Abstract
Lipolysis of white adipose tissue triacylglycerol stores results in the liberation of glycerol and nonesterified fatty acids that are released into the vasculature for use by other organs as energy substrates. In response to changes in nutritional state, lipolysis rates are precisely regulated through hormonal and biochemical signals. These signals modulate the activity of lipolytic enzymes and accessory proteins, allowing for maximal responsiveness of adipose tissue to changes in energy requirements and availability. Recently, a number of novel adipocyte triacylglyceride lipases have been identified, including desnutrin/ATGL, greatly expanding our understanding of adipocyte lipolysis. We have also begun to better appreciate the role of a number of nonenzymatic proteins that are critical to triacylglyceride breakdown. This review provides an overview of key mediators of lipolysis and the regulation of this process by changes in nutritional status and nutrient intakes.
Keywords: adipocyte, lipolysis, triacylglyceride lipase, perilipin, desnutrin/ATGL, hormone-sensitive lipase
INTRODUCTION
White adipose tissue (WAT) triacylglycerol (TAG) is the major energy reserve in higher eukaryotes. This lipid pool is in a constant state of flux, resulting from a largely futile cycle of lipolysis and re-esterification (55). During times of energy deprivation, WAT undergoes a shift toward greater net rates of lipolysis, which can be defined as the hydrolysis of TAG to generate fatty acids (FAs) and glycerol that are released into the vasculature for use by other organs as energy substrates. Lipolysis proceeds in an orderly and regulated manner, with different enzymes acting at each step. TAG is hydrolyzed sequentially to form diacylglycerol (DAG), then monoacylglycerol (MAG), with the liberation of a FA at each step. MAG is hydrolyzed to release the final FA and glycerol. The storage of energy reserves as TAG, and the ability to rapidly mobilize these reserves as FA to fuel energy demands, represents a highly adapted metabolic response. The liberation of FA from TAG by adipocyte lipolysis is also important to supply substrate for hepatic synthesis of very-low-density lipoproteins (VLDLs). Circulating FAs are a major source of substrate for the hepatic production of TAG-rich lipoproteins (34, 80), and impairment of adipose tissue lipolysis inhibits VLDL synthesis (44, 94, 134).
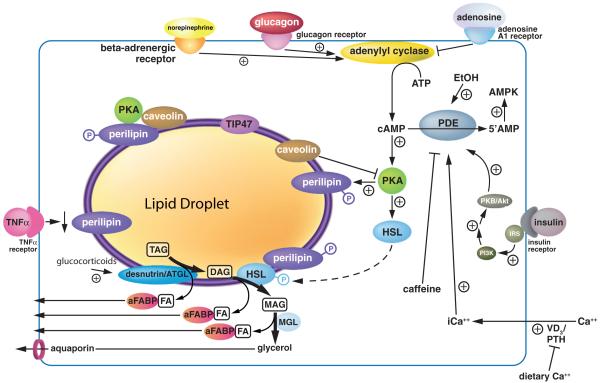
Alterations in lipolysis are frequently associated with obesity, including an increase in basal rates of lipolysis that may contribute to the development of insulin resistance, as well as an impaired responsiveness to stimulated lipolysis (65, 102). Obesity is characterized primarily by an excess of WAT and an enlargement in adipocyte size that results from increased TAG storage. Obesity has become a prevalent health problem due to its close association with a number of disorders, including type 2 diabetes, hypertension, and atherosclerosis (133). Here, we review adipocyte lipolysis and the major nutritional determinants controlling this process. Figure 1 provides an overview of the nutritional regulation of adipocyte lipolysis.
Figure 1.
Regulation of adipocyte lipolysis.
TRIACYLGLYCEROL HYDROLYSIS
Until recently, initiation of TAG hydrolysis in adipose tissue was believed to be controlled by hormone-sensitive lipase (HSL) (109). The generation of HSL-null mouse models, however, demonstrated clearly the existence of residual HSL-independent TAG lipase activity, suggesting the presence of previously unidentified adipocyte enzyme(s) with TAG hydrolase activity (44, 94, 134). In recent years, novel adipose tissue lipases have been identified and characterized. All are serine esterases that harbor a common structural element—the alpha/beta-hydrolase fold—and a conserved catalytic diad or triad that is composed of the GXSXG pentapeptide motif as well as an active aspartate or glutamate (D/E)GG tripeptide and an active histidine (H). Studies to determine the relative contribution of each enzyme to adipocyte lipolysis are ongoing.
Hormone-Sensitive Lipase
Adipose tissue HSL (E.C. 3.1.1.3) is an 84 kDa cytoplasmic protein with demonstrated activity against a wide variety of substrates including TAG, DAG, cholesteryl esters (CEs), and retinyl esters (51). Relative fatty acyl hydrolase activity of HSL in vitro is elevenfold greater against DAG than TAG, and twofold greater against CEs (51). HSL shows a preference for activity against fatty acids in the sn-1 or sn-3 position (99). Until recently, HSL was believed to be the primary enzyme responsible for virtually all TAG and DAG hydrolase activity in adipocytes, as well as neutral cholesteryl ester hydrolase (NCEH) activity. To address the functional role of HSL in vivo, several laboratories generated HSL-null mouse models (44, 94, 134). Although differences have been observed between these models, a great deal of insight has been gained regarding the relative contribution of HSL to adipocyte TAG hydrolysis. For instance, studies have demonstrated that although NCEH activity was indeed absent in HSL-null adipocytes, a substantial fraction of catecholamine stimulated lipolysis (90, 94), and most, if not all, basal lipolysis remained (30, 134). Moreover, the lipolytic response to extended fasting (>48 h) appeared to be normal in HSL-null mice that demonstrated adequate or even heightened mobilization and oxidation of FA (30). HSL-mediated lipolysis, however, did contribute an important component to adipose FA liberation. HSL-null mice displayed lower serum NEFA and TAG levels and reduced hepatic TAG storage, indicating that HSL-independent lipolysis was inadequate to maintain FA output from adipose tissue at levels that could meet normal demands for energy substrates and VLDL synthesis (45, 132).
Examination of the physiology of HSL-null mice offers additional insight into the relative importance of HSL in TAG hydrolysis in vivo. On a normal chow diet, HSL-null mice had body weights similar to wild-type mice (47), and adipocyte size was reportedly either similar or slightly larger (94)—a finding that is consistent with a role for HSL in adipocyte FA mobilization. However, WAT mass in these animals was either unchanged (94) or decreased in size (134). Furthermore, on a high-fat diet, HSL ablation was associated with a significant protective effect against the development of obesity (47). This result was unexpected, but could be explained by the finding that in the absence of HSL, adipocytes undergo a compensatory decrease in the re-esterification of FA that results in decreased resynthesis of TAG and increased liberation of FA to the vasculature (144). Although this compensatory response may have confounded measurement of the true contribution of HSL to adipose fatty acid mobilization, it is clear from a number of studies that HSL is not strictly required for the initiation of TAG hydrolysis. This fact is made even more evident by the finding that DAG, but not TAG, accumulated in adipocytes of HSL-null mice (44). The relative contribution of HSL to DAG lipolysis is further discussed below.
Desnutrin/Adipose Triglyceride Lipase
In 2004, using rat cDNA microarray analysis of adipocyte-specific genes, we identified and characterized a novel adipocyte TAG lipase that we called desnutrin (130). Desnutrin is a 54 kDa protein that contains an N-terminal patatin-like domain that is found in many plant acyl hydrolases and is characterized by a conserved serine in the GXSXG motif, an alpha/beta-hydrolase fold, a conserved aspartate belonging to the DX(G/A) motif, and a glycine-rich region (130). This same enzyme was subsequently identified by two other laboratories from database searches of proteins containing the conserved GXSXG pentapeptide motif and alpha/beta-hydrolase fold, and was named adipose triglyceride lipase (ATGL) (145) or iPLA2ζ (53, 145). Murine desnutrin/ATGL is found predominantly in adipose tissue, but it is also found at much lower levels in other tissues, notably cardiac and skeletal muscle and testis. When cells contain fat stores, such as differentiated 3T3-L1 cells (145) as well as HeLa cells grown in oleic acid–rich medium (115), desnutrin/ATGL is found both in the cytoplasm and tightly associated with the lipid droplet (115, 130, 145). In COS-7 cells that lack substantial lipid droplets, desnutrin/ATGL was found to be relatively homogenously distributed within the cytoplasm (130). Factors governing subcellular distribution of desnutrin/ATGL, including mechanisms by which the cytoplasmic enzyme accesses its substrate and potential translocation to the lipid droplet, remain to be determined.
Several lines of evidence indicate that desnutrin/ATGL is a TAG-specific lipase. We have shown that overexpression of desnutrin/ATGL in COS-7 cells increased FFA release to the medium, decreasing intracellular stores of TAG without affecting intracellular phospholipid stores (130). A similar effect on TAG level has also been reported in 293HEK cells (62), while expression of desnutrin/ATGL in 3T3-L1 adipocytes has been shown to increase both glycerol and FA release (144). In vitro, TAG hydrolase activity has been demonstrated for the enzyme purified by expression in Sf9 insect cells and 293HEK cells as well as COS-7 cells (53, 130, 145). The specificity of desnutrin/ATGL for TAG has also been investigated. Zimmermann et al. (144) prepared cytosolic extracts from HepG2 cells infected with adenoviral-desnutrin/ATGL and incubated them with radiolabeled triolein and diolein substrates. They found significantly higher TAG lipase activity (approximately six- to tenfold higher) than DAG lipase activity, indicating a primary role for the enzyme in catalyzing the first, rate-limiting step in lipolysis. This finding was supported by work in COS-7 cells demonstrating a 21-fold increased accumulation of DAG in cells transfected with desnutrin/ATGL compared with HSL, as well as a modest increase in MAG levels (144). Extracts from COS-7 cells transiently expressing desnutrin/ATGL had activity against cholesteryl esters and retinyl esters that was comparable to control values (144), further demonstrating the specific role for desnutrin/ATGL in TAG hydrolysis.
Nutritional regulation of desnutrin/ATGL further supports a role for this enzyme in the mobilization of TAG stores in response to increased energy demand. We have shown that desnutrin/ATGL is induced by fasting in mice and is suppressed by refeeding (130). Regulation of desnutrin/ATGL mRNA by glucocorticoids may explain this, since dexamethasone was found to strongly upregulate the enzyme in a concentration- and dose-dependent manner in 3T3-L1 preadipocytes (130). We have also found that desnutrin/ATGL is downregulated in ob/ob and db/db mice, further supporting a role for the enzyme in fat breakdown and suggesting a possible contributory role in the development of obesity (130).
The relative importance of desnutrin/ATGL in lipolysis is illustrated by studies of gene ablation and functional loss of the enzyme. siRNA directed against desnutrin/ATGL has been shown to significantly decrease the release of glycerol and FA from 3T3-L1 adipocytes (144), indicating impaired lipolysis that was not compensated by the presence of other lipases. In support of this, treatment of cytosolic extracts from mouse WAT and BAT with desnutrin/ATGL antibodies decreased FA release by approximately two-thirds (144). This decrease was more pronounced when adipocytes from HSL-null mice were utilized, a finding that suggests cooperativity exists between the two enzymes, and indeed, a synergistic effect has been observed on lipolysis when cells are cotransfected with both desnutrin/ATGL and HSL (144). Achievement of optimal rates of lipolysis, therefore, likely requires the expression of both acyl hydrolases. Global loss of desnutrin/ATGL gene function in mice resulted in increased weight gain and a shift in favor of carbohydrate over fat as a primary fuel source during fasting, indicating that in vivo desnutrin/ATGL likely also functions in adipose tissue lipolysis (43). Surprisingly, however, WAT fat pad weights were elevated by only approximately twofold, which suggests that other TAG lipases may be present that could partially compensate for loss of desnutrin/ATGL. Also surprising was the finding that loss of desnutrin/ATGL was associated with premature death. This resulted from ectopic storage of fat in the heart, where cardiomyocyte TAG levels were found to have increased twentyfold by 12 weeks of age. Ectopic fat storage was also evident in other tissues. This effect was observed despite a more favorable plasma lipid profile and increased insulin sensitivity. Total lipase activity was dramatically reduced in several tissues, including WAT and BAT, but also cardiac muscle, skeletal muscle, testis, and liver. This finding highlights the potential metabolic importance of intracellular TAG hydrolysis in tissues other than adipose tissue and indicates that desnutrin/ATGL may play a critical role in the liberation of FA in multiple tissues. Generation of conditional knockout models lacking desnutrin/ATGL in individual tissues will be required to verify this hypothesis.
Triacylglycerol Hydrolases
Soni et al. (116) were the first group to report discovery of a novel TAG lipase that may contribute to non-HSL-mediated TAG lipolysis in adipocytes. Others have subsequently confirmed the presence of triacylglycerol hydrolase (TGH; carboxylesterase 3; EC 3.1.1.1) in adipose tissue (10). TGH is a 60 kDa microsomal lipase that contains a catalytic triad with an active site serine located in the GXSXG motif (68). TGH displays activity against long-, medium-, and short-chain TAGs and has also been reported to hydrolyze neutral cholesteryl esters (88), but it lacks phospholipase or acyl-CoA thioesterase activity (69). The enzyme is expressed predominantly in liver, where it functions in mobilization of intracellular TAG stores and likely plays a critical role in synthesis of TAG-rich very-low-density lipoproteins (VLDLs) (39).
Recently, however, TGH has also been identified in other tissues, including kidney, heart, intestine, and adipose tissue (26). In 3T3-L1 cells, expression of TGH is upregulated tenfold upon differentiation of preadipocytes into adipocytes (25) due, at least in part, to transcriptional regulation by the adipogenic transcription factor C/EBPα (136). These findings suggest a functional role for TGH in the mature fat cell, and indeed, Soni and colleagues (116) have identified TGH as an adipocyte lipase using functional proteomics. In this study, they subjected infranatant and fat cake fractions prepared from mouse intra-abdominal WAT to oleic acid–linked agarose chromatography. One of the two major peaks of esterase activity eluted contained substantial lipase activity, and this fraction was also found to contain TGH. It is therefore likely that TGH contributes to adipose tissue lipolysis. However, additional molecular and genetic studies will be required to determine the relative contribution of this lipase to overall mobilization of FA from TAG in adipocytes.
Using a database search for serine esterases of the alpha/beta-hydrolase fold that also contain the GXSXG and His-Gly dipeptide motifs, Okazaki et al. (90) have uncovered a previously unannotated gene that is induced in 3T3-L1 cells during differentiation into adipocytes. This protein shares a high degree of sequence homology with TGH (>70%) as well as similar subcellular localization, and therefore was named TGH-2. Also similar to TGH, TGH-2 exhibits activity against mono- and tri- but not diolein, with a substantial preference for short-chain fatty acid TAG. TGH-2 was found to be expressed predominantly in liver but was also present in adipose tissue and kidney and was induced by fasting and inhibited by refeeding. Molecular studies utilizing siRNA directed against TGH-2 in 3T3-L1 adipocytes demonstrated a 10% decrease in isoproterenol-stimulated glycerol release, while TGH-2 overexpression was found to increase glycerol release by 20%. Further studies are required to determine the relative contribution of this novel lipase to basal lipolysis and to in vivo lipase activity in adipose tissue. However, these results suggest a role for TGH-2 in mobilization of stored TAG during times of increased energy demand.
Adiponutrin
Adiponutrin is a 45 kDa patatin domain–containing protein that is highly expressed primarily in adipose tissue (9). It shares a high degree of sequence homology with desnutrin, including positioning of the GXSXG and DGG active site motifs (9). Extracts from insect (53, 62) and mammalian cells (53, 62) transfected with adiponutrin possess functional TAG lipase activity that requires the active site serine (GXSXG motif) when assayed in vitro (62). However, overexpression of adiponutrin has no effect on TAG hydrolysis in 293 HEK cells, in contrast to desnutrin/ATGL and other patatin domain–containing proteins that increase TAG hydrolysis in these cells (62). Furthermore, unlike other known lipases, adiponutrin expression is dramatically upregulated in animals that have been refed following a fast, whereas adiponutrin mRNA is almost undetectable in fasted animals (9). Although desnutrin/ATGL mRNA is downregulated in obese rats (130), adiponutrin mRNA is induced 50-fold (9). This differential regulation of adiponutrin compared with other lipases suggests that in vivo this enzyme may serve a primary function other than lipolysis. In vitro, adiponutrin purified from insect cells has been shown also to have acyltransferase activity (53) consistent with an anabolic, rather than a catabolic, role in adipocyte metabolism. Clearly, additional work is required to clarify the role of this enzyme, if any, in adipocyte lipolysis.
GS2 and GS2-Like
GS2 was identified by Jenkins et al. (53) as a TAG lipase with a patatin homology domain that includes a combination of the G/AXGXXG and GXSXG motifs that are conserved in calcium-independent phospholipase A2 family members. In vitro assay has demonstrated triolein hydrolase activity for GS2 that exceeded activities for adiponutrin or desnutrin (53). GS2 lipase activity has subsequently been confirmed in keratinocytes (35) and in 293 HEK cells overexpressing the enzyme (62). Overexpression of the related protein GS2-Like in HEK 293 cells also results in decreased TAG storage, which suggests a functional role for both of these proteins in lipolysis in vivo (62). GS2 transcripts have only been identified in humans (62). The relative contribution of GS2 and GS2-Like to lipolysis in white adipose tissue remains to be determined.
DIACYLGLYCEROL HYDROLYSIS
The second step of lipolysis involves the hydrolysis of DAG to yield MAG and a nonesterified fatty acid. This reaction occurs at a rate 10- to 30-fold higher than the hydrolysis of TAG, which is the initiating and rate-limiting step in lipolysis (40). To date, the only DAG lipase identified in adipocytes is HSL.
The maximum rate of hydrolysis of DAG by HSL in vitro is 11-fold greater than that of TAG (51). As described above, studies of murine models deficient in HSL have confirmed the importance of HSL for breakdown of DAG. While mice lacking adipose HSL can achieve near normal rates of TAG hydrolysis, these animals display severely blunted DAG lipase activity, and as a result, DAG accumulates in adipose tissues (44). Downregulation of the activity of a number of CoA-dependent acyltransferases has been reported in HSL-null mice, and it is believed to contribute to maintenance of normal weight in the face of complete ablation of this important lipolytic enzyme (144). The metabolic fate of accumulated DAG in adipocytes of HSL-null mice remains to be determined. Release of glycerol and fatty acids was normal or even elevated in fasted HSL-null mice, suggesting complete breakdown of TAG (94). Impaired re-esterification of DAG by acyltransferases could result in a normalized release of FA generated from the hydrolysis of TAG. Release of glycerol, however, would be impaired. Neither TGH nor ATGL/desnutrin has been shown to exhibit significant DAG lipase activity (90, 145). Results from HSL-null mouse models suggest, therefore, that adipocytes may harbor additional, less active DAG lipases, as well as TAG lipases.
MONOACYLGLYCEROL HYDROLYSIS
Monoglyceride lipase (MGL) is a 33 kDa hydrolase, purified 2500-fold from rat adipose tissue in 1975 by Tornqvist & Belfrage (129). In 1986, the respective roles of HSL and MGL in TAG hydrolysis were clarified when isolated HSL was found to hydrolyze acylglycerol with an accumulation of MAG in vitro, whereas glycerol release was blunted through selective removal of MGL by immunoprecipitation (31).
MGL hydrolyzes the 1(3) and 2-ester bonds of MAG at equal rates and exhibits no in vitro catalytic activity against DAG, TAG, or cholsteryl esters (57). MGL contains an alpha/beta-hydrolase fold, characteristic of known lipases (57). It is predicted to be composed of 302 amino acids (57). MGL is related to a number of microbial proteins that include esterases, lysophospholipases, and haloperoxidases (58). Two lipase motifs have been identified in the primary sequence: the active site serine motif GXSXG and the HG dipeptide (57). The catalytic triad of MGL is formed by Ser-122, Asp-239, and His-269. Mutating any of these three residues has been shown to completely abolish both the lipase and esterase activities of MGL (57).
ROLE OF LIPID DROPLET–ASSOCIATED PROTEINS IN LIPOLYSIS
Perilipin is the major protein found in association with lipid droplets in adipocytes (42). Analysis of lipid droplets from Chinese hamster ovary (CHO) cells by mass spectrometry has identified, in addition to perilipin, more than 40 structural and signaling proteins, as well as enzymes involved in lipid synthesis, storage, and utilization, suggesting that the lipid droplet should be considered a metabolic organelle rather than an inert storage site (70). Lipolysis requires that soluble lipases access the highly hydrophobic TAG substrate and that hydrophobic products of this reaction be removed. A number of cytosolic as well as lipid droplet–associated proteins are known to modulate rates of basal and/or stimulated lipolysis.
Perilipin
Perilipin A and B are isoforms of perilipin that arise from differential splicing (41). Perilipin A is the predominant isoform found in mature adipocytes, and it was among the earliest lipid droplet–associated proteins to be identified. As such, it has been studied extensively. Much evidence supports a complex role for perilipin proteins in regulating both basal and stimulated adipocyte lipolysis.
Under unstimulated conditions, cell fractionation and confocal microscopy studies show that both desnutrin/ATGL (145) and a substantial proportion of HSL (up to 50% of cellular levels) are localized to the lipid droplet (84). The presence of perilipins A and B coating the lipid droplet is believed to function as a protective barrier that restricts access of TAG lipases to neutral lipid substrates in order to prevent unrestrained basal lipolysis (15). The physiologic and metabolic effects of perilipin ablation in mice support this idea. Perilipin-null mice have constitutively elevated basal lipolysis, resulting in a marked reduction in WAT mass and smaller adipocyte size (78, 106, 127). Perilipin-null mice also display a significant resistance to diet-induced obesity (78, 106, 127) that is explained, at least in part, by increased beta-oxidation of fatty acids, the products of lipolysis (106), following from a compensatory induction of genes involved in lipid and energy metabolism and a downregulation of genes involved in lipid biosynthesis (20). Ectopic expression of perilipin A, as expected, increases the storage of TAG by inhibiting hydrolysis (15, 127). 3T3-L1 preadipocytes transfected with perilipin A stored 6- to 30-fold more TAG than control 3T3-L1 cells, resulting from a 5-fold reduction in the rate of lipolysis (15). In CHO cells, perilipin A was found to inhibit TAG hydrolysis by 87% in cells maintained under unstimulated conditions (127). Although perilipin A clearly restrains the action of TAG lipases under basal conditions, this protein plays an entirely different role in stimulated adipocyte lipolysis.
Perilipin ablation is associated with near-maximal rates of lipolysis under basal conditions (78). However, loss of functional perilipin proteins also causes a dramatic attenuation of stimulated lipolytic activity (78, 127). As a target for protein kinase A (PKA; cAMP-dependent protein kinase)-mediated phosphorylation at up to six sites (Ser-81, Ser-223, Ser-277, Ser-434, Ser-492, and Ser-517), perilipin A is highly regulated by lipolytic stimuli that act through the beta-adrenergic receptor/adenylyl cyclase pathway (117, 125, 128, 140). Studies in CHO cells demonstrate that PKA-mediated phosphorylation of perilipin A alone is sufficient to increase lipolysis (126). Conversely, mutation of perilipin A phosphorylation sites blunts the contribution of this protein to stimulated lipolysis (84, 117). In adipocytes, the presence of perilipin proteins on the lipid droplet appears to be necessary for PKA-mediated stimulation of HSL translocation (84, 125). Although recent evidence indicates that these perilipins do not have to be phosphorylated in order to mediate translocation of HSL from the cytosol to the surface of the lipid droplet, phosphorylation does appear to be necessary for attainment of maximally stimulated lipolysis following PKA activation (84). PKA-dependent phosphorylation of perilipin A may facilitate interaction with HSL on the lipid droplet, thereby increasing activity of the enzyme (84). Coexpression of perilipin A and HSL in CHO cells results in a cooperative effect that produces a more rapidly accelerated lipolysis following PKA stimulation than is evident in cells that express either protein alone (125).
Perilipin A may also influence lipolysis by regulating the distribution and architecture of lipid droplets within adipocytes. Chronic stimulation of lipolysis in 3T3-L1 adipocytes causes the large perinuclear lipid droplets to fragment into many microlipid droplets coated with perilipin A (76). This dispersion is prevented by stable expression of perilipin A that has been mutated to prevent phosphorylation at serine 492 (76). Activation of perilipin A by PKA-mediated phosphorylation may, therefore, increase lipolysis by increasing the surface area of neutral lipid droplets accessible for attack by lipases (76, 85). Taken together, these studies indicate that perilipin expression and phosphorylation state are critical regulators of lipolysis in adipocytes. In nonadipocytes, the perilipin-related protein adipocyte differentiation-related protein/adipophilin replaces perilipin at the surface of neutral lipid storage droplets, although this protein is largely absent in mature adipocytes (13).
Adipose Fatty Acid–Binding Protein
Adipose fatty acid–binding protein (aFABP/ALBP/aP2) is a member of the cytosolic lipid-binding proteins that carry both fatty acids and retinoic acid in adipocytes (79). Maximal rates of lipolysis require the removal of fatty acids from the adipocyte in order to prevent accumulation of reaction products and feedback inhibition of lipases. aFABP/ALBP/aP2 is postulated to act as a molecular chaper-one, facilitating the movement of fatty acids out of adipocytes following their liberation from cellular TAG stores by lipases (22). Studies of aFABP/ALBP/aP2 gene ablation in mice provide insight into the relative role of aFABP/ALBP/aP2 in lipolysis. Basal lipolysis has been reported to be decreased in aFABP/ALBP/aP2-null mice compared with wild-type littermates (22, 49), and stimulated lipolysis in adipocytes isolated from these mice has also been shown to be attenuated (22, 110). Consistent with a decrease in the rate of efflux of fatty acids, intracellular fatty acid levels have been found to be threefold higher in adipocytes from aFABP/ALBP/aP2 nulls compared with wild types (22). However, others have observed no difference in basal or stimulated rates of lipolysis in adipocytes isolated from aFABP/ALBP/aP2-null mice compared with wild-type littermates (110). In that study, a compensatory induction of keratinocyte lipid-binding protein was observed that was reported to overcome the functional effects of loss of adipocyte-specific aFABP/ALBP/aP2 (110). Intracellular lipid-binding proteins may be important regulators of lipolysis. Further work is required to discern the relative roles of these proteins in adipocyte lipolysis.
Caveolin-1
Caveolae and caveolar proteins are implicated in the regulation of multiple functions within cells (23, 93, 103). Caveolin-1 is found in particularly high abundance in adipose tissue, and it is strongly induced during the differentiation of 3T3-L1 fibroblasts to mature adipocytes (107). Although typically found in the caveolae of plasma membranes, proteomic analysis has demonstrated that in adipocytes caveolin-1 localizes to the lipid droplet as well (14, 23, 93, 103). A role for caveolin-1 in lipolysis has been suggested from studies of caveolin-1 null mice. These animals have markedly attenuated lipolytic activity in white adipose tissue (23) and fail to show the normal increase in serum nonesterified FA that is expected to occur in fasting, suggesting that caveolin-1 plays a role in activating lipolysis (24). Caveolin-1 knockout mice also fail to properly liberate fatty acids from TAG stores in brown adipose tissue in response to fasting, resulting in impaired thermogenesis (24). Regulation of cyclic AMP (cAMP)-mediated signal transduction may contribute to the effects of caveolin-1 on lipolysis (101). Caveolin-1 has been shown to directly interact with the catalytic subunit of PKA to inhibit cAMP-dependent signaling in vivo (101). In knockout mice, however, it has been shown that whereas PKA activity was greatly increased in the absence of caveolin-1, phosphorylation of perilipin was dramatically reduced (23). Activation of the β3-adrenergic receptor results in the formation of a ligand-induced complex between perilipin, caveolin-1, and the catalytic subunit of PKA in adipocytes (23). Caveolin-1, therefore, may facilitate PKA-mediated phosphorylation of perilipin, thereby contributing to increased stimulation of lipolysis.
CGI-58
Mutations in CGI-58 (Comparative Gene Identification 58), also known as ABHD5 (alpha/beta-hydrolase domain-containing protein 5) have been found to be the cause of a rare autosomal recessive disease called Chanarin-Dorfman syndrome (CDS) (67). CDS is characterized by excessive accumulation of TAG in the cells of many organs, and the clinical manifestations include ichthyosis, cataracts, ataxia, neurosensory hearing loss, and mental retardation (67). CGI-58 resembles lipases structurally; however, it lacks the conserved active site serine, which has been replaced by an asparagine (66). In 3T3-L1 adipocytes, CGI-58 is localized to the lipid droplet and has been shown to directly interact (139) and colocalize with perilipin A (121). However, when cells are treated with agents to promote lipolysis, it disperses off the lipid droplet (121). Recently, Lass et al. (66) demonstrated that CGI-58 stimulates in vitro lipolysis and is an activator of ATGL, but not HSL. When added to cytosolic extracts of mouse adipose tissue, CGI-58 stimulated lipolysis, while purified CGI-58 exhibited no lipase activity in vitro. Furthermore, they showed that mutant forms of CGI-58 fail to activate ATGL. Although CGI-58 appears to play a role in lipolysis, more studies are needed to elucidate the molecular mechanism of its activation of ATGL as well as its physiological role in living organisms.
Other Proteins Implicated in TAG Hydrolysis
Aquaporin 7 is a water- and glycerol-transporting protein expressed in the plasma membrane of adipocytes (46, 50, 75). Mice deficient in aquaporin 7 have impaired glycerol release in response to fasting and treatment with beta-adrenergic agonists, although FA release from adipocytes and plasma FFA levels are comparable to those observed in wild-type littermates (75). These animals develop age-associated obesity caused by an induction of glycerol kinase and increased storage of TAG (53).
Lipotransin was identified by yeast two-hybrid screening of a 3T3-L1 adipocyte cDNA library as an HSL-interacting protein (123). It is believed to function as a docking protein that mediates the hormonally induced translocation of HSL from cytoplasm to the lipid droplet (123).
TIP47 is a lipid droplet–associated PAT family protein of unknown function (36). TIP47 inhibits the hydrolysis of retinyl esters by GS2 and by HSL in human keratinocytes, suggesting that in adipocytes it may share a similar antilipolytic function with other PAT family proteins, such as perilipin or adipose differentiation-related protein/adipophilin (36).
NUTRITIONAL REGULATION OF LIPOLYSIS
Nutritional regulation of lipolysis occurs at multiple levels in response to changing metabolic conditions and nutrient intakes. Acute, rapid regulation of adipose tissue lipolysis occurs in order to maintain the supply of energy substrates during the postabsorptive state and to allow for efficient storage of excess fuels following a meal. Chronic exposure to extreme nutritional states, such as obesity or starvation, also induces metabolic adaptations that include changes in lipolysis. And, finally, a growing body of evidence indicates that exposure to specific metabolically active nutrients in the diet can also regulate lipolysis.
STIMULATION OF LIPOLYSIS DURING FASTING
Fasting acutely stimulates lipolysis, upregulating the serum concentration of fatty acids and glycerol that act as oxidative substrates to maintain energy requirements for other metabolic tissues. Catecholamines are the primary activators of fasting-induced lipolysis. The metabolic pathways through which these molecules act to stimulate TAG hydrolysis and FA release have been studied extensively and reviewed in detail (6, 17, 18, 29, 63, 64, and references contained therein). The catecholamine norepinephrine binds beta-adrenergic receptors on the plasma membrane of adipocytes. These receptors are coupled with Gs-proteins that transmit a stimulatory signal to adenylyl cyclase to generate cyclic AMP (cAMP). cAMP binds PKA, causing the regulatory subunits to dissociate from the catalytically active subunits, resulting in increased activity of the enzyme (60).
PKA catalyzes the polyphosphorylation of HSL on multiple sites, including a nonactivating site (Ser-563) as well as two additional sites (Ser-659 and Ser-660) that cause activation and subsequent translocation of this lipase from the cytosol to the lipid droplet (4, 51, 120). As discussed above, PKA also phosphorylates perilipin on the lipid droplet, resulting in changes that enhance stimulated lipolysis, including movement of perilipin away from the lipid droplet (21), perilipin-mediated remodeling of lipid droplets that increases the surface area available for lipolytic attack (75), and perilipin-mediated activation of HSL activity at the surface of the lipid droplet (84, 125). Findings from studies in HSL-null mice suggest that catecholamine-stimulated lipolysis may also involve other TAG lipases. Treatment of adipocytes isolated from HSL-null mice with the beta-adrenergic agonist isoproterenol causes enhanced lipolysis, albeit at a blunted level compared with adipocytes from wild-type animals (91). This suggests that some or all of the newly discovered adipocyte TAG-lipases may be direct targets for PKA-mediated phosphorylation and activation, and indeed, Zimmerman et al. (145) have shown previously that desnutrin/ATGL is phosphorylated, although it was not a target for PKA. This also suggests that PKA may indirectly activate non-HSL-mediated TAG lipolysis. Perilipin expression alone has been shown to be sufficient to confer PKA-mediated lipolysis in CHO cells that lack HSL (126). Phosphorylation of perilipin by PKA may result in changes that increase the activity of multiple lipid droplet–associated lipases. Potential mechanisms include promoting access of soluble lipases to hydrophobic substrates, facilitating the formation of complexes between lipases and lipolysis-associated proteins, and stabilizing lipases at the surface of the lipid droplet. Further studies are required to elucidate the nature of PKA-mediated lipolysis in the absence of HSL.
Glucagon stimulates lipolysis in isolated mouse (48, 114) and human (96) adipocytes, independent of antagonistic effects on insulin action. Glucagon treatment causes an increase in adenylyl cyclase activity, resulting in an increase in intracellular adipocyte cAMP levels (95, 96). Gastric inhibitory polypeptide competes with glucagon for binding to the glucagon receptor and can inhibit glucagon-stimulated lipolysis in adipocytes, indicating that glucagon mediates lipolysis, at least in part, through direct activation of its receptor (27). Although glucagon action is primarily liver specific, glucagon receptors have been reported to be present in membranes of human adipose tissue (83), suggesting that direct action of glucagon likely plays an important role in regulating human, as well as rodent, lipolysis.
INHIBITION OF LIPOLYSIS DURING REFEEDING
Refeeding attenuates adipocyte lipolysis, primarily through the potent antilipolytic actions of insulin. This regulatory pathway has also been studied and reviewed extensively (6, 17, 18, 29, 63, and references contained therein). Rapid, acute regulation of lipolysis by insulin involves both cAMP-dependent and cAMP-independent mechanisms. cAMP-dependent suppression of lipolysis by insulin involves activation of phosphodiesterase 3B (63). Insulin binding causes autophosphorylation of its receptor, resulting in activation and subsequent tyrosine phosphorylation of insulin receptor substrates and binding of the p85 regulatory subunit of phosphatidyl inositol kinase-3 (PI3K). Activated PI3K autophosphorylates its inositol ring and the p85 regulatory subunit and p110 catalytic subunits, which is followed by phosphorylation and activation of protein kinase B/Akt (PKB/Akt). PKB/Akt phosphorylates and activates phosphodiesterase 3B, which degrades cAMP in adipocytes, releasing PKA from activation and decreasing lipolysis through a reduction in the phosphorylation-mediated activation of HSL and perilipin. cAMP-independent regulation of lipolysis by insulin involves the stimulation of protein phosphatase-1 through phosphorylation of its regulatory subunit (100). Activated protein phosphatase-1 rapidly dephosphorylates and deactivates HSL, causing a fall in the rate of lipolysis (73, 92, 100, 119, 143).
Insulin may also suppress lipolysis in 3T3-L1 adipocytes through downregulation of desnutrin/ATGL mRNA (59). In contrast, HSL mRNA expression is not regulated by short-term changes in nutritional status (124). In addition to inhibitory effects on the enzymes in TAG hydrolysis, insulin also decreases measurable rates of lipolysis (as indicated by the release of free fatty acids and glycerol from intact cells) by promoting the re-esterification of fatty acids (16). This serves to magnify the apparent suppression of TAG hydrolysis by insulin and the physiological effect of the hormone.
AMP-activated protein kinase (AMPK) as a master intracellular energy sensor is implicated in the regulation of both glucose and lipid metabolism (33, 86). Activation of AMPK in adipocytes by 5-aminoimidazole-4-carboxyamide-1-beta-D-ribofuranoside (AICAR) inhibits lipolysis (32, 33, 112). Infusion of obese Zucker rats as well as lean littermates with AICAR decreases plasma TAG and FA concentrations, as well as glycerol turnover (37), whereas mice lacking the AMPK-alpha2 subunit have been shown to have increased adiposity and weight gain (98, 131). Others have found, however, that AMPK activation may also play a role in the stimulation of maximal rates of lipolysis by cAMP (86). The molecular events and targets underlying regulation of lipolysis by AMPK are yet to be understood.
LIPOLYSIS IN OBESITY
Obesity is associated with an increase in basal lipolysis (102) but a decrease in catecholamine-stimulated lipolysis (65). Impaired sensitivity of adipocytes to insulin signaling, including the antilipolytic effects of this hormone, may contribute to enhanced basal lipolysis in obesity. Decreased insulin-mediated suppression of adipocyte lipolysis has been reported in obese rats (118) as well as in women with visceral obesity (3, 54), although several other studies in humans report that the antilipolytic effects of insulin are well conserved, even in subjects with highly impaired insulin-mediated glucose regulation (7, 8, 11, 52). In both obese and nonobese subjects, fasting and weight reduction cause a significant enhancement of sensitivity to the antilipolytic effects of insulin (7, 28, 71).
Overexpression of the leptin gene in adipocytes and increased circulating levels of leptin may also contribute to enhanced basal lipolysis in obesity (74). Treatment of adipocytes isolated from lean mice with leptin stimulates lipolytic activity (33). Treatment of adipocytes isolated from obese ob/ob mice that are deficient in leptin results in an even greater stimulation of lipolysis (33). Moreover, chronic (112) or acute (32) peripheral administration of leptin also stimulates adipose TAG hydrolysis in rats, resulting in a 9- to 16-fold increase in rates of lipolysis. Lipolytic actions of leptin are dependent on the leptin receptor, since obese fa/fa Zucker rats (112) and db/db mice (32) that have an inactivating mutation of the long form of the leptin receptor are resistant to leptin-induced lipolysis. Increased circulating leptin may also enhance lipolysis by counteracting the antilipolytic effects of insulin (86). Leptin impairs several metabolic effects of insulin, including the ability of the hormone to inhibit beta-adrenergic receptor-mediated lipolysis and PKA activation (86).
Beta-adrenergic receptor-stimulated lipolysis is impaired in obesity (65). Adipocytes from obese subjects have lower levels of adenylyl cyclase activity under hormone-stimulated conditions when compared with adipocytes from nonobese controls (77). Alterations in the adrenergic signaling pathways may contribute to this effect. Obesity is associated with a decreased lipolytic effect of catecholamines in adipose tissue (65). Adipocytes from obese Zucker rats have higher levels of antilipolytic α2 adrenoceptors compared with adipocytes from lean littermates (19). Conversely, adipocytes from obese mice express twofold lower levels of Gsα, a subunit of the GTP-binding protein through which beta-adrenergic receptors stimulate adenylyl cyclase (38). Post-receptor defects may also contribute to defects in hormone-stimulated lipolysis. The maximum lipolytic capacity has been shown to be reduced in adipocytes isolated from obese subjects compared with adipocytes from nonobese control subjects following stimulation with the phosphodiesterase-resistant cAMP analogue dibutyryl cAMP (65). This finding indicates impairment in the actions of cAMP downstream of effects of obesity on adrenergic receptor signaling, G-protein coupled activation of adenylyl cyclase, or cAMP levels. HSL and perilipin A are major targets for cAMP-dependent PKA activation. Decreased levels of HSL (65) and perilipin (135) in adipose tissue from obese subjects may contribute to the impairment of catecholamine-mediated lipolysis through a postreceptor defect. Weight reduction in obese subjects causes a substantial increase and normalization of sensitivity to catecholamine stimulation of lipolysis (77) without changing the number of β-adrenergic receptors (102).
Tumor necrosis factor-alpha (TNFα) production is increased in adipocytes from obese individuals and may contribute to enhanced basal lipolysis in obesity (97, 104, 105). This cytokine signals in an autocrine/paracrine manner through the TNFα receptor to activate the mitogen-activated protein kinases p44/42 and JNK that, in turn, downregulate perilipin mRNA and protein expression (104, 105). Studies with specific inhibitors of p44/42 and JNK support that the TNFα-mediated increase in lipolysis is largely attributed to a reduction in perilipin levels in adipocytes (104, 105), and lower levels of perilipin have been found in adipose tissues from obese subjects (135).
REGULATION OF ADIPOCYTE LIPOLYSIS BY DIETARY COMPOUNDS
Calcium
Higher intakes of calcium are associated with decreased adiposity and a reduced risk of obesity in a variety of epidemiological studies (108, and references therein). Moreover, calcium supplementation has been shown to aid in weight loss in obese humans consuming a calorie-deficient diet (142) and in calorie-restricted obese mice (111), and has also been reported to inhibit weight regain during refeeding in mice (122). Increased lipolysis is believed to contribute to these findings, and indeed, acute intakes of calcium have been reported to correlate significantly with fat oxidation in humans (82). Several studies have investigated the molecular mechanisms underlying potentiation of adipocyte lipolysis by dietary calcium. Increasing dietary calcium feedback inhibits the secretion of parathyroid hormone (PTH) and, subsequently, the activation of 25 hydroxycholecalciferol to 1,25 dihydroxycalciferol (vitamin D3; VD3) (87). Adipocytes are targets for the action of these hormones (141). PTH stimulates a dose-dependent rise in adipocyte intracellular calcium levels that is due to both increased influx and mobilization of intracellular stores (89). VD3 has also been shown to elicit an increase in intracellular calcium levels (111). Increased intracellular calcium in human adipocytes inhibits lipolysis stimulated by the β-adrenergic receptor pathway (111, 138), resulting in decreased cAMP levels and reduced HSL phosphorylation (138). These effects appear to be mediated primarily through activation of phosphodiesterase 3B (138). Low dietary calcium intakes and increased circulating VD3 may also have indirect inhibitory effects on adipocyte lipolysis by regulating the use of lipolytic substrates for energy metabolism (111, 122). Inverse regulation of intracellular calcium levels in adipocytes by calcitropic hormones may contribute to effects of dietary calcium on adiposity.
Caffeine
The lipolytic effects of caffeine and other methylxanthines derived from tea and coffee are well established and well characterized. These compounds stimulate lipolysis by increasing cellular levels of cAMP through two principal mechanisms. The first is antagonism of A1-adenosine receptors (72). These receptors predominate on differentiated mature adipocytes, where they inhibit adenylyl cyclase activity and suppress lipolysis (12). Antagonism of A1-receptors results in a derepression of adenylyl cyclase activity and increased lipolysis (72). Methylxanthines also inhibit phosphodiesterase activity, preventing the breakdown of cAMP and stimulating lipolysis in fat cells (2, 95). Caffeine ingestion increases lipid turnover (2) and the concentration of serum FFAs (5, 61). As such, high intakes of methylxanthines may also contribute to improved weight loss and weight maintenance through enhanced fat oxidation and thermogenesis (137).
Ethanol
Acute ethanol ingestion has antilipolytic effects that cause a significant fall in serum FFAs (1) and a decrease in whole-body lipid oxidation (113). Increased plasma acetate may contribute to this effect (1, 113). Chronic ethanol feeding in rats has been reported to suppress beta-adrenergic receptor-mediated lipolysis in adipocytes, likely through increased activation of phosphodiesterase 4 (56). This resulted in a decrease in beta-adrenergic receptor-stimulated PKA activation and decreased activating phosphorylation of perilipin A and HSL (56). Chronic ethanol consumption has also been reported to be associated with decreased PTH secretion, which may also contribute to increased lipolysis by decreasing intracellular calcium levels in adipocytes (81).
SUMMARY AND FUTURE DIRECTIONS
Adipocyte lipolysis is a complex process that is precisely controlled through integration of multiple and diverse hormonal and biochemical signals. Breakdown of this regulation may contribute to the development of obesity and associated pathologies. Many exciting advances have been made recently, including the discovery of major TAG lipases that contribute to in vivo adipocyte lipolysis. However, much remains to be elucidated regarding the in vivo functioning and relative contribution of these lipases to overall adipocyte lipolysis. As genetic mouse models of these enzymes are generated, our understanding of adipocyte lipolysis is likely to increase dramatically in the near future.
SUMMARY POINTS.
Lipolysis is precisely regulated by multiple hormonal and biochemical signals that converge on adipocytes to regulate the function of lipases and nonenzymatic accessory proteins.
Hydrolysis of TAG is rate limiting in lipolysis and is catalyzed by one or more novel lipases that include desnutrin/ATGL and TGH.
Major regulators of lipolysis include the lipolytic activators glucagon and the catecholamines, and the antilipolytic agent insulin.
Dietary components may also regulate lipolysis. These include dietary calcium, ethanol, and caffeine.
FUTURE ISSUES.
Generation of mice deficient in desnutrin/ATGL specifically in the adipose tissue will help to clarify the in vivo role of this lipase in adipose tissue lipolysis.
Genetic mouse models deficient in other TAG lipases will also be needed to determine their relative contribution to lipolysis.
The role of the energy sensor AMPK in mediating lipolysis remains unclear. Further work is required to resolve this issue.
Several dietary nutrients have been found to regulate lipolysis. Given the burden of chronic disease that is associated with obesity, discovery of dietary nutrients that stimulate lipolysis would be of great interest.
Glossary
- WAT
white adipose tissue
- TAG
triacylglyceride
- FA
fatty acid
- DAG
diacylglyceride
- MAG
monoacylglyceride
- Lipolysis
the hydrolysis of TAG to generate nonesterified fatty acids (FAs) and glycerol that are released into the vasculature for use by other organs as energy substrates
- HSL
hormone-sensitive lipase
- ATGL
adipose triglyceride lipase
- TGH
triacylglyceride hydrolase
- MGL
monoglyceride lipase
- PKA
protein kinase A
- cAMP
cyclic AMP
- PKB
protein kinase B
- PTH
parathyroid hormone
- VD3
1,25(OH)2 vitamin D3
LITERATURE CITED
- 1.Abramson EA, Arky RA. Acute antilipolytic effects of ethyl alcohol and acetate in man. J. Lab. Clin. Med. 1968;72:105–17. [PubMed] [Google Scholar]
- 2.Acheson KJ, Gremaud G, Meirim I, Montigon F, Krebs Y, et al. Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am. J. Clin. Nutr. 2004;79:40–46. doi: 10.1093/ajcn/79.1.40. [DOI] [PubMed] [Google Scholar]
- 3.Albu JB, Curi M, Shur M, Murphy L, Matthews DE, Pi-Sunyer FX. Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am. J. Physiol. 1999;277:E551–60. doi: 10.1152/ajpendo.1999.277.3.E551. [DOI] [PubMed] [Google Scholar]
- 4.Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998;273:215–21. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 5.Arciero PJ, Gardner AW, Calles-Escandon J, Benowitz NL, Poehlman ET. Effects of caffeine ingestion on NE kinetics, fat oxidation, and energy expenditure in younger and older men. Am. J. Physiol. 1995;268:E1192–98. doi: 10.1152/ajpendo.1995.268.6.E1192. [DOI] [PubMed] [Google Scholar]
- 6.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract. Res. Clin. Endocrinol. Metab. 2005;19:471–82. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Arner P, Bolinder J, Engfeldt P, Hellmer J, Ostman J. Influence of obesity on the antilipolytic effect of insulin in isolated human fat cells obtained before and after glucose ingestion. J. Clin. Invest. 1984;73:673–80. doi: 10.1172/JCI111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arner P, Engfeldt P, Skarfors E, Lithell H, Bolinder J. Insulin receptor binding and metabolic effects of insulin in human subcutaneous adipose tissue in untreated noninsulin dependent diabetes mellitus. Ups. J. Med. Sci. 1987;92:47–58. doi: 10.3109/03009738709178677. [DOI] [PubMed] [Google Scholar]
- 9.Baulande S, Lasnier F, Lucas M, Pairault J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J. Biol. Chem. 2001;276:33336–44. doi: 10.1074/jbc.M105193200. [DOI] [PubMed] [Google Scholar]
- 10.Birner-Gruenberger R, Susani-Etzerodt H, Waldhuber M, Riesenhuber G, Schmidinger H, et al. The lipolytic proteome of mouse adipose tissue. Mol. Cell. Proteomics. 2005;4:1710–17. doi: 10.1074/mcp.M500062-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Bolinder J, Lithell H, Skarfors E, Arner P. Effects of obesity, hyperinsulinemia, and glucose intolerance on insulin action in adipose tissue of sixty-year-old men. Diabetes. 1986;35:282–90. doi: 10.2337/diab.35.3.282. [DOI] [PubMed] [Google Scholar]
- 12.Borglum JD, Vassaux G, Richelsen B, Gaillard D, Darimont C, et al. Changes in adenosine A1- and A2-receptor expression during adipose cell differentiation. Mol. Cell. Endocrinol. 1996;117:17–25. doi: 10.1016/0303-7207(95)03728-4. [DOI] [PubMed] [Google Scholar]
- 13.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 1997;38:2249–63. [PubMed] [Google Scholar]
- 14.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 2004;279:46835–42. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 15.Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 2000;275:38486–93. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 16.Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Regulation of free fatty acid metabolism by insulin in humans: role of lipolysis and reesterification. Am. J. Physiol. 1992;263:E1063–69. doi: 10.1152/ajpendo.2006.263.6.E1063. [DOI] [PubMed] [Google Scholar]
- 17.Carey GB. Mechanisms regulating adipocyte lipolysis. Adv. Exp. Med. Biol. 1998;441:157–70. doi: 10.1007/978-1-4899-1928-1_15. [DOI] [PubMed] [Google Scholar]
- 18.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–8. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Carpene C, Rebourcet MC, Guichard C, Lafontan M, Lavau M. Increased alpha 2-adrenergic binding sites and antilipolytic effect in adipocytes from genetically obese rats. J. Lipid Res. 1990;31:811–19. [PubMed] [Google Scholar]
- 20.Castro-Chavez F, Yechoor VK, Saha PK, Martinez-Botas J, Wooten EC, et al. Coordinated upregulation of oxidative pathways and downregulation of lipid biosynthesis underlie obesity resistance in perilipin knockout mice: a microarray gene expression profile. Diabetes. 2003;52:2666–74. doi: 10.2337/diabetes.52.11.2666. [DOI] [PubMed] [Google Scholar]
- 21.Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J. Biol. Chem. 2000;275:5011–15. doi: 10.1074/jbc.275.7.5011. [DOI] [PubMed] [Google Scholar]
- 22.Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J. Lipid Res. 1999;40:967–72. [PubMed] [Google Scholar]
- 23.Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–70. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AW, Schubert W, Brasaemle DL, Scherer PE, Lisanti MP. Caveolin-1 expression is essential for proper nonshivering thermogenesis in brown adipose tissue. Diabetes. 2005;54:679–86. doi: 10.2337/diabetes.54.3.679. [DOI] [PubMed] [Google Scholar]
- 25.Dolinsky VW, Gilham D, Hatch GM, Agellon LB, Lehner R, Vance DE. Regulation of triacylglycerol hydrolase expression by dietary fatty acids and peroxisomal proliferator-activated receptors. Biochim. Biophys. Acta. 2003;1635:20–28. doi: 10.1016/j.bbalip.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Dolinsky VW, Sipione S, Lehner R, Vance DE. The cloning and expression of a murine triacylglycerol hydrolase cDNA and the structure of its corresponding gene. Biochim. Biophys. Acta. 2001;1532:162–72. doi: 10.1016/s1388-1981(01)00133-0. [DOI] [PubMed] [Google Scholar]
- 27.Dupre J, Greenidge N, McDonald TJ, Ross SA, Rubinstein D. Inhibition of actions of glucagon in adipocytes by gastric inhibitory polypeptide. Metabolism. 1976;25:1197–99. doi: 10.1016/s0026-0495(76)80002-9. [DOI] [PubMed] [Google Scholar]
- 28.Engfeldt P, Bolinder J, Ostman J, Arner P. Influence of fasting and refeeding on the antilipolytic effect of insulin in human fat cells obtained from obese subjects. Diabetes. 1985;34:1191–97. doi: 10.2337/diab.34.11.1191. [DOI] [PubMed] [Google Scholar]
- 29.Fain JN, Garcija-Sainz JA. Adrenergic regulation of adipocyte metabolism. J. Lipid Res. 1983;24:945–66. [PubMed] [Google Scholar]
- 30.Fortier M, Wang SP, Mauriege P, Semache M, Mfuma L, et al. Hormone-sensitive lipase-independent adipocyte lipolysis during beta-adrenergic stimulation, fasting, and dietary fat loading. Am. J. Physiol. Endocrinol. Metab. 2004;287:E282–88. doi: 10.1152/ajpendo.00203.2003. [DOI] [PubMed] [Google Scholar]
- 31.Fredrikson G, Tornqvist H, Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 1986;876:288–93. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- 32.Fruhbeck G, Aguado M, Gomez-Ambrosi J, Martinez JA. Lipolytic effect of in vivo leptin administration on adipocytes of lean and ob/ob mice, but not db/db mice. Biochem. Biophys. Res. Commun. 1998;250:99–102. doi: 10.1006/bbrc.1998.9277. [DOI] [PubMed] [Google Scholar]
- 33.Fruhbeck G, Aguado M, Martinez JA. In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem. Biophys. Res. Commun. 1997;240:590–94. doi: 10.1006/bbrc.1997.7716. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda N, Ontko JA. Interactions between fatty acid synthesis, oxidation, and esterification in the production of triglyceride-rich lipoproteins by the liver. J. Lipid Res. 1984;25:831–42. [PubMed] [Google Scholar]
- 35.Gao JG, Simon M. Identification of a novel keratinocyte retinyl ester hydrolase as a transacylase and lipase. J. Invest. Dermatol. 2005;124:1259–66. doi: 10.1111/j.0022-202X.2005.23761.x. [DOI] [PubMed] [Google Scholar]
- 36.Gao JG, Simon M. Molecular screening for GS2 lipase regulators: inhibition of keratinocyte retinylester hydrolysis by TIP47. J. Invest. Dermatol. 2006;126:2087–95. doi: 10.1038/sj.jid.5700327. [DOI] [PubMed] [Google Scholar]
- 37.Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–57. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 38.Gettys TW, Ramkumar V, Uhing RJ, Seger L, Taylor IL. Alterations in mRNA levels, expression, and function of GTP-binding regulatory proteins in adipocytes from obese mice (C57BL/6J-ob/ob) J. Biol. Chem. 1991;266:15949–55. [PubMed] [Google Scholar]
- 39.Gilham D, Alam M, Gao W, Vance DE, Lehner R. Triacylglycerol hydrolase is localized to the endoplasmic reticulum by an unusual retrieval sequence where it participates in VLDL assembly without utilizing VLDL lipids as substrates. Mol. Biol. Cell. 2005;16:984–96. doi: 10.1091/mbc.E04-03-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giudicelli H, Combes-Pastre N, Boyer J. Lipolytic activity of adipose tissue. IV. The diacylglycerol lipase activity of human adipose tissue. Biochim. Biophys. Acta. 1974;369:25–33. doi: 10.1016/0005-2760(74)90188-x. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg AG, Egan JJ, Wek SA, Moos MCJ, Londos C, Kimmel AR. Isolation of cDNAs of perilipins A and B: sequence and expression of lipid droplet–associated proteins of adipocytes. Proc. Natl. Acad. Sci. USA. 1993;90:12035–39. doi: 10.1073/pnas.90.24.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991;266:11341–46. [PubMed] [Google Scholar]
- 43.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–37. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 44.Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002;277:4806–15. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 45.Haemmerle G, Zimmermann R, Strauss JG, Kratky D, Riederer M, et al. Hormone-sensitive lipase deficiency in mice changes the plasma lipid profile by affecting the tissue-specific expression pattern of lipoprotein lipase in adipose tissue and muscle. J. Biol. Chem. 2002;277:12946–52. doi: 10.1074/jbc.M108640200. [DOI] [PubMed] [Google Scholar]
- 46.Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, et al. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005;280:15493–96. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- 47.Harada K, Shen WJ, Patel S, Natu V, Wang J, et al. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1182–95. doi: 10.1152/ajpendo.00259.2003. [DOI] [PubMed] [Google Scholar]
- 48.Heckemeyer CM, Barker J, Duckworth WC, Solomon SS. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Endocrinology. 1983;113:270–76. doi: 10.1210/endo-113-1-270. [DOI] [PubMed] [Google Scholar]
- 49.Hertzel AV, Smith LA, Berg AH, Cline GW, Shulman GI, et al. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am. J. Physiol. Endocrinol. Metab. 2006;290:E814–23. doi: 10.1152/ajpendo.00465.2005. [DOI] [PubMed] [Google Scholar]
- 50.Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA. 2005;102:10993–98. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 2003;31:1120–24. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 52.Howard BV, Klimes I, Vasquez B, Brady D, Nagulesparan M, Unger RH. The antilipolytic action of insulin in obese subjects with resistance to its glucoregulatory action. J. Clin. Endocrinol. Metab. 1984;58:544–48. doi: 10.1210/jcem-58-3-544. [DOI] [PubMed] [Google Scholar]
- 53.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 2004;279:48968–75. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JA, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am. J. Physiol. Endocrinol. Metab. 2001;280:E40–49. doi: 10.1152/ajpendo.2001.280.1.E40. [DOI] [PubMed] [Google Scholar]
- 55.Kalderon B, Mayorek N, Berry E, Zevit N, Bar-Tana J. Fatty acid cycling in the fasting rat. Am. J. Physiol. Endocrinol. Metab. 2000;279:E221–27. doi: 10.1152/ajpendo.2000.279.1.E221. [DOI] [PubMed] [Google Scholar]
- 56.Kang L, Nagy LE. Chronic ethanol feeding suppresses beta-adrenergic receptor-stimulated lipolysis in adipocytes isolated from epididymal fat. Endocrinology. 2006;147:4330–38. doi: 10.1210/en.2006-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J. Biol. Chem. 1997;272:27218–23. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 58.Karlsson M, Reue K, Xia YR, Lusis AJ, Langin D, et al. Exon-intron organization and chromosomal localization of the mouse monoglyceride lipase gene. Gene. 2001;272:11–18. doi: 10.1016/s0378-1119(01)00559-5. [DOI] [PubMed] [Google Scholar]
- 59.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–57. [PMC free article] [PubMed] [Google Scholar]
- 60.Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307:690–96. doi: 10.1126/science.1104607. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi-Hattori K, Mogi A, Matsumoto Y, Takita T. Effect of caffeine on the body fat and lipid metabolism of rats fed on a high-fat diet. Biosci. Biotechnol. Biochem. 2005;69:2219–23. doi: 10.1271/bbb.69.2219. [DOI] [PubMed] [Google Scholar]
- 62.Lake AC, Sun Y, Li JL, Kim JE, Johnson JW, et al. Expression, regulation, and triglyceride hydrolase activity of adiponutrin family members. J. Lipid Res. 2005;46:2477–87. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Langin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C. R. Biol. 2006;329:598–607. doi: 10.1016/j.crvi.2005.10.008. discussion 653–55. [DOI] [PubMed] [Google Scholar]
- 64.Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004;30:294–309. doi: 10.1016/s1262-3636(07)70121-0. [DOI] [PubMed] [Google Scholar]
- 65.Large V, Reynisdottir S, Langin D, Fredby K, Klannemark M, et al. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J. Lipid Res. 1999;40:2059–66. [PubMed] [Google Scholar]
- 66.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 2006;3:309–19. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 2001;69:1002–12. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lehner R, Vance DE. Cloning and expression of a cDNA encoding a hepatic microsomal lipase that mobilizes stored triacylglycerol. Biochem. J. 1999;343(Pt. 1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 69.Lehner R, Verger R. Purification and characterization of a porcine liver microsomal triacylglycerol hydrolase. Biochemistry. 1997;36:1861–68. doi: 10.1021/bi962186d. [DOI] [PubMed] [Google Scholar]
- 70.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 2004;279:3787–92. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 71.Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia. 2005;48:2334–42. doi: 10.1007/s00125-005-1961-6. [DOI] [PubMed] [Google Scholar]
- 72.Londos C, Cooper DM, Schlegel W, Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc. Natl. Acad. Sci. USA. 1978;75:5362–66. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Londos C, Honnor RC, Dhillon GS. cAMP-dependent protein kinase and lipolysis in rat adipocytes. III. Multiple modes of insulin regulation of lipolysis and regulation of insulin responses by adenylate cyclase regulators. J. Biol. Chem. 1985;260:15139–45. [PubMed] [Google Scholar]
- 74.Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat. Med. 1995;1:950–53. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 75.Maeda N, Funahashi T, Hibuse T, Nagasawa A, Kishida K, et al. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. USA. 2004;101:17801–6. doi: 10.1073/pnas.0406230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin A directs lipid droplet fragmentation and dispersion. J. Biol. Chem. 2006;281:11901–9. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- 77.Martin LF, Klim CM, Vannucci SJ, Dixon LB, Landis JR, LaNoue KF. Alterations in adipocyte adenylate cyclase activity in morbidly obese and formerly morbidly obese humans. Surgery. 1990;108:228–34. discussion 234–35. [PubMed] [Google Scholar]
- 78.Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, et al. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 2000;26:474–79. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 79.Matarese V, Bernlohr DA. Purification of murine adipocyte lipid-binding protein. Characterization as a fatty acid- and retinoic acid-binding protein. J. Biol. Chem. 1988;263:14544–51. [PubMed] [Google Scholar]
- 80.Mayes PA, Topping DL. Regulation of hepatic lipogenesis by plasma free fatty acids: simultaneous studies on lipoprotein secretion, cholesterol synthesis, ketogenesis and gluconeogenesis. Biochem. J. 1974;140:111–14. doi: 10.1042/bj1400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarty MF, Thomas CA. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis—implications for the impact of calcium, vitamin D, and alcohol on body weight. Med. Hypotheses. 2003;61:535–42. doi: 10.1016/s0306-9877(03)00227-5. [DOI] [PubMed] [Google Scholar]
- 82.Melanson EL, Sharp TA, Schneider J, Donahoo WT, Grunwald GK, Hill JO. Relation between calcium intake and fat oxidation in adult humans. Int. J. Obes. Relat. Metab. Disord. 2003;27:196–203. doi: 10.1038/sj.ijo.802202. [DOI] [PubMed] [Google Scholar]
- 83.Merida E, Delgado E, Molina LM, Villanueva-Penacarrillo ML, Valverde I. Presence of glucagon and glucagon-like peptide-1-(7-36)amide receptors in solubilized membranes of human adipose tissue. J. Clin. Endocrinol. Metab. 1993;77:1654–57. doi: 10.1210/jcem.77.6.8263154. [DOI] [PubMed] [Google Scholar]
- 84.Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 2006;281:15837–44. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- 85.Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J. Biol. Chem. 2005;280:43109–20. doi: 10.1074/jbc.M506336200. [DOI] [PubMed] [Google Scholar]
- 86.Muller G, Ertl J, Gerl M, Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J. Biol. Chem. 1997;272:10585–93. doi: 10.1074/jbc.272.16.10585. [DOI] [PubMed] [Google Scholar]
- 87.Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s Biochemistry. Appleton & Lange; Stamford, CT: 2000. p. 927. [Google Scholar]
- 88.Natarajan R, Ghosh S, Grogan W. Catalytic properties of the purified rat hepatic cytosolic cholesteryl ester hydrolase. Biochem. Biophys. Res. Commun. 1996;225:413–19. doi: 10.1006/bbrc.1996.1188. [DOI] [PubMed] [Google Scholar]
- 89.Ni Z, Smogorzewski M, Massry SG. Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology. 1994;135:1837–44. doi: 10.1210/endo.135.5.7525254. [DOI] [PubMed] [Google Scholar]
- 90.Okazaki H, Igarashi M, Nishi M, Tajima M, Sekiya M, et al. Identification of a novel member of the carboxylesterase family that hydrolyzes triacylglycerol: a potential role in adipocyte lipolysis. Diabetes. 2006;55:2091–97. doi: 10.2337/db05-0585. [DOI] [PubMed] [Google Scholar]
- 91.Okazaki H, Osuga J, Tamura Y, Yahagi N, Tomita S, et al. Lipolysis in the absence of hormone-sensitive lipase: evidence for a common mechanism regulating distinct lipases. Diabetes. 2002;51:3368–75. doi: 10.2337/diabetes.51.12.3368. [DOI] [PubMed] [Google Scholar]
- 92.Olsson H, Belfrage P. The regulatory and basal phosphorylation sites of hormone-sensitive lipase are dephosphorylated by protein phosphatase-1, 2A and 2C but not by protein phosphatase-2B. Eur. J. Biochem. 1987;168:399–405. doi: 10.1111/j.1432-1033.1987.tb13433.x. [DOI] [PubMed] [Google Scholar]
- 93.Ostermeyer AG, Paci JM, Zeng Y, Lublin DM, Munro S, Brown DA. Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J. Cell Biol. 2001;152:1071–78. doi: 10.1083/jcb.152.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl. Acad. Sci. USA. 2000;97:787–92. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peers DG, Davies JI. Significance of the caffeine-like effect of various purines, pyrimidines and derivatives on adipose-tissue phosphodiesterase. Biochem. J. 1971;124:P8–9. doi: 10.1042/bj1240008p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perea A, Clemente F, Martinell J, Villanueva-Penacarrillo ML, Valverde I. Physiological effect of glucagon in human isolated adipocytes. Horm. Metab. Res. 1995;27:372–75. doi: 10.1055/s-2007-979981. [DOI] [PubMed] [Google Scholar]
- 97.Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, et al. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes. 1997;46:1939–44. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- 98.Qian H, Hausman GJ, Compton MM, Azain MJ, Hartzell DL, Baile CA. Leptin regulation of peroxisome proliferator-activated receptor-gamma, tumor necrosis factor, and uncoupling protein-2 expression in adipose tissues. Biochem. Biophys. Res. Commun. 1998;246:660–67. doi: 10.1006/bbrc.1998.8680. [DOI] [PubMed] [Google Scholar]
- 99.Raclot T, Leray C, Bach AC, Groscolas R. The selective mobilization of fatty acids is not based on their positional distribution in white-fat-cell triacylglycerols. Biochem. J. 1995;311(Pt. 3):911–16. doi: 10.1042/bj3110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ragolia L, Begum N. Protein phosphatase-1 and insulin action. Mol. Cell. Biochem. 1998;182:49–58. [PubMed] [Google Scholar]
- 101.Razani B, Rubin CS, Lisanti MP. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J. Biol. Chem. 1999;274:26353–60. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- 102.Reynisdottir S, Langin D, Carlstrom K, Holm C, Rossner S, Arner P. Effects of weight reduction on the regulation of lipolysis in adipocytes of women with upper-body obesity. Clin. Sci. (Lond.) 1995;89:421–29. doi: 10.1042/cs0890421. [DOI] [PubMed] [Google Scholar]
- 103.Robenek MJ, Severs NJ, Schlattmann K, Plenz G, Zimmer KP, et al. Lipids partition caveolin-1 from ER membranes into lipid droplets: updating the model of lipid droplet biogenesis. FASEB J. 2004;18:866–68. doi: 10.1096/fj.03-0782fje. [DOI] [PubMed] [Google Scholar]
- 104.Ryden M, Arvidsson E, Blomqvist L, Perbeck L, Dicker A, Arner P. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem. Biophys. Res. Commun. 2004;318:168–75. doi: 10.1016/j.bbrc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 105.Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, et al. Mapping of early signaling events in tumor necrosis factor alpha-mediated lipolysis in human fat cells. J. Biol. Chem. 2002;277:1085–91. doi: 10.1074/jbc.M109498200. [DOI] [PubMed] [Google Scholar]
- 106.Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J. Biol. Chem. 2004;279:35150–58. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]
- 107.Scherer PE, Lisanti MP, Baldini G, Sargiacomo M, Mastick CC, Lodish HF. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol. 1994;127:1233–43. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schrager S. Dietary calcium intake and obesity. J. Am. Board Fam. Pract. 2005;18:205–10. doi: 10.3122/jabfm.18.3.205. [DOI] [PubMed] [Google Scholar]
- 109.Schwartz JP, Jungas RL. Studies on the hormone-sensitive lipase of adipose tissue. J. Lipid Res. 1971;12:553–62. [PubMed] [Google Scholar]
- 110.Shaughnessy S, Smith ER, Kodukula S, Storch J, Fried SK. Adipocyte metabolism in adipocyte fatty acid binding protein knockout mice (aP2−/−) after short-term high-fat feeding: functional compensation by the keratinocyte [correction of keritinocyte] fatty acid binding protein. Diabetes. 2000;49:904–11. doi: 10.2337/diabetes.49.6.904. [DOI] [PubMed] [Google Scholar]
- 111.Shi H, Dirienzo D, Zemel MB. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001;15:291–93. doi: 10.1096/fj.00-0584fje. [DOI] [PubMed] [Google Scholar]
- 112.Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, et al. Direct effects of leptin on brown and white adipose tissue. J. Clin. Invest. 1997;100:2858–64. doi: 10.1172/JCI119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siler SQ, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am. J. Clin. Nutr. 1999;70:928–36. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- 114.Slavin BG, Ong JM, Kern PA. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J. Lipid Res. 1994;35:1535–41. [PubMed] [Google Scholar]
- 115.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7:106–13. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Soni KG, Lehner R, Metalnikov P, O’Donnell P, Semache M, et al. Carboxylesterase 3 (EC 3.1.1.1) is a major adipocyte lipase. J. Biol. Chem. 2004;279:40683–89. doi: 10.1074/jbc.M400541200. [DOI] [PubMed] [Google Scholar]
- 117.Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J. Biol. Chem. 2002;277:8267–72. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 118.Stevens J, Green MH, Kaiser DL, Pohl SL. Insulin resistance in adipocytes from fed and fasted obese rats: dissociation of two insulin actions. Mol. Cell. Biochem. 1981;37:177–83. doi: 10.1007/BF02354886. [DOI] [PubMed] [Google Scholar]
- 119.Stralfors P, Honnor RC. Insulin-induced dephosphorylation of hormone-sensitive lipase. Correlation with lipolysis and cAMP-dependent protein kinase activity. Eur. J. Biochem. 1989;182:379–85. doi: 10.1111/j.1432-1033.1989.tb14842.x. [DOI] [PubMed] [Google Scholar]
- 120.Su CL, Sztalryd C, Contreras JA, Holm C, Kimmel AR, Londos C. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J. Biol. Chem. 2003;278:43615–19. doi: 10.1074/jbc.M301809200. [DOI] [PubMed] [Google Scholar]
- 121.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J. Biol. Chem. 2004;279:42062–71. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 122.Sun X, Zemel MB. Calcium and dairy products inhibit weight and fat regain during ad libitum consumption following energy restriction in aP2-agouti transgenic mice. J. Nutr. 2004;134:3054–60. doi: 10.1093/jn/134.11.3054. [DOI] [PubMed] [Google Scholar]
- 123.Syu LJ, Saltiel AR. Lipotransin: a novel docking protein for hormone-sensitive lipase. Mol. Cell. 1999;4:109–15. doi: 10.1016/s1097-2765(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 124.Sztalryd C, Kraemer FB. Regulation of hormone-sensitive lipase during fasting. Am. J. Physiol. 1994;266:E179–85. doi: 10.1152/ajpendo.1994.266.2.E179. [DOI] [PubMed] [Google Scholar]
- 125.Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, et al. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 2003;161:1093–103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, et al. Functional studies on native and mutated forms of perilipins: a role in protein kinase A-mediated lipolysis of triacylglycerols. J. Biol. Chem. 2003;278:8401–6. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- 127.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2001;98:6494–99. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin A in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004;56:379–85. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- 129.Tornqvist H, Belfrage P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J. Biol. Chem. 1976;251:813–19. [PubMed] [Google Scholar]
- 130.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: Ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 2004;279:47066–75. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 131.Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53:2242–49. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- 132.Voshol PJ, Haemmerle G, Ouwens DM, Zimmermann R, Zechner R, et al. Increased hepatic insulin sensitivity together with decreased hepatic triglyceride stores in hormone-sensitive lipase-deficient mice. Endocrinology. 2003;144:3456–62. doi: 10.1210/en.2002-0036. [DOI] [PubMed] [Google Scholar]
- 133.Walley AJ, Blakemore AI, Froguel P. Genetics of obesity and the prediction of risk for health. Hum. Mol. Genet. 2006;15(Suppl. 2):R124–30. doi: 10.1093/hmg/ddl215. [DOI] [PubMed] [Google Scholar]
- 134.Wang SP, Laurin N, Himms-Hagen J, Rudnicki MA, Levy E, et al. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes. Res. 2001;9:119–28. doi: 10.1038/oby.2001.15. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Sullivan S, Trujillo M, Lee MJ, Schneider SH, et al. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes. Res. 2003;11:930–36. doi: 10.1038/oby.2003.128. [DOI] [PubMed] [Google Scholar]
- 136.Wei E, Lehner R, Vance DE. C/EBPalpha activates the transcription of triacylglycerol hydrolase in 3T3-L1 adipocytes. Biochem. J. 2005;388:959–66. doi: 10.1042/BJ20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 2005;13:1195–204. doi: 10.1038/oby.2005.142. [DOI] [PubMed] [Google Scholar]
- 138.Xue B, Greenberg AG, Kraemer FB, Zemel MB. Mechanism of intracellular calcium ([Ca2+]i) inhibition of lipolysis in human adipocytes. FASEB J. 2001;15:2527–29. doi: 10.1096/fj.01-0278fje. [DOI] [PubMed] [Google Scholar]
- 139.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J. Biol. Chem. 2004;279:30490–97. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 140.Yamaguchi T, Omatsu N, Omukae A, Osumi T. Analysis of interaction partners for perilipin and ADRP on lipid droplets. Mol. Cell. Biochem. 2006;284:167–73. doi: 10.1007/s11010-005-9045-y. [DOI] [PubMed] [Google Scholar]
- 141.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J. Am. Coll. Nutr. 2002;21:146–51S. doi: 10.1080/07315724.2002.10719212. [DOI] [PubMed] [Google Scholar]
- 142.Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes. Res. 2004;12:582–90. doi: 10.1038/oby.2004.67. [DOI] [PubMed] [Google Scholar]
- 143.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signaling to protein kinase A in adipocytes. Nature. 2005;437:569–73. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 144.Zimmermann R, Haemmerle G, Wagner EM, Strauss JG, Kratky D, Zechner R. Decreased fatty acid esterification compensates for the reduced lipolytic activity in hormone-sensitive lipase-deficient white adipose tissue. J. Lipid Res. 2003;44:2089–99. doi: 10.1194/jlr.M300190-JLR200. [DOI] [PubMed] [Google Scholar]
- 145.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–86. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]