Abstract
Attention and memory deficits are among the most prominent cognitive disturbances observed in schizophrenia. It has been suggested that a disruption in anatomical connectivity between areas involved in attentional control might be responsible for these abnormalities. We used Diffusion Tensor Tractography and Color Stroop/Negative Priming(NP) paradigm to investigate integrity of Cingulum Bundle(CB), the main white matter tract interconnecting these regions, and its relationship with executive functions in patients with schizophrenia and matched controls. The Fractional Anisotropy(FA), was calculated along the CB pathways, and correlated with reaction times for each Stroop item, and both Stroop, and NP effects. Patients with schizophrenia demonstrated decreased CB integrity and diminished NP effect, compared with controls, but both groups showed Stroop effect. For patients only, reaction times for every item, as well as for Stroop effect, correlated with left CB FA. These findings suggest that CB integrity disruptions might compromise the executive processes in schizophrenia.
Keywords: diffusion, white matter, schizophrenia, attention, memory
INTRODUCTION
Deficits in attention and memory, two core mental processes involved in executive control, are prominent cognitive disturbances in schizophrenia (Kraepelin 1919/1971). Brain regions involved in executive control are located predominantly in the “frontal” part of the brain, and form a “network” that includes the dorsolateral prefrontal cortex (DLPFC), anterior cingulate gyrus (ACC), and medial parietal, as well as lateral parietal regions. The two main functions of these areas are: 1) evaluation and monitoring (i.e., detecting conflicts in information processing) (e.g., Botvinick et al. 2001, Carter et al. 2001, Cohen and Shoup 2000, Gehring and Knight 2000), and 2) regulation and control (often referred to as executive-attention) (e.g., Fan and Posner 2004).
The Color Stroop paradigm is one of the most frequently used tasks to evaluate the two aforementioned main functions of executive control (e.g., MacLeod and MacDonald 2000, Harrison et al. 2005, Kerns et al. 2004, McNeely et al. 2003, van Veen and Carter 2005, Posner and Petersen 1990). In this task, color words are typically printed in color fonts. The task is to name the color of the font in which a word is typed. In the congruent condition, a color word is printed in the color font corresponding to the name of the color word, e.g., RED printed in red font. In the incongruent Stroop condition, word-reading competes with the color-naming response (i.e., when the word RED is written in blue ink), causing interference, which results in slower response time to the target stimuli (i.e., ink color).
Brain imaging studies of healthy controls suggest that the ACC, and to lesser extent DLPFC and parietal regions, are more active in incongruent trials compared with congruent trials (e.g., Carter et al. 1995, Gruber et al. 2002, Pardo et al. 1990) or neutral trials (e.g., Bench et al. 1993, Carter et al. 1995, George et al. 1994).
Such differential activity is reduced in patients with schizophrenia, suggesting a disease-related disturbance in conflict monitoring in this brain circuit (fMRI study-Carter et al. 1997). Behavioral studies using the Stroop paradigm in a single-trial version in schizophrenia have, however, produced mixed results, with some studies reporting increased facilitation in schizophrenia patients, with normal levels of interference, while other studies, especially those using long inter-stimulus interval (ISI), reporting either increased Stroop interference or increased Stroop error rates (see MacLeod and MacDonald 2000, Henik and Salo 2004 for a review).
Attention itself has generally been described as an active process where the “to be attended” information is actively processed, while unattended information (such as incongruent color font in the Stroop paradigm) is either ignored, or processed at a superficial level. Recently, however, it has been suggested that unattended information is actually actively processed as well, and thus can have a sustained effect on the processing of subsequent, “to be attended” information. The notion that selective attention processes rely on short term “working” memory, has often been tested within the context of the Stroop paradigm using a negative priming condition. In this context, negative priming (NP) refers to slowed response to a target that was a distractor and has been ignored in a previous trail, such as RED printed in blue preceded by BLUE printed in green (i.e. BLUE distractor turns to blue target) (e.g., Neill and Kaufman 1977, Tipper 1985). This effect has been studied extensively to understand attention selection mechanisms in healthy controls (e.g., Tipper 1985, Neill et al. 1992, Park and Kanwisher 1994, MacDonald et al. 1999). Negative priming has also been studied in schizophrenia, with most studies reporting a lack of this effect (e.g., Salo et al. 1996, Salo et al. 1997, Salo et al. 2002, Laplante et al. 1992, MacQueen et al. 2003, Beech et al. 1989).
Early interpretations of NP effects pointed to the active inhibition of the distractor, leading to slowed reaction to distractors when they became targets (Tipper 1985, Tipper et al. 1994). According to this view, the lack of NP in schizophrenia would be the effect of either inefficient inhibition of the distractor, or fading of the inhibitory tag during the inter trial interval (e.g., MacQueen et al. 2003, Salo et al. 1996, Salo et al. 1997, Salo et al. 2002). Recently this idea has been challenged, as it has been pointed out that NP exists even when distractors are attended to (MacDonald et al. 1999). Thus rather than being the result of actively inhibiting previous stimulus, NP is thought to be the effect of additional interference between “incongruent” attended to and recently encoded information (MacDonald and Joordens 2000), thereby implicating episodic memory mechanisms. The only existing NP event related fMRI experiment in healthy subjects also points to the episodic memory retrieval mechanism localized to dorsolateral prefrontal cortex as the region responsible for NP effects (Egner and Hirsch 2005). Thus, according to this view, a lack of NP in schizophrenia would be related to deficits in short-term episodic memory (Neill et al., 1992).
As reviewed above, fMRI Stroop/NP experiments suggest that executive control relies on efficient communication between substrates of the anterior attentional network. The presence of functional abnormalities in schizophrenia in all substrates of this network (e.g., Kerns et al. 2005, Weiss et al. 2003) is also consistent with theories of anatomical and functional disconnectivity in schizophrenia (e.g., Weinberger et al. 1992, Frith et al. 1995, Fletcher et al. 1999, Ford et al. 2001). According to this theory, many of the cognitive abnormalities observed in schizophrenia can be related to faulty anatomical connections (provided by white matter fiber tracts) between brain areas constituting functional networks. Thus cognitive deficits observed in schizophrenia during Stroop/NP experiment, could be attributed to decreased integrity of the cingulum bundle, the main fiber bundle interconnecting the anterior attentional network.
The ability to evaluate such connections, however, had to await the advent of appropriate neuroimaging tools. It has only been recently that diffusion tensor imaging (DTI) has emerged as one of the most efficient in vivo tools to investigate anatomical connections in schizophrenia. The technique is based on detecting the motion of water molecules in the presence of directionally oriented magnetic gradients, thus making it sensitive to the direction and orientation of water diffusion in the brain. Fractional Anisotropy, a measure of anisotropy of water diffusion, is sensitive to axonal as well as myelin loss, in multiple clinical populations (e.g., Ge et al. 2002, Grossman et al. 1994, Rovaris et al. 2000). In addition, DTI- physiology experiments demonstrate that diffusion is restricted (anisotropic), in both myelinated and nonmyelinated fibers (Beaulieu and Allen 1994), but the degree of diffusion anisotropy in white matter increases during myelination processes (Huppi et al. 1998, Baratti et al. 1999), and decreases in pathological demyelination processes (Filippi et al. 2001).
Multiple DTI schizophrenia studies, to-date, have demonstrated decreased integrity of the cingulum bundle (CB), the main white matter fiber tract interconnecting regions mediating executive control (Kubicki et al. 2003, Wang et al. 2004, Hubl et al. 2004). The Cingulum Bundle is a 5-7 mm in diameter fiber tract that interconnects all parts of the Limbic System. It originates within the white matter of the temporal pole, and runs posterior and superior into the parietal lobe, then turns, forming a “ring-like belt” around the corpus callosum, into the frontal lobe, terminating anterior and inferior to the genu of the corpus callosum in the orbital-frontal cortex (Schmahmann 2006). Moreover, the CB consists of long, association fibers that directly connect temporal and frontal lobes, as well as shorter fibers radiating into their own gyri. The CB also includes most afferent and efferent cortical connections of cingulate cortex, including those of prefrontal, parietal and temporal areas, and the thalamostriatae bundle (Domesick 1970). In addition, lesion studies (e.g., Laplane et al. 1981) document a variety of neurobehavioral deficits resulting from a lesion located in this area, including akinetic mutism, apathy, transient motor aphasia, attentional deficits, motor activation, and memory deficits. The role of this fiber bundle, and the relationship between abnormalities in this bundle and executive control in schizophrenia, however, remains to be determined.
In the current study, we investigated the relationship between the anterior cingulum bundle integrity, as measured by DTI, and performance on the single-trial Color Stroop with negative priming, in patients with chronic schizophrenia and healthy controls. Given the fact that CB is an integral part of the anterior attentional network, and that these experiments involve the cooperation of all parts of this network, we predicted that CB integrity would be associated with conduction speed, i.e., thus affecting reaction times to the presented stimuli, with more impact on processes requiring extensive communication between involved areas (i.e., conflict resolution and memory encoding/retrieval). Accordingly, we predicted that CB integrity would be decreased in schizophrenia, and that this abnormality would predict increase of reaction times and affect patients’ performance on Stroop and Negative Priming paradigms. The relationship between white matter integrity and cognitive performance investigated here, namely between attention, working memory, reaction times and cingulum bundle integrity has been suspected, or even assumed, as discussed above, but despite preliminary findings regarding some of its components (see related work on CB and attentional control (Nestor et al., 2008), as well as attention and executive function (Lim et al., 2006)), never clearly demonstrated. This is, to our knowledge, one of the first schizophrenia studies to test directly such a relationship (relationship between FA within the frontostriatal WM and Stroop interference was also demonstrated in patients with geriatric depression (Murphy et al., 2007)), and the first to use advanced analytic techniques, such as fiber tractography, to achieve this goal.
METHODS
Subjects
Eighteen patients with schizophrenia were recruited from in-patient, day treatment, out-patient, and foster care programs at the VA Boston Healthcare System, Brockton, MA. SCID-P interviews were administered to make DSM-IV diagnoses, and SCID-NP interviews were completed for 18 normal comparison subjects. All participants were tested on behavioral measures. Fifteen patients and fifteen comparison subjects undewent DTI-MRI.
Comparison subjects were recruited from the general community and group-matched to patients on age, sex, handedness, and parental social economic-status (PSES).
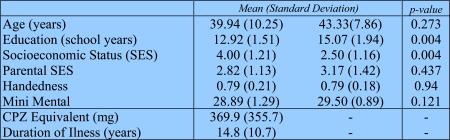
Inclusion criteria for all subjects were: right-handedness, ages between 18 and 55 years, no history of electroconvulsive shock treatment, no history of neurological illness, no alcohol or drug dependence in the last 5 years and no abuse in the past year, verbal IQ above 70, no medication with deleterious effects on neurological or cognitive functions and an ability and desire to cooperate with the procedures confirmed by a written informed consent. In addition, normal comparison subjects were screened to exclude individuals who had a first degree relative with an Axis I disorder. The study was approved by the local IRB committee, and all subjects signed informed consent prior to study participation. The demographic and clinical characteristics of the two study groups are included in table 1.
|
MRI Protocol
For all the subjects, DTI data were acquired on a 1.5 Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, WI), with a quadrature head coil, using line scan diffusion imaging (LSDI), and the acquisition protocol described previously (Kubicki et al. 2002, Kubicki et al. 2003). Coronal oblique 1.7 × 1.7 × 4 mm slices covering the entire brain (32-35 slices depending on head size) were acquired perpendicular to both interhemispheric fissure and AC-PC line, with TE (echo time) 70 ms; TR (repetition time) 80 ms (effective TR 2500 ms). We acquired 6 independent diffusion directions (B=1000) and baseline images (B=5). Data were analyzed using in-house software: slicer 2.7 (www.slicer.org), and dodti (http://neuroimage.yonsei.ac.kr/~dodti, Park et al. 2004).
Image Analysis
After tensor reconstruction, which involved eddy current distortion correction, as well as movement correction, maps of eigenvectors, eigenvalues, FA and diffusivity were calculated. Out-of-plane directional diffusion maps were filtered (flux diffusion filter, Krissian 2002), and CB regions of interest (ROIs) were automatically detected on two slices, first one that included genu of the corpus callosum, and the last one still including splenium of the corpus callosum, using a surface evolution automated segmentation method (levelsets, Krissian and Westin 2003, Krissian in press). This algorithm is a fast and efficient way of finding contours of the white matter structures, based on the directional diffusion differences between white matter tract and surrounding brain tissue. Regions of interest were then used in the next step to guide fiber tractography. Fiber tractography, a post-processing method for propagating streamline points by following the local fiber orientation, as defined by the diffusion tensor field (Mori et al. 1999, Basser et al. 2000) was then used to generate cingulum bundle fiber tracts. We used the fourth order Runge-Kutta method for the integration solver (Press et al. 1992). Instead of tracking fiber bundles starting from an ROI seed points, which is more prone to the partial volume effects and can limit the number of fiber tracts included in the analysis (Mori and Van Zijl 2002), first, we reconstructed entire white matter fiber bundles from seed points assigned to all voxels inside the white matter segmentation of B0-images. Stopping criteria for fiber tracking included a low fractional anisotropy (FA) (0.15) and a rapid change of direction (20° per 1 mm) (Jones et al. 2005). In addition, in order to account for nonisotropic voxels, and avoid rapid change of direction due to the low out-of-plane data resolution and noise, a trilinear interpolation method was used for obtaining subvoxel tensor estimation with a 1-mm step size.
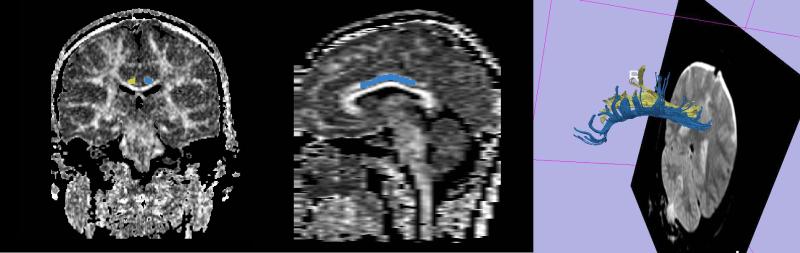
The next step included fiber extraction, which was done using ROIs defined previously. After the algorithm automatically excluded fibers that did not travel through two ROIs, mean FA averaged over all the voxels belonging to the CB fiber bundle (Figure 1) was calculated separately for left and right sides, and compared between groups, as well as subjected to correlational analysis.
1.
Left- Fractional Anisotropy map containing left and right cingulum bundle regions of interest, Middle- Sagittal view of FA map and cingulum bundle, showing extension of CB region of interest, right- 3D model of the cingulum bundle tractography generated from ROIs (yellow –right, blue-left).
Color Stroop Paradigm
All subjects were tested using a single-trial computerized version of the Color Stroop Paradigm, a classic behavioral task of attention control and conflict monitoring (Stroop 1935). The original Stroop experiment consisted of incongruent and neutral trials (Stroop 1935), however since the introduction of the congruent condition (Dalrymple-Alford and Budayer 1966), many experiments use this condition as a baseline (Pardo et al. 1990, Carter et al. 1995, Posner et al. 2002) for calculating the Stroop effect. Since the facilitation effect is still debatable [viewed sometimes as a byproduct of faster color reading (in congruent condition) than color naming (in neutral condition)] (Dunbar and MacLeod 1984), and minimal compared to interference effect, and since the functional experiments using neutral and congruent conditions as baseline give virtually the same results of Stroop effect, for the sake of experiment simplicity we decided to use congruent and incongruent conditions to calculate “Stroop effect” as a difference between incongruent and congruent reaction times. Subjects were asked to name the ink color of colored words (e.g., RED in blue ink). For the incongruent trials, dominant lexical representation (name of the color) entries interfere, compete, or conflict with the perceptual representation (ink color) requiring control, overriding, or inhibition. In addition, we measured negative priming effect, reflected behaviorally by particularly slow responses to the ink color of an item (e.g., GREEN printed in red ink) that is preceded by the presumably previously encoded and inhibited color word (e.g., RED printed in yellow) of the previous item (Table 2). All subjects completed 1 practice run and 1 test run of 73 stimuli each; 22 congruent and 51 incongruent (21 with negative priming effect). Each stimulus stayed on the screen until the subject made a response (button press) (with the maximum exposure duration of 4 seconds). The next stimulus appeared on the screen 2.5 seconds after the response. Stimuli were the words RED, GREEN, YELLOW, and BLUE, printed in one of these four colors in 45 Arial font, against a white background, and were presented sequentially in the middle of a computer screen. Stimuli were delivered using Presentation software (version 9.0 Neurobehavioral Systems, www.neurobs.com). Subjects were asked to identify the font color in which each word was printed, using one of four arrow keys on a standard keyboard. Reaction times were recorded for each stimulus type (condition). Mean reaction times for correct responses only and for each condition separately were calculated for each subject, and analyzed using SPSS software.
| Stimulus |
Distractor |
Target |
Type of Trial |
|---|---|---|---|
| BLUE |
blue |
blue |
Congruent |
| RED |
red |
yellow |
Incongruent |
| BLUE | blue | blue | Stroop Effect |
| RED |
red |
yellow |
|
| RED | red | yellow | Negative Priming Effect |
| GREEN | green | red |
Statistical Analysis
Mean FA values for the CB fiber tracts were computed separately for the left and right sides for each subject and entered into analyses with group as a between variable (controls and patients) and FA values for right and left CB as within variables. Likewise, reaction times (correct responses only) for the congruent and incongruent Stroop trials, as well as for the incongruent with negative priming and incongruent without negative priming trials were entered separately for analyses using the SPSS statistical software package (SPSS 11). Between group comparisons (two levels: controls and patients) were performed using ANOVAs with trial type (incongruent total and congruent for Stroop and incongruent with and without negative priming for NP) as within group factors. Follow-up within group comparisons were conducted using paired t-tests to estimate Stroop (congruent and incongruent trials) and Negative Priming effects (incongruent with and without negative priming) for each group. Errors, due to the relatively long inter-stimulus interval, as well as training session, were at a very low level (97.7% correct responses for controls, and 97.2% correct responses in patients); nonetheless, trials with errors were excluded from the analyses. In addition, correlations between FA values and reaction times for each condition, as well as for the Stroop (RT difference between incongruent and congruent stimuli) and NP (RT difference between incongruent stimuli without and with negative priming) effects were conducted separately for control and for schizophrenic subjects. Given that based on Kolmogorov-Smirnov test, behavioral data did not have a normal distribution, especially in the patient group, Spearman correlations were used.
RESULTS
Groups did not differ in age, parental socio-economic status or handedness, there were, however, expected differences in other indices including years of education, and personal socioeconomic status (see Table 1 for demographic data).
DTI results
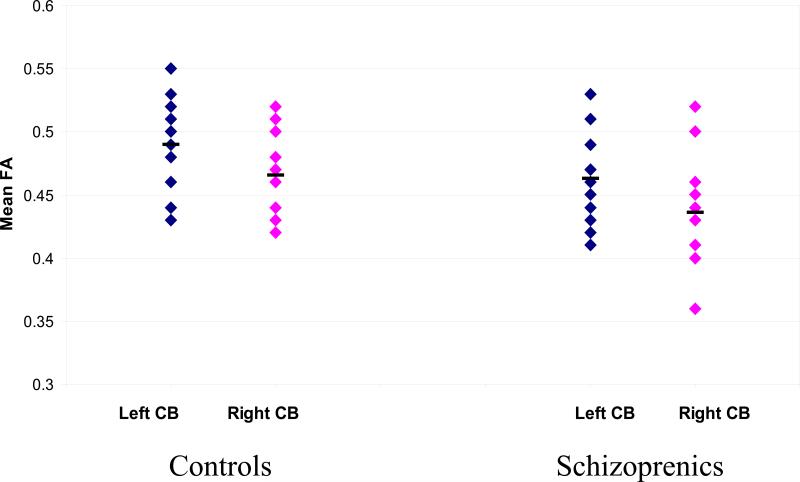
Regarding the FA values, the results replicated our previous study (Kubicki et al. 2003) where, using a different subject sample (only one control and one patient participated in both studies), and region of interest approach, we reported a bilateral FA decrease within the CB in the patient group. In the current study, independent sample t-tests revealed decrease of FA in schizophrenic patients relative to control subjects on the left side (mean FA=0.49, SD=0.034 for controls and FA=0.46, SD=0.037 for schizophrenics; t=2.10; P(1,28)=0.045), and on the right side (mean FA=0.46, SD=0.029 for controls and FA=0.44, SD=0.041 for schizophrenics; t=2.24; P(1,28)=0.034) (Figure 2).
2.
Scatter plots of mean FA values for left and right Cingulum Bundle obtained from DT Tractography.
Behavioral results
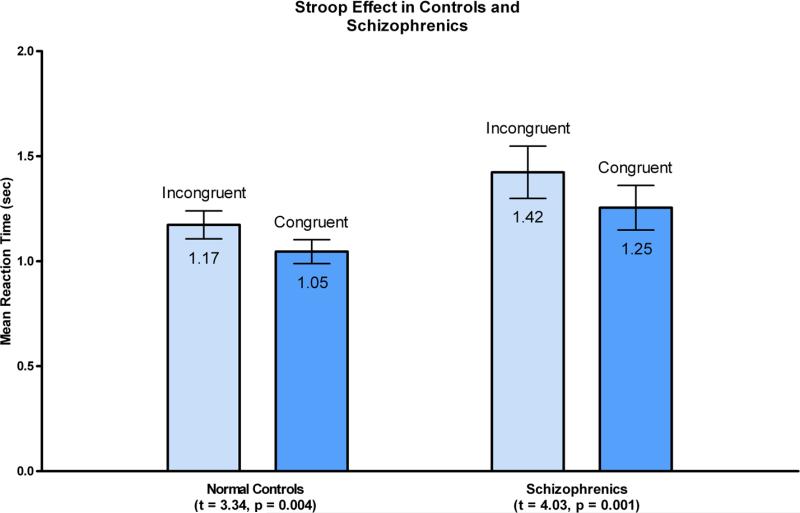
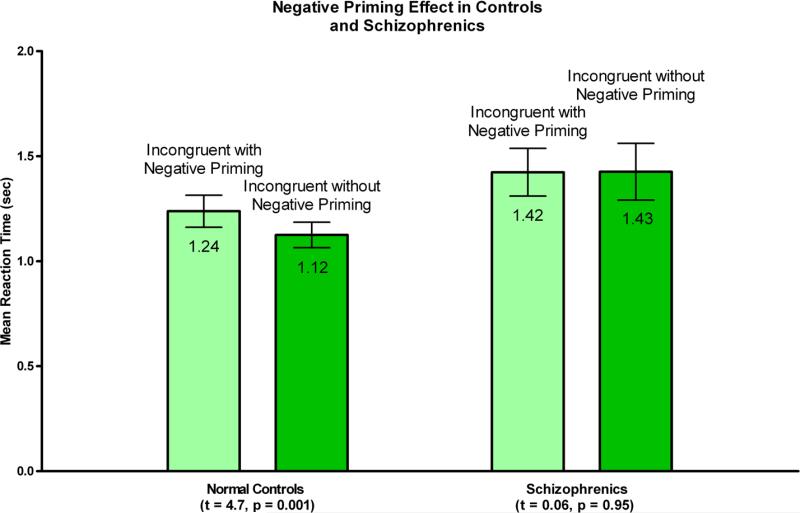
In behavioral results, there was a strong main effect of Stroop interference (F (1, 34) = 27.35, p < 0.0001), no group by condition interaction (F (1, 34) = 0.54, p < 0.47) and a main effect of group at a trend level (F(1,34) = 3.22, p<0.082) with schizophrenia individuals somewhat slower than normal control individuals. For negative priming, there was a main effect of negative priming (F(1,34)=5.16, p <0.03), a significant group by NP interaction (F(1,34)=5.65), p <0.023), and trend level main effect (F(1,34) = 3.00, p < 0.092) suggesting somewhat slower responses in the patient group. To follow-up on the interaction effect, we have conducted paired within-sample t-tests for each group for the NP effect. While normal controls showed a strong NP effect (p < t= −4.709, p < 0.0001), the patient group did not show this effect (t=0.06, p < 0.95).
Correlation analyses
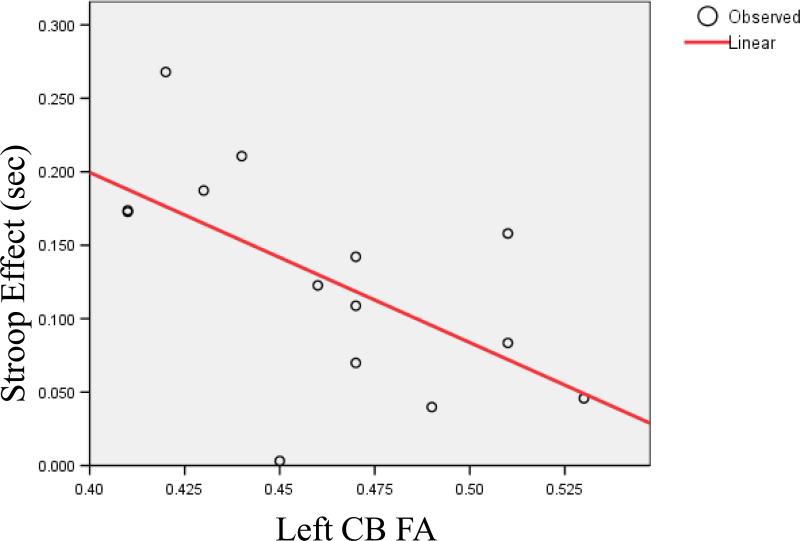
Reaction times for all types of stimuli (congruent, incongruent with and incongruent without negative priming) and left CB FA were significantly negatively correlated in schizophrenic patients (congruent- rho=−.601; P=0.018; incongruent without NP- rho=−.607; P=0.017; incongruent with NP- rho=−.619; P=0.014). Finally, Stroop effect in patients only correlated with left CB integrity- rho=−.531; P=0.042 (See figure 5). Neither right CB in schizophrenics, nor left and right CB in controls correlated with any of the behavioral measures. Behavioral and DTI measures did not significantly correlate with any demographic variables. Also medication, calculated as Chlorpromazine equivalent did not correlate with any diffusion and behavioral measures.
5.
Correlation between Stroop Effect (in seconds) and Left CB Fractional Anisotropy in patients with chronic schizophrenia.
DISCUSSION
In the current study, reduced fractional anisotropy in schizophrenia was found in the cingulum bundle, a major limbic white matter fiber tract connecting prefrontal, cingulate, parietal and temporal regions. Furthermore, for the incongruent stimuli with negative priming, patients did not show increased RT, as was observed in the control group. Finally, and importantly, in the schizophrenia population only, RTs associated with all conditions of the Stroop paradigm, as well as the Stroop effect itself, were negatively correlated with left cingulum bundle fractional anisotropy, a measure of white matter integrity.
These findings are interesting and important on many levels. First, they emphasize the importance of always suspected, but never directly demonstrated relationship between attention, working memory, reaction times and cingulum bundle integrity. Second, these results underlie the significance of abnormalities of connectivity, and specifically the role of CB integrity disruptions, in at least some aspects of cognitive functioning abnormalities observed in schizophrenia.
Previous reports have demonstrated white matter abnormalities in schizophrenia, as measured by DTI, in multiple brain regions, including cingulum bundle, uncinate fasciulus, arcuate fasciculus, corpus callosum and internal capsule (Burns et al. 2003, Kubicki et al. 2001, Kubicki et al. 2003, Hubl et al. 2004, Minami et al. 2003, Sun et al. 2003, Steel et al. 2001), and this study provides further evidence for such abnormalities. It has been further suggested, by both microscopic (Uranova et al. 2001) and genetic studies (Hakak et al. 2001), that abnormalities in oligodendrocytes, cells that play a major role in protecting axons traveling within white matter fiber tracts by forming myelin sheaths, might be the source of observed DTI changes in schizophrenia. So far, however, a direct relationship between white matter myelin pathology and cognitive performance in a clinical population has not been well established.
Since diffusion anisotropy indices are sensitive to white matter integrity disruptions and to fiber myelination abnormalities and, in turn, white matter abnormalities are likely associated with abnormal communication between the brain areas supporting different aspects of cognition, there should exist a direct relationship between diffusion abnormalities and behavioral task performance. It has been challenging, however, to find such a relationship, as specific fiber tracts are difficult to quantify, and their role in brain processes has not been well understood. Some recent publications elucidate aspects of this relationship. For example, in one recent publication, it has been shown that the RTs in a choice performance task in healthy subjects were correlated with the white matter integrity (as measured by DTI) of visual tracts, including right optic radiation, right posterior thalamus and right medial precuneus white matter (Tuch et al. 2005). As inter-individual differences in RT have been linked previously to white matter physiology such as myelination and axonal diameter (Jack et al. 1983) (reflected in DTI measures), these investigators postulated that their DTI findings provided evidence of DTI being sensitive to the physiological differences in white matter.
Our results further elucidate the relationship between physiological properties of white matter and cognitive function by demonstrating a relationship between reaction times to the Stroop stimuli, and Stroop effect itself, and cingulum bundle white matter integrity in schizophrenia. Functional MRI studies demonstrate that the Stroop effect, as well as the NP effect, activate similar regions of the brain, specifically cingulate gyrus, dorsolateral prefrontal cortex, parietal and temporal regions (MacLeod and MacDonald 2000, Steel et al. 2001). Since the cingulum bundle is the single largest white matter fiber tract interconnecting these regions, its integrity might play a crucial role in Stroop/NP performance.
As reviewed in the Introduction, the Stroop effect has been associated with attentional and executive control, while negative priming effects have been associated with both inhibitory mechanisms and short-term memory function. In fact, recently researchers have attempted to integrate inhibition and semantic retrieval (Tipper 2001), suggesting that while inhibitory mechanisms prevail during processing of prime stimulus, semantic retrieval becomes more relevant when the prime is presented as a probe. In view of this finding, as well as in view of a recent functional MRI experiment (Egner and Hirsch 2005) showing prefrontal region involvement in the NP effect, episodic memory likely plays a crucial role in NP effects. If, as suggested before, FA diffusion measures are related to myelin dysfunction, our data indicate that the CB integrity abnormalities observed in schizophrenia affect more the speed, or efficacy of information processing when attentional and executive control is required (Nestor et al. 2004).
On the other hand, in the NP condition, the subject retrieves information about color name and color itself as well as additional, previously encoded information related to distractor that has become a target. In controls, this information, when retrieved, causes additional conflict, and is arguably responsible for the NP effect. Since encoding a distractor properties relies on fast and effective processing within episodic memory, in schizophrenia, where information is transferred slower, encoding may be weaker and, as a result, the memory trace of the distractor is weaker and either can not effectively interfere with proper information, or can not be fully retrieved. This hypothesis is in line with work of Brebion and coauthors (Brebion et al. 2006), who recently demonstrated that slowing in processing speed strongly predicts verbal memory performance in schizophrenia.
The significant correlations found in this study between measures derived from the Stroop task and CB integrity measures should not be treated as narrow indicators of the relationship between CB and the specific operations examined in the Stroop task. Rather, they should be viewed as indicators of the role of CB in the type of cognitive tasks exemplified by the Stroop: these would be such tasks as conflict monitoring, control and response selection. One can speculate that CB specificity relative to functions that its integrity may affect is conferred by the types of brain regions that it connects and their particular role in the cognitive functions listed above. The unique contribution of the CB may be in efficient transfer of information from one region to another. Where such efficiency fails as in cases of compromised integrity of the fibers, the quality of the transferred information is impacted and the cognitive operations dependent on it are impaired.
We note here that, with the exception of NP effect, performance in all Stroop conditions was correlated with CB measures of integrity where lower FA values were associated with longer RTs. In addition, in spite of the necessarily reduced difference RT range reflected in the Stroop effect, and the fact that the Stroop effect was found in both normal control and schizophrenia individuals, the correlation was also found between CB and the Stroop effect in the patient group. Thus, admittedly normal range Stroop interference effect was associated with reduced CB FA values. One can speculate here that sub-normal CB function might necessitate compensatory strategies in the schizophrenia group, such as using larger portions of brain areas devoted to operations involved in the effective Stroop performance or additional brain areas relative to normal control group. These questions may be addressed in future studies, especially using fMRI techniques to map the extent of activations in relevant brain areas.
A separate problem to be addressed in future studies is the question concerning the origins of CB abnormalities of conductivity. Are these abnormalities a consequence of cortex organization abnormalities in schizophrenia, or are they independent of such abnormalities, perhaps originating from a common pathological process?
We did not find a significant correlation between RTs in control subjects and the FA measures within the CB. This is not unexpected given the fact that relationships between anatomical substrates and function are often found in circumstances where a disease process eliminated a normal redundancy in the system.
The method of extracting diffusion data for analysis requires some discussion as well. In this investigation we used fiber tractography, instead of a region of interest approach used previously (Kubicki et al. 2003). We believe that tractography, unlike the ROI method, gives us the ability to extract the entire fiber bundle, and more efficiently exclude voxels that do not belong to the bundle. It has also been demonstrated that tractography results are more sensitive to white matter schizophrenia pathology (Kanaan et al. 2006, Jones et al. 2005). In addition, even though we used an almost entirely different population of subjects (only 2 subjects participated in both studies), we were able to replicate the results of our previous investigation that used an ROI method (Kubicki et al. 2003).
There are several limitations to this study. First, even though fiber tractography was used, the data resolution was relatively low, and thus partial volume artifacts could be significant. Also, our clinical study participants were chronic schizophrenic patients, and even though medication effects were not correlated with DTI measures, it is not possible to rule out the confounding effect of medication. Additionally, all our subjects were males, thus we were not able to investigate diffusion and gender interactions in schizophrenia. Previous studies suggest that the percentage of incongruent trials to congruent trials influences the Stroop effect such that the smaller percentage of incongruent trials, the greater the Stroop effect. In the current experiment, we used a relatively large percentage of incongruent trials, given the fact that we were interested in the NP effect in addition to the Stroop effect. In addition, compared to other, behavioral investigations, we used a relatively small number of stimuli. However, a robust Stroop effect was found in both groups, and we are thus confident that the results observed in this study do not suffer from a lack of statistical power. Finally, the cognitive performance data were limited to one task: Stroop and we do not have data from a different task that would not rely on cognitive control. Thus, while we did observe correlations between CB integrity and indices of behavioral performance, we cannot claim that these correlations were exclusive to tasks requiring cognitive control. Future studies will address the issue of differential sensitivity of CB bundle integrity to attentional performance.
Therefore, we conclude that our study, using fiber DTI, tractography and single-trial computerized version of Color Stroop/Negative Priming (NP) paradigm provides supportive evidence for an association between Cingulum Bundle fiber tract integrity disruptions and attention deficits in schizophrenia. A further understanding of the role of fiber tracts and their integrity in cognitive performance can help us understand neuropathological processes underlying clinical, as well as neuropsychological symptoms observed in diseases such as schizophrenia, and bring us closer to developing drugs that would preserve specific neuroanatomical units.
3.
Boxplots of means (in seconds) and error bars for reaction times to incongruent (light blue), and congruent (darker blue) stimuli. Difference between these reaction times constitute behavioral Stroop effect, present both in controls (P=0.004) as well as in schizophrenia subjects (P=0.001) (standard deviations: 0.28sec for incongruent and 0.24sec for congruent stimuli in controls, 0.53sec for incongruent and 0.45 sec for congruent stimuli in schizophrenics).
4.
Boxplots of means (in seconds) and standard deviations for reaction times to incongruent stimuli with (light green), and without (darker green) negative priming. Difference between these reaction times constitute behavioral Negative Priming effect, present in controls (P=0.001) but not in schizophrenia subjects (P=0.95) (standard deviations: 0.32 sec for incongruent stimuli with negative priming and 0.26 sec for incongruent stimuli without negative priming in controls, 0.48 sec for incongruent stimuli with negative priming and 0.57 sec for incongruent stimuli without negative priming in schizophrenics).
REFERENCES
- 1.Baratti C, Barnett AS, Pierpaoli C. Comparative MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology. 1999;210(1):133–142. doi: 10.1148/radiology.210.1.r99ja09133. [DOI] [PubMed] [Google Scholar]
- 2.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu C, Allen P. Determinants of Anisotropic Water Diffusion in Nerves. MRM. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 4.Beech A, Powell T, McWilliam J, Claridge G. Evidence of reduced ‘cognitive inhibition’ in schizophrenia. Br J Clin Psychol. 1989;28(Pt 2):109–116. doi: 10.1111/j.2044-8260.1989.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 5.Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31(9):907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- 6.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 7.Brebion G, David AS, Bressan RA, Pilowsky LS. Processing speed: a strong predictor of verbal memory performance in schizophrenia. J Clin Exp Neuropsychol. 2006;28(3):370–382. doi: 10.1080/13803390590935390. [DOI] [PubMed] [Google Scholar]
- 8.Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- 9.Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 10.Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage. 1995;2(4):264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- 11.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154(12):1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Shoup R. Response selection processes for conjunctive targets. J Exp Psychol Hum Percept Perform. 2000;26(1):391–411. doi: 10.1037//0096-1523.26.1.391. [DOI] [PubMed] [Google Scholar]
- 13.Dalrymple-Alford EC, Budayer B. Examination of some aspects of the Stroop Color-Word Test. Percept Mot Skills. 1966;23(3):1211–1214. doi: 10.2466/pms.1966.23.3f.1211. [DOI] [PubMed] [Google Scholar]
- 14.Domesick VB. The fasciculus cinguli in the rat. Brain Res. 1970;20(1):19–32. doi: 10.1016/0006-8993(70)90150-2. [DOI] [PubMed] [Google Scholar]
- 15.Dunbar K, MacLeod CM. A horse race of a different color: Stroop interference patterns with transformed words. J Exp Psychol Hum Percept Perform. 1984;10(5):622–639. doi: 10.1037//0096-1523.10.5.622. [DOI] [PubMed] [Google Scholar]
- 16.Egner T, Hirsch J. Where memory meets attention: neural substrates of negative priming. J Cogn Neurosci. 2005;17(11):1774–1784. doi: 10.1162/089892905774589226. [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Posner M. Human attentional networks. Psychiatr Prax. 2004;31(Suppl 2):S210–214. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- 18.Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56(3):304–311. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9(3):337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 20.Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158(12):2069–2071. doi: 10.1176/appi.ajp.158.12.2069. [DOI] [PubMed] [Google Scholar]
- 21.Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RS, Liddle PF. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry. 1995;167(3):343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- 22.Ge Y, Grossman RI, Udupa JK, Babb JS, Mannon LJ, McGowan JC. Magnetization transfer ratio histogram analysis of normal-appearing gray matter and normal-appearing white matter in multiple sclerosis. J Comput Assist Tomogr. 2002;26(1):62–68. doi: 10.1097/00004728-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3(5):516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 24.George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Casey BJ, Trimble MR, Horwitz B, Hervscovitch P, Post RM. Regional brain activity when selecting a response despite interference: An H2-015 PET study of the Stroop and emotional Stroop. Human Brain Mapping. 1994;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- 25.Grossman RI, Gomori JM, Ramer KN, Lexa FJ, Schnall MD. Magnetization transfer: theory and clinical applications in neuroradiology. Radiographics. 1994;14(2):279–290. doi: 10.1148/radiographics.14.2.8190954. [DOI] [PubMed] [Google Scholar]
- 26.Gruber SA, Rogowska J, Holcomb P, Soraci S, Yurgelun-Todd D. Stroop performance in normal control subjects: an fMRI study. Neuroimage. 2002;16(2):349–360. doi: 10.1006/nimg.2002.1089. [DOI] [PubMed] [Google Scholar]
- 27.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98(8):4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison BJ, Shaw M, Yucel M, Purcell R, Brewer WJ, Strother SC, Egan GF, Olver JS, Nathan PJ, Pantelis C. Functional connectivity during Stroop task performance. Neuroimage. 2005;24(1):181–191. doi: 10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Henik A, Salo R. Schizophrenia and the stroop effect. Behav Cogn Neurosci Rev. 2004;3(1):42–59. doi: 10.1177/1534582304263252. [DOI] [PubMed] [Google Scholar]
- 30.Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61(7):658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 31.Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44(4):584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Jack JJB, Noble D, Tsien RW. Electrical Current Flow in Excitable Cells. Oxford University Press; Oxford: 1983. [Google Scholar]
- 33.Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'Sullivan M, Maguire P, Horsfield MA, Simmons A, Williams SC, Howard RJ. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am J Geriatr Psychiatry. 2005;13(12):1092–1099. doi: 10.1176/appi.ajgp.13.12.1092. [DOI] [PubMed] [Google Scholar]
- 34.Kanaan RA, Shergill SS, Barker GJ, Catani M, Ng VW, Howard R, McGuire PK, Jones DK. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. 2006;146(1):73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 36.Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 37.Kraepelin E. Dementia Praecox, Churchill Livingstone Inc.; New York: 1919/1971. [Google Scholar]
- 38.Krissan KW, C-F. Fast sub-voxel re-initialization of the distance map for level set methods. . Pattern Recognition Letters. in press. [Google Scholar]
- 39.Krissian K. Flux-based anisotropic diffusion applied to enhancement of 3-D angiogram. IEEE Trans Med Imaging. 2002;21(11):1440–1442. doi: 10.1109/TMI.2002.806403. [DOI] [PubMed] [Google Scholar]
- 40.Krissian K, Westin CF. Fast and accurate redistancing for level set methods.. Paper presented at the Computer Aided Systems Theory (EUROCAST '03); Spain. Las Palmas de Gran Canaria; 2003. [Google Scholar]
- 41.Kubicki M, Shenton ME, David E, Frumin M, Salisbury D, Hirayasu Y, McCarley RW. A Comparison of Voxel-Based Morphometry (VBM) with Region-of-Interest (ROI) Analysis of Gray Matter in Control and First Episode Schizophrenia Subjects.. Paper presented at the Biological Psychiatry; New Orlean. 2001. [Google Scholar]
- 42.Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54(11):1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laplane D, Degos JD, Baulac M, Gray F. Bilateral infarction of the anterior cingulate gyri and of the fornices. Report of a case. J Neurol Sci. 1981;51(2):289–300. doi: 10.1016/0022-510x(81)90107-6. [DOI] [PubMed] [Google Scholar]
- 45.Laplante L, Everett J, Thomas J. Inhibition through negative priming with Stroop stimuli in schizophrenia. Br J Clin Psychol. 1992;31(Pt 3):307–326. doi: 10.1111/j.2044-8260.1992.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald PA, Joordens S. Investigating a memory-based account of negative priming: support for selection-feature mismatch. J Exp Psychol Hum Percept Perform. 2000;26(4):1478–1496. doi: 10.1037//0096-1523.26.4.1478. [DOI] [PubMed] [Google Scholar]
- 47.MacDonald PA, Joordens S, Seergobin KN. Negative priming effects that are bigger than a breadbox: attention to distractors does not eliminate negative priming, it enhances it. Mem Cognit. 1999;27(2):197–207. doi: 10.3758/bf03211405. [DOI] [PubMed] [Google Scholar]
- 48.MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci. 2000;4(10):383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- 49.MacQueen GM, Galway T, Goldberg JO, Tipper SP. Impaired distractor inhibition in patients with schizophrenia on a negative priming task. Psychol Med. 2003;33(1):121–129. doi: 10.1017/s0033291702006918. [DOI] [PubMed] [Google Scholar]
- 50.McNeely HE, West R, Christensen BK, Alain C. Neurophysiological evidence for disturbances of conflict processing in patients with schizophrenia. J Abnorm Psychol. 2003;112(4):679–688. doi: 10.1037/0021-843X.112.4.679. [DOI] [PubMed] [Google Scholar]
- 51.Minami T, Nobuhara K, Okugawa G, Takase K, Yoshida T, Sawada S, Ha-Kawa S, Ikeda K, Kinoshita T. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47(3):141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- 52.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 53.Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7-8):468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 54.Neill DB, Kaufman JL. Deficits in behavioral responding to regulatory challenges after lesions of ventrobasal thalamus in rats. Physiol Behav. 1977;19(1):47–52. doi: 10.1016/0031-9384(77)90157-3. [DOI] [PubMed] [Google Scholar]
- 55.Neill WT, Valdes LA, Terry KM, Gorfein DS. Persistence of negative priming: II. Evidence for episodic trace retrieval. J Exp Psychol Learn Mem Cogn. 1992;18(5):993–1000. doi: 10.1037//0278-7393.18.5.993. [DOI] [PubMed] [Google Scholar]
- 56.Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, Shenton ME. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18(4):629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park HJ, Kubicki M, Westin CF, Talos IF, Brun A, Peiper S, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Method for combining information from white matter fiber tracking and gray matter parcellation. AJNR Am J Neuroradiol. 2004;25(8):1318–1324. [PMC free article] [PubMed] [Google Scholar]
- 59.Park J, Kanwisher N. Negative priming for spatial locations: identity mismatching, not distractor inhibition. J Exp Psychol Hum Percept Perform. 1994;20(3):613–623. doi: 10.1037/0096-1523.20.3.613. [DOI] [PubMed] [Google Scholar]
- 60.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 61.Posner MI, Rothbart MK, Vizueta N, Levy KN, Evans DE, Thomas KM, Clarkin JF. Attentional mechanisms of borderline personality disorder. Proc Natl Acad Sci U S A. 2002;99(25):16366–16370. doi: 10.1073/pnas.252644699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C. Cambridge University Press; Cambrigde: 1992. [Google Scholar]
- 63.Rovaris M, Filippi M, Minicucci L, Iannucci G, Santuccio G, Possa F, Comi G. Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. AJNR Am J Neuroradiol. 2000;21(2):402–408. [PMC free article] [PubMed] [Google Scholar]
- 64.Salo R, Henik A, Nordahl TE, Robertson LC. Immediate versus sustained processing in schizophrenia. J Int Neuropsychol Soc. 2002;8(6):794–803. doi: 10.1017/s1355617702860076. [DOI] [PubMed] [Google Scholar]
- 65.Salo R, Robertson LC, Nordahl TE. Normal sustained effects of selective attention are absent in schizophrenic patients withdrawn from medication. Psychiatry Res. 1996;62(2):121–130. doi: 10.1016/0165-1781(96)02804-1. [DOI] [PubMed] [Google Scholar]
- 66.Salo R, Robertson LC, Nordahl TE, Kraft LW. The effects of antipsychotic medication on sequential inhibitory processes. J Abnorm Psychol. 1997;106(4):639–643. doi: 10.1037//0021-843x.106.4.639. [DOI] [PubMed] [Google Scholar]
- 67.Schmahmann JP, DN . Fiber Pathways of the Brain. Oxford University Press; 2006. [Google Scholar]
- 68.Steel RM, Bastin ME, McConnell S, Marshall I, Cunningham-Owens DG, Lawrie SM, Johnstone EC, Best JJK. Diffusion tensor imaging (DTI) and prton magnetic resonance spectroscopy (H MRS) in schizophrenic subjects and normal controls. Psychiatry Research: Neuroimaging Section. 2001;106:161–170. doi: 10.1016/s0925-4927(01)00080-4. [DOI] [PubMed] [Google Scholar]
- 69.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- 70.Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, Cong Z, Zhang H, Li B, Hong N, Zhang D. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003;14(14):1833–1836. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- 71.Tipper SP. The negative priming effect: inhibitory priming by ignored objects. Q J Exp Psychol A. 1985;37(4):571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- 72.Tipper SP. Does negative priming reflect inhibitory mechanisms? A review and integration of conflicting views. Q J Exp Psychol A. 2001;54(2):321–343. doi: 10.1080/713755969. [DOI] [PubMed] [Google Scholar]
- 73.Tipper SP, Weaver B, Jerreat LM, Burak AL. Object-based and environment-based inhibition of return of visual attention. J Exp Psychol Hum Percept Perform. 1994;20(3):478–499. [PubMed] [Google Scholar]
- 74.Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102(34):12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55(5):597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 76.van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage. 2005;27(3):497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 77.Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H, Cong Z, Hong N, Zhang D. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161(3):573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- 78.Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149(7):890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 79.Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, Kremser C, Brinkhoff C, Felber SR, Fleischhacker WW. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. 2003;123(1):1–15. doi: 10.1016/s0925-4927(03)00019-2. [DOI] [PubMed] [Google Scholar]