Abstract
The dopamine transporter (DAT) substrates dopamine, d-amphetamine (AMPH), and methamphetamine are known to rapidly and transiently reduce DAT activity and/or surface expression in dorsal striatum and heterologous expression systems. We sought to determine if similar substrate-induced regulation of DATs occurs in rat nucleus accumbens. In dorsal striatum synaptosomes, brief (15-min) in vitro substrate pre-exposure markedly decreased maximal [3H]dopamine uptake velocity whereas identical substrate pre-exposure in nucleus accumbens synaptosomes produced a smaller, non-significant reduction. However, 45 min after systemic AMPH administration, maximal ex vivo [3H]dopamine uptake velocity was significantly reduced in both brain regions. Protein kinase C inhibition blocked AMPH’s down-regulation of DAT activity. DAT synaptosomal surface expression was not modified following either the brief in vitro or in vivo AMPH pre-exposure but was reduced after a longer (1-h) in vitro pre-exposure in both brain regions. Together, our findings suggest that relatively brief substrate exposure results in greater down-regulation of DAT activity in dorsal striatum than in nucleus accumbens. Moreover, exposure to AMPH appears to regulate striatal dopamine transporters in a biphasic manner, with an initial protein kinase C-dependent decrease in DAT-mediated uptake velocity and then, with longer exposure, a reduction in DAT expression.
Keywords: Biotinylation, dopamine transporter, dopamine uptake, dorsal striatum, nucleus accumbens, psychostimulant
The neurotransmitter dopamine (DA) plays an essential role in normal CNS functions such as cognition, locomotion, and reward (Iversen and Iversen 2007). The duration and extent of DA neurotransmission is largely limited by DA transporters (DATs) (Jaber et al. 1997). The DAT is a member of the neurotransmitter:sodium symporter family, which also includes GABA, norepinephrine, and serotonin transporters (Chen et al. 2004). DATs are localized to dopaminergic neurons (Nirenberg et al. 1996), where they are functional when expressed on the plasma membrane. In particular, DATs located within striatal brain reward circuitry help to mediate the psychostimulant actions of d-amphetamine (AMPH) and methamphetamine (METH) (Koob and Nestler 1997; Wise and Bozarth 1987).
DAT function can be rapidly regulated by exposure to DAT substrates (e.g. DA, AMPH, and METH), DAT inhibitors (e.g. cocaine), ligands for various presynaptic receptors (e.g. DA D2 receptors), and several signaling systems (e.g. protein kinase C (PKC)) (for reviews see: Gulley and Zahniser 2003; Mortensen and Amara 2003). Here, we focused on further understanding the relatively rapid and transient down-regulation of DAT activity and cell surface expression induced by its substrates. This has been shown to occur following their initial uptake by DAT, inhibition/reversal of DAT, and ultra rapid up-regulation of DATs in dorsal striatum (dSTR) and cells expressing cloned DATs (Fleckenstein et al. 1997; Saunders et al. 2000; Chi and Reith 2003; Johnson et al. 2005; Kahlig et al. 2006; Boudanova et al. 2008). Although it may be counterintuitive why exposure to DAT substrates results in not only increased DA in the synapse, but also down-regulation of DAT uptake activity, this latter effect may be a protective mechanism since cytoplasmic DA can be toxic to neurons (Ziv et al. 1994).
Interestingly, two published studies have suggested that DAT activity may be differentially regulated by substrates within subregions of striatum (Kokoshka et al. 1998; Gulley et al. 2002;). Specifically, systemic administration of METH decreased DAT activity measured ex vivo in well-washed synaptosomes prepared from rat dSTR but not from nucleus accumbens (NAc) (Kokoshka et al. 1998). Additionally, brief, rapidly repeated local application of DA decreased in vivo DA clearance, consistent with loss of DATs, in rat dSTR but not in NAc (Gulley et al. 2002). Since NAc has 40-60% fewer DATs than dSTR (Marshall et al. 1990), it could be important for DATs in NAc to be more resistant to substrate-induced down-regulation. However, whether this is the case and the explanation for this putative brain regional difference are not known. The DAT protein is a single gene product, and to date only one regional difference has been identified (i.e. glycosylation of DATs differs in rat dSTR and NAc) (Lew et al. 1992). Although lack of N-glycosylation reduces DAT activity and surface expression, it is not essential for DAT surface expression (Li et al. 2004). Post-translational modifications and accessory proteins involved in DAT trafficking could also differ between dSTR and NAc, but these have not been reported.
It is important to understand if DATs in dSTR and NAc differ in substrate-induced regulation because this could contribute to the differential dopaminergic neuronal function reported for these two brain regions. For instance, AMPH- and cocaine-induced increases in extracellular DA are greater in NAc than in dSTR (Carboni et al. 1989; Cass et al. 1992; Kuczenski and Segal 1992), but the vulnerability to the neurotoxic effects of METH is greater in dSTR than in NAc (Haughey et al. 1999; Wallace et al. 1999). Furthermore, for psychostimulants (e.g. AMPH, METH, and cocaine), dSTR and NAc are suggested to play differing roles in the transition from initial drug use to drug addiction, with NAc being particularly important during the early stages and dSTR being more important in the later stages (Everitt and Robbins 2005).
Therefore, the purpose of the present study was to compare directly the effect of relatively brief in vitro pre-exposure to DAT substrates, with an emphasis on AMPH, on DAT uptake activity, kinetics, and cell surface expression in synaptosomes prepared from rat dSTR versus NAc. Since PKC has been previously shown to play a role in substrate-mediated regulation of DATs in dSTR and cells expressing cloned DATs (Giambalvo 1992a,b; Vaughan et al. 1997; Zhang et al. 1997; Zhu et al. 1997; Daniels and Amara 1999; Blakely and Bauman 2000; Chi and Reith 2003), follow-up experiments were performed to assess if this was also the case in NAc synaptosomes. Lastly, the effects of systemic administration of AMPH were compared on ex vivo DAT kinetics and surface expression to explore further regionally selective substrate-induced DAT regulation.
Materials and methods
Animals
Research was performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and was approved by the University of Colorado Denver Anschutz Medical Campus Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (150-200 g; Charles River Laboratories, Wilmington, MA, USA) were used. Rats were housed 3-4 per cage and maintained on a 12-h light/dark cycle (lights on at 0600) with food and water available ad libitum.
Materials
Drugs used included: AMPH (d-amphetamine sulfate salt), DA (dopamine hydrochloride), and METH [(+)-methamphetamine hydrochloride] from Sigma-Alrich (St. Louis, MO, USA); bisindolylmaleimide I hydrochloride (Bis-I) from EMD Chemicals (Gibbstown, NJ, USA); and (−)-cocaine hydrochloride from National Institute on Drug Abuse (Research Triangle Institute International, Research Triangle Park, NC, USA). Antibodies used included: mouse monoclonal protein phosphatase 2A (PP2A) primary antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA); mouse monoclonal DAT primary antibody, a generous gift from Dr. Roxanne Vaughan (University of North Dakota, Grand Forks, ND, USA); and goat anti-mouse horseradish peroxidase-conjugated secondary antibody from Bio-Rad Laboratories (Hercules, CA, USA). [3H]DA (specific activity 39-60 Ci/mmol) was purchased from Perkin-Elmer Life Sciences (Boston, MA, USA). EZ-Link sulfo-NHS-biotin and immobilized monomeric avidin were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA). All other chemicals were purchased from either Sigma-Aldrich or Thermo Fisher Scientific.
Preparation of Synaptosomes
Synaptosomes were prepared as previously described by Hoover et al. (2007). Briefly, following decapitation, brains were rapidly removed from the rats and sectioned (700-1000 microns) with an ice-cooled Vibratome; dSTR and NAc were dissected out. Tissue was homogenized in ice-cold phosphate buffer (3.3 mM NaH2PO4 + 12.7 mM Na2HPO4) containing 0.32 M sucrose (pH 7.4) with a glass/Teflon homogenizer. For DAT biotinylation experiments, the phosphate buffer also contained 1 mM EDTA, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 0.1 mM aminoethyl-benzenesulfonyl fluoride hydrochloride. The homogenate was centrifuged at 1,000g for 12 min at 4°C, and the resulting supernatant was centrifuged at 12,500g for 15 min to isolate the P2 pellet (synaptosomes). The P2 pellet was re-suspended either at 15 mg/ml (wet weight of tissue) in assay buffer (134 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 1.4 mM MgSO4, 3.3 mM NaH2PO4, 12.7 mM Na2HPO4, 11 mM glucose, and 1 mM ascorbic acid, pH 7.4) for [3H]DA uptake experiments or at 100 mg/ml in Krebs bicarbonate/CaCl2 buffer (in mM: 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 0.045 EDTA, 1.7 CaCl2, 25 NaHCO3, 10 glucose, 0.1 pargyline, 0.1 ascorbic acid, pH 7.4) for DAT biotinylation experiments.
Synaptosomal tissue was then pre-incubated with either assay buffer (control) or drug at 37°C (see Results for time periods and drug concentrations). Following pre-incubation, samples were washed twice by centrifugation at 12,500g for 15 min at 4°C. The resulting P4 pellets were re-suspended at the same concentrations as the P2 pellets, and uptake or biotinylation assays were initiated. For ex vivo experiments, rats were injected with 2 mg/kg AMPH (intraperitoneal, (i.p.)), decapitated after 45 min, and synaptosomes (P2 pellet) were made as described above.
[3H]DA uptake into synaptosomes
Synaptosomal tissue was pre-incubated with assay buffer containing 1 μM pargyline for 10 min at 37°C. Specific uptake of 0.5 nM [3H]DA was then measured for 3 min at 37°C as the difference between uptake in the absence and presence of 100 μM cocaine. For kinetic analysis, unlabeled DA (0, 10, 50, 100, 250, or 500 nM) was added concomitantly with the [3H]DA. The assay was halted by placing the samples on ice and filtering through Whatman GF/C glass microfiber filters with a cell harvester. The filters were washed 3 times with ice-cold 0.32 M sucrose solution before being placed into 4 ml of scintillation cocktail. Radioactivity was determined by liquid scintillation spectrometry. Sample protein concentrations were determined using bovine serum albumin as the standard (Bradford 1976).
Cell surface biotinylation of DATs in synaptosomes
The method used is based on those published by Zhu et al. (2005), as modified by Hoover et al. (2007). Synaptosomal tissue pooled from four rats was incubated with 2 mg/ml EZ-Link sulfo-NHS-biotin in 1X phosphate buffered saline (PBS)/Ca/Mg buffer (in mM: 138 NaCl, 2.7 KCl, 1.5 KH2PO4, 9.6 Na2HPO4, 1 MgCl2, 0.1 CaCl2, pH 7.3) for 60 min at 4°C with continual shaking. The free sulfo-NHS-biotin was removed by incubation with 1 mM glycine in PBS/Ca/Mg buffer (pH 7.3) for 10 min on ice and centrifugation at 8000g for 4 min at 4°C. The pellet was re-suspended in 0.1 mM glycine in PBS/Ca/Mg buffer (pH 7.3) and centrifuged at 8000g for 4 min at 4°C and re-suspension and centrifugation was repeated one more time. The pellet was then re-suspended and incubated in 0.1 mM glycine in PBS/Ca/Mg buffer (pH 7.3) for 30 min at 4°C with continual shaking. The samples were centrifuged at 8000g for 4 min at 4°C, and then the pellets were re-suspended in PBS/Ca2+/Mg2+ buffer and centrifuged again. The re-suspension and centrifugation steps were repeated two more times. The resulting pellets were lysed with 1% Triton X-100 buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% Na deoxycholate, 0.1% sodium dodecyl sulfate (SDS)) for 30 min at 4°C. Total lysates were centrifuged at 20,000g for 30 min at 4°C, and the supernatant was incubated with 100 μl freshly prepared monomeric avidin beads overnight at 4°C with continual shaking. Biotinylated proteins were eluted with Laemmeli buffer (62.5 mM Tris, pH 6.8, 20% glycerol, 5% β-mercaptoethanol, 2% SDS). Total, biotinylated, and non-biotinylated samples were prepared for Western blot analysis by adding sample buffer (except for biotinylated sample; 62.5 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.1% dithiothreitol (DTT), trace of bromophenol blue), heating for 5 min at 75°C, and storing at −20°C until use.
Western blot analysis of synaptosomes
7.5 μl of the total and non-biotinylated samples and 10 μl of the biotinylated sample were separated by 7.5% SDS-polyacrylamide gel electrophoresis and transferred to a polyscreen polyvinylidene difluoride transfer membrane (Perkin-Elmer Life Sciences, Boston, MA, USA) using the Mini Trans-Blot Cell apparatus (Bio-Rad Laboratories, Hercules, CA, USA). After transfer, membrane blots were blocked for 60 min at 22°C in 5% nonfat dry milk in 0.1% Tween-20/Tris-buffered saline/ (TTBS; 140 mM NaCl, 20 mM Tris, pH 7.6, 0.01% Tween 20). After blocking, membranes were incubated with an antibody directed against DAT or PP2A overnight at 4°C. The next day, membranes were rinsed in TTBS, incubated with goat anti-mouse horseradish peroxidase-conjugated secondary antibody for 2 h at 22°C, and rinsed again with TTBS. Blots were then developed with Supersignal Pico Chemiluminescent Substrate using either a film developer or the FluorChem SP Imager (Alpha Innotech, San Leandro, CA) and analyzed using the gel analyzer function in ImageJ (Rasband, NIH, Bethesda, MD, USA).
Since protein quantitation assays were not performed on the samples, the area under the curve values were normalized. This was done by determining the percent of the sample loaded onto the gel (i.e. 2.5% (7.5 μl/300 μl), total samples; 13.3% (10 μl/75 μl), biotinylated samples). The percentage of the total sample was then normalized to the biotinylated sample, resulting in a correction factor of 5.3 for the total samples; and the area under the curve value for each sample was multiplied by its correction factor.
Data analysis
Data are presented as mean ± standard error of the mean (SEM), with N = number of animals. The time courses of drug-induced alterations in [3H]DA uptake were analyzed by two-way repeated measures analysis of variance (RMANOVA); paired t-test post hoc tests were performed only if the interaction between treatment and time reached significance. The concentration-response and PKC experiments were analyzed by one-way RMANOVA followed by Fisher LSD post hoc tests (SigmaStat 2.03, San Jose, CA). Affinity (Km) and maximal transport velocity (Vmax) values were estimated by nonlinear regression analysis using Prism software (GraphPad, La Jolla, CA) and analyzed by paired t-tests (SigmaStat). Biotinylation samples were analyzed as percent biotinylated/total by paired t-tests (SigmaStat). Significant differences were defined as those with p < 0.05.
Results
Regulation of DAT activity by brief in vitro DAT substrate pre-exposure in rat dSTR and NAc synaptosomes
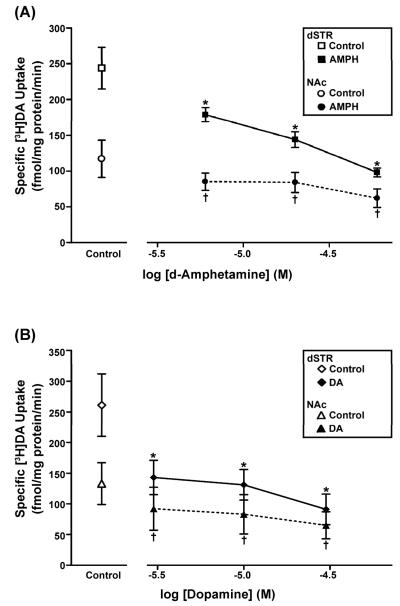
To investigate whether relatively brief pre-exposure to DAT substrates differentially regulates DATs in synaptosomes prepared from rat dSTR or NAc, the temporal and concentration characteristics of pre-incubation with AMPH, DA, or METH were examined on subsequent specific [3H]DA uptake into the well-washed synaptosomes. As expected, in controls, dSTR [3H]DA uptake was higher (by 54%) than in NAc. In dSTR synaptosomes, pre-incubation with 20 μM AMPH, 10 μM DA or 10 μM METH significantly reduced DAT-mediated uptake activity by an average of 53%, 33%, or 52%, respectively, compared to control (Fig. 1). Similar results were seen with 20 μM AMPH, 10 μM DA or 10 μM METH in NAc synaptosomes (decreased by 50%, 45%, or 46%, respectively, compared to control) (Fig. 1). Two-way RMANOVA showed a main effect of drug treatment in both brain regions [dSTR: AMPH: F(1,3) = 98.7, p < 0.01; DA: F(1,3) = 15.3, p < 0.05; METH: F(1,3) = 21.2, p < 0.05 and NAc: AMPH: F(1,3) = 79.2, p < 0.001; DA: F(1,3) = 12.8, P < 0.05; METH: F(1,3) = 38.4, P < 0.01]. However, since this effect was not consistently time-dependent nor was there a consistent interaction between treatment and time, post hoc analysis was not conducted. Pre-incubation for 15 min was chosen for use in further analyses. Concentration-response studies and one-way RMANOVA with Fisher LSD post hoc tests revealed that in dSTR synaptosomes, a 15-min pre-incubation with all tested concentrations of AMPH or DA (3 – 60 μM) significantly decreased [3H]DA uptake by 27-60% [F(3,6) = 12.7, p < 0.01] or 45-65% [F(3,9) = 10.6, p < 0.01], respectively, compared to control (Fig. 2). Comparable significant results were observed with all concentrations of AMPH or DA tested in NAc synaptosomes (decreased by 27-47% [F(3,6) = 8.0, p < 0.05] or 31-51% [F(3,9) = 8.8, p < 0.01], respectively, compared to control) (Fig. 2).
Fig. 1.
Temporal characteristics of the effect of in vitro pre-incubation with DAT substrates on [3H]DA uptake into synaptosomes prepared from rat dSTR or NAc. After pre-incubation of synaptosomes with (A) 20 μM AMPH, (B) 10 μM DA, or (C) 10 μM METH for 5, 15, or 30 min and subsequent drug wash out, specific uptake of 0.5 nM [3H]DA was measured (see Methods for details). Mean value ± SEM shown for N = 4 per treatment and brain region. In both brain regions, two-way RMANOVA showed a main effect of drug treatment, but not a main effect of time or a treatment-time interaction.
Fig. 2.
Concentration characteristics of the effect of in vitro pre-incubation with DAT substrates on [3H]DA uptake into synaptosomes prepared from rat dSTR or NAc. After pre-incubation of synaptosomes with either (A) 6, 20, or 60 μM AMPH or (B) 3, 10, or 30 μM DA for 15 min and subsequent drug wash out, specific uptake of 0.5 nM [3H]DA was measured. Mean value ± SEM shown for N = 3-4 per treatment and brain region. * p < 0.05 versus dSTR control; † p < 0.05 versus NAc control; Fisher LSD post-hoc tests.
Next, analyses of DAT kinetic parameters were performed. AMPH was used as a prototypic DAT substrate to determine if substrate-induced decreases in [3H]DA uptake were due to decreased Vmax and/or Km and if these were regionally specific. Pre-incubation of synaptosomes prepared from dSTR with 20 μM AMPH for 15 min significantly reduced DAT Vmax in the washed synaptosomes by 78%, with no change in Km (Fig. 3a, Table 1). The results in NAc synaptosomes after similar AMPH pre-incubation were more variable, and AMPH-induced reductions in Vmax were smaller (Fig. 3b). Overall in NAc, no significant changes in either DAT Vmax or Km were observed, compared to control (Table 1). Moreover, paired t-tests revealed that there were significant differences between dSTR and NAc in the percentages of AMPH-induced changes in both Vmax (78% versus 32%, respectively) and Km (32% versus 215%, respectively) values.
Fig. 3.
Decreased DAT Vmax in synaptosomes prepared from dSTR, but not from NAc, following in vitro pre-incubation with 20 μM AMPH for 15 min. Representative results are shown from analysis of DAT kinetic parameters in synaptosomes prepared from (A) dSTR or (B) NAc. After drug wash out, specific uptake of 0.5 nM [3H]DA uptake + unlabeled DA (0, 10, 50, 100, 250, or 500 nM) was measured. Group results are given in Table 1.
Table 1.
Effect of a 15-min in vitro pre-incubation with 20 μM AMPH on DAT kinetic parameters in rat dSTR or NAc synaptosomes
| Condition | Vmax (pmol/min/mg protein) | Km (nM) |
|---|---|---|
| dSTR, Control | 76 ± 9.2 | 160 ± 32 |
| dSTR, AMPH | 17 ± 2.5* | 110 ± 11 |
| NAc, Control | 57 ± 11 | 200 ± 42 |
| NAc, AMPH | 39 ± 4.6 | 430 ± 110 |
N = 6 per condition and brain region.
p < 0.05 versus dSTR control; paired t-test.
Involvement of PKC in AMPH-induced down-regulation of DAT activity in rat dSTR and NAc synaptosomes
Next, we investigated whether the AMPH-induced down-regulation of DAT activity was blocked by first pre-incubating synaptosomes with the PKC inhibitor Bis-I (1 μM) for 5 min, prior to pre-incubation with AMPH (20 μM; 15 min). In synaptosomes prepared from both dSTR and NAc, AMPH significantly reduced DAT activity (one-way RMANOVA; [dSTR: F(3,6) = 389.2, p < 0.001 and NAc: F(3,6) = 15.2, p < 0.01], and Bis-I blocked both of these AMPH-induced decreases (Fig. 4).
Fig. 4.
Blockade by the PKC inhibitor Bis-I of AMPH-induced down-regulation of [3H]DA uptake into synaptosomes prepared from rat dSTR or NAc. After pre-incubation of synaptosomes with 1 μM Bis-I for 5 min and 20 μM AMPH for an additional 15 min and subsequent drug wash out, specific uptake of 0.5 nM [3H]DA uptake was measured. Mean value ± SEM shown for N = 3 per treatment and brain region. * p < 0.05 versus dSTR AMPH; † p < 0.05 versus NAc AMPH; Fisher LSD post-hoc tests.
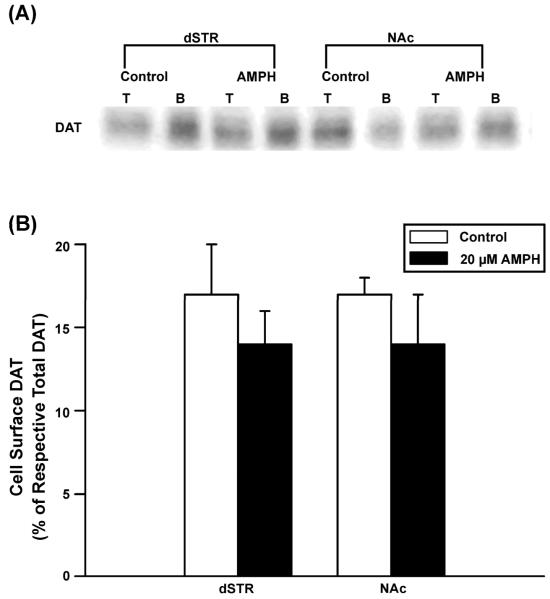
Effect of brief in vitro DAT substrate pre-exposure on DAT cell surface expression in rat dSTR and NAc synaptosomes
To determine if the decrease in Vmax induced by pre-incubation with AMPH (20 μM; 15 min) was due to a reduction in DATs in the plasma membrane, DAT cell surface biotinylation assays were performed. [3H]DA uptake assays were also performed in the synaptosomal tissue to verify the viability of the preparation and that AMPH had indeed reduced DAT activity, as observed previously. These assays confirmed that uptake following AMPH pre-incubation was decreased by 42% in dSTR and by 23% in NAc, versus respective controls (data not shown). In controls, cell surface DATs were found to be similar in the two striatal subregions: 17 ± 3% (dSTR; biotinylated/total) and 17 ± 3% (NAc). However, no change in cell surface expression of DATs was observed after pre-incubation with 20 μM AMPH for 15 min in synaptosomes prepared from either dSTR or NAc (Fig. 5). Western blots were also probed with PP2A, an intracellular protein, to confirm the integrity of the synaptosomes and quality of the biotinylation assays. PP2A was not detected in the synaptosomal samples containing biotinylated proteins indicating that the synaptosomes were not “leaky” and that the biotinylation reaction was restricted to cell surface proteins (data not shown).
Fig. 5.
No change in DAT cell surface expression induced by in vitro pre-incubation with AMPH in synaptosomes prepared from rat dSTR or NAc. After pre-incubation of synaptosomes with 20 μM AMPH for 15 min, biotinylation assays were performed. Total (T) and biotinylated (B) samples were probed for the presence of DAT using Western Blot analysis (see Methods for details). (A) Representative western blots. (B) Group results. Mean value ± SEM shown for N = 4 per treatment and brain region. No significant differences by paired t-test.
Ex vivo analysis of AMPH-induced regulation of DAT kinetic parameters and cell surface expression in rat dSTR and NAc synaptosomes
Next, we examined DAT kinetic parameters and cell surface expression in synaptosomes prepared from dSTR and NAc of rats 45 min after a 2 mg/kg AMPH (i.p.) injection. Based on previous studies in our lab (Briegleb et al. 2004), this dose of AMPH primarily increases locomotor activity of rats, with maximal behavioral activation reached at approximately 45 min. Ex vivo [3H]DA uptake assays revealed that systemic administration of AMPH significantly reduced DAT Vmax by 61% and 52%, with no change in Km, in synaptosomes prepared from dSTR and NAc, respectively (Fig. 6a,b; Table 2). However, similar to the in vitro AMPH pre-incubation results (Fig. 5), no change in cell surface expression of DATs in either dSTR or NAc synaptosomes was observed 45 min after the systemic treatment with AMPH (Fig. 6c,d).
Fig. 6.
Decreased ex vivo DAT Vmax, but no change in DAT cell surface expression, in synaptosomes prepared from dSTR or NAc 45 min after treatment of rats with 2 mg/kg AMPH (i.p.). (A, B) For kinetic analysis specific [3H]DA uptake was measured as in Fig. 3. Representative curves for (A) dSTR and (B) NAc. Group results are given in Table 2. (C, D) Cell surface expression was measured by biotinylation assays as in Fig. 5. (C) Representative western blots. (D) Group results. Mean value ± SEM shown for N = 4 per treatment and brain region. No significant differences by paired t-test.
Table 2.
Effect of systemic pre-treatment with AMPH (2 mg/kg, i.p.) on DAT kinetic parameters measured ex vivo 45 min later in rat dSTR or NAc synaptosomes
| Condition | Vmax (pmol/min/mg protein) | Km (nM) |
|---|---|---|
| dSTR, Control | 46 ± 4.5 | 57 ± 4.5 |
| dSTR, AMPH | 18 ± 1.0* | 35 ± 8.2 |
| NAc, Control | 18 ± 3.6 | 81 ± 21 |
| NAc, AMPH | 8.7 ± 1.9† | 120 ± 30 |
N = 4-5 per condition and brain region.
p < 0.05 versus dSTR control
p < 0.05 versus NAc control; paired t-test.
Regulation of DAT activity and cell surface expression by longer in vitro DAT substrate pre-exposure in rat dSTR and NAc synaptosomes
Since neither a 15-min in vitro pre-exposure to AMPH nor a 45-min in vivo pre-treatment with AMPH altered DAT surface expression, we investigated whether a longer (1-h) in vitro pre-exposure to DAT substrates would alter both DAT activity and cell surface expression in synaptosomes prepared from rat dSTR and NAc. [3H]DA uptake, measured in washed striatal synaptosomes after 1 h of pre-exposure to 20 μM AMPH, was significantly reduced by 74% in dSTR and 50% in NAc, compared to control (data not shown). Interestingly, after this longer in vitro pre-exposure to AMPH, cell surface expression of DATs in dSTR synaptosomes was significantly decreased by 37%, compared to control (Fig. 7). In agreement with Chi and Reith (2003), we also observed a significant reduction in cell surface DATs in dSTR synaptosomes after a 1-h pre-incubation with DA (100 μM; decreased by 49%, compared to control) (Fig. 7). Significant decreases in DAT cell surface expression were also observed in NAc synaptosomes after a 1-h pre-incubation with both 20 μM AMPH (decreased 25%, compared to control) and 100 μM DA (decreased 22%, compared to control) (Fig. 7).
Fig. 7.
Reduced DAT cell surface expression induced by a longer, 1-hr in vitro pre-exposure with AMPH or DA in rat dSTR and NAc synaptosomes. After pre-incubation of synaptosomes prepared from dSTR or NAc with 20 μM AMPH or 100 μM DA for 1 hr, biotinylation assays were performed as in Fig. 5. (A) Representative western blots. (B) Average of all trials. Mean value ± SEM shown for N = 4-5 per treatment. * p < 0.05 versus respective control; paired t-test.
Discussion
We utilized primarily AMPH as a prototypic DAT substrate to examine whether DAT substrates regulate striatal DAT function and trafficking in a regionally specific manner. Our results confirm that relatively brief in vitro pre-exposure of rat dSTR synaptosomes to DAT substrates like AMPH resulted in a marked decrease in Vmax for [3H]DA uptake but that identical in vitro pre-exposure in NAc synaptosomes produced a smaller, non-significant reduction in the DAT-mediated Vmax. In contrast, following systemic injection of AMPH, the DAT-mediated Vmax was significantly reduced in both dSTR and NAc synaptosomes. Thus, while substrate-induced DAT regulation was more readily observed in dSTR than in NAc, there was not an absolute regional difference. Moreover, mechanisms in addition to trafficking appear to play a significant role in this relatively rapid substrate-induced regulation because DAT cell surface expression was not modified following either brief in vitro or in vivo AMPH pre-exposure but was significantly reduced after a longer in vitro pre-exposure.
Rapid regulation of DAT activity by DAT substrates in rat dSTR versus NAc synaptosomes
Our results in rat dSTR synaptosomes pre-incubated in vitro with DA, AMPH and METH showed that even relatively brief exposure (≥ 5 min) to relatively high concentrations (≥ 3 μM) of these DAT substrates markedly reduced [3H]DA uptake activity. Likewise, a 15-min pre-exposure to 20 μM AMPH decreased DAT Vmax without altering its Km. It is unlikely that this reduction in DAT function was due to residual drug being present during the [3H]DA uptake assays because the synaptosomes were washed twice prior to the assays. Furthermore, a similar reduction in DAT Vmax was seen in dSTR synaptosomes prepared from rats pre-treated with 2 mg/kg (i.p.) AMPH for 45 min. Because of competition between AMPH and [3H]DA for DAT, if residual AMPH were present, the kinetic analyses would have been expected to show a lower DAT affinity, which they did not. Additionally, in striatal synaptosomes made from rats pre-exposed to 15 mg/kg METH, Fleckenstein et al. (1997) continued to observe decreased [3H]DA uptake even when brain METH levels were negligible. Our findings agree with several previous reports in which reduced DAT activity was seen after pre-incubation with various concentrations of AMPH, DA, or METH (1-100 μM) for a range of times (5-60 min) in striatal synaptosomes or cells expressing cloned DATs (Fleckenstein et al. 1997; Saunders et al. 2000; Chi and Reith 2003).
In NAc synaptosomes, in vitro pre-incubation with substrates produced an overall significant effect on DAT-mediated uptake in our temporal and concentration studies. However, analysis of kinetic parameters in NAc showed no significant changes in DAT Vmax or Km. Additionally, there were significant differences in the magnitudes of AMPH-induced changes in DAT Vmax and Km between dSTR and NAc. However, a significant reduction in DAT Vmax was observed in NAc synaptosomes prepared from rats treated acutely with AMPH (2 mg/kg, i.p.; 45 min). The discrepancy between our in vitro and ex vivo results may reflect better viability of the synaptosomes in the later experiments because of shorter tissue preparation times, resulting in less experimental variability. Alternatively, the ex vivo experiment should more closely mimic the in vivo situation with intact neuronal circuits present during the drug exposure. Additionally, precisely how treatment of rats with 2 mg/kg AMPH for 45 min corresponds to the in vitro pre-incubation of synaptosomes with 20 μM AMPH for 15 min, in terms of substrate-induced DAT down-regulation, is not known. In any case, the regional differences we observed (dSTR>NAc) in the ability of DAT substrates to regulate DAT activity are similar to those reported by Kokoshka et al. (1998) and Gulley et al. (2002).
Mechanisms underlying rapid regulation of DAT activity by DAT substrates in rat dSTR and NAc synaptosomes
DAT substrate-induced reductions in DAT Vmax are thought to be due, at least in part, to decreased DAT cell surface expression. This has been shown in a number of studies using cells expressing cloned DATs ( Saunders et al. 2000; Chi and Reith 2003; Kahlig et al. 2006; Boudanova et al. 2008), as well as in rat striatal synaptosomes (Chi and Reith 2003; Johnson et al. 2005). Here, we used rat synaptosomes containing endogenously expressed DATs because they allow for regional comparisons, namely between dSTR and NAc. We detected no change in cell surface DATs after either a 15-min in vitro or 45-min in vivo pre-exposure to AMPH. In agreement, no change in DAT cell surface expression was seen in rat striatal synaptosomes pre-incubated with 3 μM AMPH for 2.5-30 min using a biotinylation assay similar to ours, although a rapid and transient (<1 min) AMPH-induced increase in surface DATs was observed (Johnson et al. 2005). These findings of a lack of down-regulation could reflect a limitation in the sensitivity of the cell surface biotinylation assay in synaptosomes and/or a “basement” effect (i.e., control surface DAT was only ~15% of total DAT). An argument against this conclusion, however, is the fact that we have previously shown that DAT cell surface expression in striatal synaptosomes is significantly decreased after a 15-min pre-incubation with protein tyrosine kinase inhibitors (Hoover et al. 2007). Also, Chi and Reith (2003) found that a 1-h in vitro pre-exposure with 100 μM DA decreased DAT surface expression by 21-27% in rat striatal synaptosomes. Likewise, after a 1-h in vitro pre-exposure with 100 μM DA, as well as with 20 μM AMPH, we observed significant decreases of 37-49% in cell surface expression of DATs in dSTR synaptosomes. Although smaller (22-25%), significant reductions in cell surface expression of DATs were also seen in NAc synaptosomes with both AMPH and DA after the longer pre-exposure time. Taken together, these findings suggest that acute DAT substrate-induced regulation of DATs may occur biphasically, i.e. relatively brief exposure (15-45 min) may selectively decrease DAT kinetic activity whereas longer exposures (>1 h) may alter both DAT activity and trafficking. Interestingly, similar biphasic regulation of the serotonin transporter, which is in the same family of neurotransmitter transporters as DAT, has previously been observed (Jayanthi et al. 2005; Steiner et al., 2008).
It is not known how or why DAT substrates produce greater DAT down-regulation in dSTR versus NAc, but a potential mechanistic difference between the two brain regions could involve differential activity of PKC. Initially, AMPH appears to regulate DAT uptake function through a PKC-dependent mechanism. In agreement with many studies (Giambalvo 1992a,b; Vaughan et al. 1997; Zhang et al. 1997; Zhu et al. 1997; Daniels and Amara 1999; Blakely and Bauman 2000; Chi and Reith 2003), we found that pre-treatment of the synaptosomes with the PKC inhibitor Bis-1 abolished the AMPH-induced decrease in DAT activity in both dSTR and NAc synaptosomes. In addition, AMPH and METH-induced N-terminal phosphorylation of DAT is PKC-dependent (Foster et al. 2002; Cervinski et al. 2005). Furthermore, AMPH-induced DA efflux, which involves a change in DAT conformation from “outward facing” to “inward facing”, requires DAT phosphorylation (Khoshbouei et al. 2004; Kahlig et al. 2005; Sulzer 2005; Kniazeff et al. 2008). More DATs in the “inward facing” conformation would likely reduce [3H]DA uptake. Although our data indicate that the decreased DAT uptake activity resulting from brief pre-exposure to AMPH is PKC-dependent in both regions, future experiments are needed to elucidate if PKC-induced DAT phosphorylation and altered DAT conformation are involved, and if so, how they might differ between dSTR and NAc.
More prolonged AMPH exposure may decrease DAT function by altering DAT surface expression via either a PKC-dependent or -independent mechanism. In this regard, a recent study found that in PC12 cells transfected with human DAT, AMPH-mediated decreases in DAT cell surface expression were not dependent on PKC activation (Boudanova et al. 2008). One possible PKC-independent mechanism by which AMPH could alter DAT trafficking is through the activation of Ca2+/calmodulin-dependent kinase II and subsequent inactivation of Akt (Garcia et al. 2005; Wei et al. 2007). Interestingly, longer time periods of AMPH exposure (at least 30 min) are needed to fully employ this signaling pathway (Wei et al. 2007). Alternatively, AMPH-induced alteration of DAT trafficking could still be a PKC-dependent mechanism involving activation of Nedd4-2 and subsequent DAT ubiquitination leading to increased DAT internalization (Sorkina et al. 2006). Future experiments are needed to determine which mechanism(s) is(are) occurring in rat striatal synaptosomes and how these mechanisms may differ between dSTR and NAc.
Conclusions
Our findings suggest that relatively brief exposure to high concentrations of substrates results in a greater down-regulation of DAT-mediated uptake in dSTR than in NAc. In addition, exposure to AMPH appears to regulate striatal DATs in a biphasic manner, with an initial PKC-dependent decrease in DAT-mediated uptake velocity and then, with longer exposure, a reduction in DAT surface expression.
Acknowledgements
The authors would like to thank Dr. Alexander Sorkin for his valuable discussions; Dr. Richard M. Allen for his help with the statistical analyses; and Gaynor Larson, Bruce Mandt, and Dorothy Yamamoto for their technical assistance. The authors would also like to thank Dr. Roxanne Vaughan (University of North Dakota) for the generous gift of the DAT monoclonal antibody. This research was supported by NIH grants: DA004216, DA014204, AA007464, and DA015050.
Abbreviations used
- AMPH
amphetamine
- Bis-I
bisindolylmaleimide I hydrochloride
- DA
dopamine
- DAT
dopamine transporter
- dSTR
dorsal striatum
- DTT
dithiothreitol
- i.p.
intraperitoneal
- Km
affinity
- METH
methamphetamine
- NAc
nucleus accumbens
- PBS
phosphate buffered saline
- PKC
protein kinase c
- PP2A
protein phosphatase 2A
- RMANOVA
repeated measures analysis of variance
- SDS
sodium dodecyl sulfate
- SEM
standard error of the mean
- TTBS
0.1% Tween-20/Tris-buffered saline
- Vmax
maximal transport velocity
References
- Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr. Opin. Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Melikian HE. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase c-independent. Neuropharmacology. 2008;54:605–612. doi: 10.1016/j.neuropharm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology. 2004;29:2168–2179. doi: 10.1038/sj.npp.1300536. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J. Neurochem. 1992;59:259–266. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase c-dependent mechanisms. J. Biol. Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J. Pharmacol. Exp. Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J. Biol. Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J. Pharmacol. Exp. Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J. Biol. Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol. Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT. Protein kinase c and dopamine transport--1. Effects of amphetamine in vivo. Neuropharmacology. 1992a;31:1201–1210. doi: 10.1016/0028-3908(92)90048-t. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT. Protein kinase c and dopamine transport--2. Effects of amphetamine in vitro. Neuropharmacology. 1992b;31:1211–1222. doi: 10.1016/0028-3908(92)90049-u. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Doolen S, Zahniser NR. Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J. Neurochem. 2002;83:400–411. doi: 10.1046/j.1471-4159.2002.01133.x. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur. J. Pharmacol. 2003;479:139–152. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Hanson GR. Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J. Neurochem. 1999;72:661–668. doi: 10.1046/j.1471-4159.1999.0720661.x. [DOI] [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J. Neurochem. 2007;101:1258–1271. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Jaber M, Jones S, Giros B, Caron MG. The dopamine transporter: a crucial component regulating dopamine transmission. Mov. Disord. 1997;12:629–633. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase c on serotonin transporter function, endocytosis, and phosphorylation. Mol. Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, Galli A. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol. Pharmacol. 2006;70:542–548. doi: 10.1124/mol.106.023952. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:0387–0393. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J. Biol. Chem. 2008;283:17691–17701. doi: 10.1074/jbc.M800475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur. J. Pharmacol. 1998;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J. Neuropsychiatry Clin. Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Differential effects of amphetamine and dopamine uptake blockers (cocaine, nomifensine) on caudate and accumbens dialysate dopamine and 3-methoxytyramine. J. Pharmacol. Exp. Ther. 1992;262:1085–1094. [PubMed] [Google Scholar]
- Lew R, Patel A, Vaughan RA, Wilson A, Kuhar MJ. Microheterogeneity of dopamine transporters in rat striatum and nucleus accumbens. Brain Res. 1992;584:266–271. doi: 10.1016/0006-8993(92)90905-o. [DOI] [PubMed] [Google Scholar]
- Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 2004;279:21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- Marshall JF, O’Dell SJ, Navarrete R, Rosenstein AJ. Dopamine high-affinity transport site topography in rat brain: major differences between dorsal and ventral striatum. Neuroscience. 1990;37:11–21. doi: 10.1016/0306-4522(90)90187-9. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur. J. Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J. Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of nedd4-2 in dopamine transporter ubiquitination and endocytosis. J. Neurosci. 2006;26:8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase c-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J. Biol. Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J. Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wei Y, Williams JM, Dipace C, Sung U, Javitch JA, Galli A, Saunders C. Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol. Pharmacol. 2007;71:835–842. doi: 10.1124/mol.106.026351. [DOI] [PubMed] [Google Scholar]
- Zhang L, Coffey LL, Reith ME. Regulation of the functional activity of the human dopamine transporter by protein kinase c. Biochem. Pharmacol. 1997;53:677–688. doi: 10.1016/s0006-2952(96)00898-2. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J. Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR. Activation of protein kinase c inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1997;282:1358–1365. [PubMed] [Google Scholar]
- Ziv I, Melamed E, Nardi N, Luria D, Achiron A, Offen D, Barzilai A. Dopamine induces apoptosis-like cell death in cultured chick sympathetic neurons--a possible novel pathogenetic mechanism in Parkinson’s Disease. Neurosci. Lett. 1994;170:136–140. doi: 10.1016/0304-3940(94)90258-5. [DOI] [PubMed] [Google Scholar]