Abstract
Selectivity to insects over mammals is one of the important characteristics for a chemical to become a useful insecticide. Fipronil was found to block cockroach GABA receptors more potently than rat GABAA receptors. Furthermore, glutamate-activated chloride channels (GluCls), which are present in cockroaches but not in mammals, were very sensitive to the blocking action of fipronil. The IC50s of fipronil block were 30 nM in cockroach GABA receptors and 1600 nM in rat GABAA receptors. Moreover, GluCls of cockroach neurons had low IC50s for fipronil. Two types of glutamate-induced chloride current were obswerved: desensitizing and non-desensitizing, with fipronil IC50s of 800 and 10 nM, respectively. We have developed methods to separately record these two types of GluCls. The non-desensitizing and desensitizing currents were selectively inhibited by trypsin and polyvinylpyrrolidone, respectively. In conclusion, in addition to GABA receptors, GluCls play a crucial role in selectivity of fipronil to insects over mammals. GluCls form the basis for development of selective and safe insecticides.
Introduction
Selectivity for insects over mammals is a desirable characteristics for an insecticide. It was previously thought that the ability of mammals to detoxify insecticides more effectively than insects is a major mechanism responsible for selectivity. This is certainly true for some insecticides such as malathion [1]. However, it has now become increasingly clear that the sensitivity of target sites to insecticides is in many cases higher in insects than in mammals.
We previously developed a method whereby the percentage of sodium channels modified by pyrethroids can be measured [2]. This percentage turned out to be astonishingly small for pyrethroids: modification of only 0.6% of sodium channels by tetramethrin was enough to induce hyperactivity in animals [3]. Using this technique, the selectivity of pyrethroids to insects over mammals could be largely explained on the basis of a 1000-fold difference in sodium channel sensitivity between insects and mammals [4-6].
Fipronil is a phenylpyrazole insecticide that was introduced commercially in 1993. It exhibits a high selectivity to insects over mammals: the LD50 values in houseflies and rat were 0.13 and 41 mg/kg, respectively, a 315-fold difference [7, 8]. Fipronil is a potent GABA receptor blocker: the IC50 values in cockroach and rat were estimated to be 30 nM and 1600 nM, respectively, a 53-fold difference [9, 10]. However, the mechanism of fipronil's selectivity is not limited to GABA receptors. It turned out that fipronil potently inhibits glutamate-activated chloride channels (GluCls), which are present in invertebrates such as insects, but not in mammals [6, 11, 12]. Because GluCls are not present in mammals, chemicals that selectively or potently block them could be excellent insecticides. This chapter summarizes our recent work on fipronil block of GluCls.
Methods
American cockroaches, Periplaneta americana, were used as a representative insect preparation. The methods of isolation and culture of thoracic ganglion pyriform neurons are given in detail in our previous paper [12]. The dorsal root ganglion neurons of the rat were also used, to compare with cockroach neurons, and the methods for isolation and primary cultures are described in our previous publication [10]. Whole-cell currents induced by application of GABA or L-glutamate via a U-tube system were recorded by patch clamp techniques as described before [10, 12]. Data acquisition and analysis methods are given in our previous papers 10, 12].
Results
Target site sensitivity as a basis for selectivity
Whereas the selectivity of certain insecticides (e.g. organophosphates) is due primarily to differences in metabolic detoxication [1, 13-16], in many other cases it is due to the higher sensitivity of targets to insecticides in insects than in mammals, as exemplified by pyrethroids [3, 17].
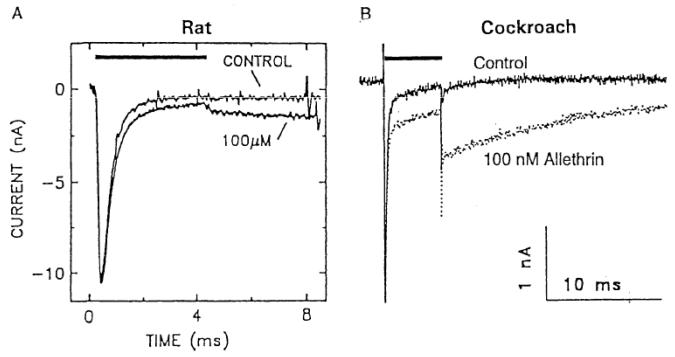
Allethrin was much more potent on cockroach sodium channels than on rat sodium channels (Figure 1). Allethrin at 100 nM modified 14% of sodium channels in cockroach neurons, whereas 100 μM allethrin modified only 4% of sodium channels in rat neurons. Thus, the difference in allethrin sensitivity is little over 1000-fold. Including some other factors, such as the temperature dependence of pyrethroid sensitivity of sodium channels, the difference in LD50s can be accounted for [3].
Fig. 1.
Allethrin modulates the activity of tetrodotoxin-sensitive sodium channels >1000 times more potently in cockroach neurons than in rat dorsal root ganglion neurons. See text for further explanation. Rat data from Ginsburg and Narahashi [4] and cockroach data from Narahashi [5].
Fipronil
Fipronil is known to block GABAA receptors in mammals [10]. It also inhibits GABA receptor activity in the cockroach [12]. As described below, cockroach GABA receptors are more sensitive than rat GABAA receptors to the blocking action of fipronil. This selective sensitivity of the GABAergic system certainly contributes to the selectivity of fipronil to insects over mammals. However, it has been discovered that an additional factor significantly contributes to the selectivity: GluCls, which are present in invertebrates including insects, but absent from mammals [18, 19]. Much of our knowlwdge of GluCls comes from studies on C. elegans, particularly as a target site of the anthelminthic/insecticide ivermectin [20-22]. Studies of GluCls using insects in connection with ivermectin were limited [23, 24], but recent work has focused increasingly on insect GluCls [11, 12, 25-28].
Fipronil block of GABA receptors
In order to compare with fipronil actions on cockroach GluCls, fipronil block of rat and cockroach GABA receptors is briefly described here. GABA-induced currents of rat were blocked by fipronil with IC50s of 1.66 and 1.61 μM for the closed and open channels, respectively [10].
GABA-induced currents were recorded from pyriform cockroach neurons [9]. With symmetric chloride concentrations in external and internal solutions, inward currents were evoked by GABA at a holding potential of −60 mV. GABA-induced currents reversed their polarity at the equilibrium potential for chloride, indicating that the currents were carried by chloride ions. Similar to GABA-induced currents in rats, the currents in cockroaches were inhibited by picrotoxinin. By contrast, cockroach GABA currents were unaffected by bicuculline, which blocks GABA-induced currents in rat. Similar to rat GABAA receptors, cockroach GABA receptors were blocked by fipronil in both resting and activated states. The IC50 values were similar in both states, being 28 nM and 35 nM for the resting and activated receptors, respectively. Thus, the affinity of fipronil is more than 50 times higher for cockroach than for rat GABA receptors.
Glutamate-induced currents are carried by chloride
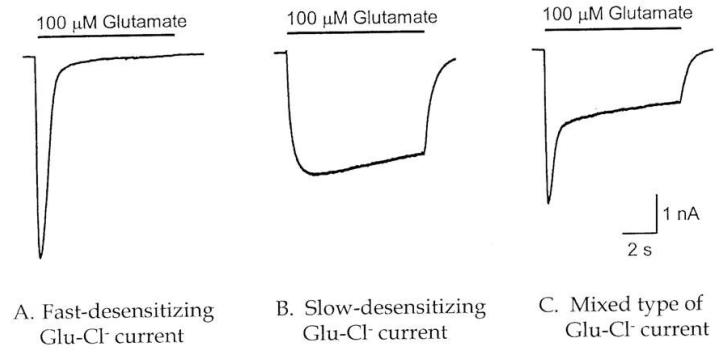
Two types of currents were evoked in cockroach neurons by application of 100 μM glutamate via a U-tube: desensitizing and non-desensitizing. Some neurons generated a mixed-type of current, containing both desensitizing and non-desensitizing components (Figure 2) [25]. In order to demonstrate that glutamate-induced currents were carried by chloride ions, the reversal potentials for currents were measured with two different chloride concentrations in the external solution. With symmetrical chloride concentration at 188 mM in external and internal solutions, both desensitizing and non-desensitizing GluCls reversed polarity at a membrane potential of +2.5 mV, which was close to the calculated chloride equilibrium potential of −0.1 mV after correction for a 3.1 mV liquid junction potential between internal and external solutions. Furthermore, when the external chloride concentration was reduced to one quarter (47 mM) of the normal value, using sodium gluconate to replace sodium chloride, the reversal potentials were shifted by 35.0 mV towards depolarization for both type of GluCls, which was close to the calculated shift of 34.5 mV. Thus, it was concluded that both desensitizing and non-desensitizing GluCls evoked by glutamate were carried by chloride ions [25].
Fig. 2.
Two components of glutamate-activated chloride currents in cockroach neurons. Desensitizing (left), non-desensitizing (middle), and mixed types (right) [32].
Differential sensitivities of two GluCls to picrotoxinin and bicuculline
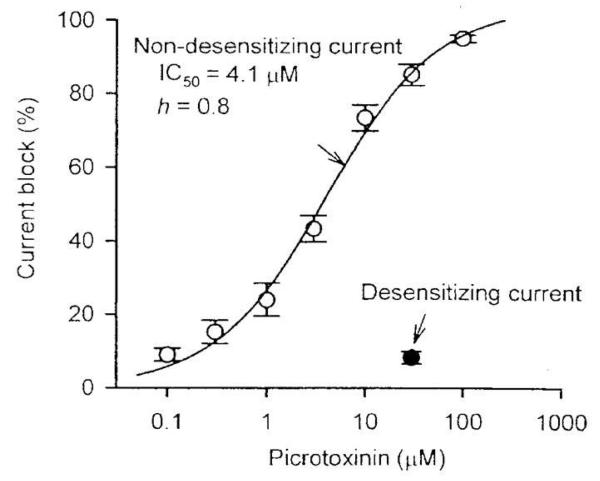
The desensitizing and non-desensitizing GluCls were found to exhibit totally different sensitivities to picrotoxinin, a GABA receptor blocker. While the desensitizing GluCls were inhibited only 8% by 30 μM picrotoxinin, the non-desensitizing GluCls were inhibited by picrotoxinin with an IC50 of 4.1 μM (Figure 3) [25]. Neither type of GluCl was affected by 100 μM bicuculline [25].
Fig. 3.
Differential sensitivities of the desensitizing and non-desensitizing GluCls to the blocking action of picrotoxinin. Dose-response relationships for picrotoxinin inhibition show an IC50 of 4.1 ± 1.0 μM for the non-desensitizing component, and only 8.2 ± 2.9% inhibition of the desensitizing component by 30 μM picrotoxinin [12].
Fipronil blocks two GluCls differentially
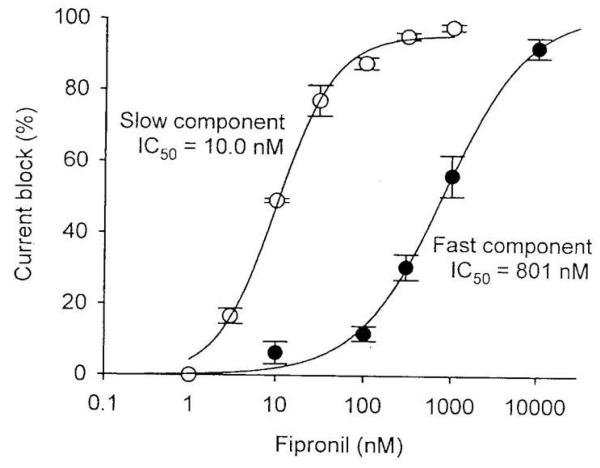
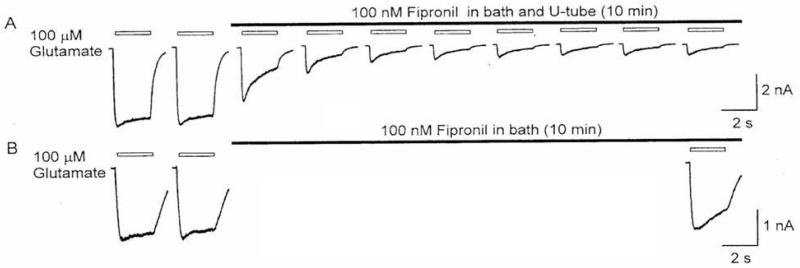
Whereas fipronil blocked both desensitizing and non-desensitizing GluCls, the former was much less sensitive than the latter, with IC50s of 800 nM and 10 nM, respectively (Figure 4) [12]. Fipronil block of non-desensitizing GluCls was use dependent when the currents were evoked by 100 μM glutamate for 2 s; bath and U-tube applications of 100 nM fipronil inhibited the currents gradually over a period of 10 min (Figure 5A). However, exposure to the same concentration of fipronil for the same period of time without glutamate activation resulted in very little block (Figure 5B). The percentage of block was 90% with, but only 10% without activation[12]. Thus, it was concluded that fipronil blocked non-desensitizing GluCls in a highly use-dependent manner, requiring opening of the channels for block.
Fig. 4.
Dose-response relationships of fipronil block of the desensitizing and non-desensitizing GluCls. The peak currents and the steady-state currents were measured for the desensitizing and non-desensitizing currents, respectively. The IC50 values are 801 ± 207 nM and 10.0 ± 0.9 nM for the desensitizing and non-desensitizing currents, respectively [12].
Fig. 5.
Use-dependent block of non-desensitizing GluCls by fipronil. Two protocols were used. A. Currents were induced by 2-s applications of 100 μM glutamate at an interval of 30 s. Fipronil at 100 nM was bath-perfused and co-applied with glutamate for 10 min. B. Similar to A but no glutamate was applied during a 10 min bath perfusion of 100 nM fipronil. A test pulse of glutamate was applied to determine unblocked GluCls [12].
GluCls and GABA chloride channels are different entities
GABA-gated chloride channels (GABA-Cls) and GluCls are closely related ligand-gated chloride channels and there is much overlap of pharmacological properties between the two channels. Both GluCls and GABA-Cls were sensitive to picrotoxin [29-31], and the amino acid sequences of the Drosophila GABA-Cls subunit (Rdl) and the Drosophila GluCls show many similarities [19].
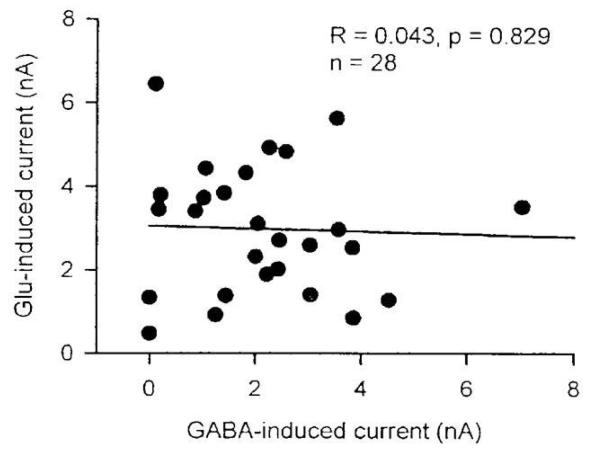
Four types of experiments were performed to test the hypothesis that GluCls and GABA-Cls are different entities [25]. First, some neurons responded to glutamate application without producing responses to GABA and vice versa, and there was no correlation between the glutamate and GABA responses in the same neurons (Figure 6). Second, in neurons responsive to both GABA and glutamate, block of GABA-Cls by dieldrin or by fipronil did not prevent the glutamate response. Thus, the GABA-induced chloride current and the glutamate-induced chloride current are generated by separate receptors. Third, a corollary of this conclusion is that the glutamate-induced current and the GABA-induced current will be additive in the same neuron. This was indeed the case. Fourth, experiments were conducted to test whether there is cross-desensitization between the GABA- and glutamate-induced currents. This was not the case. These four types of experiments are in keeping with the conclusion that GluCls and GABA-Cls function independently.
Fig. 6.
Lack of correlation between glutamate-induced and GABA-induced currents in individual neurons. In 28 different neurons tested, no significant correlation was obtained (p>0.05) with a correlation coefficient of only 0.043 [25].
Separation of desensitizing and non-desensitizing GluCls
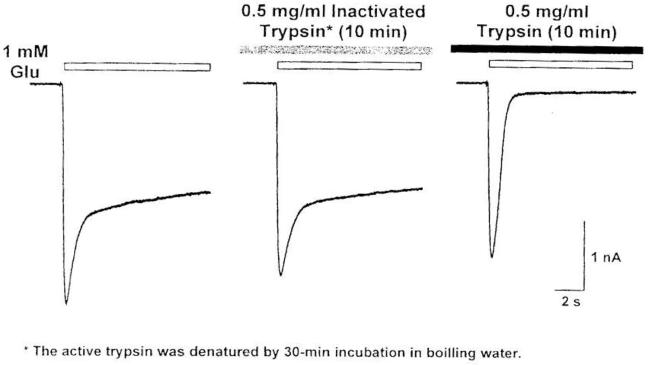
It is difficult to separately record desensitizing and non-desensitizing currents from cockroach neurons. In order to facilitate experiments, several pharmacological means have recently been developed to separate desensitizing and non-desensitizing GluCls [32]. Bath application of trypsin at a concentration of 0.5 mg/ml for 10 min completely eliminated the non-desensitizing GluCl component, leaving the desensitizing component intact (Figure 7). This effect of trypsin was enzymatic and irreversible.
Fig. 7.
Trypsin eliminates the non-desensitizing component of the GluCl current, leaving the desensitizing component intact, but heat-inactivated trypsin loses this ability [32].
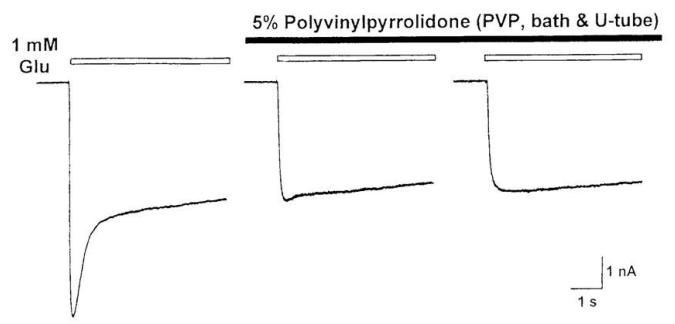
Papain at 0.5 mg/ml or bovine serum albumin at 0.5 mg/ml had no effect on either desensitizing or non-desensitizing GluCls. The desensitizing GluCls could be selectively and reversibly blocked by either 0.5 mg/ml soybean trypsin inhibitor or 5% polyvinylpyrrolidone (Figure 8). Therefore, trypsin and soybean trypsin inhibitor or polyvinylpyrrolidone can be used to selectively record desensitizing or non-desensitizing GluCl currents, respectively.
Fig. 8.
Polyvinylpyrrolidone reversibly blocks the desensitizing GluCl current without affecting the non-desensitizing GluCl current [32].
Conclusions
The high sensitivity of insect GABA receptors and glutamate-activated chloride channels to fipronil is responsible for the selectivity in insects over mammals.
The glutamate-activated chloride channels, which are present in insects but not in mammals, are an excellent target for selective and safe insecticides.
Acknowledgement
This work was supported by NIH grant R01 NS014143.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Brien RD. Insecticides: Action and Metabolism. Academic Press; New York: 1967. p. 332. [Google Scholar]
- 2.Tatebayashi H, Narahashi T. Differencial mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- 3.Song JH, Narahashi T. Modulation of sodium channels of rat cerebellar Purkinje neurons by the pyrethroids tetramethrin. J. Pharmacol. Exp. Ther. 1996;277:445–453. [PubMed] [Google Scholar]
- 4.Ginsburg KS, Narahashi T. Differential sensitivity of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels to the insecticide allethrin in dorsal root ganglion neurons. Brain Res. 1993;627:239–248. doi: 10.1016/0006-8993(93)90326-i. [DOI] [PubMed] [Google Scholar]
- 5.Narahashi T. Recent progress in the mechanism of action of insecticides: pyrethroids, fipronil and indoxacarb. J. Pesicide Sci. 2001;26:277–285. [Google Scholar]
- 6.Narahashi T, Zhao X, Ikeda T, Nagata K, Yeh JZ. Differential actions of insecticidas on target sites: basis for selective toxicity. Human Exp. Toxicol. 2007;26:361–366. doi: 10.1177/0960327106078408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hainzl D, Casida JE. Fipronil insecticide: novel phochemical desulfinylation with reten of neurotoxicity. Proc. Natl. Acad. Sci. USA. 1996;93:12764–12767. doi: 10.1073/pnas.93.23.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharf M, Siegfried B. Toxicity and neurophysiological effects of fipronil and fipronil sulfone on the western corn rootworm (Coleoptera: chrysomelidae) Archiv. Insect Biochem. Physiol. 1999;40:150–156. [Google Scholar]
- 9.Zhao X, Salgado VL, Yeh JZ, Narahashi T. Differential actions of fipronil and dieldrin insecticides on GABA-gated chloride channels in cockroach neurons. J. Pharmacol. Exp. Ther. 2003;306:914–924. doi: 10.1124/jpet.103.051839. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda T, Zhao X, Nagata K, Kono Y, Yeh JZ, Narahashi T. Fipronil modulation of γ-aminobutyric acidA receptors in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2001;296:914–921. [PubMed] [Google Scholar]
- 11.Ikeda T, Zhao X, Kono Y, Yeh JZ, Narahashi T. Fipronil modulation of glutamate-induced chloride currents in cockroach thoracic ganglion neurons. Neurotoxicology. 2003;24:807–815. doi: 10.1016/S0161-813X(03)00041-X. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Yeh JZ, Salgado VL, Narahashi T. Fipronil is a potent open channel blocker of glutamate-activated chloride channels in cockroach neurons. J. Pharmacol. Exp. Ther. 2004;310:192–201. doi: 10.1124/jpet.104.065516. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura F. Toxicology of Insecticides. 2nd ed. Plenum Press; New York: 1985. p. 588. [Google Scholar]
- 14.Brooks GT. Insecticide metabolism and selective toxicity. Xenobiotica. 1986;16:989–1002. doi: 10.3109/00498258609038978. [DOI] [PubMed] [Google Scholar]
- 15.Mahajna M, Quistad GB, Casida JE. Acephate insecticide toxicity: safety conferred by inhibition of the bioactivating carboxyamidase by the metabolite methamidophos. Chem. Res. Toxicol. 1997;10:64–69. doi: 10.1021/tx9601420. [DOI] [PubMed] [Google Scholar]
- 16.Hainzl D, Cole LM, Casida JE. Mechanisms of selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem. Res. Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- 17.Warmke JW, Reenan RA, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Ploeg LH, et al. Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology. J. gen. Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleland TA. Inhibitory glutamate receptor channels. Mol. Neurobiol. 1996;13:97–136. doi: 10.1007/BF02740637. [DOI] [PubMed] [Google Scholar]
- 19.Raymond V, Sattelle DB. Novel animal-health drug targets from ligang-gated chloride channels. Nat.Rev. Drug Discov. 2002;1:427–436. doi: 10.1038/nrd821. [DOI] [PubMed] [Google Scholar]
- 20.Hejmadi MV, Jagannathan S, Delany NS, Coles GC, Wolstenholme AJ. Lglutamate binding sites of parasitic nematodes: an association with ivermectin resistance? Parasitology. 2000;120:535–545. doi: 10.1017/s0031182099005843. [DOI] [PubMed] [Google Scholar]
- 21.Burkhart CN. Ivermectin: an assessment of its pharmacology, microbiology and safety. Vet. Hum. Toxicol. 2000;42:30–35. [PubMed] [Google Scholar]
- 22.Arena JP, Liu KK, Paress PS, Frazier EG, Cully DF, Mrozik H, Schaeffer JM. The mechanism of action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. J. Parasitol. 1995;81:286–294. [PubMed] [Google Scholar]
- 23.Kane NS, Hirschberg B, Qian S, Hunt D, Thomas B, Brochu R, Ludmerer SW, Zheng Y, Smith M, Arena JP, Cohen CJ, Schmatz D, Warmke J, Cully DF. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc. Natl. Acad. Sci. USA. 2000;97:13949–13954. doi: 10.1073/pnas.240464697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MM, Warren VA, Thomas BS, Brochu RM, Ertel EA, Rohrer S, Schaeffer J, Schmatz D, Petuch BR, Tang YS, Meinke PT, Kaczorowski GJ, Cohen CJ. Nodulisporic acid opens insect glutamate-gated chloride channels: identification of a new high affinity modulator. Biochemistry. 2000;39:5543–5554. doi: 10.1021/bi992943i. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Salgado VL, Yeh JZ, Narahashi T. Kinetic and pharmacological characterization of desensitizing and non-desensitizing glutamate-gated chloride channels in cockroach neurons. Neurotoxicology. 2004;25:967–980. doi: 10.1016/j.neuro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Ihara M, Ishida C, Okuda H, Ozoe Y, Matsuda K. Differential blocking actions of 4′-ethynyl-4-n-propylbicycloorthobenzoate (EBOB) and γ-hexachlorocyclohexane (γ-HCH) on γ-aminobutyric acid- and glutamate-induced responses of American cockroach neurons. Invert. Neurosci. 2005;5:157–164. doi: 10.1007/s10158-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 27.Eguchi Y, Ihara M, Ochi E, Shibata Y, Matsuda K, Fushiki S, Sugama H, Hamasaki Y, Niwa H, Wada M, Ozoe F, Ozoe Y. Functional characterization of Musca glutamate- and GABA-gated chloride channels expressed independently and coexpressed in Xenopus oocytes. Insect Mol Biol. 2006;15:773–783. doi: 10.1111/j.1365-2583.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 28.Janssen D, Derst C, Buckinx R, Van den Eynden J, Rigo J-M, Van Kerkhove E. Dorsal unpaired median neurons of Locusta migratoria express ivermectin- and fipronil-sensitive glutamate-gated chloride channels. J. Neurophysiol. 2007;97:2642–2650. doi: 10.1152/jn.01234.2006. [DOI] [PubMed] [Google Scholar]
- 29.Etter A, Cully DF, Liu KK, Reiss B, Vassilatis DK, Schaeffer JM, Arena JP. Picrotoxin blockade of invertebrate glutamate-gated chloride channel subunits; subunit dependence and evidence for binding within pore. J. Neurochem. 1999;72:318–326. [PubMed] [Google Scholar]
- 30.Raymond V, Sattelle DB, Lapied B. Co-existence in DUM neurons of two GluCl channels that differ in their picrotoxin sensitivity. NeuroReport. 2000;11:2695–2701. doi: 10.1097/00001756-200008210-00018. [DOI] [PubMed] [Google Scholar]
- 31.Wafford KA, Sattelle D,B. Effects of amino acid neurotransmitter candidates on an identified insect motorneurone. Neurosci. Lett. 1986;63:135–140. doi: 10.1016/0304-3940(86)90050-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Salgado VL, Yeh JZ, Narahashi T. Mechanism of selective toxicity of α-endosulfan in insects and mammals. Soc. Toxicol. Ann. Mtg. Abstr. #895. [Google Scholar]