Abstract
Chlorpyrifos (CPF) an organophosphate pesticide causes persisting behavioral dysfunction in rat models when exposure is during early development. In earlier work zebrafish were used as a complementary model to study mechanisms of CPF-induced neurotoxicity induced during early development. We found that developmental (first five days after fertilization) chlorpyrifos exposure significantly impaired learning in zebrafish. However, this testing was time and labor intensive. In the current study we tested the hypothesis that persisting effects of developmental chlorpyrifos could be detected with a brief automated assessment of startle response and that this behavioral index could be used to help determine the neurobehavioral mechanisms for persisting CPF effects. The swimming activity of adult zebrafish was assessed by a computerized video-tracking device after a sudden tap to the test arena. Ten consecutive trials (1/min) were run to determine startle response and its habituation. Additionally, habituation recovery trials were run at 8, 32 and 128 min after the end of the initial trial set. CPF-exposed fish showed a significantly (p<0.025) greater overall startle response during the 10-trial session compared to controls (group sizes: Control N=40, CPF N=24). During the initial recovery period (8 min) CPF-exposed fish showed a significantly (p<0.01) greater startle response compared to controls. To elucidate the contributions of nicotinic and muscarinic acetylcholine receptors to developmental CPF-mediated effects, the effects of developmental nicotine and pilocarpine exposure throughout the first five days after fertilization were determined. Developmental nicotine and pilocarpine exposure significantly increased startle response, though nicotine (group sizes: Control N=32, 15 mM N=12, 25 mM N=20) was much more potent than pilocarpine (group sizes: Control N=20, 100 μM N=16, 1000 μM N=12). Neither was as potent as CPF for developmental exposure increasing startle response in adulthood. Lastly, developmental CPF exposure decreased dopamine and serotonin levels and increased transmitter turnover in developing zebrafish larvae (N=4 batches of 50 embryos/treatment). Only the decline in dopamine concentrations persisted into adulthood (group sizes: Control N=14, CPF N=13). This study shows that a quick automated test of startle can detect persisting neurobehavioral impairments caused by developmental exposure to CPF. This may be helpful in screening for persisting neurobehavioral defects from a variety of toxicants.
Keywords: Zebrafish, Chlorpyrifos, Nicotine, Pilocarpine, Startle response, Development
1. Introduction
The organophosphate pesticide chlorpyrifos (CPF) has been one of the most widely used insecticides in the world [14]. Developmental exposure to low levels of CPF during different phases of pre and postnatal development has been shown to cause a variety of persisting neurotoxic effects in adolescent and adult rats [2,17,25,26,39]. While the toxic effects of CPF involve the inhibition of acetylcholinesterase and the consequent hyperactivation of cholinergic receptors, CPF-mediated neurotoxicity also involves additional cellular mechanisms and transmitter systems [34,35]. Molecular defects that have been linked to CPF exposure include, but are not limited to, cellular differentiation and synaptogenesis [10,11,13]. Transmitter systems that have been shown to be impacted by CPF exposure include dopaminergic, serotonergic, and noradrenergic systems [1,4,12,31,34,37].
Rodents have traditionally been used as the model system to study the neurobehavioral effects of environmental toxicants. We have found that early developmental exposure to CPF (first five days after fertilization) caused a persisting impairment in cognitive function in rats [3,17,25,26]. Prenatal CPF exposure has been shown to cause behavioral defects that persist long after the initial exposure. Developmental CPF exposure has been shown to significantly alter locomotor activity [17,25,26] and impair cognitive functioning in both adolescent and adult rats [2,17,25,26]. While rodent studies have been important to the fields of toxicity and teratology; these studies are extremely time consuming, expensive and difficult to work with at early developmental periods. Thus it would be helpful to develop animal/behavioral models in which the critical neurodevelopmental processes impacted by CPF and other environmental toxicants can be rapidly analyzed in more cost effective protocols. Zebrafish offer such a model [29,32].
Zebrafish with all of their embryonic development occurring outside the mother and their clear chorion are becoming widely used to study neurodevelopmental defects associated with toxicant exposure [33] and neurological diseases [6]. As with rodents, proper cholinergic functioning in developing zebrafish is critical for normal development of the nervous system [6]. Other transmitter systems important for behavioral function such as the monoamines, dopamine, norepinepherine and serotonin are also fully present in zebrafish [15,21,30,46]. Zebrafish have an extensive behavioral repertoire and will learn spatial and color discrimination [5,9,23,24,27,28,45]. Zebrafish models of the neurobehavioral teratology could be particularly relevant and represent an important complementary model, which together with rodent models could help elucidate the mechanistic bases for neurotoxicant-induced behavioral impairment.
Our laboratory has developed methods for assessing behavioral functioning in adult zebrafish. Spatial discrimination learning can be effectively assessed in a three-chambered task [24,27,28]. This method is particularly effective at differentiating response latency from choice accuracy. With this task we have shown that adult zebrafish which were developmentally exposed to CPF (10 or 100 ng/ml on days 0–5 post-fertilization), exhibited persisting defects in both spatial discrimination and response latency [27]. With response latency, there was a biphasic effect with the 10 ng/ml significantly increasing latency and the 100 ng/ml exposure significantly decreasing it. This study was important in demonstrating persisting neurobehavioral effects after early development; however, the delayed spatial alternation procedure is quite labor intensive and the assessment takes considerable time to complete. Higher throughput behavioral tests sensitive to developmental neurotoxic effects are needed.
To increase the throughput we have developed an automated assessment of startle response and its habituation. Startle response was chosen because it provides a quick measure of sensory and motor integration and shows a rapid habituation curve with repeated trials, which gives information concerning neuroplasticity. Startle response and its adaptations in rodents has very well characterized neural mechanisms and is a sensitive indicator of pharmacological and toxicological treatments [20,42]. In particular postnatal exposure of rats to the OP pesticide parathion was shown in our earlier study to significantly decrease startle response [43]. In zebrafish startle response and habituation have been studied in embryos [7,8,44]. Developmental mercury exposure was found to increase startle reactivity [44]. We developed a startle response test for adult zebrafish to assess the persisting effects of developmental toxicant exposure. We found that ten trials at the 1 min interval provided a rapid but reliable measure of startle response and its habituation. The hypothesis of this study was that the automated characterization of startle response in zebrafish would provide a rapid and sensitive indicator of persisting neurobehavioral impairment caused by early developmental exposure to chlorpyrifos at a dose that previously had been shown to cause a learning impairment [27]. The benchmark 0.29 μM dose (100 ng/ml) of CPF was chosen because it had been previously shown to cause marked impairment in memory function without causing increases in mortality or overt dismorphogenesis. CPF inhibits acetylcholinesterase, which provides indirect agonist effects at nicotinic and muscarinic acetylcholine receptors. Therefore, we tested the effects of developmental exposure to direct agonists at these receptors to determine whether one or the other receptor type was the substrate for the CPF effect on startle. Effects of developmental exposure to nicotine and pilocarpine were tested at doses subthreshold for causing dysmorphogenesis and lethality in embryos. Finally, the effects of developmental CPF exposure on the monoaminergic neurotransmitters dopamine, norepinepherine and serotonin were tested both immediately after exposure (day 6) and long after exposure (approximately 20 weeks) since monoaminergic systems have been shown to be affected by developmental CPF exposure in rats [1,4,18,36–38]. This neurochemical analysis was conducted to determine the possible involvement of these transmitters in the neurobehavioral impairments.
2. Methods
2.1. Design
This set of experiments examined the persisting effects of early developmental exposure (0–5 dfp) to CPF on later (2 months of age) neurobehavioral function (startle response). Nicotine and pilocarpine exposures were used to determine the contribution of nicotinic and muscarinic cholinergic receptor systems to the CPF-induced effects since CPF indirectly simulates both of these receptor types by inhibiting acetylcholinesterase and increasing the persistence of acetylcholine in the synapse. CPF effects on the monoamine neurotransmitters (dopamine, norepinepherine and serotonin) were examined because of the demonstration of the CPF effects on these transmitters in rodent models and the important involvement of these transmitters in neurobehavioral development.
| Event | CPF, nicotine or pilocarpine exposure | Neurotransmitter analysis | Startle response testing | Neurotransmitter analysis |
| Age | 0–5 dpf | 6 dpf | 20 weeks of age | >22 weeks of age |
2.2. Subjects
The experimental protocol was approved by the Duke University Institutional Committee for the use of animal subjects. Embryonic to larval and adult zebrafish (Danio rerio) were used for the current study. Zebrafish embryos were collected at the beginning of the 14-h light cycle on the morning following pairing of AB* strain adult breeders. Embryos were viewed under a dissecting scope, all appeared healthy and were between the two and four cell stage.
2.3. Chemical treatments
Selected embryos were separated into five treatment groups: 0.29 μM CPF (O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate) (≥98% pure, Chem Service, West Chester, PA)/DMSO (100 ng/ml), 15 μM nicotine hydrogen tartrate (≥98% pure, Sigma, St. Louis, MO)/DMSO, 25 μM nicotine/DMSO, 100 μM pilocarpine HCl (≥98% pure, Sigma, St. Louis, MO)/DMSO, 1 mM pilocarpine/DMSO and control (0.1% DMSO). From our experience with pilot experiments we chose concentrations that did not increase embryonic fatalities or malformations above control rates. Increased rates of death or overt malformations of the embryos were not seen with the doses of the exposure chemicals used. Treatment was started within 2 h post-fertilization (designated day 0). Each treatment group of approximately 30–50 embryos was kept in a total volume of 25 ml of egg water (60 mg/ml Instant Ocean, Spectrum Brands, Atlanta, GA, USA) including DMSO with or without CPF mixed to above dilutions in an incubator set at 28.5 °C. CPF dilutions or vehicle were changed daily with exposure ending on Day 5 pf (post-fertilization). On Day 3 pf, 30 hatched larvae were selected (10 from each treatment condition), placed in 250 ml beakers, and returned to the 28.5 °C incubator. No apparent growth differences were observed. Beginning on Day 5, zebrafish larvae were all transferred from treatment solution to 150 ml of egg water. Then they were moved to tanks in the flow-through chambers at 2 weeks of age. All treatments were performed on the first five days of development even though the behavioral assessments were done in adulthood. This is the initial study of a series in which we will in the future identify the critical window of exposure.
Adult zebrafish (D. rerio) were kept at approximately 28.5 °C on a 12:12-hour light\dark cycle. Behavioral testing of drug effects took place during the light phase between 8:00 AM and 5:00 PM. The tank water used de-ionized H2O and sea salts (Instant Ocean, 1.2 g/20 l of water). The tanks with the groups of adult fish were maintained with constant filtration and aeration. Fish were fed daily with lab grown brine shrimp and flake fish food (TetraMin, Blacksburg, VA).
2.4. Behavioral assessment
Startle responses were determined in adult zebrafish that were developmentally exposed to CPF, nicotine, or pilocarpine. Fish were tested in groups of eight unless noted otherwise. Subjects were brought from the colony room to the test room in their home tanks. In the test room they were netted and placed in test arenas where they were left for a ten-minute acclimatization before testing (introduction to the test arena produced 1 or 2 min of rapid swimming, followed by a stable pattern of swim bouts).
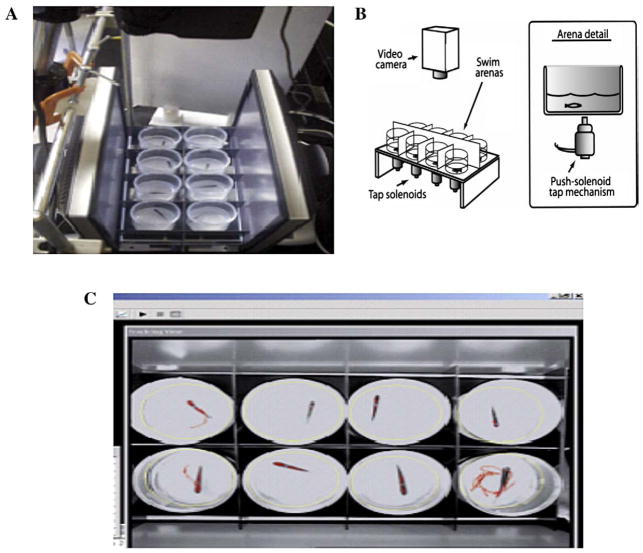
The experimental apparatus is illustrated in Fig. 1. Eight fish were simultaneously tested in a 2×4 array of swim arenas. Arenas were 60 mm in diameter and 90 mm tall, filled with system water to a height of 20 mm. As seen in panel 1B, below each arena was a centrally located 24-volt DC push solenoid that provided a sudden tap when activated. Opaque-white screens separated the arenas so that subjects were isolated from each other and from the experimenter’s movements (outer screens are not shown). The sides of the custom-built arenas were angled slightly to all be directed toward the lens of the camera so that the walls did not provide a visual barrier to the camera; however, the floors of all the arenas were horizontal.
Fig. 1.
Diagram of the zebrafish startle testing method. A) Top view showing the orientation of the vibrational startle apparatus. This fish are tested in eight cylindrical chambers arranged between computer monitors (monitors are use to elicit visual startle). B) Schematic diagram illustrating the testing chambers above that tap solenoids which are computer driven to deliver a vibrational startle at 1 min intervals (ten total trials) and at 8, 32, and 128 min after the initial session trial. C) View of the computer screen showing the video tracking of eight zebrafish during the startle task.
A digital-video camera (Sony 8 mm cassette recorder) was centrally positioned above the arena display, 75 cm above the water level. Fluorescent ceiling lights provided light for video recording. The video output from the camera was fed to a computer running tracking software (Noldus EthoVision™). The location of each fish was determined six times per second. Timing of experimental events was done with the tracking software that sent logic pulses at scheduled times to a second experiment–control computer via a parallel port connection. Software running in the control computer was used to activate the eight-solenoid battery (using solid-state relays). Event timing by the control computer was done with the multimedia hardware clock. Motor startle responses were assessed for 10 trials with 1 min intertrial intervals to determine the initial startle response and habituation with repeated testing. Further trials at 8, 32 and 128 min (intertrial intervals were 8, 24 and 96 min) after the end of the ten-trial sequence were to measure reestablishment of the startle response without closely spaced trials. The dependent measure for tap-elicited swimming was swim rate (distance/time) for 5 s following each tap. In pilot tests for development of the paradigm we found that the integrated measure of activity for 5 s after the startling stimulus provided a stable and sensitive measure. The responses were log-transformed to normalize the data for statistical analysis.
2.5. Neurochemistry
For immediate effects of CPF exposure on, 0–5 days post-fertilization, neurochemical levels (six days post-fertilization), larvae were grouped approximately 50/group. The larvae from both CPF-exposed and control groups used for the neurochemical analyses were alive and swimming. Any dead or obviously deformed embryos were removed. No CPF treatment differences in mortality or overt deformity were seen. The tissue harvest was conducted during the light phase of the diurnal cycle. The embryos were poured through a screen to remove aquarium water. The larvae were rapidly killed by the homogenization in perchlorate to preserve accurate neurotransmitter levels. They were weighed and placed in 10× vol/weight homogenization solution (one part perchloric acid (0.1 N) to ten parts mobile phase). The larvae were than homogenized with an ultrasonic homogenizer. After column purification samples were diluted in mobile phase (1:10) and 20 μl were analyzed for monoamine levels.
For adult fish neurochemical levels were assayed at least two weeks after completion of the startle habituation task. Specifically, fish were anesthetized by submersion in 4 °C aquarium water and euthanized by decapitation. The brains were rapidly excised and homogenized 25× volume per weight in homogenization solution. After column purification samples were diluted in mobile (1:10) and 20 μl were analyzed for monoamine levels.
The HPLC system used consisted of an isocratic pump (model LC1120, GBC Separations), a Rheodyne injector (model 7725i) with a 20 μl PEEK loop, and an INTRO amperometric detector (Antec Leyden). The electrochemical flow cell (model VT 03, Antec Leyden) had a 3 mm glassy carbon working electrode with a 25 μm spacer, and a Ag/AgCl reference electrode. The cell potential was set at 700 mV. The signal was filtered with a low pass in-line noise killer, LINK (Antec Leyden) set at a 14 s peak width and a cut off frequency of 0.086 Hz. The signal is integrated using the EZChrom elite chromatography software (Scientific Software Inc). The injector, flow cell, and analytical column were placed in the Faraday shielded compartment of the detector where the temperature is maintained at 30 °C. The stationary phase was a reverse phase BDS Hypersil C18 column 100 mm × 2.1 mm, with 5 μm particle size and 120 Å pore size (Keystone Scientific). The mobile phase was 50 mM H3PO4, 50 mM citric acid, 100 mg/l 1-octanesulfonic acid (sodium salt), 40 mg/l EDTA, 2 mM KCl and 3% methanol, corrected to pH 3.0 with NaOH. The mobile phase was continually degassed with a Degasys Populaire on-line degasser (Sanwa Tsusho Co. Ltd.) and delivered at a flow rate of 0.26 ml/min. The limit of quantitation was approximately 1.56 pg/mg tissue. The limit of detection was approximately 1.07 pg/mg tissue. There were external standards run with the analyses. The standard curve was run for concentrations of 2.5, 10, 40 and 160 pg/20 μl.
2.6. Statistical analysis
The statistical model was a mixed design analysis of variance with the between subjects factor of developmental exposure and the within subjects factor of repeated trials on the startle test. A main effect of treatment (CPF, nicotine and pilocarpine) indicated that the treatment condition had an effect on response. When there was more than a single treatment dose as in the nicotine and pilocarpine experiments, planned comparisons of controls vs. each of the treatment doses were necessary to determine which of the doses caused significant changes. The swimming activity data were log-transformed for analysis as a standard way to make activity data more normal in distribution to make it more appropriate for the analysis of variance. Interactions of the treatment factors and the repeated measure of trials were assessed.
The main effect of trial was analyzed to determine if there was habituation that reduced startle response with repeated trials. The linear trend (slope) of decreasing startle response over the consecutive trials was analyzed to determine exposure effects on habituation. The linear trend analysis is a statistical evaluation of the slope of the line across trials being non-zero, that is, whether the effect changes in a consistent direction with repeated testing. This shows that the test of startle habituation was successful, and important internal validation of the test. The treatment × trial interactions tested whether the effect of the treatment significantly differed across trials. With the adult behavior and neurochemistry each fish has a separate observation. The embryos were assayed for neurochemistry as batches of approximately 50. Batch was considered an observation for the embryos. Each neurochemical analyte was separately analyzed. The between subjects factor was chlorpyrifos exposure and the within subjects factors were minute and trial of testing. Repeated measures consisted of the 10 trials of the startle habituation phase. The linear trend of habituation was assessed within the analysis of variance over the 10 trials [22]. Further startle trials were given at 8, 32 and 128-minute intervals after the end of the 10-trial habituation phase. The intertrial intervals were 8, 24 and 96 min. A value of p<0.05 was used as the threshold for significance. For interaction terms at p<0.1, values were separated according to the interactive factors and the data reexamined for lower-order main effects as recommended by Snedecor and Cochran [40]; that is, the p<0.1 criterion for interactions was not defined as significant, but rather was used to trigger the lower-order testing. For all the final analyses the threshold for significance of the factor tested was p<0.05.
3. Results
3.1. Effects of chlopyrifos on startle response
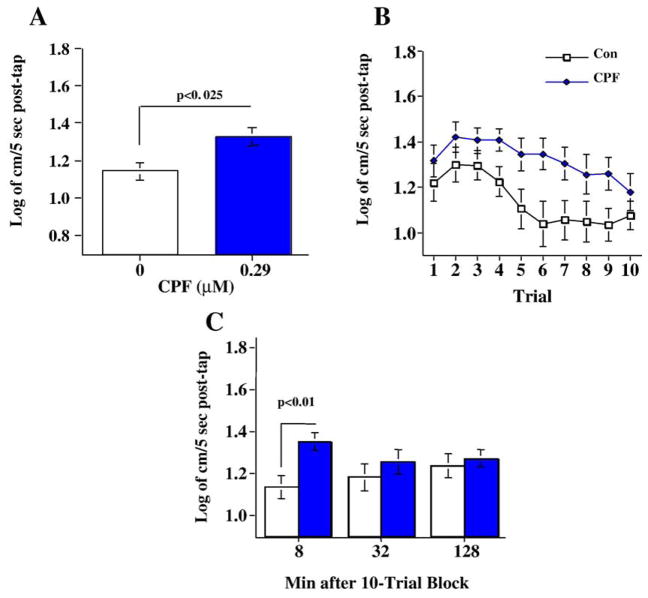
Persisting neurobehavioral defects as a result of CPF exposures were determined by assessing startle responses in adult zebrafish (20 weeks) that had been exposed to CPF from days 0–5 post-fertilization (group sizes: Control N=40, CPF N=24). Developmental chlorpyrifos exposure caused a significant main effect of (F(1,62)=6.44, p<0.025) overall increase in startle response in adult fish (Fig. 2A). There was a significant main effect of trial (F(9,558)=3.24, p<0.001) with a significant (p<0.0001) linear trend of decreasing response with repeated trials (Fig. 2B). The interaction of chlorpyrifos × trial was not significant (p=0.81). Following the ten-trial startle response sequence the fish were retested for startle response after delays of 8, 32 and 128 min. The chlorpyrifos exposure × delay interaction (F(2,124)=2.61, p<0.08) led to tests of the simple main effects of chlorpyrifos at each of the delays. The simple main effect tests showed that the chlorpyrifos-exposed fish had a significantly greater startle response with the 8 min delay (F(1,62)=7.64, p<0.01), while at the 32 and 128 time points there was no significant difference (Fig. 2C).
Fig. 2.
Developmental chlorpyrifos effects on A) startle response and habituation over ten trials (mean±sem). B) Comparison of developmental nicotine exposure with developmental chlorpyrifos exposure on average startle reaction over 10 trials (mean±sem). C) Developmental chlorpyrifos exposure effects on startle response 8, 32 and 128 min after the 10-trial habituation sequence (mean±sem) (group sizes: Control N=40, CPF N=24).
3.2. Effects of nicotine on startle response
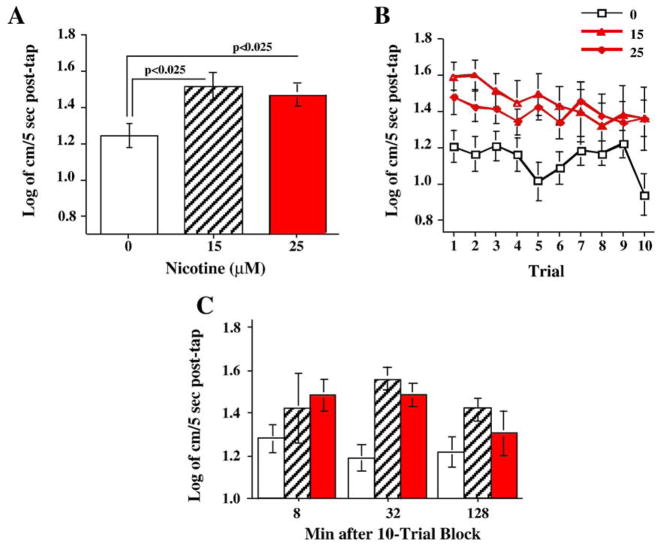
To access the contribution of nicotinic ACh receptors to developmental CPF neurotoxicity, zebrafish larvae were exposed to nicotine on days 0–5 post-fertilization (group sizes: Control N=32, 15 μM N=12, 25 μM N=20) and the resultant effects on startle responses were determined at 20 weeks. Developmental nicotine exposure (15 and 25 μM) caused a significant main effect of (F(1,61)=4.58, p<0.025) overall increase in startle response in adult fish (Fig. 3A). Both nicotine doses significantly (p<0.025) increased average startle responses across the ten trials. There was a significant main effect of trial (F(9,549)=2.45, p<0.01) with a significant (p<0.0005) linear trend of decreasing response with repeated trials (Fig. 3B). The interaction of nicotine × trial was not significant (p=0.27). During the period after the ten-trial sequence the fish were retested for startle response with 8, 32 and 128 min later. The nicotine exposure main effect was significant (F(2,61)=4.35, p<0.025). Both nicotine doses significantly (p<0.05) increased average startle responses across all delays. There was no significant interaction of nicotine exposure and delay (Fig. 3C).
Fig. 3.
Developmental nicotine effects on A) startle response and habituation over ten trials (mean±sem). B) Comparison of developmental nicotine exposure on average startle reaction over 10 trials (mean±sem). C) Developmental nicotine exposure effects on startle response 8, 32 and 128 min after the 10-trial habituation sequence (mean±sem) (group sizes: Control N=32, 15 μM N=12, 25 μM N=20).
3.3. Effects of pilocarpine on startle response
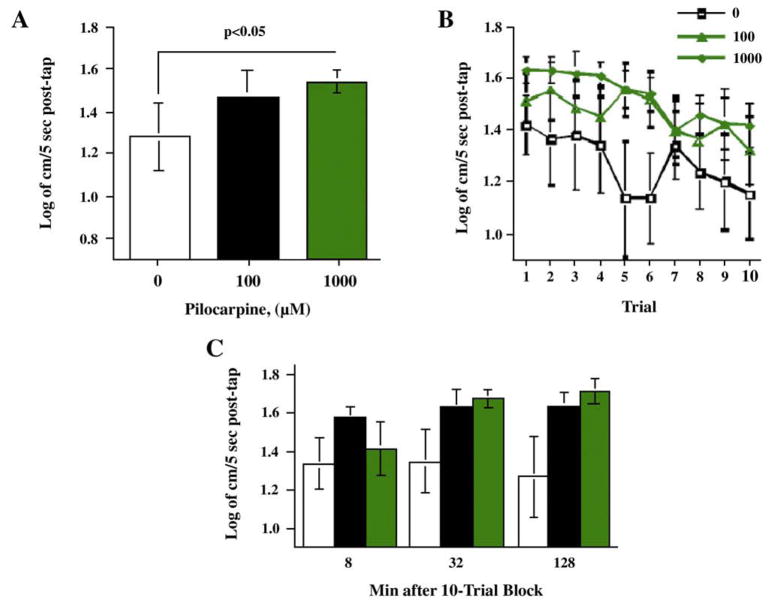
In Fig. 4A, there was a significant main effect of trial (F(9,279)=4.33, p<0.0001) with a significant (p<0.0001) linear trend of decreasing response with repeated trials. The interaction of pilocarpine × trial was not significant. After the ten-trial sequence the fish were retested for startle response with 8, 32 and 128 min later. The developmental pilocarpine exposure did not have a significant effect during this test phase.
Fig. 4.
Developmental pilocarpine effects on A) startle response and habituation over ten trials (mean±sem). B) Comparison of developmental pilocarpine exposure on average startle reaction over 10 trials. (mean±sem). C) Developmental pilocarpine exposure effects on startle response 8, 32 and 128 min after the 10-trial habituation sequence (mean± sem) (group sizes: Control N=20, 100 μM N=16, 1000 μM N=12).
3.4. Effect of chlorpyrifos on neurochemical levels
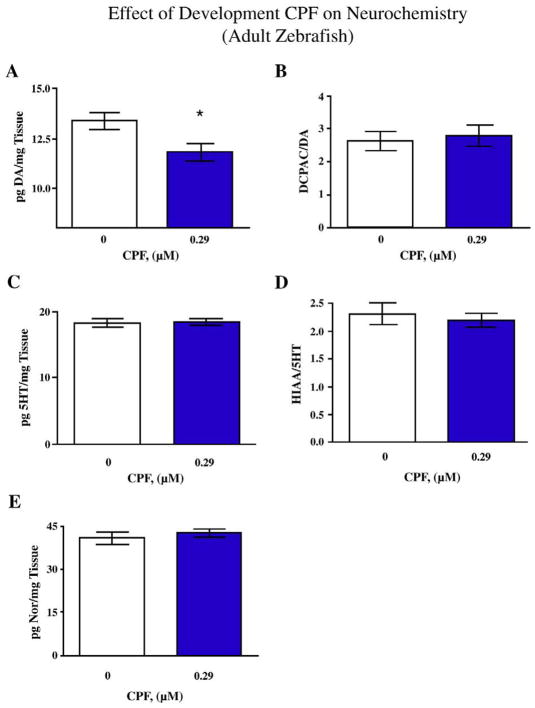
After the completion of the behavioral task the adult fish derived from 0–5 dpf (days post-fertilization) exposures were euthanized and whole brain neurochemical levels were determined. Dopamine (DA) levels were significantly (F(1,25)=7.08, p<0.025) decreased in CPF-exposed zebrafish compared to DMSO-exposed controls (Fig. 5A). There were no significant differences in the levels of the DA metabolite DOPAC (not shown) or the DA turnover (Fig. 5B). The levels of whole brain serotonin (5HT) or the 5HT metabolite 5HIAA did not significantly differ between DMSO-exposed and CPF-exposed fish (Fig. 5C and D). Lastly there were no significant differences in the levels of norepinephrine (NE) between DMSO-exposed and CPF-exposed fish (Fig. 5E).
Fig. 5.
Effects of developmental CPF exposure on neurochemical measures in the adult zebrafish brain. A) Effect of CPF on DA levels *p<0.025 CPF vs. Control, B) DOPAC/DA ratios, C) 5HT levels, D) 5HIAA/5HT ratios, and E) Norepinephrine levels. (mean±sem), (group sizes: Control N=14, CPF N=13).
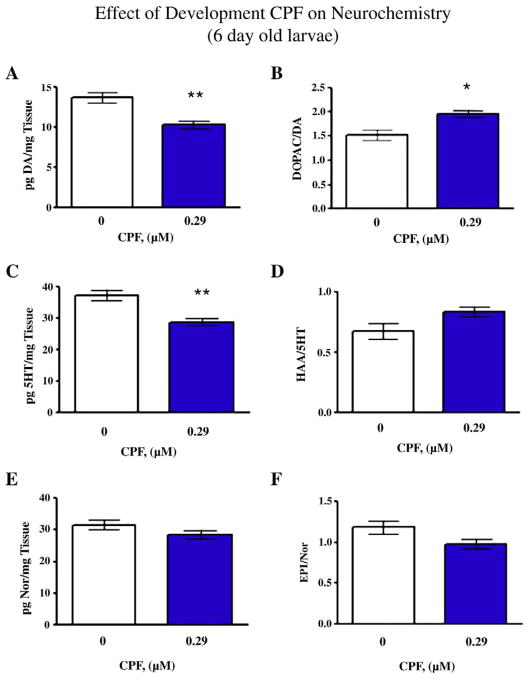
Developmental CPF (i.e. 0–5 dpf exposure and tissue extraction and HPLC at 6 dpf) caused a global decrease in catecholamine levels in zebrafish larvae. Fig. 6A shows that DA levels were decreased to approximately 66% of control levels in CPF-exposed larvae compared to DMSO controls (F(1,6)=16.36, p<0.01). There were no significant differences in DOPAC levels between CPF-exposed larvae and DMSO controls (not shown). However there is a significant (F(1,6)=10.98, p<0.025) increase in DA turnover in CPF-exposed larvae compared to DMSO controls (Fig. 6B). Serotonin levels were significantly (F(1,6)= 16.26, p<0.01) decreased in CPF-exposed larvae (Fig. 6C) and 5HT turnover was not significantly increased (Fig. 6D). Lastly, NE levels were not significantly changed in CPF-exposed larvae (Fig. 6E) and NE turnover was not significantly changed compared to DMSO-exposed controls (Fig. 6F).
Fig. 6.
Effects of developmental CPF exposure on neurochemical measures in the larvae (6 days post-fertilization). A) Effect of CPF on DA levels **p<0.01 CPF vs. Control, B) DOPAC/DA ratios *p<0.025 CPF vs. Control, C) 5HT levels **p<0.01 CPF vs. Control, D) 5HIAA/5HT ratios, E) Norepinephrine levels and E/NE ratio (mean±sem), N=4 batches of 50 embryos/exposure group.
4. Discussion
These results demonstrated that persisting behavioral impairments caused by early developmental exposure to low levels of CPF during first five days after fertilization can be detected in a simple, rapid test of vibrational startle response (Fig. 1). Specifically, developmental CPF exposure caused a significant increase in startle responses in adult zebrafish during the first ten repetitions of the startling stimulus, and the CPF-exposed fish continued to exhibit increased startle response 8 min later (Fig. 2). Developmental exposure to nicotine also caused long-lasting increased startle response (Fig. 3). Developmental exposure to the muscarinic agonist pilocarpine caused a very similar effect as nicotine on startle responses, albeit at much higher doses (Fig. 4). The data also showed that developmental CPF exposure significantly decreased basal levels of dopamine (DA) and serotonin (5HT) and significantly increased transmitter turnover of these transmitters in zebrafish larvae and caused long-term suppression of dopamine levels in the adult zebrafish brain after developmental exposure.
This study showed that early developmental CPF exposure has robust and persisting effects of enhancing startle response of adult zebrafish. CPF exposure at this level (0.29 mM) has been shown in our earlier study to inhibit acetylcholinesterase in zebrafish [29]. This would increase acetylcholine levels causing greater net stimulation of nicotinic and muscarinic cholinergic receptors. This study also found that early developmental exposure to the direct nicotinic and muscarinic agonists, nicotine and pilocarpine, caused similar increases in startle response. However, CPF (0.29 μM) was far more potent at increasing startle responses than either nicotine (15 μM and 25 μM) or pilocarpine (100 μM and 1 mM), suggesting either a pronounced synergistic interaction between nicotinic and muscarinic systems mediating the effects of developmental CPF exposure or involvement of additional non-cholinergic mechanisms in the neurotoxic actions of CPF. These may involve neural replication and differentiation effects as well as cascading effects on other transmitter systems such as the monoamines, dopamine, norepinepherine and serotonin and amino acid transmitters GABA and glutamate.
There was little if any apparent dose–response relationship of developmental (0–5 dpf) nicotine and pilocarpine treatment with regard to startle response. With developmental nicotine exposure the 25 μM dose was not significantly greater in effect than the 15 μM dose in potentiating startle responses. Both caused significantly greater responses than control. This may be due to a ceiling effect in the magnitude of response obtainable or to insufficient dose separation. With pilocarpine the higher dose had a significantly greater response relative to control whereas the lower dose did not; however, there was no significant difference between the response levels with the two pilocarpine doses.
The relative doses of nicotine and pilocarpine needed to alter startle responses (15 μM vs. 1 mM) were quite different. One interpretation of this observation could be that nicotinic systems are more important for the development of neural circuits involved in the startle response and for the persistent effects of developmental CPF exposure. However, differences in binding constant of the two compounds to their respective receptors, and/or differences in the pharmacokinetics of the compounds to get to their molecular targets in zebrafish embryos could also explain this concentration difference in effect dose.
A behavioral interpretation of the startle response data is that developmental CPF exposure induces hyperactivity in adult fish in response to a sensory startle. Developmental CPF exposure has been shown to induce hyperactivity in rats. When administered during neurogenesis (gestational days 17–20) CPF caused an initial hyperactivity response in rats as assessed in the T-maze task [26]. Zebrafish exposed to 100 ng/ml CPF showed a significant increase in response latency in a three-chambered task that test spatial discrimination learning [27]. In the current study we again tested the hyperactivity in CPF-exposed fish in a startle task that focuses on motor responsiveness without the response choice aspect assessed in the three-chambered spatial discrimination task.
Developmental CPF exposure had significant effects on neurochemical levels in larval and adult zebrafish. In the larvae these effects were more pervasive with decreases seen in DA, and 5HT levels (Fig. 6). Additionally there were significant increases in larval DA and 5HT turnover (Fig. 6). In the adult zebrafish the effect of developmental CPF on neurochemistry was restricted to a decrease in DA content (Fig. 5). Rodent models have also shown effects of developmental CPF exposure on neurochemical levels/metabolism at adolescent and adult time points [1,4,12,19,31,39]. Neonatal CPF treatment in rats decreased cortical and hippocampal DA levels in adulthood [4,39]. Rats also show developmental CPF-induced changes in 5HT systems [4]. CPF doses, which decreased cholinesterase activity significantly decreased 5HT levels [4]. These findings were similar to the current effects seen in zebrafish. However, the effects of CPF on 5HT levels were not sustained as it is in rodents.
Spieler et al. [41] found that the circadian changes in neurotransmitters in fathead minnows were altered by lead exposure. It is not immediately clear how this translates to zebrafish. But it is quite likely that zebrafish like other species also have circadian rhythms in their neurotransmitter levels and that these can be affected by toxicant exposure. In the current study, the fish were all killed and their brains were removed during the midst of the light phase of the diurnal cycle. The fish with different exposures were killed in an interspersed pattern so there would not have been a confound of circadian changes with treatment effects. This study showing persisting CPF effects on dopamine levels provides a basis for future work to determine the detailed changes in circadian cycling of this transmitter.
In this study we found persisting behavioral defects in adult zebrafish after early developmental CPF exposure. This was accompanied by persisting CPF-induced depletions of DA. Depleting DA during neurodevelopment has been shown to induce the expression of supersensitive DA receptors [16]. The expression of supersensitive receptors is a long-lasting event with expression of levels remaining elevated long after the initial depletion of DA. Decreased DA levels in the brains of adult fish could be important for long-term behavioral defect, though further research is needed to determine the mechanistic importance of DA depletion for the increased startle response in the CPF-exposed fish.
The current study assayed the effects of developmental CPF exposure on startle response, startle habituation, and the recovery from habituation. This and other studies highlight the utility of using zebrafish as a complimentary animal model in the fields of pharmacology and toxicology. This rapid startle response neurobehavioral assay can be useful for rapid assessment of the functional effects of a broad array of potential neurotoxic compounds. This is the initial study of a series in which we will in the future identify the critical window of exposure. The more rapid throughput computerized startle response assessment in zebrafish may prove valuable for thoroughly assessing the critical windows of developmental CPF exposure as well as other toxicants.
Acknowledgments
We thank D. Wassenberg, L. Upchurch, S. Donerly, and J. Yen for providing information about exposure concentrations, conditions and providing fish for this study. Research Support by Duke University Superfund Basic Research Center (NIH ES10356).
Footnotes
Conflict of interest
Nothing declared.
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environmental Health Perspectives. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environmental Health Perspectives. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environmental Health Perspectives. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environmental Health Perspectives. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur D, Levin ED. Spatial and non-spatial discrimination learning in zebrafish (Danio rerio) Animal Cognition. 2001;4:125–131. [Google Scholar]
- 6.Best JD, Alderton WK. Zebrafish: an in vivo model for the study of neurological diseases. Neuropsychiatric Disease and Treatment. 2008;4:567–576. doi: 10.2147/ndt.s2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Best JD, Berghmans S, Hunt JJ, Clarke SC, Fleming A, Goldsmith P, Roach AG. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- 8.Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. Journal of Neuroscience. 2007;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behavioural Processes. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Developmental Brain Research. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- 11.Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Developmental Brain Research. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 12.Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos releases norepinephrine from adult and neonatal rat brain synaptosomes. Developmental Brain Research. 1999;118:129–133. doi: 10.1016/s0165-3806(99)00139-x. [DOI] [PubMed] [Google Scholar]
- 13.Das KP, Barone S., Jr Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicology and Applied Pharmacology. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- 14.DowAgroSciences. 2008;2008 www.dowagro.com/chlorp/na/about/
- 15.Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid JRBE. Development of the locomotor network in zebrafish. Progress in Neurobiology. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. Journal of Neuroscience. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicology & Teratology. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DE, Seidler FJ, Slotkin TA. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chlorpyrifos. Brain Research Bulletin. 1998;45:143–147. doi: 10.1016/s0361-9230(97)00329-8. [DOI] [PubMed] [Google Scholar]
- 19.Karen DJ, Li W, Harp PR, Gillette JS, Bloomquist JR. Striatal dopaminergic pathways as a target for the insecticides permethrin and chlorpyrifos. Neurotoxicology. 2001;22:811–817. doi: 10.1016/s0161-813x(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 20.Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Experimental and Toxicologic Pathology. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- 21.Kaslin J, Panula P. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio) Journal of Comparative Neurology. 2001;440:342–377. doi: 10.1002/cne.1390. [DOI] [PubMed] [Google Scholar]
- 22.Keppel G. Design and Analysis: a Researcher’s Handbook. 2. Prentice-Hall, Inc; Englewood Cliffs, New Jersey: 1982. p. 699. [Google Scholar]
- 23.Levin ED, Cerutti DT. Behavioral neuroscience of zebrafish. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; New York: 2008. [PubMed] [Google Scholar]
- 24.Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicology and Teratology. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Developmental Brain Research. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 26.Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicology and Teratology. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 27.Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicology & Teratology. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 28.Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- 29.Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicology & Teratology. 2004;26:709–718. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 30.McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. Journal of Comparative Neurology. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- 31.Moreno M, Cañadas F, Cardona D, Suñol C, Campa L, Sánchez-Amate MC, Flores P, Sanchez-Santed F. Long-term monoamine changes in the striatum and nucleus accumbens after acute chlorpyrifos exposure. Toxicology Letters. 2008;176:162–167. doi: 10.1016/j.toxlet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Scalzo FM, Levin ED. The use of zebrafish (Danio rerio) as a model system in neurobehavioral toxicology. Neurotoxicology & Teratology. 2004;26:707–708. doi: 10.1016/j.ntt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Scholz S, Fischer S, Gündel U, Küster E, Luckenbach T, Voelker D. The zebrafish embryo model in environmental risk assessment—applications beyond acute toxicity testing. Environmental Science and Pollution Research International. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- 34.Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environmental Health Perspectives. 1999;107(Suppl 1):65–69. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, and organophosphates. Toxicology and Applied Pharmacology. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Developmental Brain Research. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reproductive Toxicology. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Developmental Brain Research. 2002;287:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- 39.Slotkin TA, Freibaum BD, Tate CA, Thillai I, Ferguson SA, Cada AM, Seidler FJ. Long-lasting CNS effects of a short-term chemical knockout of ornithine decarboxylase during development: nicotinic cholinergic receptor upregulation and subtle macromolecular changes in adulthood. Brain Research. 2003;981:118–125. doi: 10.1016/s0006-8993(03)02993-7. [DOI] [PubMed] [Google Scholar]
- 40.Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; Ames, Iowa: 1967. [Google Scholar]
- 41.Spieler RE, Russo AC, Weber DN. Waterborne lead affects circadian variations of brain neurotransmitters in fathead minnows. Bulletin of Environmental Contamination and Toxicology. 1995;55:412–418. doi: 10.1007/BF00206680. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 43.Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Research Bulletin. 2008;30:38–45. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber DN. Dose-dependent effects of developmental mercury exposure on C-start escape responses of larval zebrafish Danio rerio. Journal of Fish Biology. 2006;69:65–94. [Google Scholar]
- 45.Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behavioural Processes. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 46.Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. Journal of Comparative Neurology. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]