Abstract
Background
The androgen pathway remains biologically relevant even in castration resistant prostate cancer (CRPC). In preclinical models, androgen therapy for CRPC leads to growth arrest, apoptosis, and tumor shrinkage. This study determined the toxicity and feasibility of a testosterone therapy in early CRPC.
Methods
Prostate cancer patients with progressive disease following androgen ablation, antiandrogen therapy and withdrawal, with none to minimal metastatic disease were randomized to treatment with transdermal testosterone, at 2.5, 5.0 or 7.5 mg/day. Toxicity, PSA, imaging, quality of life, and strength were monitored. Treatment was discontinued for significant toxicity, clinical progression, or a 3-fold increase in PSA.
Results
Fifteen men, median age 73 (62–92), median PSA 11.1 ng/ml (5.2–63.6), were treated. Testosterone increased from castrate, to median concentrations of 305, 308, 297 ng/dL for 2.5mg (n=4), 5.0mg (n=5), and 7.5mg (n=5) doses, respectively. One patient was taken off study at 53 weeks due to grade 4 cardiac toxicity. There were no other grade 3 or 4 toxicities related to the study medication, and the grade 2 toxicities were minimal. Only one patient experienced symptomatic progression, and 3 (20%) demonstrated a decrease in PSA (largest =43%). Median time to progression was 9 weeks (range 2–96), with no detectable difference in the three dose cohorts. There was no significant change in QOL or hand-grip strength with treatment.
Conclusion
Testosterone is a feasible and reasonably well tolerated therapy for men with early CRPC. A larger, randomized trial is underway to further characterize efficacy and impact on QOL measures.
Introduction
Prostate cancer (PC) is the most common cancer diagnosis in men and, despite increasing diagnosis at earlier stages, remains the second leading cause of cancer related mortality for men in the United States1. Although the 15 year survival for men with newly diagnosed prostate cancer remains high (75%), for patients with advanced, castrate resistant disease, the median survival ranges from 7.5 to 24 months, depending on risk characteristics2,3.
Since the sentinel work by Huggins and Hodges in the 1940’s, it has been well known that prostate cancer is driven by androgens and can be treated with androgen ablation 4,5. As such, medical or surgical castration aimed at lowering systemic androgen levels is the mainstay for initial management of systemic PC6. Although typically very effective initially, in most cases hormone responsiveness is finite. On average, patients with systemic PC fail first line androgen deprivation within 2–3 years, progressing to castration resistant prostate cancer (CRPC), also termed androgen independent or hormone refractory prostate cancer7,8. Moreover, with routine prostate specific antigen (PSA) testing, which can capture progression of prostate cancer often well before clinical or radiological progression is evident, an increasing number of patients are being labeled as castration resistant after primary hormone therapy9. Regardless, early progression is a harbinger of clinical metastatic disease, PC mortality and morbidity. Recently, docetaxel based chemotherapy has been show to provide a modest survival benefit in men with CRPC10,11. Nonetheless, there remains a subset of CRPC patients, often those with low disease burden, in whom further hormonal maneuvers, including addition and withdrawal of antiandrogens, ketoconazole, and estrogens, can be beneficial8,12. Depending on the agent, these therapies can be associated with significant PSA (from 25–50%) and radiologic (10–20%) responses. However, none have demonstrated an improvement in survival or quality of life9. Furthermore, androgen ablation is typically maintained throughout the course of therapy and is associated with significant side effects such as persistent hot flashes, osteoporosis, sexual dysfunction, metabolic/cardiac toxicities, and diminished overall quality of life (QOL)13–15.
The complexity of PC hormonal therapy reflects the intricacies of PC at the cellular and molecular levels. Much of our evolving understanding of prostate cancer pathophysiology revolves around significant biological changes at the molecular level, in particular, those involving the androgen receptor (AR) pathway. Upregulation of AR expression has been linked to progression from hormone sensitive to CRPC under selective pressure from androgen ablation in multiple preclinical and clinical studies 16–18. It is postulated that this amplification can make PC more sensitive to extremely low levels of circulating or intra-tumoral androgens, and in some instances lead to antiandrogens acting paradoxically as AR agonists19.
Interestingly, this AR upregulation may lead to recapitulation of normal prostate epithelial cell growth arrest following androgen exposure 20,21 Liao and colleagues have demonstrated that certain androgen insensitive LNCaP human prostate cancer cell lines adapted to an androgen deprivation are inhibited by physiologic levels of androgen in both in vitro and in vivo models 20,22–24. Similar pre-clinical observations have been demonstrated by other investigators25–27.
In addition, there have been reports of PC patients responding to exogenous testosterone28,29. The most commonly reported study was a retrospective analysis of 52 patients in the pre-PSA era with metastatic prostate cancer of mixed clinical characteristics. The majority (87%) had an “unfavorable” response, however, 7 patients did achieve symptomatic benefit. Based on these data, it has been postulated that there is a subgroup of patients with CRPC who may benefit from exogenous testosterone30,31.
We have therefore conducted a phase I clinical trial of transdermal testosterone treatment for low risk CRPC patients. The primary endpoint of the study was to determine the safety of testosterone treatment in patients with early CRPC. The secondary objectives were to determine the effects of this therapy on systemic testosterone levels and PSA, and on QOL.
Methods
Patient Eligibility
Eligible patients were men 18 years or older, ECOG performance status of 2 or less, with PC displaying evidence of castration resistance with rising PSA after antiandrogen and antiandrogen withdrawal in accordance with standard definitions32, with a minimum absolute PSA value of 3.0 ng/ml. At the time of screening, the patient must have had no evidence of visceral metastasis, and no more than minimal bone metastasis, defined by a bone scan index of ≤1.4%33. Previous cytotoxic or radionuclide therapy was prohibited. All patients provided written informed consent and the clinical trial was approved by the institutional review board of The University of Chicago Medical Center.
Treatment Plan
Medical castration therapy was continued throughout the study. Patients were treated with Androderm® transdermal testosterone system and were randomized to 2.5 mg/day, 5.0mg/day, or 7.5mg/day and patients instructed on patch use in accordance with manufacturers’ specifications34,35. Triamcinolone acetatonide cream (0.1%) was provided with the Androderm patches to decrease the incidence and severity of skin irritation.
Patient Monitoring and Response Evaluation
All patients had baseline laboratory testing, including hormone levels, PSA, basic chemistry, liver function and blood counts. Laboratory studies were repeated at regular intervals. Imaging studies (CT scans of chest/abdomen/pelvis, bone scan) were performed within 4 weeks of study initiation, and repeated every 8 weeks. Patients underwent clinical examination with toxicity evaluation every 2–4 weeks. All adverse events were graded according to the revised NCI Common Toxicity Criteria version 3.0.
Patients were continued on study until disease progression, either by PSA or imaging, unacceptable adverse events, or patient withdrawal of consent. Patients could also be taken off study at the physician’s discretion for clinical progression of disease. Because androgen was expected to increase PSA levels, even in the absence of any effect on tumor burden, a study specific definition of PSA progression of an increase in level to three times the nadir PSA was utilized. All other PSA endpoints were defined per original PSA Working Group criteria32. If a patient developed unequivocal new lesions on imaging, they were considered to have progressive disease, and were taken off study.
In addition, quality of life and strength were monitored throughout the study. QOL measures used included the UCLA Prostate Cancer Index and the Rand SF-36 36,37. Physical performance was assessed using the short physical performance battery, and muscle strength was tested using hand grip strength evaluation38,39.
Statistical Analysis
Subjects were randomly assigned to the three dosing levels of Androderm in a 1:1:1 fashion, according to a computer generated randomization list. A total of 18 patients (6 per dose group) were to be entered into the trial. The primary objective was to determine the safety of testosterone treatment at these dosing levels. A dose level was considered to have acceptable safety if ≤1 patient per dosing group experienced a dose limiting toxicity, defined as CTCAE grade 3 or higher toxicity, or the inability to administer the study medication due to toxicity for >7 consecutive days. The first of two secondary aims was to determine the effect of transdermal testosterone patches on bioavailable serum testosterone levels in men with PC on castration therapy. Based on previous pharmokinetic studies34, testosterone patches can elevate serum testosterone to normal levels from castrate levels with a coefficient of variation in measured testosterone of approximately 45%. There was thus a 94% power to detect a hypothesized testosterone level of 300ng/dl in the 2.5mg (low dose) group, compared to 600ng/dl in the 7.5 mg (high dose) group, under the assumption of equivalent standard deviations of 150ng/dl (1-sided α = 0.05). The effects of transdermal testosterone on QOL, sexual function and muscle strength were tabulated and changes from baseline to on treatment were analyzed using a paired t-test. In addition, changes from baseline were to be compared between dose groups using a linear regression analysis, and the effects of dose and other factors on time to disease progression were assessed using Cox regression analysis 40. Due to small sample number no statistical modeling or imputation for missing data was performed.
Results
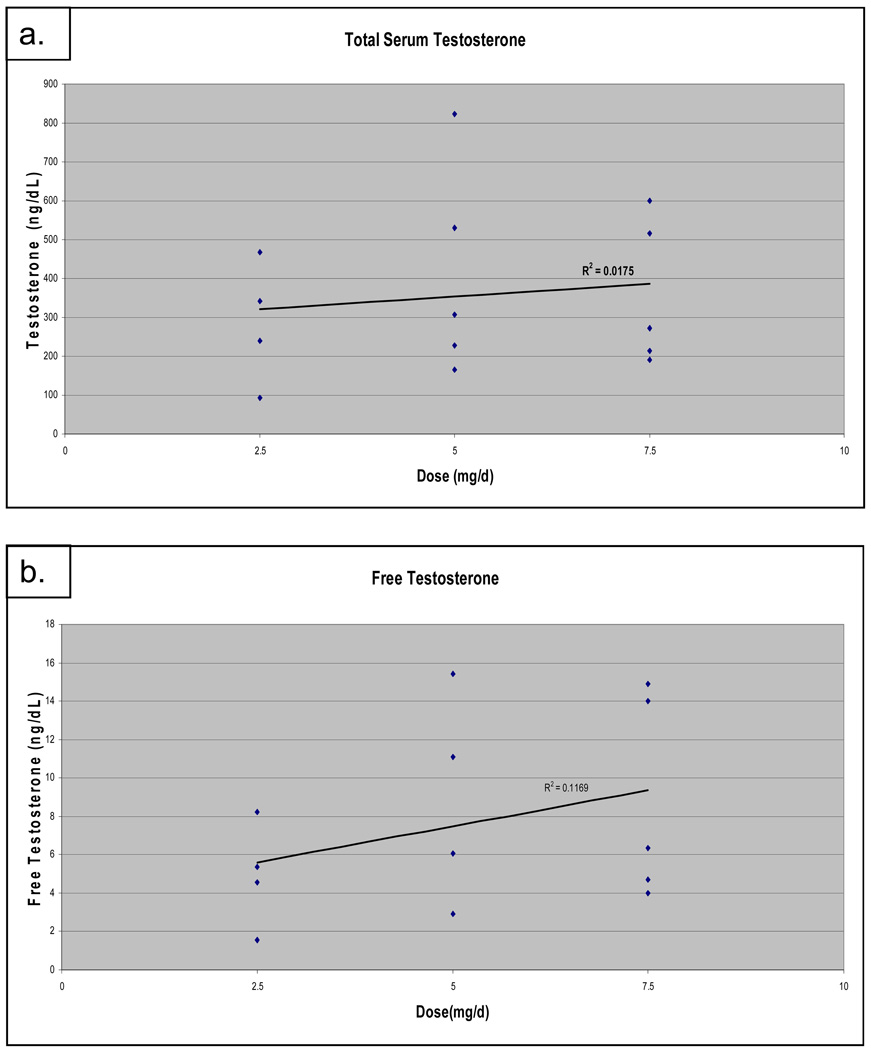
Because another study reported testosterone replacement to be safe41 and a followup phase II trial was opened (see discussion), 16 of the planned 18 patients were consented to participate between August 2004, and March 2007, when the study was closed to accrual. One patient withdrew consent prior to initiating treatment with the study medication and was not included in the analysis, leaving 15 patients randomized to the three treatment arms 2.5mg (n=4), 5.0mg (n =5), 7.5 mg (n=6) (Table 1). The median patient age was 73 years (range 61–92) and the average number of prior hormonal therapies was 2.8 (range 2–4). The median PSA at enrollment was 11.1 ng/ml, with a wide range of 5.3–63.6 ng/ml, and 6/15 patients had evidence of bone metastases prior to enrollment. On treatment, the serum testosterone increased from castrate levels (<30ng/dl), to median concentrations of 291 (range 94–468), 308 (164–824), and 271 (191–599) ng/dL for 2.5mg (n=4), 5.0mg (n=5), and 7.5mg (n=5) doses, respectively (figure 1). These levels reflect the first post treatment testosterone level available for each patient at each dose level. One patient in the 7.5mg/day group had no available post treatment level. For those patients that had more than one measurement post treatment, no large variation was seen amongst those levels. Using a linear regression model, there was no significant dose response (R2=0.018, p=0.65). There were slight variations in SHBG and albumin, however, calculation of free testosterone levels generated similar results (R2=0.117, p=0.20)42. As expected for patients continued on LHRH agonist therapy, LH and FSH levels were suppressed, and did not vary significantly between any of the treatment groups.
Table 1.
Baseline Patient Characteristics
| 2.5mg/d (n=4) |
5.0mg/d (n=5) |
7.5mg/d (n=6) |
Total† (n=15) |
|
|---|---|---|---|---|
| Median Age (range) |
66.5 (61–81) |
81 (69–81) |
72.5 (62–92) |
73 (61–92) |
| Median PSA ng/ml (range) |
8.4 (5.4–23.6) |
14.3 (7.0–63.6) |
13.2 (5.3–46.4) |
17.7 (5.3–63.6) |
| Mean Prior Hormonal Therapies* (range) |
2.75 (2–3) |
3.0 (2–3) |
3.0 (2–4) |
2.8 (2–4) |
| Bicalutamide | 3 | 4 | 5 | 12 |
| Flutamide | 1 | 0 | 2 | 3 |
| Nilutamide | 1 | 2 | 1 | 4 |
| Ketoconazole | 1 | 1 | 2 | 4 |
| Finasteride** | 1 | 1 | 1 | 3 |
| Diethylsilbestrol | 0 | 1 | 0 | 1 |
| Baseline Testosterone (range) |
19 (<10–22) |
16 (<10–23) |
17 (<10–20) |
18 (<10–23) |
| # with Bone Metastases | 3 | 3 | 0 | 6 |
16 of planned 18 patients consented (1 withdrew consent prior to therapy) when study closed due to presentation of similarly designed phase I trial showing safety41, and opening of phase II trial
Prior hormonal therapies including castration (medical or surgical), not counting antiandrogen withdrawal
In combination with an antiandrogen
Figure 1.
Testosterone levels on treatment by treatment group. Total (a) and free (b) testosterone levels for all patients on study who had at least one post treatment level. Using a linear regression model, there was no significant dose response for either total (p=.65) or free (p=.20) testosterone on treatment.
Overall, testosterone therapy was well tolerated at all doses tested. There was one grade 4 cardiac toxicity, a myocardial infarction, in the 7.5mg/day group at week 53, that was deemed possibly related to study medication. There were no other cardiac toxicities noted, nor were there any other grade 3 or 4 toxicities reported throughout the trial. The grade 2 toxicities were minimal and included hot flashes (n=2), hyperglycemia (n=2), skin rash (n=1), and hypertension (n=1). None of these toxicities required medical intervention or dose adjustment.
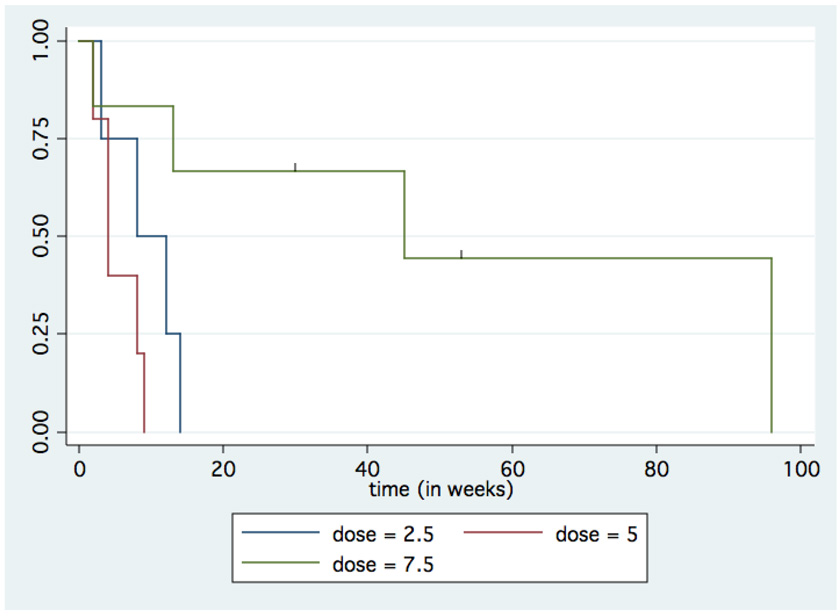
The majority of patients were eventually taken off study due to progression of disease by either PSA (n = 9) or both imaging and PSA (n = 3) criteria. In addition to the patient who was taken off study due to his cardiac event, one patient was taken off study due to insurance issues precluding regular follow-up, and one patient was taken off study due to a decline in performance status, possibly due to symptomatic progression of disease. The median time to progression was 9 weeks, with a range from 2–96 weeks, and an estimated mean of 26 weeks. Per dosing level, the median times to progression were 10 (3–14), 4 (2–9), and 45 (2–96; two censored at 30 and 53) weeks for the 2.5mg, 5.0mg, and 7.5mg dose groups, respectively (Figure 2). There was no statistically significant relationship between dose or testosterone level and time to progression using a Cox proportional hazards model (p=0.072 and p=0.14, respectively). Baseline PSA and presence/absence of bone metastases were not associated with time to progression (p=0.59 and p=0.13).
Figure 2.
Time to progression (TTP) by treatment group. Kaplan-Meier curve showing TTP on treatment for each treatment dose group. Using a Cox proportional hazards model the relationship between dose and TTP was not statistically significant (p=0.072).
Three patients demonstrated a decrease in PSA while on treatment (maximum of 16%, 20%, 43%), with the largest decrease being from 41.3 ng/ml to 23.4 ng/ml. Of these, 1 had an initial PSA increase of 69% (from 8.5 to 12.3), followed by a decrease of 55% (from 12.3 to 6.75). In addition, 7 patients without a PSA decline remained on treatment for at least 8 weeks, with the longest duration of therapy in this group being 53 weeks. One of these patients had an initial rise in PSA of 68% (from 10.6 ng/ml to 15.5 ng/ml), followed by at 44% decline, after which the PSA fluctuated from 12 ng/ml–18 ng/ml for another 47 weeks. In 12 of the 14 patients with available follow up PSA measurements, the PSA fell after cessation of study medication, with a median percent decline of 47% (range 14–71%) from the maximum. Only one patient returned to his pretreatment PSA after cessation of study medication. As noted above, only one patient had any evidence of possible symptomatic progression.
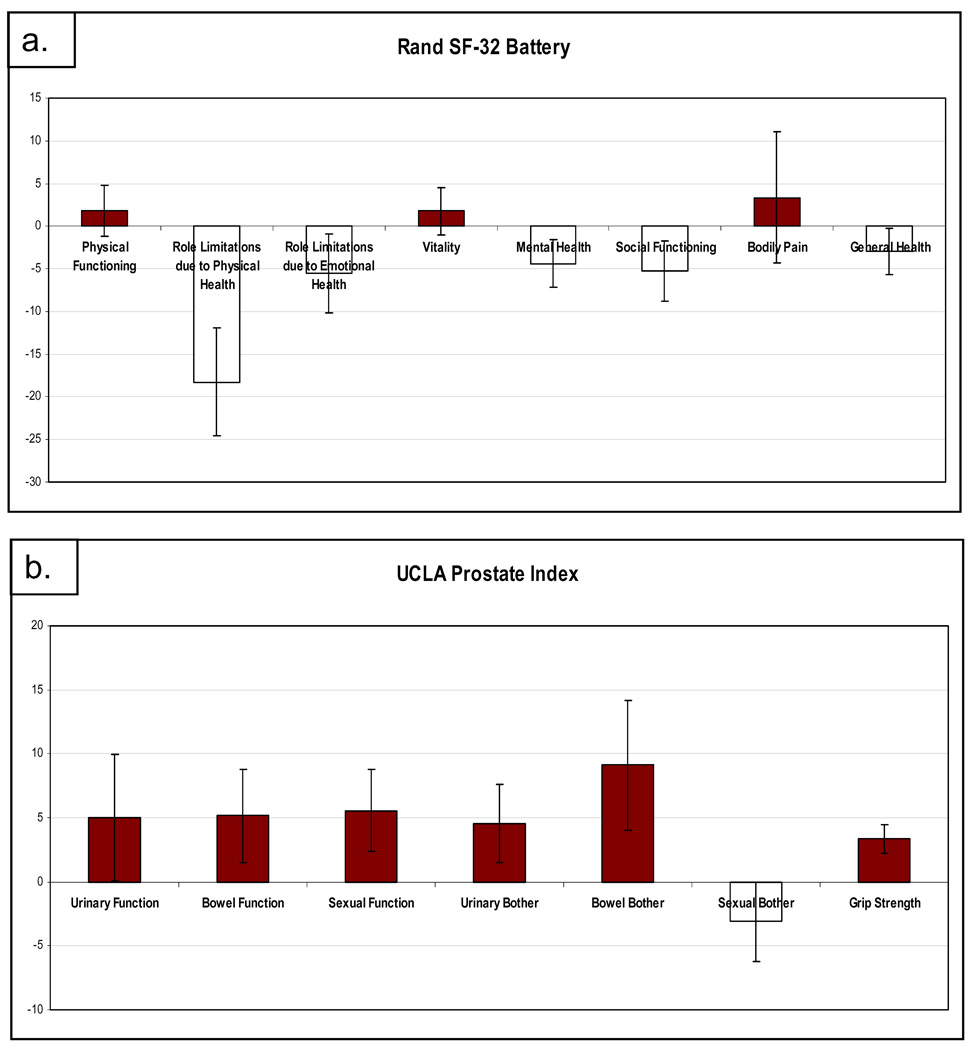
Quality of life was measured along with muscle strength throughout the study. As shown in figure 3, when all patients treated are analyzed together, the measures of quality of life varied from baseline to first assessment post treatment. There were no significant changes seen in role limitations due to emotional health (p=.26), social functioning (p=0.17), mental health (p=0.14) general health (p=0.31), physical functioning (p=0.56), bowel bother (p=0.10), urinary bother (p=0.17), sexual function (p=0.11), urinary function (p=0.33), vitality (p=0.55), bodily pain (p=0.71), bowel function (p=0.19), or sexual bother (p=0.35). There was a statistically significant decline in role limitations due to physical health (mean −18.25%, 95% confidence interval (−32.3, −4.3) p=0.015). Only 5 patients had grip strength measured pre and post treatment. The mean percent change in grip strength was +3.3%, range 0.7–6%. While this reached statistical significance (p=0.042), the result could be biased due to missing data for 10 of the patients.
Figure 3.
Quality of Life using the SF-32 (a), and UCLA Prostate Index (b). Graphs depict cumulative mean change in QOL with treatment with standard error bars.
Conclusions
This study represents the second trial of testosterone treatment for castration resistant prostate cancer in the current era of PSA monitoring and improved imaging modalities. Men with early, low burden castrate resistant disease were treated with 3 different doses of transdermal testosterone, with the primary purpose being to determine the toxicity and feasibility of testosterone therapy in this patient population. As described, the therapy was very well tolerated. There was one grade 4 cardiac event possibly related to study drug, and one possible symptomatic disease progression. Cardiovascular disease is the most common cause of death in men with prostate cancer who do not die of the cancer itself. Furthermore, there is a growing body of evidence that androgen deprivation therapy is associated with increased cardiovascular morbidity and mortality 43–45. As all of our patients are on long term androgen suppression, it is possible that androgen deprivation, along with the more common and predictable cardiovascular risks associated with elderly men contributed to the cause of this patients myocardial infarction. Whether testosterone supplementation can increase cardiovascular risk is unclear 46.
This trial used three escalating doses of transdermal testosterone with the hypothesis that there would be a dose response effect in measured serum testosterone levels. Based on pharmacokinetic studies of transdermal testosterone patches, it is well established that measured serum testosterone levels can vary greatly depending on time of patch application, time of blood draw, and metabolism. Peak levels are typically achieved 4–6 hours after application, after which, testosterone levels fall steadily34,47. Although all our patients were instructed to apply the patch at night, variations in timing of patch application and corresponding blood draw could have accounted for the lack of dose response of testosterone levels within this trial.
There was no statistically significant correlation of measured testosterone level with time to progression, but this is not unexpected for such a small trial in which time to progression is dominated by prognostic disease characteristics, even if the underlying hypothesis of testosterone being growth inhibitory in certain patients is true. Interestingly, the relationship between dose and time to progression approached significance (p=0.072) with delayed progression in the high dose group, but this should be interpreted very guardedly, again due to the small sample size.
In support of the underlying hypothesis that testosterone may have growth inhibitory effects in patients with castrate resistant disease, there were 3 men whose PSA declined, and several others who had apparent disease stability while on study, with four patients staying on study for over 6 months. These results are similar to a preliminary report of a phase I trial of testosterone therapy in castrate resistant metastatic prostate cancer from Memorial Sloan Kettering Cancer Center41. This observation is particularly interesting in light of the fact that PSA is an androgen regulated gene and would thus be expected to be upregulated in response to increased testosterone levels. The patients with an initial PSA increase followed by a decrease or stabilization is exactly what would have been predicted from the preclinical models20. Nevertheless, PSA changes in the context of an uncontrolled trial must be interpreted with caution and cannot be definitively linked to patient benefit.
There was no statistically significant change in QOL noted with testosterone treatment in this trial, except for a slight decline in the physical role limitations scale. Whether this is due to the small study size, the insensitivity of the measurement instruments, or lack of testosterone effect in patients with long term prior androgen ablation cannot be determined at this time. It is expected that QOL measures would decline in an untreated control group with progressive prostate cancer on continuous androgen deprivation. A controlled study would thus be necessary to determine whether testosterone could minimize or ameliorate such a decline. Grip strength did improve, but this was assessed in only five of the men and a bias due to missing data cannot be ruled out. Furthermore, it will be interesting to note any changes in these measures in patients who remain on testosterone therapy for longer time periods. In this context it is notable that a recent study of oral testosterone supplementation for 6 months in hypogonadsal, but not castrate, men, did demonstrate modest effects on muscle mass, but was unable to demonstrate any significant changes in measures of strength or QOL, including the SF-3648.
In sum, this phase I trial of testosterone treatment in low risk CRPC demonstrates that the therapy is well tolerated and safe. There is furthermore a suggestion of anti-tumor effect based on a few patients with PSA decreases and long term disease stabilization in a subset of patients. A larger, placebo controlled, randomized study of testosterone in CRPC patients has thus been initiated to more accurately determine the effects of testosterone on disease progression and quality of life.
Acknowledgments
Support for Research: Internal funds from the University of Chicago
Footnotes
Prior Presentation of Data:
Poster presentation, ASCO Genitourinary Symposium, 2008
Disclaimers: None
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 3.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges C. Studies on prostatic cancer. I. The effets of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–297. [Google Scholar]
- 5.Huggins C, Stevens RJ, Hodges C. Studies on prostatic cancer. II. The effects of castration on advanced carcinoma of the prostate gland. Archives of Surgery. 1941;43:209–223. Archives of Surgery 43:209–223, 1941. [Google Scholar]
- 6.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 7.Daskivich TJ, Oh WK. Recent progress in hormonal therapy for advanced prostate cancer. Curr Opin Urol. 2006;16:173–178. doi: 10.1097/01.mou.0000193392.77469.e2. [DOI] [PubMed] [Google Scholar]
- 8.Vogelzang N. Comprehensive textbook of genitourinary oncology. ed 3rd. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 9.Lam JS, Leppert JT, Vemulapalli SN, et al. Secondary hormonal therapy for advanced prostate cancer. Journal of Urology. 2006;175:27–34. doi: 10.1016/S0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- 10.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 11.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 12.Mohler J, Babaian RJ, Bahnson RR, et al. Prostate cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2007;5:650–683. [PubMed] [Google Scholar]
- 13.Gomella LG. Contemporary use of hormonal therapy in prostate cancer: managing complications and addressing quality-of-life issues. BJU Int. 2007;99 Suppl 1:25–29. doi: 10.1111/j.1464-410X.2007.06598.x. discussion 30. [DOI] [PubMed] [Google Scholar]
- 14.Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–504. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 16.Edwards J, Krishna NS, Grigor KM, et al. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89:552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Furihata M, Tsunoda T, et al. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67:5117–5125. doi: 10.1158/0008-5472.CAN-06-4040. [DOI] [PubMed] [Google Scholar]
- 19.Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 20.Umekita Y, Hiipakka RA, Kokontis JM, et al. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci U S A. 1996;93:11802–11807. doi: 10.1073/pnas.93.21.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitacre DC, Chauhan S, Davis T, et al. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1–11. [PubMed] [Google Scholar]
- 22.Kokontis JM, Hay N, Liao S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12:941–953. doi: 10.1210/mend.12.7.0136. [DOI] [PubMed] [Google Scholar]
- 23.Chuu CP, Hiipakka RA, Fukuchi J, et al. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082–2084. doi: 10.1158/0008-5472.CAN-04-3992. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Kokontis J, Tang F, et al. Androgen and its receptor promote Baxmediated apoptosis. Mol Cell Biol. 2006;26:1908–1916. doi: 10.1128/MCB.26.5.1908-1916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heisler LE, Evangelou A, Lew AM, et al. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 26.Joly-Pharaboz MO, Ruffion A, Roch A, et al. Inhibition of growth and induction of apoptosis by androgens of a variant of LNCaP cell line. J Steroid Biochem Mol Biol. 2000;73:237–249. doi: 10.1016/s0960-0760(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 27.Wolf DA, Schulz P, Fittler F. Synthetic androgens suppress the transformed phenotype in the human prostate carcinoma cell line LNCaP. Br J Cancer. 1991;64:47–53. doi: 10.1038/bjc.1991.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prout GR, Jr, Brewer WR. Response of men with advanced prostatic carcinoma to exogenous administration of testosterone. Cancer. 1967;20:1871–1878. doi: 10.1002/1097-0142(196711)20:11<1871::aid-cncr2820201112>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Fowler JE, Jr, Whitmore WF., Jr The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J Urol. 1981;126:372–375. doi: 10.1016/s0022-5347(17)54531-0. [DOI] [PubMed] [Google Scholar]
- 30.Prehn RT. On the prevention and therapy of prostate cancer by androgen administration. Cancer Res. 1999;59:4161–4164. [PubMed] [Google Scholar]
- 31.Soto AM, Sonnenschein C. The two faces of janus: sex steroids as mediators of both cell proliferation and cell death. J Natl Cancer Inst. 2001;93:1673–1675. doi: 10.1093/jnci/93.22.1673. [DOI] [PubMed] [Google Scholar]
- 32.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 33.Imbriaco M, Larson SM, Yeung HW, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the Bone Scan Index. Clin Cancer Res. 1998;4:1765–1772. [PubMed] [Google Scholar]
- 34.Rolf C, Gottschalk I, Behre HM, et al. Pharmacokinetics of new testosterone transdermal therapeutic systems in gonadotropin-releasing hormone antagonist-suppressed normal men. Exp Clin Endocrinol Diabetes. 1999;107:63–69. doi: 10.1055/s-0029-1212075. [DOI] [PubMed] [Google Scholar]
- 35.Dienstmann R, Da Silva Pinto C, Tutungi Pereira M, et al. Palliative percutaneous nephrostomy in 26 patients with recurrent cervical cancer. J Clin Oncol. 2005;23:8235. (Meeting Abstracts) [Google Scholar]
- 36.Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Lubeck DP, Grossfeld GD, Carroll PR. The effect of androgen deprivation therapy on health-related quality of life in men with prostate cancer. Urology. 2001;58:94–100. doi: 10.1016/s0090-4295(01)01250-x. [DOI] [PubMed] [Google Scholar]
- 38.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 39.Kenny AM, Prestwood KM, Gruman CA, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–M272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- 40.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methologolical) 1972;34:187–220. [Google Scholar]
- 41.Morris MJ, Kelly WK, Slovin S, et al. Phase I trial of exogenous testosterone (T) for the treatment of castrate metastatic prostate cancer (PC) J Clin Oncol. 2004;22:4560. (Meeting Abstracts) [Google Scholar]
- 42.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 43.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 44.Tsai HK, D'Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 45.D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 46.Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 47.Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with biweekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84:3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 48.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]