Abstract
Pulsed ultrasound exposures of brain tissue in the presence of microbubble contrast agents have been shown to achieve transient focal disruption of the blood−brain barrier without significant damage to surrounding brain tissue. The effects of overall exposure duration on the extent of blood−brain barrier disruption was studied in these experiments to determine operating conditions for increasing the amount of therapeutic agents delivered to the brain. Exposures at 1.08 MHz ranging from 0.2 to 0.8 MPa in amplitude were delivered transcranially to the brains of rabbits and rats for durations ranging from 30 to 1200 s. The amount of signal enhancement on contrast-enhanced T1-weighted MR images were used to quantify the extent of blood−brain barrier disruption, and histological evaluation of the exposed regions was performed to evaluate the impact on brain tissue. A subset of four rats underwent weekly exposures for 3 weeks to evaluate the feasibility of repeat sonications to the brain. The results suggest that exposures less than 180 s in duration are associated with a low probability of irreversible damage to brain tissue at pressure amplitudes of 0.38 MPa. Although exposures greater than 300 s were associated with an increase in the proportion of irreversible damage, this may be acceptable for chemotherapy delivery, where the therapeutic goal is tissue destruction. Repeat exposures to the brain were feasible but resulted in evidence of focal brain damage in 50% of animals.
Keywords: Ultrasound, blood−brain barrier, MRI, focused ultrasound, microbubbles
A physiological barrier to achieving effective delivery of therapeutic agents into the brain is the blood−brain barrier (BBB) (1,2). A number of strategies are being investigated to overcome this obstacle including nanoparticles (3) and intra-arterial injection of mannitol (4); however, these approaches are either invasive or unable to achieve localized opening of the BBB in the region of interest. Pulsed ultrasound exposures of brain tissue in the presence of microbubble contrast agents have been shown to achieve local disruption of the BBB without significant damage to brain tissue (5−7). Under appropriate exposure conditions, the disruption of the BBB has been observed to be transient, providing a window of a few hours for delivery of therapeutic agents into the brain at the site of exposure (8,9). Within this window, a number of agents including MR contrast agents, chemotherapeutic drugs, dyes, antibodies, and contrast agents have been shown to traverse the BBB and accumulate within brain tissue in animal studies (10−13).
The acoustic parameters employed during focused ultrasound exposures in brain have a strong influence on the resulting biological effect. The effect of acoustic pressure, burst length, frequency, contrast agent type and dose, and repetition frequency on the magnitude of BBB disruption as well as the histological effects on brain tissue has been investigated previously (7,9,14−16). These studies indicate that there is a fine balance between transient BBB disruption and tissue damage, emphasizing the importance of characterizing and understanding these relationships.
In a recent study, BBB disruption with focused ultrasound demonstrated delivery of therapeutic concentrations of doxorubicin in the brains of rats (13). This result was achieved with repeat exposures of the target region in the brain over multiple minutes. Investigation of the impact of increased exposure durations on the extent of BBB disruption and the impact on brain tissue has not been studied extensively. In addition, the feasibility of performing multiple ultrasound exposures of the same location for repeat BBB opening, as would be required, for example, for chemotherapy delivery, has not been investigated. The purpose of this study was to investigate the impact of exposure time on the extent of BBB opening and the resulting biological effects in brain tissue. The motivation for this work was to evaluate whether longer exposures could result in achieving increased BBB opening for improved delivery of therapeutic agents without causing undesirable brain damage.
Results and Discussion
The prospect of achieving local delivery of drugs and other therapeutic agents to the brain through noninvasive disruption of the blood−brain barrier could have wide applications in medicine. Initial studies have demonstrated the safety of this approach under appropriate ultrasound exposure conditions. The nature of the ultrasound exposure has an influence on the size and type of molecules that will pass through the BBB into brain parenchyma (8,12,17). Attempts to increase the delivery of therapeutic quantities of drugs such as doxorubicin have involved delivering multiple exposures spaced in time to the same location in the brain, although these values were not necessarily optimal (13). The goal of this study was to evaluate the influence of overall exposure time on the amount of MR contrast agent that passed through the BBB to investigate the influence of this parameter on BBB disruption.
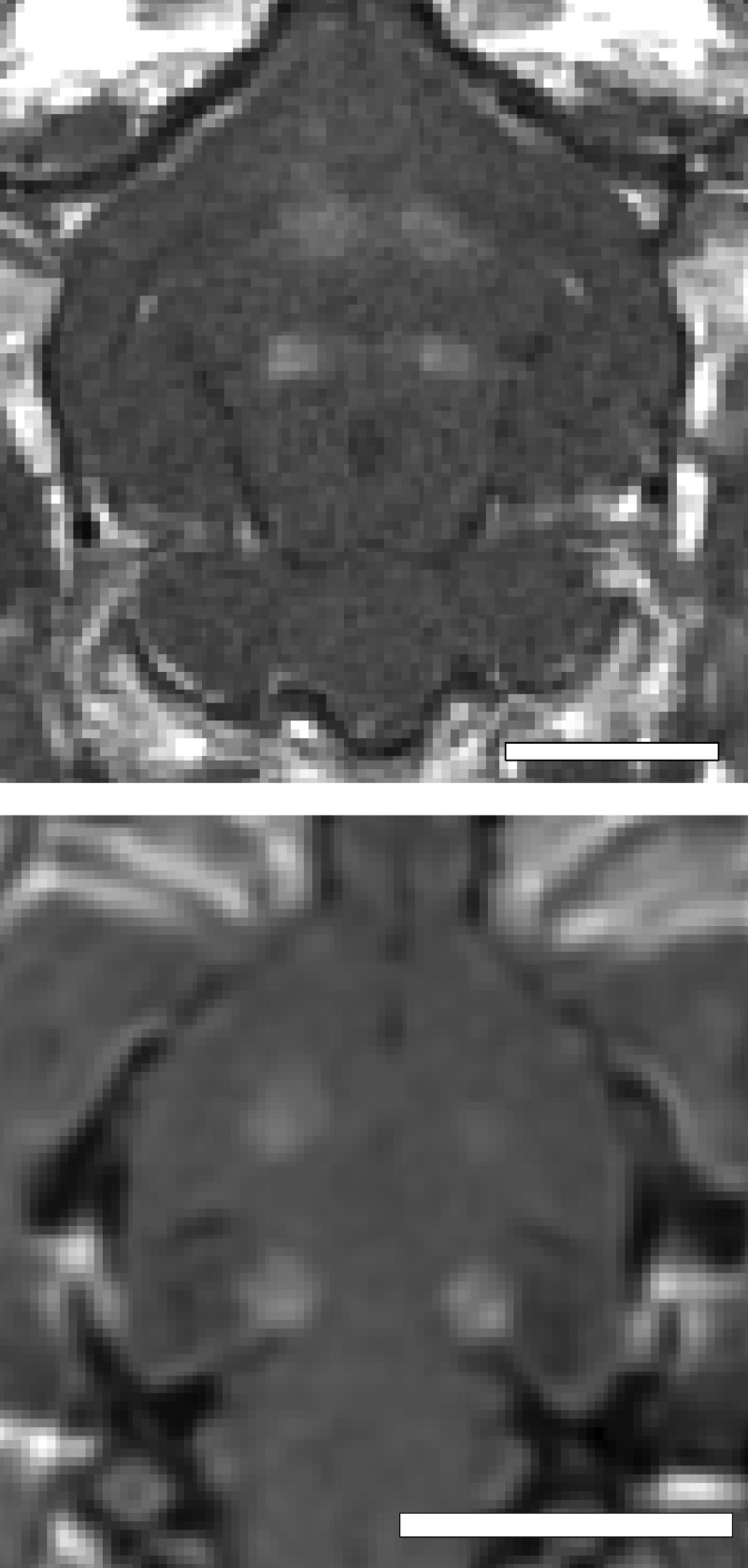
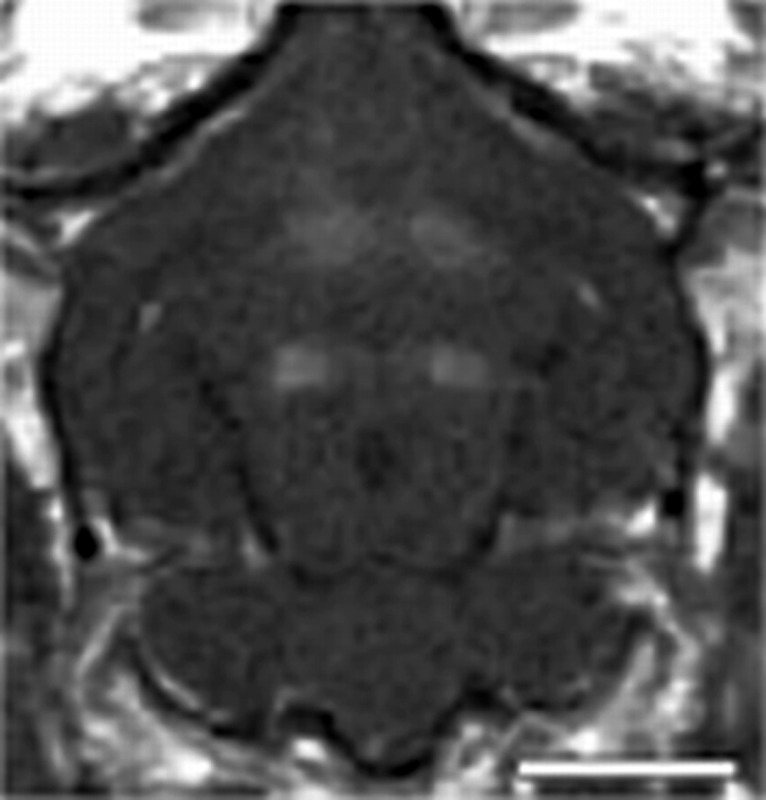
The spatial location of BBB disruption in the rabbit and rat brain is shown in the coronal contrast-enhanced T1-weighted MR images in Figure 1. The exposures were spaced approximately 5−9 mm apart depending on the size of the brain. Clear solitary enhancement in the images caused by leakage of the MR contrast agent through the BBB is observed in the sonicated locations. The enhancement was quantified by taking the mean signal over a 3 × 3 region of interest (ROI) centered on the region of enhancement and normalizing the change relative to an unexposed area of the brain in close proximity to the sonication.
Figure 1.
Coronal contrast-enhanced T1-weighted images in the rabbit (top) and rat (bottom) brains showing the spatial location of ultrasound exposures and BBB disruption. The spacing between the four exposures was adjusted based on the size of the brain. All exposures were performed through intact skull.
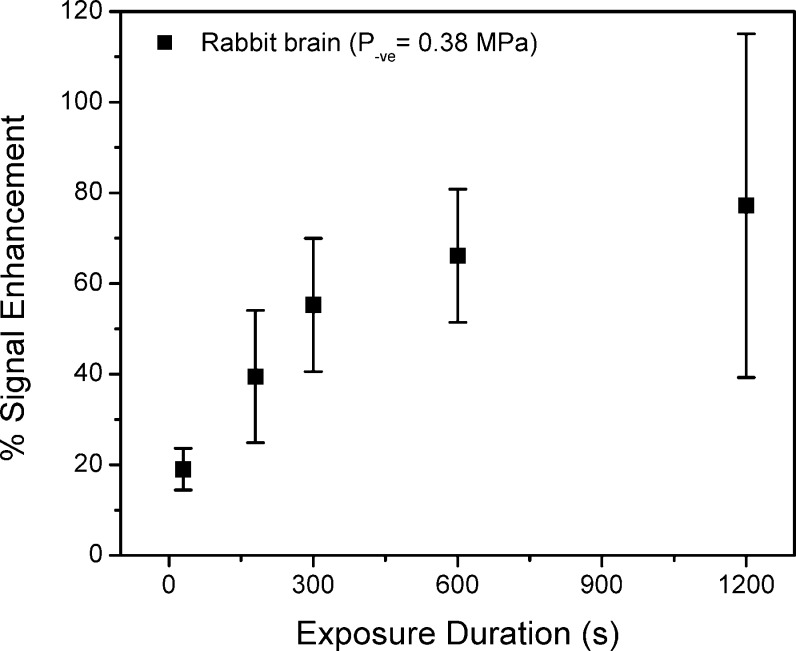
The relative increase in signal enhancement with increasing exposure time is shown in Figure 2 for the exposures in rabbit brain. An increasing trend was observed with increasing exposure duration with some evidence of the enhancement leveling off above approximately 600 s. At these long exposures, there was also evidence of irreversible tissue damage on T2-weighted images acquired after sonication.
Figure 2.
An increase in the signal enhancement after contrast agent administration on T1-weighted images is observed with increasing exposure duration in rabbits. The increase levels off for exposures greater than 600 s.
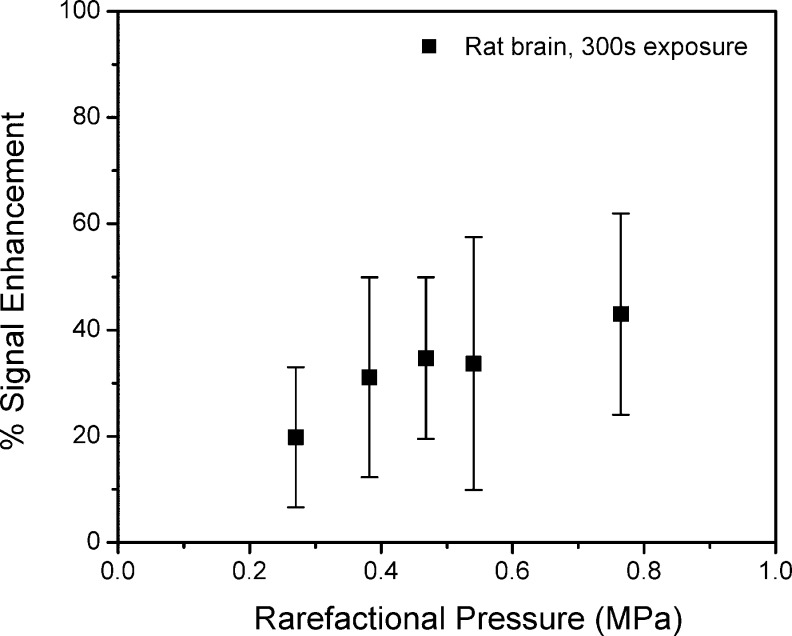
The relative change in signal enhancement with increasing exposure amplitude for 300 s exposures is shown in Figure 3 for the rat exposures. An increase in the mean enhancement was observed up to about 0.47 MPa, with evidence of leveling off above this level. Exposures of 0.54 and 0.76 MPa (1 and 2 W acoustic power) were associated with clear evidence of irreversible changes on T2-weighted images after sonications.
Figure 3.
Increasing pressure amplitude for exposure durations of 300 s has a minor influence on signal enhancement on T1-weighted MR images and also appears to have diminishing influence above 0.5 MPa.
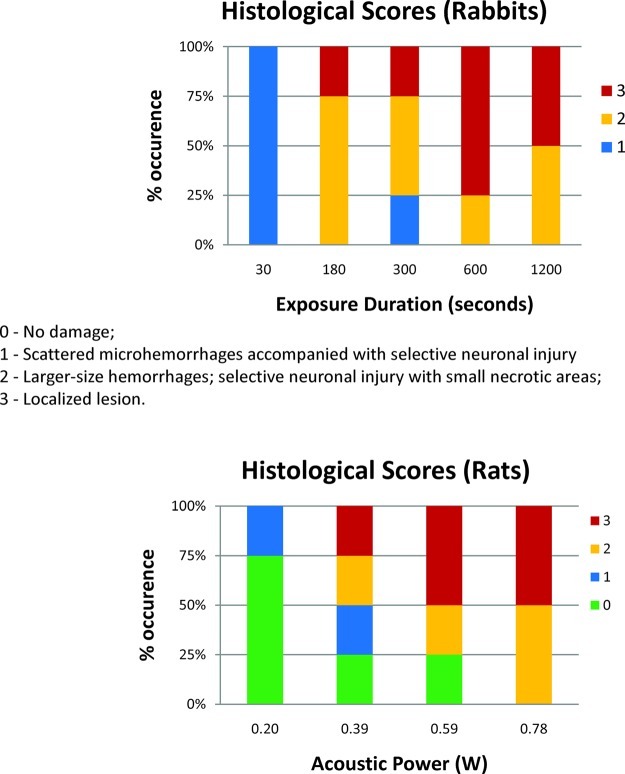
The frequency of histological patterns in the exposed locations is shown as a function of exposure duration and amplitude in Figure 4. In both cases, there was a trend to higher levels of damage with increasing exposure time and amplitude. Exposures of 30 s at 0.38 MPa or 300 s at 0.27 MPa exhibited minimal damage in the sonicated regions of the brain. For exposures greater than 600 s at 0.38 MPa, or greater than 0.47 MPa for 300 s, at least 50% of the sonicated locations exhibited localized lesions. These findings were consistent with the observations on the T2-weighted MR images acquired during the experiments.
Figure 4.
In both the rabbit and rat exposures, a trend toward increasing damage was observed with increased exposure duration and pressure amplitude. Little or no damage was observed for exposure durations of 30 s at 0.38 MPa and 300s at 0.27 MPa. At least 50% of sonicated locations resulted in localized lesions for exposure durations greater than 600 s and pressures greater than 0.47 MPa.
For exposure durations greater than 300 s, consistent changes were seen in the sonicated locations on T2-weighted images, which are often associated with damage to brain tissue. The histological evaluation of these exposures confirmed that localized lesions were present in at least 25% of sonications greater than 180 s in duration. It should be pointed out that the pressure amplitude used in the experiments in rabbit brain was 0.38 MPa, which is sufficient to open the BBB with a 30 s exposure. Perhaps reduced amplitude exposures would be able to achieve greater BBB opening without significant damage at these extended exposure durations. The influence of pressure amplitude for 300 s exposures was investigated in the rat brain, and a trend toward increased signal enhancement was observed with increasing pressure. There was significant variability in the results, which was probably because the sonications were performed through intact skull, and there may have been experimental issues related to the method of injection of microbubbles in some experiments (18). Nonetheless, the results still depict a trend that is similar to similar investigations of pressure amplitude on the extravasation of MR contrast agents in the brain (14,19). Histological evaluation of the rat brains indicated that for pressure amplitudes of 0.38 MPa and greater; 300 s exposures resulted in localized lesions in 25% of sonicated locations.
Figure 5 shows examples of the types of biological effects in the brain used to classify the extent and nature of damage. Score 1 was characterized by scattered microhemorrhages due to small blood vessel leaks into brain parenchyma (a). Score 2 was characterized by more extensive hemorrhages associated with selective neuronal degeneration (b). Score 3 involved a wider range of effects including small areas of ischemic necrosis (c,d). Also present in some instances were extensive hemorrhagic lesions (e−h). Within these lesions, there were areas around the blood vessels showing erythrocyte dispersion into the surrounding tissue in regions adjacent to the extensive hemorrhagic lesion (i,j).
Figure 5.

Tissue sections (hematoxylin−eosin stain) depicting the criteria used to quantify the biological effect of ultrasound exposures in the brain parenchyma: (a) score 1, scattered microhemorrhages due to small blood vessel leaks into brain parenchyma; (b) score 2, more extensive hemorrhages are associated with selective neuronal degeneration (arrows); (c, d) score 3, small area of ischemic necrosis (shown by empty arrows); (d) magnified view of necrotic area from panel c demonstrates ischemic dark-stained neurons; (e−h) score 3, extensive hemorrhagic lesion; (f) magnified view of the central part of the lesion shows severely damaged blood vessels surrounded by ball (g) or ring hemorrhages (h); (i, j) satellite areas around the blood vessels showing erythrocyte dispersion into the surrounding tissue in regions adjacent to the extensive hemorrhagic lesion.
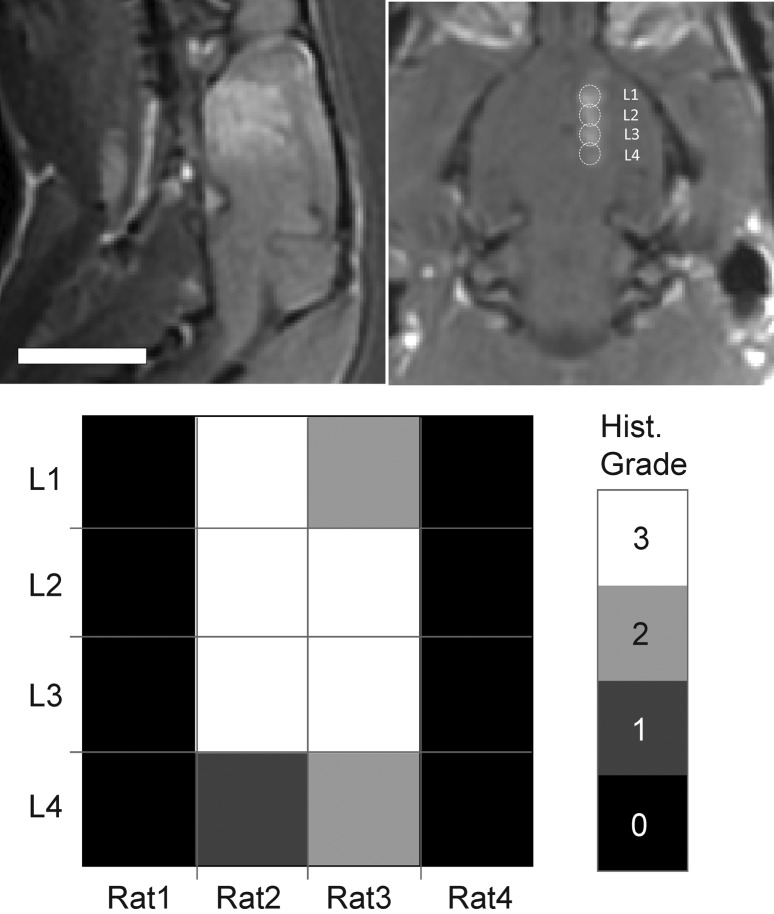
The final set of pilot experiments described in this study was related to the feasibility of performing repeat sonications in the brain, and the histological impact of these exposures. As shown in Figure 6, a large and continuous region of BBB disruption was achieved by delivering a linear set of four overlapping sonications. An example of a linear exposure in the rat brain consisting of four sonications is shown in the sagittal and coronal contrast-enhanced images of Figure 6. The images depict a contiguous and large region of signal enhancement in the brain after the exposure. The region of signal enhancement is localized to the locations of the sonications. The extent of histological effects for each of the four rats is summarized in the false-color map in Figure 6, where the histological grade of each sonicated location was evaluated and represented on a four level grayscale. Two of the four rats exhibited no evidence of damage (grade 0) at all sonicated locations after three consecutive exposures, while two exhibited damage ranging from slight hemorrhage (grade 1) to localized lesions (grade 3). The results of this initial study were mixed: 50% of rats exhibited no signs of damage, while the other 50% exhibited damage ranging from minor hemorrhage to localized lesions. The variability in these results was in part due to the limitations of the experimental setup. It was noticed that the pressure amplitude required for BBB disruption changed for the same rats each week. This was likely due to changes in the skull orientation that occurred in the time between exposures but may also have been due to changes in the skin from repeat removal of hair using depilatory cream, or to the brain parenchyma itself. The incorporation of acoustic feedback to monitor for activation of contrast agents could improve the ability to overcome this variability (20). In addition, there may also have been effects due to the nature of the microbubble injection that were present in these exposures, which could have had an impact on the results18.
Figure 6.
A sagittal (left) and coronal (right) contrast-enhanced T1-weighted image in the rat brain after linear exposure of four locations in the brain following a single injection of ultrasound microbubbles. The region of signal enhancement is continuous and extends over a large area in the brain. The histological grade observed at each sonicated location across all rats is shown in the false colormap in the bottom panel.
Overall, the results of this study demonstrate that the relative signal enhancement on contrast-enhanced MR images increases with increasing exposure duration and pressure amplitude. Previous studies have shown that the increase in signal enhancement on contrast-enhanced MR images is related to the extent of BBB disruption in the brain. This study also showed that there is an increase in damage to brain tissue that must be considered when attempting longer exposure durations. Nonetheless, the relative signal enhancement that was achieved with exposure durations greater than 300 s was greater than 60% over unexposed regions of the brain, which has been shown to achieve therapeutic levels of doxorubicin in the brain13.
Conclusions
These initial experiments suggest that exposures less than 180 s in duration are associated with a low probability of irreversible damage to brain tissue at pressure amplitudes of 0.38 MPa. The increase in contrast agent leakage with exposure time was more significant than for increased pressure amplitude, suggesting that lower power sonications delivered over a longer exposure time may result in increased delivery of therapeutic agents to the brain. Although exposures greater than 300 s were associated with an increase in the proportion of irreversible damage, this may be acceptable for chemotherapy delivery, where the therapeutic goal is tissue destruction.
Methods
Ultrasound Exposures
Ultrasound exposures were generated using a spherically focused, air-backed piezoceramic transducer (f0 = 1.08 MHz) with a diameter of 7 cm and a focal length of 5.6 cm. The transducer was driven by a function generator (model 395, Wavetek, San Diego, CA) and RF amplifier (model 240 L, ENI Inc., Rochester, NY). The electrical power was measured with a power meter (model 438A, Hewlett-Packard, Palo Alto, CA) connected to the forward and reserve signal of a dual directional coupler (C173, Werlatone, Brewster, NY). The transducer’s electrical impedance was matched to the output impedance of the amplifier (50 Ω) with a custom-made passive matching circuit. The transducer was calibrated using a radiation force method for the acoustic power and calibrated hydrophone for the acoustic pressure amplitude. Measurements though ex vivo rabbit and rat skulls were performed to estimate the pressure amplitudes in vivo. The pressure through the rat skull and rabbit skull was found to be 51% and 16%, respectively, of the pressure measured in water.
The transducer was mounted in a three-dimensional mechanical positioning device in an acrylic tank filled with deionized water. The transducer was pointed up and through an acoustic window on the top of the tank such that the beam could enter the skull placed over the opening. Translation of the ultrasound transducer to localize the focal volume within a desired brain location was achieved using a manual three-axis linear positioning system (Velmex, Inc.) modified to be nonmagnetic.
Animals
Both rabbits and rats were used to investigate the influence of ultrasound exposure duration on blood−brain barrier opening. Institutional approval from the Animal Care Committee was obtained for all experiments.
Twelve rabbits with four sonications in each brain were used to study the effect of exposure duration at a fixed acoustic power. New Zealand white rabbits with an approximate weight of 4 kg were used, and the hair at the top of the skull was shaved and depilated to ensure good coupling of the ultrasound beam into the brain. The rabbits were anesthetized with a combination of 50 mg/kg of ketamine and 5 mg/kg of xylazine administered intramuscularly and maintained with top-up injections with the same dose as required during the experiment. All ultrasound and MR contrast agents were injected into a cannulated ear vein in the rabbits. Four sonications were delivered in a rectangular grid to each brain with two exposures in each hemisphere (one in the forebrain and the other in the midbrain). The distance between sonications was approximately 7 mm. The ultrasound focus was aimed midway through the brain for all exposures to minimize interactions with the skull base or surface.
After the initial experiments studying the influence of exposure duration, subsequent experiments were performed in a rat model to reduce the cost and the influence of the skull bone on the variability of results. The rats used in the study were male Wistar rats with an approximate weight of 300−400 g. The hair at the top of the skull was shaved and depilated similarly to procedure with the rabbits for acoustic coupling. The rats were anesthetized with a combination of 100 mg/kg of ketamine and 10 mg/kg of xylazine administered intramuscularly and maintained with top-up injections of the same dose as required during the experiment. All ultrasound and MR contrast agents were injected into a cannulated tail vein in the rats. A total of 90 sonications were delivered to the brains of 25 rats. Two groups of sonications were delivered to the rats in this study. In the first group (n = 21), four sonications were delivered to the brain in a square grid pattern similar to that in the rabbits, except the spacing between points was approximately 5 mm due to the reduced size of the organ. In the second group (n = 4), four sonications were delivered in a linear fashion to one hemisphere spaced approximately 1.5 mm apart from each other. The linear exposure was performed by sonicating a location after an injection of microbubbles and waiting for 5 min for the bubbles to clear the system before sonicating the next location. The exposures were repeated in approximately the same location once every week for three weeks in these animals.
All animals were positioned supine on a platform on top of a water tank containing the ultrasound transducer and degassed, deionized water, as shown in Figure 7. The heads of the animals were placed over an opening in the platform and were coupled to the transducer with water. A custom-made single-loop RF receive coil was used to acquire MR images of the brain. The coil diameter was approximately 8 cm for the rabbit experiments and 3 cm for the rat experiments to optimize signal-to-noise ratio (SNR). The head was positioned such that the ultrasound beam entered the brain at approximately normal incidence to the skull bone to reduce refraction of the beam. The animals were maintained at a constant temperature with a circulating water blanket, and the rectal temperature of the animals was monitored during the experiment.
Figure 7.
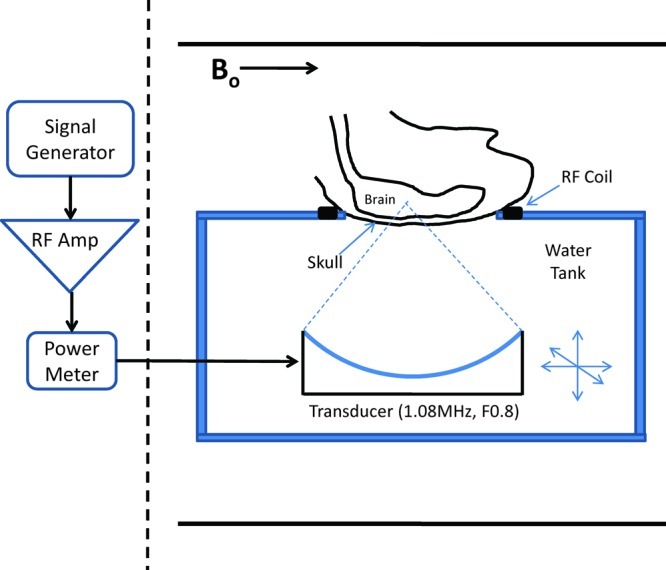
Experimental arrangement for transcranial sonications. Animals were placed supine over a focused ultrasound transducer, with a single-loop RF receive coil around the head. The experiments were performed in a closed-bore MRI.
Sonications
An initial set of experiments was performed in rabbits (n = 12) in order to investigate the influence of overall exposure duration on BBB disruption. The acoustic power delivered during the ultrasound burst by the transducer was fixed at 3.9 W with exposures ranging from 30 to 1200 s. Based on prior calibration of the transducer in a water tank, this corresponded to a peak negative pressure amplitude of 0.38 MPa in the brain. Each burst was 10 ms long with a repetition frequency of 1 Hz. This combination of pulse duration and repetition frequency parameters has been shown to produce repeatable blood−brain barrier opening in previous studies14. Prior to each sonication, a bolus injection of ultrasound contrast agent at a clinical diagnostic dose of 10 μL/kg (Definity, Bristol-Myers Squibb) was injected through the ear vein. A saline bolus of 0.5 mL was injected subsequently to flush the bubbles through the needles and tubing into the body. The sonications started immediately after the injection of the contrast agent into the body.
A second set of experiments was performed in rats (n = 21) to evaluate the influence of exposure amplitude at a given exposure duration. The overall exposure was 300 s, with the same burst duration (10 ms) and repetition frequency (1 Hz) as implemented in the first experiments. The acoustic power delivered from the transducer was varied from 0.2 to 0.78 W in these animals, corresponding to a peak negative pressure in the brain ranging from 0.27 to 0.54 MPa. The same ultrasound contrast agent dose of 10 μL/kg was administered through the tail vein, but the saline flush volume was reduced to 0.1 mL.
A final set of experiments was performed in four rats to investigate the feasibility of repeat opening of the BBB in a series of locations in the brain. For these exposures, the overall duration was kept constant at 300 s, and the acoustic power delivered from the transducer was increased until leakage of MR contrast agent was observed in the brain. The lowest exposure level was 0.2 W, and all exposures were below 0.6 W. The sonications were repeated weekly for three weeks, and MR imaging was performed after each exposure to evaluate the feasibility of opening the BBB.
MR Imaging
All experiments in this study were performed in a 3 T closed bore MR imager (Signa, GE Healthcare). Co-registration of the ultrasound focus in the coordinate system of the MR images was accomplished by first heating a tissue-mimicking phantom and locating the region of signal reduction due to heating.
Anatomical images were acquired in multiple planes prior to and after sonication using a T2-weighted sequence (FSE-XL, TE = 75 ms, TR = 2000 ms, ETL = 4, BW = 6.9 kHz, 256 × 256/128 × 128, slice = 1 mm, NEX = 2, FOV = 10 cm/5 cm) to evaluate whether signs of tissue damage were present after the exposures. After all sonications, a bolus injection of MR contrast agent (0.1 mmol/kg, Omniscan, GE Healthcare) was administered, followed by a saline flush, in order to visualize BBB disruption in the brain. The contrast agent was typically administered after all the target locations in the brain had been sonicated. Contrast-enhanced images were acquired transverse to the ultrasound beam direction using a T1-weighted imaging sequence (FSE-XL, TE = 14 ms, TR = 500 ms, ETL = 4, BW = 15 kHz, 256 × 256/128 × 128, slice = 1.5 mm, NEX = 5, FOV = 10 cm/5 cm) to evaluate the presence and extent of BBB opening.
Histology
All animals were kept under anesthesia for approximately 2−4 h after the sonications. After this time point, the animals were perfused with saline and formalin, and the brains were subsequently removed from the carcass and submerged in 10% neutral buffered formalin. After fixation for greater than 4 weeks, the brains were manually sliced using a brain matrix along a coronal direction into a 4 mm thick slab and placed in a standard cassette. The slab of brain tissue was perpendicular to the direction of the ultrasound beam, and the thickness was on the order of the beam focal length. The slabs were processed histologically; 4 μm sections obtained every 0.25 mm in depth through the brain were stained with hematoxylin and eosin (H&E). The stained sections were evaluated at the location of each sonication, guided by the MR images. The person evaluating the sections did not have any knowledge of the exposure parameters used for the sonications. The histological findings on H&E stained sections were ranked as following: 0 = no damage; 1 = scattered microhemorrhages due to small blood vessel leaks into brain tissue with minimal neuronal damage; 2 = larger-size hemorrhages, selective neuronal injury, and small necrotic areas; 3 = localized lesion.
Data Analysis
The MR images for all experiments were analyzed off-line using MATLAB. At the location of each sonication, the change in signal intensity over time on the contrast-enhanced images was measured to quantify the extent of BBB disruption. The average signal intensity change across a 3 × 3 voxel ROI was measured at the target location and a nearby region of unexposed brain tissue. A relative change in signal intensity was calculated by using the unexposed region to normalize the change observed in the target location.
Acknowledgments
The authors wish to thank Ms. Shawna Rideout for her assistance with the experiments performed in rats and rabbits.
This work was supported by the National Institutes of Health (Grants R01EB003268 and R33EB000705), and the Canada Research Chair Program.
R. Chopra contributed to execution of experiments, analysis of data, and manuscript preparation and editing. N. Vykhodtseva contributed to analysis of tissue specimens and manuscript preparation. K. Hynynen contributed to study design, execution of experiments, and manuscript preparation and editing.
Funding Statement
National Institutes of Health, United States
References
- Abbott N. J.; Romero I. A. (1996) Transporting Therapeutics across the Blood-Brain Barrier. Mol. Med. Today 2, 106–113. [DOI] [PubMed] [Google Scholar]
- Misra A.; Ganesh S.; Shahiwala A.; Shah S. P. (2003) Drug Delivery to the Central Nervous System: A Review. J. Pharm. Pharm. Sci. 6, 252–273. [PubMed] [Google Scholar]
- Silva G. A. (2008) Nanotechnology Approaches to Crossing the Blood-Brain Barrier and Drug Delivery to the CNS. BMC Neurosci. 9(Suppl 3), S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll R. A., Neuwelt E. A. (1998) Outwitting the Blood-Brain Barrier for Therapeutic Purposes: Osmotic Opening and Other Means. Neurosurgery 42, 1083−1099; discussion 1099−1100. [DOI] [PubMed] [Google Scholar]
- Hynynen K.; McDannold N.; Vykhodtseva N.; Jolesz F. A. (2001) Noninvasive MR Imaging-Guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 220, 640–646. [DOI] [PubMed] [Google Scholar]
- Choi J. J.; Pernot M.; Small S. A.; Konofagou E. E. (2007) Noninvasive, Transcranial and Localized Opening of the Blood-Brain Barrier using Focused Ultrasound in Mice. Ultrasound Med. Biol. 33, 95–104. [DOI] [PubMed] [Google Scholar]
- Yang F. Y.; Fu W. M.; Yang R. S.; Liou H. C.; Kang K. H.; Lin W. L. (2007) Quantitative Evaluation of Focused Ultrasound with a Contrast Agent on Blood-Brain Barrier Disruption. Ultrasound Med. Biol. 33, 1421–1427. [DOI] [PubMed] [Google Scholar]
- Hynynen K.; McDannold N.; Sheikov N. A.; Jolesz F. A.; Vykhodtseva N. (2005) Local and Reversible Blood-Brain Barrier Disruption by Noninvasive Focused Ultrasound at Frequencies Suitable for Trans-Skull Sonications. Neuroimage 24, 12–20. [DOI] [PubMed] [Google Scholar]
- Wang F.; Cheng Y.; Mei J.; Song Y.; Yang Y. Q.; Liu Y.; Wang Z. (2009) Focused Ultrasound Microbubble Destruction-Mediated Changes in Blood-Brain Barrier Permeability Assessed by Contrast-Enhanced Magnetic Resonance Imaging. J. Ultrasound Med. 28, 1501–1509. [DOI] [PubMed] [Google Scholar]
- Hynynen K. (2007) Focused Ultrasound for Blood-Brain Disruption and Delivery of Therapeutic Molecules into the Brain. Expert Opin. Drug Delivery 4, 27–35. [DOI] [PubMed] [Google Scholar]
- Choi J. J.; Pernot M.; Brown T. R.; Small S. A.; Konofagou E. E. (2007) Spatio-Temporal Analysis of Molecular Delivery through the Blood-Brain Barrier using Focused Ultrasound. Phys. Med. Biol. 52, 5509–5530. [DOI] [PubMed] [Google Scholar]
- Kinoshita M.; McDannold N.; Jolesz F. A.; Hynynen K. (2006) Targeted Delivery of Antibodies through the Blood-Brain Barrier by MRI-Guided Focused Ultrasound. Biochem. Biophys. Res. Commun. 340, 1085–1090. [DOI] [PubMed] [Google Scholar]
- Treat L. H.; McDannold N.; Vykhodtseva N.; Zhang Y.; Tam K.; Hynynen K. (2007) Targeted Delivery of Doxorubicin to the Rat Brain at Therapeutic Levels Using MRI-Guided Focused Ultrasound. Int. J. Cancer. 121, 901–907. [DOI] [PubMed] [Google Scholar]
- McDannold N.; Vykhodtseva N.; Hynynen K. (2008) Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption. Ultrasound Med. Biol. 34, 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N.; Vykhodtseva N.; Hynynen K. (2008) Blood-Brain Barrier Disruption Induced by Focused Ultrasound and Circulating Preformed Microbubbles Appears to be Characterized by the Mechanical Index. Ultrasound Med. Biol. 34, 834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N. J.; Vykhodtseva N. I.; Hynynen K. (2006) Microbubble Contrast Agent with Focused Ultrasound to Create Brain Lesions at Low Power Levels: MR Imaging and Histologic Study in Rabbits. Radiology 241, 95–106. [DOI] [PubMed] [Google Scholar]
- Choi J. J.; Wang S.; Tung Y. S.; Morrison B.; Konofagou E. E. (2010) Molecules of various Pharmacologically-Relevant Sizes can Cross the Ultrasound-Induced Blood-Brain Barrier Opening in Vivo. Ultrasound Med. Biol. 36, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talu E.; Powell R. L.; Longo M. L.; Dayton P. A. (2008) Needle Size and Injection Rate Impact Microbubble Contrast Agent Population. Ultrasound Med. Biol. 34, 1182–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K.; McDannold N.; Vykhodtseva N.; Jolesz F. A. (2003) Non-Invasive Opening of BBB by Focused Ultrasound. Acta Neurochir. 86(Suppl.), 555–558. [DOI] [PubMed] [Google Scholar]
- McDannold N.; Vykhodtseva N.; Hynynen K. (2006) Targeted Disruption of the Blood-Brain Barrier with Focused Ultrasound: Association with Cavitation Activity. Phys. Med. Biol. 51, 793–807. [DOI] [PubMed] [Google Scholar]