Abstract
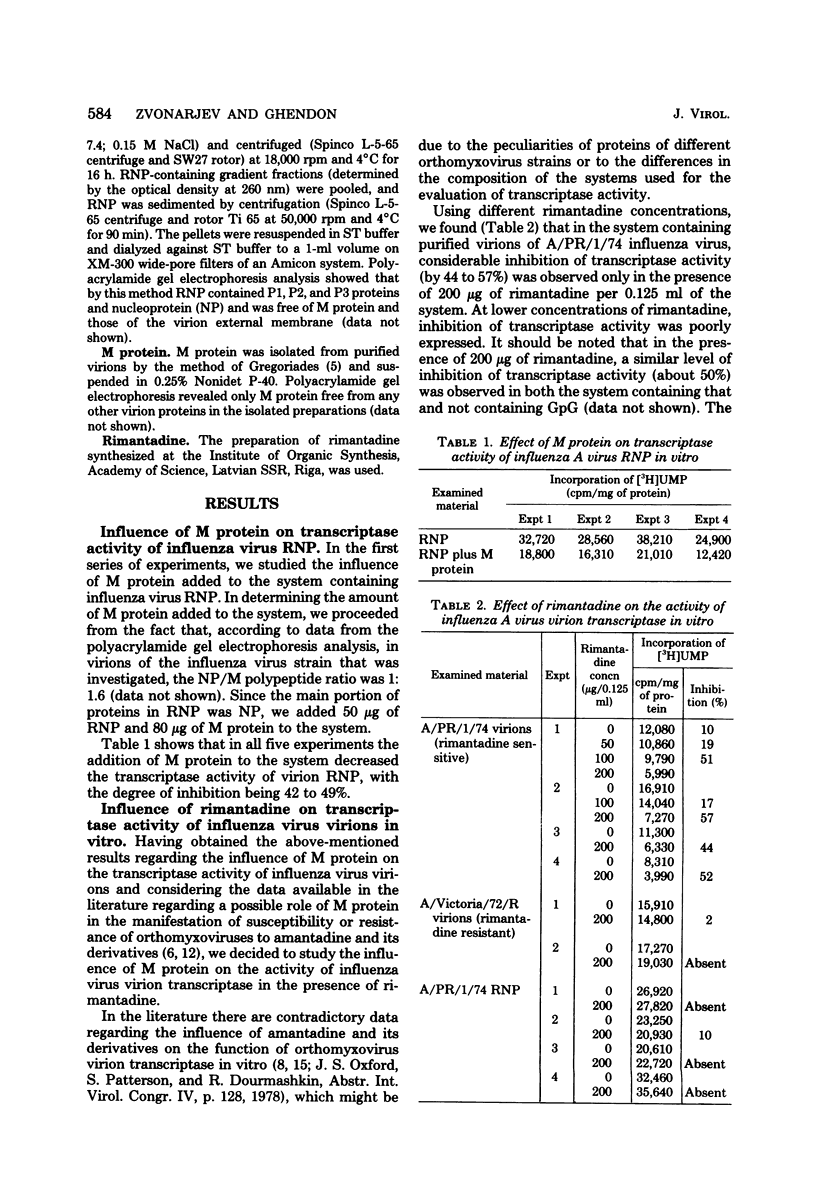
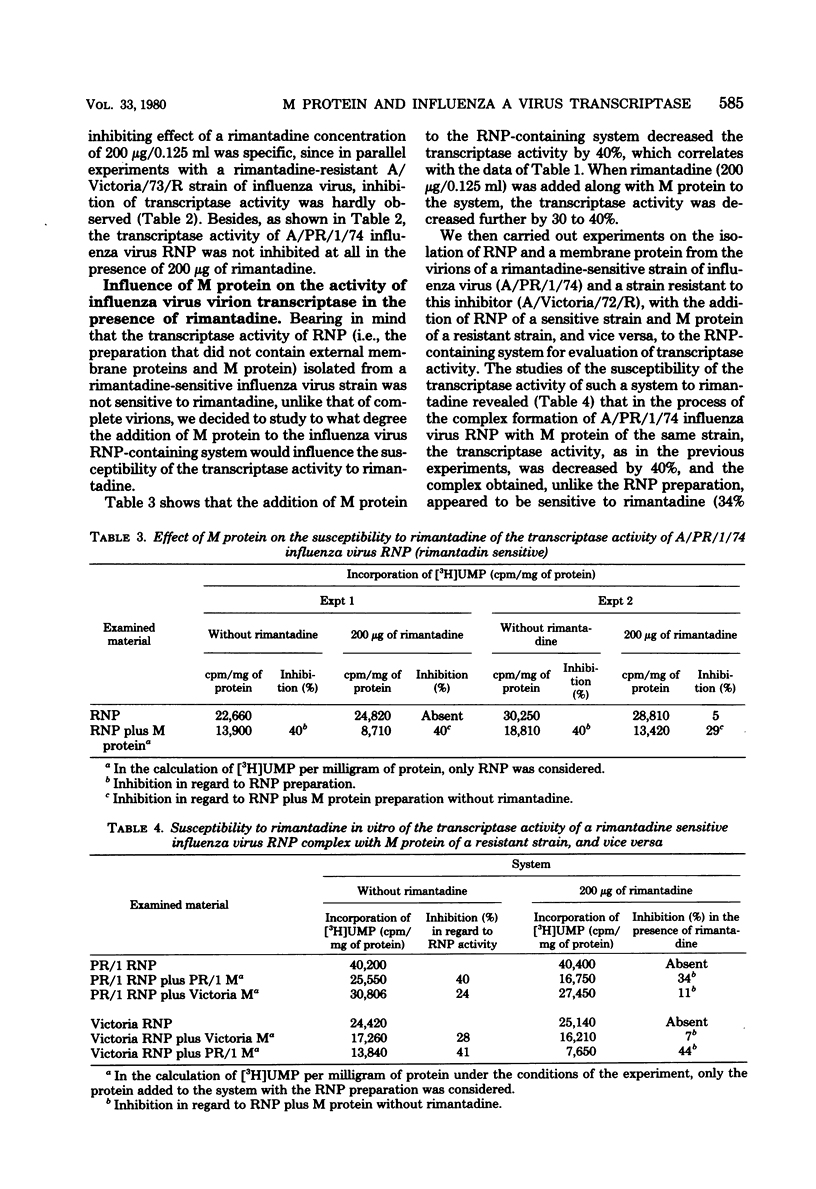
The transcriptase activity of influenza A virus ribonucleoproteins was inhibited by 42 to 49% in vitro in the presence of membrane (M) protein. The addition of M protein to the system of ribonucleoprotein preparations isolated from rimantadine-sensitive or rimantadine-resistant influenza virus strains, as well as the addition of M protein isolated from a sensitive strain, in the presence of rimantadine further inhibited the transcriptase activity of such complexes by approximately 40%. In the system containing the same ribonucleoprotein preparations, but with M protein isolated from a rimantadine-resistant influenza virus strain, the transcriptase activity was not sensitive to rimantadine. The data show that M protein can influence the activity of influenza A virus virion transcriptase and that the susceptibility of influenza virus to rimantadine may be due to the peculiarities of M protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya O. V., Ghendon Y. Z. Comparative study of virion transcriptase of some influenza virus strains. Acta Virol. 1978 Mar;22(2):97–103. [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmashkin R. R., Tyrrell D. A. Electron microscopic observations on the entry of influenza virus into susceptible cells. J Gen Virol. 1974 Jul;24(1):129–141. doi: 10.1099/0022-1317-24-1-129. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. The membrane protein of influenza virus: extraction from virus and infected cell with acidic chloroform-methanol. Virology. 1973 Aug;54(2):369–383. doi: 10.1016/0042-6822(73)90150-5. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Kennedy N. C., Skehel J. J., Appleyard G. The matrix protein gene determines amantadine-sensitivity of influenza viruses. J Gen Virol. 1979 Jan;42(1):189–191. doi: 10.1099/0022-1317-42-1-189. [DOI] [PubMed] [Google Scholar]
- Indulen M. K., Kalninya V. A. Study on the mechanism of inhibiting action of aminoadamantane on the reproduction of fowl plague virus. Acta Virol. 1973 Jul;17(4):273–280. [PubMed] [Google Scholar]
- Kalninya V. A., Indulen M. K. Effect of adamantane derivatives on the activity of orthomyxovirus RNA-dependent RNA polymerase. Acta Virol. 1976 Aug;20(4):343–346. [PubMed] [Google Scholar]
- Kato N., Eggers H. J. Inhibition of uncoating of fowl plague virus by l-adamantanamine hydrochloride. Virology. 1969 Apr;37(4):632–641. doi: 10.1016/0042-6822(69)90281-5. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. I. Isolation and preliminary characterization of RNA from virus particles. J Mol Biol. 1966 Jun;18(1):195–203. doi: 10.1016/s0022-2836(66)80085-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lubeck M. D., Schulman J. L., Palese P. Susceptibility of influenza A viruses to amantadine is influenced by the gene coding for M protein. J Virol. 1978 Dec;28(3):710–716. doi: 10.1128/jvi.28.3.710-716.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D., Kitron N. Influenza virion RNA-dependent RNA polymerase: stimulation by guanosine and related compounds. J Virol. 1975 Apr;15(4):686–695. doi: 10.1128/jvi.15.4.686-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P. Identification of the defective genes in three mutant groups of influenza virus. J Virol. 1977 Mar;21(3):1196–1204. doi: 10.1128/jvi.21.3.1196-1204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J., Armstrong J. A. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J Gen Virol. 1978 Jan;38(1):97–110. doi: 10.1099/0022-1317-38-1-97. [DOI] [PubMed] [Google Scholar]



