These calculations are in close agreement to the LC50 ratios between KS119, Onrigin™ and 90CE under oxygen deficiency where the highest difference was found to be 28-fold (Table 1), close to the calculated maximum using HPLC measurements.
3.5. O6-benzylguanine enhancement of KS119 cytotoxicity
The inactivation of AGT by O6-BG has been tested clinically for its ability to enhance the therapeutic efficacy of the DNA O6-guanine alkylating agent carmustine; although O6-BG depleted tumor AGT without host toxicity, the combination of O6-BG and carmustine failed clinically because the depletion of AGT and subsequent enhanced alkylation of DNA guanine occurred in both tumor and normal tissue. This action resulted in an unacceptable increase in alkylating agent toxicity to patients, necessitating a large reduction in carmustine dosage such that the level of nitrosourea tolerated in the presence of O6-BG in this clinical trial had little anticancer activity (see for example Ref. [28]). The use of a primary DNA O6-guanine targeted prodrug such as KS119 which is preferentially activated in oxygen-deficient cells, as one arm of a combined treatment, with a pretreatment arm which non-selectively depletes AGT such as O6-BG, may eliminate this drawback.
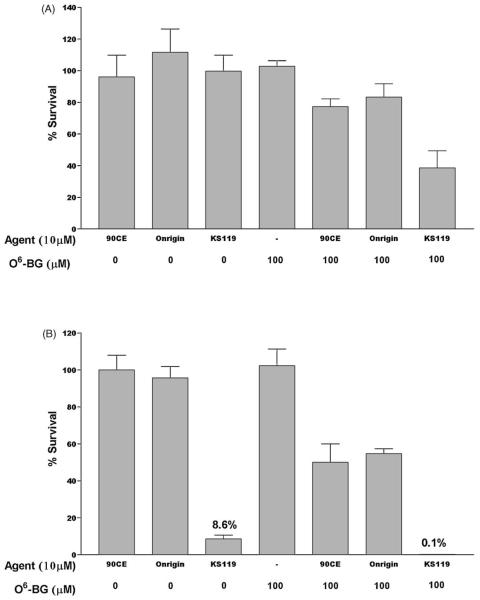
We have also evaluated the capacity of KS119 in combination with O6-BG to produce antineoplastic activity against DU145 human prostate carcinoma cells. As shown in Fig. 7B, pretreatment of DU145 cells with 100 μM O6-BG for 4 h prior to 10 μM KS119 resulted in an almost two log drop in survival, from 8.6% to 0.1%, under conditions of oxygen deficiency, with little or no toxicity to these cells under oxygenated conditions (Fig. 7A). In contrast, the use of 10 μM 90CE or Onrigin™ following 100 μM O6-BG resulted in little or no cytotoxicity under oxygenated conditions and considerably less cytotoxicity compared to KS119 in oxygen-deficient cells.
Fig. 7.
Survival of DU145 cells following pretreatment with O6-benzylguanine (O6-BG), followed by exposure to 90CE, Onrigin, or KS119. Cells were pretreated for 4 h with 100 μMO6-BG, then exposed to 10 μM KS119, Onrigin, or 90CE for 24 h under conditions of oxygenation (panel A) or oxygen deficiency (panel B). All values are the result of at least three independent experimental determinations ±SEM.
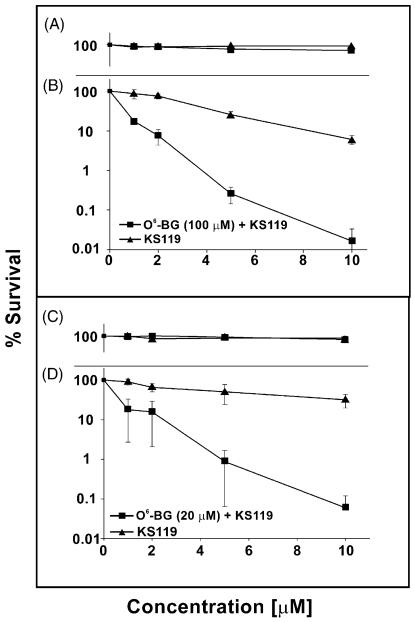
Pretreatment with a lower concentration of 20 μM O6-BG or with 100 μM O6-BG followed by exposure to graded concentrations of KS119 resulted in a pronounced degree of cytotoxicity for the combination under conditions of oxygen deficiency (Fig. 8B and D); whereas, in the presence of oxygen little or no significant antineoplastic activity occurred (Fig. 8A and C).
Fig. 8.
Survival of DU145 cells in clonogenic experiments after pretreatment with O6-benzylguanine (O6-BG), followed by treatment with KS119. Cells were pretreated for 4 h with 100 and 20 μM O6-BG, then exposed to 1–10 μM KS119 (oxygenated, panel A and panel C) (oxygen-deficient, panel B and panel D). All points are the result of at least three independent experimental determinations ±SEM.
4. Discussion
KS119 is a second generation prodrug designed to produce 90CE, the initial DNA O6-guanine chloroethylating moiety of Onrigin™. Onrigin™, following base catalyzed activation, generates 90CE in both intracellular and extracellular compartments; whereas, KS119 only produces the active chloroethylating agent 90CE inside tumor cells, since its net activation requires enzymatic reduction in oxygen-deficient cells [19]. Thus, KS119 is a rationally conceived prodrug designed to be activated within hypoxic/anoxic areas of solid tumors, where net reduction can be readily triggered by cellular reductase enzymes, with KS119 exploiting the presence of areas of aberrant vascularization that result in partial (hypoxic) or complete (anoxic) oxygen deficiencies in cancerous tissue [1-4].
The therapeutic action of KS119 is not only facilitated by differences from Onrigin™ in the activation mechanisms, but also in the short half life (30 s) of 90CE, which is believed to generate a series of exceedingly reactive and short-lived cross-linking species, with little time to escape from the tumors. The findings in this report show that KS119 is markedly more cytotoxic than a molar equivalent concentration of 90CE itself or of Onrigin™ under conditions of oxygen deficiency, even when much of the KS119 prodrug is not metabolically activated.
The intracellular activation in neoplastic tissue allows KS119 to exert lethality even in cell lines with very high levels of AGT, thereby producing a cytotoxic effect not observed with related agents such as Onrigin™, which generates 90CE both intracellularly and extracellularly. The potency of KS119 may also be augmented by properties of the DNA O6-guanine chloroethyl lesion that it produces, since the chloroethyl adduct that forms, rapidly produces the cyclic product N1,O6-ethanoguanine, which is then slowly converted to a G-C cross-link (half life = 2 h; [29]), a lesion not susceptible to repair by AGT. However, even in the presence of exceedingly large concentrations of AGT, a small portion of the initial DNA O6-guanine chloroethyl adducts will “slip through” to an AGT non-reparable G-C cross-link prior to AGT effecting a repair, due to the competitive nature of these two reactions. This results in a small yet lethal number of cross-link lesions accumulating in a time dependent manner [29]. Thus, there are two competing fates for the initial lesion, i.e., AGT repair, or progression to a highly toxic cross-link. Greater AGT levels will result in a greater proportion of the initial lesions being intercepted; however, the sensitivity of the tumors containing exceedingly large quantities of AGT to KS119 makes it conceivable that some of the AGT may be in a form or cellular site not available to compete with the formation of the G-C cross-link. In contrast, greater targeting of KS119 will result in the formation of more initial lesions and eventual cross-links. Hence, cells expressing AGT have enhanced resistance to O6-guanine targeted chloroethylating agents while little AGT depletion is observed. This contrasts markedly to methylating agents where significant AGT depletion is seen at cytotoxic concentrations [29,30]. Since relatively few G-C cross-links appear to be required for cell lethality [29] the combination of oxygen-deficient cell selective activation of KS119 coupled with the chloroethylating agent “slip through” reaction may make this dual mechanism an unusually effective one in AGT containing cells, thereby explaining the ability of KS119 to eradicate oxygen-deficient neoplastic cells expressing extraordinarily large amounts of AGT compared, for example, to DNA guanine O6-alkylating prodrugs which are activated by mechanisms other than by the reductive properties of cells, such as Onrigin™ which employs base catalyzed fragmentation for activation. The enhanced delivery of the chloroethylating species produced by KS119 may also allow minor relatively non-toxic alkylations that most probably occur to reach lethal levels. This would be most apparent in the presence of very high levels of AGT where lethality from chloroethylation of the O-6 position of DNA guanine would be diminished. Secondary DNA lesions other than alkylation of the O-6 position of DNA guanine, such as the alkylation of the N-7 position of DNA guanine, the N-3 position of DNA adenine, or the alkylation of phosphate molecules, all of which are not susceptible to repair by AGT could be significant contributors to toxicity derived therefrom, as could non-DNA alkylations [29]. At the highest concentrations of KS119 these expected secondary effects may become more important, but based upon the findings presented in this report and in others [22], it is obvious that AGT induced repair is the primary mechanism of resistance to agents of the 1,2-bis(sulfonyl)hydrazine class, confirming the preeminence of O6-DNA guanine lesions in the generation of cytotoxicity by O6-DNA guanine targeted agents, and reinforcing the importance of developing strategies to overcome AGT mediated resistance to therapeutic agents of this type. Direct demonstration of the cross-links generated in AGT expressing cells by KS119 is desirable, but limitations in the sensitivity of our cross-linking assay [29], and in the solubility of KS119 currently do not allow detection of these hypothesized “slip through” cross-links. We are hopeful that either through enhancement of the sensitivity of our cross-link assay or better formulation of KS119 we can overcome these difficulties in future experiments.
The use of O6-BG followed by KS119 resulted in synergistic cytotoxicity to all of the cell lines evaluated under conditions of oxygen deficiency. The O6-BG was employed to deplete intracellular pools of AGT and was relatively non-toxic by itself, indicating that both arms of a combination need not be independently therapeutic, if one of the components of the admixture interferes with a resistance mechanism or by some other action enhances the efficacy of the primary therapeutic component. The finding that O6-BG combined with carmustine required an 80% decrease in the dosage of the nitrosourea in clinical trials because of intolerable myelosuppression [28] indicates that O6-guanine lesions are the primary contributors to both host and neoplastic toxicity despite the fact that DNA N-7 alkylations represent 75% of the alkylations produced by carmustine; whereas, KS119 and its chemical design progenitors, 90CE and Onrigin™, are distinguished from carmustine by their significantly higher ratio of O-6 to N-7 DNA alkylations, making them substantially more selective O6-guanine targeted agents than this clinically approved nitrosourea [27].
The methodology employed herein to deplete oxygen in cell culture, enzyme generated oxygen deficiency, does not precisely mimic in vivo conditions, but studies in mice using a combined radiation/KS119 treatment protocol suggest that KS119 can target hypoxic cells in AGT non-expressing animal tumors [19]. Further animal studies are necessary to determine if KS119 can demonstrate antineoplastic activity in tumors that express AGT.
The role of oxygen-deficient tumor regions in promoting cancer metastasis is becoming increasingly clear (4,6,7). Prodrugs that exploit oxygen-deficient regions in malignant tumors by undergoing reductive activation to form a cytotoxic moiety may not only attack therapeutic resistant hypoxic tumors but may also reduce or impede tumor metastasis by preventing the establishment of oxygen-deficient niches that facilitate the formation of nascent cancer metastases. The general structural design used for the development of KS119 can be readily extended to deliver other types of small molecule prodrugs, including enzyme inhibitors and other classes of cytotoxic agents.
Acknowledgments
This work was supported by U.S. Public Health Service Grants CA090671, CA122112 and CA129186 from the National Cancer Institute, and a Grant from the National Foundation for Cancer Research.
Abbreviations
- AGT
O6-alkylguanine-DNA alkyltransferase
- 90CE
1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine
- KS119
1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine
- Onrigin™
1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-(methylaminocarbonyl)hydrazine
- O6-BG
O6-benzylguanine
Footnotes
Conflict of interest statement
The potential anticancer agents Onrigin™ and KS119, designed and synthesized in Dr. Sartorelli's laboratory, have been licensed to Vion Pharmaceuticals, Inc. by Yale University. Dr. Sartorelli currently is a Director and Chairman of the Scientific Advisory Board of this company, has common stock in Vion and, in the past, his laboratory has received gift monies in support of research. In addition to Dr. Sartorelli, two of the other authors P.G. Penketh and K. Shyam also own stock in Vion.
References
- 1.Vaupel PW, Frinak S, Bicher HI. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 1981;41:2008–13. [PubMed] [Google Scholar]
- 2.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- 3.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;9:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 4.Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 5.Bristow R, Hill R. Hypoxia. DNA repair and genetic instability. Nature. 2008;8:180–92. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 6.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le Q, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 7.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signaling in cancer and approaches to enforce tumor regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 9.Denko N. Hypoxia. HIF1 and glucose metabolism in the solid tumor. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 10.Bertout J, Patel S, Simon M. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–70. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Mazzone M, Dettori D, Oliveira R, Loges S, Schimdt T, Jonckx B, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:1–13. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giles F, Thomas D, Garcia-Manero G, Faderl S, Cortes J, Verstovsek S, et al. A phase I and pharmacokinetic study of VNP40101M, a novel sulfonylhydrazine alkylating agent, in patients with refractory leukemia. Clin Cancer Res. 2004;10:2908–17. doi: 10.1158/1078-0432.ccr-03-0738. [DOI] [PubMed] [Google Scholar]
- 14.Giles F. Laromustine: the return of alkylators to non-myeloablative therapy for AML. Leukemia Res. 2009;33:1022–3. doi: 10.1016/j.leukres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Pigneux A. Laromustine, a sulfonyl hydrolyzing alkylating prodrug for cancer therapy. IDrugs. 2009;12:39–53. [PubMed] [Google Scholar]
- 16.Mekhail T, Gettinger S, Blumenschein G, Axelrod R, Haingentz M, Guarino MJ, et al. A phase II trial of VNP40101M in patients with relapsed or refractory small cell lung cancer (SCLC) with or without brain metastases. J Clin Oncol. 2007;25:7724. [Google Scholar]
- 17.Sartorelli AC. Therapeutic attack of hypoxic cells of solid tumors: presidential address. Cancer Res. 1988;48:775–8. [PubMed] [Google Scholar]
- 18.Shyam K, Penketh PG, Shapiro M, Belcourt MF, Loomis RH, Rockwell S, et al. Hypoxia-selective nitrobenzyloxycarbonyl derivatives of 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazines. J Med Chem. 1999;42:941–6. doi: 10.1021/jm9805891. [DOI] [PubMed] [Google Scholar]
- 19.Seow HA, Penketh PG, Shyam K, Rockwell S, Sartorelli AC. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine: an anticancer agent targeting hypoxic cells. Proc Natl Acad Sci. 2005;102:9282–7. doi: 10.1073/pnas.0409013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann R, Penketh P, Seow H, Shyam K, Sartorelli A. Generation of oxygen deficiency in cell culture using a two-enzyme system to evaluate agents targeting hypoxic tumor cells. Radiation Res. 2008;170:651–60. doi: 10.1667/RR1431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pegg AE, Dolan ME, Moschel RC. Structure, function and inhibition of O6-alkylgunanine-DNA alkyltransferase. In: Cohn WE, Moldave K, editors. Progress in nucleic acid research and molecular biology. Academic Press; San Diego: 1995. pp. 167–223. [DOI] [PubMed] [Google Scholar]
- 22.Baumann RP, Seow HA, Shyam K, Penketh PG, Sartorelli AC. The antineoplastic efficacy of the prodrug cloretazine is produced by the synergistic interaction of carbamoylating and alkylating products of its activation. Oncol Res. 2005;15:313–25. doi: 10.3727/096504005776404553. [DOI] [PubMed] [Google Scholar]
- 23.Ishiguro K, Shyam K, Penketh PG, Sartorelli A. Development of an O6-alkylguanine-DNA alkyltransferase assay based on covalent transfer of the benzyl moiety from [benzene-(3)H]O6-benzylguanine to the protein. Anal Biochem. 2008;383:44–51. doi: 10.1016/j.ab.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shyam K, Penketh PG, Divo AA, Loomis RH, Patton CL, et al. Synthesis and evaluation of 1,2,2-tris(sulfonyl)hydrazines as antineoplastic and trypanocidal agents. J Med Chem. 1990;33:2259–64. doi: 10.1021/jm00170a033. [DOI] [PubMed] [Google Scholar]
- 25.Rockwell S. In vivo–in vitro tumor systems: new models for studying the response of tumors to therapy. Lab Anim Sci. 1977;27:831–51. [PubMed] [Google Scholar]
- 26.Belcourt MF, Hodnick WF, Rockwell S, Sartorelli AC. The intracellular location of NADH:cytochrome b5 reductase modulates the cytotoxicity of the mitomycins to Chinese hamster ovary cells. J Biol Chem. 1998;273:8875–81. doi: 10.1074/jbc.273.15.8875. [DOI] [PubMed] [Google Scholar]
- 27.Penketh PG, Shyam K, Sartorelli AC. Comparison of DNA lesions produced by tumor-inhibitory 1,2-bis(sulfonyl)hydrazines and chloroethylnitrosoureas. Biochem Pharmacol. 2000;59:283–91. doi: 10.1016/s0006-2952(99)00328-7. [DOI] [PubMed] [Google Scholar]
- 28.Schilsky RL, Dolan ME, Bertucci D, Ewesuedo RB, Vogelzang NJ, Mani S, et al. Phase I clinical and pharmacological study of O6-benzylguanine followed by carmustine in patients with advanced cancer. Clin Cancer Res. 2000;6:3025–31. [PubMed] [Google Scholar]
- 29.Penketh PG, Baumann RP, Ishiguro K, Shyam K, Seow HA, Sartorelli AC. Lethality to leukemia cell lines of DNA interstrand cross-links generated by Cloretazine derived alkylating species. Leukemia Res. 2008;32:1546–53. doi: 10.1016/j.leukres.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiguro K, Seow HA, Penketh PG, Shyam K, Sartorelli AC. Mode of action of the chloroethylating and carbamoylating moieties of the prodrug cloretazine. Mol Cancer Ther. 2006;5:969–76. doi: 10.1158/1535-7163.MCT-05-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]