Summary
Porphyromonas gingivalis is an oral bacterium that causes pathology in a number of dental infections that are associated with increased fibroblast cell death. Studies presented here demonstrated that P. gingivalis stimulates cell death by apoptosis rather than necrosis. Unlike previous studies apoptosis was induced independent of proteolytic activity and was also independent of caspase activity because a pan-caspase inhibitor, Z-VAD-fmk, had little effect. Moreover, P. gingivalis downregulated caspase-3 mRNA levels and caspase-3 activity. The consequence of this downregulation was a significant reduction in tumour necrosis factor-α-induced apoptosis, which is caspase-3-dependent. Immunofluorescence and immunoblot analysis revealed P. gingivalis-induced translocation of apoptosis-inducing factor (AIF) from the cytoplasm to the nucleus. siRNA studies were undertaken and demonstrated that P. gingivalis stimulated cell death was significantly reduced when AIF was silenced (P < 0.05). Treatment of human gingival fibroblasts with H-89, a protein kinase A inhibitor that blocks AIF activation also reduced P. gingivalis-induced apoptosis (P < 0.05). These results indicate that P. gingivalis causes fibroblast apoptosis through a pathway that involves protein kinase A and AIF, is not dependent upon bacterial proteolytic activity and is also independent of the classic apoptotic pathways involving caspase-3.
Introduction
Porphyromonas gingivalis is a Gram-negative anaerobic organism that is usually absent or detected at low levels in health but is frequently found at elevated levels in the periodontal pockets of patients with periodontitis, an inflammatory disease of tooth-supporting tissues (Lamont and Jenkinson, 1998; Graves et al., 2001). P. gingivalis produces a variety of virulence factors that affect the host response including lipopolysaccharide (LPS), fimbriae and proteases (Graves et al., 2000). These virulence factors may promote colonization, stimulate inflammation, or enhance survival of P. gingivalis through direct and indirect mechanisms (O’Brien-Simpson et al., 2005; Takii et al., 2005). Much attention has recently been focused on P. gingivalis proteases, most importantly, gingipains, which degrade proteins at arginine or lysine residues. These proteases may enable the bacterium to evade the host response by degrading host proteins, enhance invasion and colonization and provide nutritional support (Sheets et al., 2005). Furthermore, gingipain proteases may affect the host by inducing apoptosis of cells (Wang et al., 1999).
Emerging evidence indicates that bacteria-induced apoptosis is an important phenomenon in several pathologic conditions. For example, H. pylori contributes to the development of gastric ulcers by inducing apoptosis of gastric epithelial cells (Basak et al., 2005). Similarly, the morbidity of bacterial meningitis is aggravated by bacteria-induced neuronal cell apoptosis (Braun et al., 2001). In the periodontium the loss of fibroblasts is a characteristic features associated with progressive periodontal disease (Zappa et al., 1992). Apoptosis of fibroblasts is associated with inflammation in human gingiva (Tonetti et al., 1998; Koulouri et al., 1999). Moreover, P. gingivalis-induced fibroblast apoptosis is mediated to a large extent by tumour necrosis factor (TNF) and is aggravated by conditions such as diabetes, which is associated with more severe periodontal disease (Graves et al., 2001; Graves et al., 2006; Liu et al., 2006). However, there is evidence that P. gingivalis can directly induce apoptosis through bacterial cell wall components, particularly gingipain proteases (Kurita-Ochiai et al., 1998; Imamura et al., 2003). Gingipain proteases from bacterial culture supernatant have been reported to cause cell rounding and death by apoptosis (Wang et al., 1999; Chen et al., 2001; Sheets et al., 2005). In contrast, live P. gingivalis has been reported to be antiapoptotic for epithelial cells (Nakhjiri et al., 2001) while heat killed P. gingivalis is pro-apoptotic (Brozovic et al., 2006).
Two widely recognized mechanisms by which apoptosis occur involve the extrinsic and intrinsic apoptotic pathways. The extrinsic pathway is triggered by death activators (ligands) that bind to receptors at the cell surface. Death promoting signals are frequently associated with molecules termed death activators, such as cytokines belonging to the TNF family, namely TNF-α, lymphotoxin, FasL (fas ligand), Apo3L and TRAIL (TNF-related apoptosis-inducing ligand) (Thorburn, 2004; Thomas et al., 2004). The intrinsic pathway is generated by signals arising from within the cell in response to cell damage. For example, it is induced by conditions that cause cell damage such as exposure to reactive oxygen species, ionizing radiation or chemotherapeutic agents (Sordet et al., 2004). A third less well-understood mechanism involves molecules such as apoptosis-inducing factor (AIF). AIF is a protein that is normally located in the intermembrane space of mitochondria. When the cell receives signals for death, AIF is released from the mitochondria, similar to the release of cytochrome c in the intrinsic pathway and translocates to the nucleus where it facilitates DNA fragmentation. Interestingly, AIF does not require caspases to induce apoptosis (Cande et al., 2002; Lipton and Bossy-Wetzel, 2002). Based on previous reports we investigated mechanisms by which P. gingivalis induced apoptosis focusing initially on bacterial proteases and caspase-mediated cell death since it had been reported that P. gingivalis protesases induce fibroblast apoptosis through caspase-3 (Sheets et al., 2005). Contrary to expectations P. gingivalis-induced apoptosis of human gingival fibroblasts was largely independent of proteolytic enzymes or caspase-3. Further studies demonstrated that P. gingivalis downregulated caspase-3 activity and as a result reduced TNF-α-induced apoptosis. P. gingivalis, however, induced activation of the AIF pathway as demonstrated by enhanced nuclear translocation of AIF and P. gingivalis stimulated apoptosis was significantly inhibited by AIF siRNA, and by H-89, a protein kinase A inhibitor that has been shown to block AIF activation.
Results
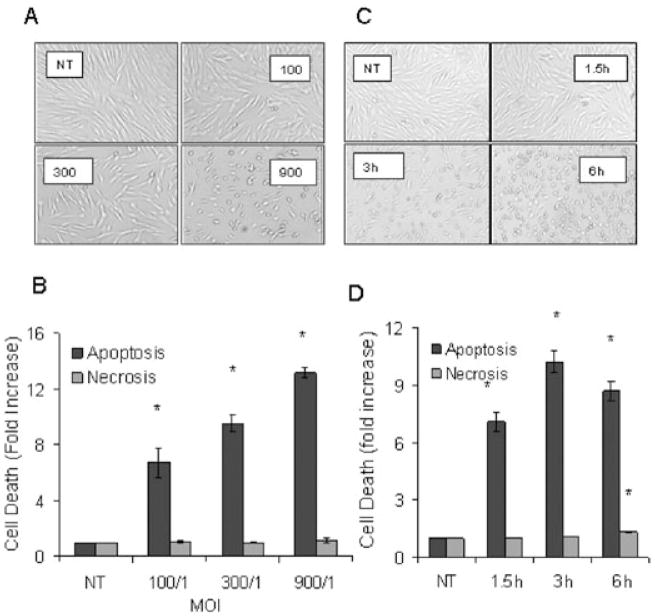
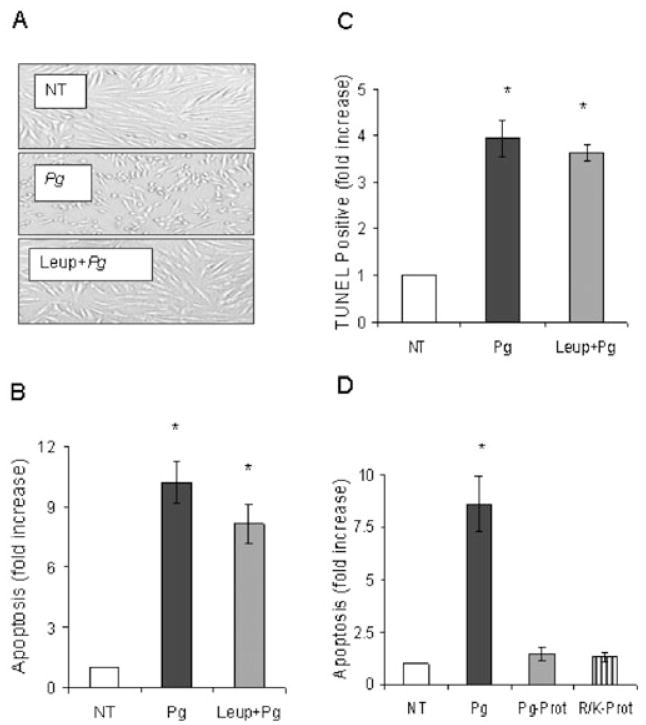
Time and dose–response experiments were undertaken to examine the impact of P. gingivalis on cell rounding and induction of apoptosis in human gingival fibroblasts. Morphological alterations and apoptosis of fibroblasts were examined after exposure to live P. gingivalis [multiplicity of infection (moi) 100:1, 300:1 and 900:1] for 3 h (Fig. 1). At the lowest concentration P. gingivalis had no effect on cell rounding but did induce apoptosis (P < 0.05). Both parameters were increased at higher concentrations. Cell rounding is consistent with the degradation of cell-extracellular matrix adhesion molecules by bacterial proteolytic enzymes (Chen et al., 2001; Sheets et al., 2005). Only small amounts of LDH were released into culture media for all doses tested indicating that cell death occurred via apoptosis but not necrosis (P > 0.05). A similar pattern was obtained when a time-course experiment was carried out. At the initial time point, 1.5 h, P. gingivalis caused little cell rounding but did enhance apoptosis (P < 0.05). With longer time points there was an increase in cell rounding and apoptosis but only a very small increase in necrosis. Thus, temporal separation of P. gingivalis-induced cell rounding and apoptosis was noted at early time points and low concentrations of bacteria. To investigate a mechanism by which P. gingivalis-induced apoptosis protease inhibitors were used. Leupeptin (an Arg-gingipain inhibitor) abolished P. gingivalis-induced morphological rounding (Fig. 2A) consistent with published data (Johansson and Kalfas, 1998; Wang et al., 1999; Chen et al., 2001). Leupeptin did not significantly alter the level of apoptosis (P > 0.05) (Fig. 2B). When cells were incubated with leupeptin plus cathepsin B inhibitor-II, a lys-gingipain inhibitor (Houle et al., 2003) no further reduction in apoptosis occurred (data not shown). When apoptosis was assessed by the TUNEL assay proteolytic inhibitors did not significantly reduce apoptosis (P > 0.05) (Fig. 2C). Similar results were obtained with proteins released by P. gingivalis that have proteolytic activity and commercially available endonucleases. Both caused extensive cell rounding (data not shown) but neither induced significant levels of fibroblast apoptosis (Fig. 2D). Thus, proteolytic enzyme contributed little to P. gingivalis-induced apoptosis.
Fig. 1.
P. gingivalis induces a dose- and time-dependent increase in fibroblast apoptosis but not necrosis that is distinct from cell rounding.
A. Human gingival fibroblasts were incubated with P. gingivalis (moi 100:1, 300:1 and 900:1) for 3 h. Original magnifications 40×.
B. Cells from A were tested for apoptosis by the presence of cytoplasmic histone-associated DNA fragments by ELISA (dark bars) or for necrosis by the release of LDH (light bars).
C. Human gingival fibroblasts were incubated with P. gingivalis (moi 300:1) for 1.5, 3 or 6 h. Original magnifications 40×.
D. Cells from C were tested for apoptosis (dark bars) and necrosis (light bars) as described above. Each value represents the mean of three separate experiments ± SEM. An asterisk indicates significant increase (P < 0.05).
Fig. 2.
P. gingivalis induces fibroblast apoptosis independent of its proteolytic enzymes. Human gingival fibroblasts were exposed to P. gingivalis (moi 300:1) with or without leupeptin for 3 h.
A. Original magnifications 40×.
B. Apoptosis was measured by ELISA.
C. The TUNEL assay was performed and TUNEL positive cells were counted.
D. Human gingival fibroblasts were also exposed to P. gingivalis-supernatant (Pg-prot) or arg/lys-specific endopeptidases (R/K-prot). Apoptosis was measure by ELISA. Each value represents the mean of three separate experiments ± SEM. An asterisk indicates significant increase (P < 0.05).
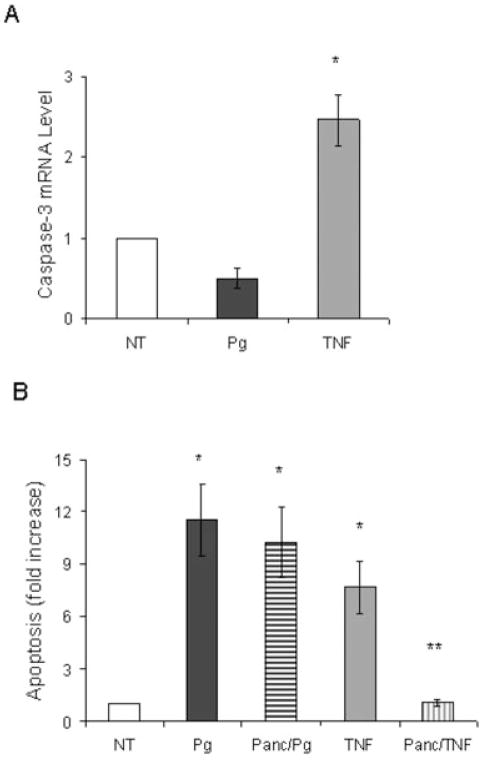
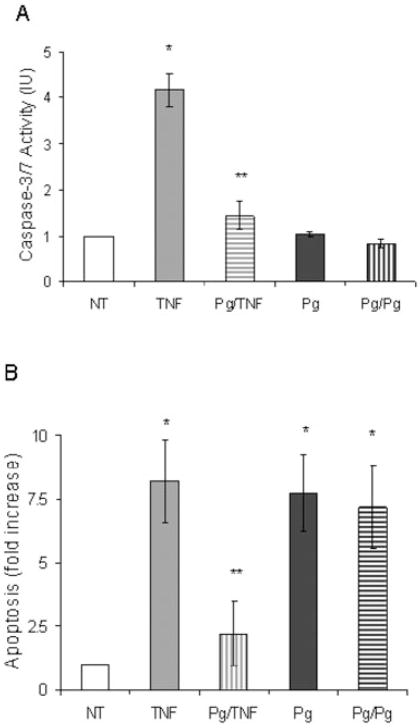
Apoptotic cell death typically occurs by stimulating caspases (Thorburn, 2004). Caspase-3 is the principle effector caspase of the well-defined cytosolic and mitochondrial pathways (Green, 2000). To examine the effect of P. gingivalis on caspase-3 expression real-time polymerase chain reaction (PCR) was carried out and compared with a positive control, TNF-α. TNF-α increased caspase-3 mRNA levels by 2.3-fold (Fig. 3A). In contrast, P. gingivalis did not enhance caspase-3 mRNA levels (P > 0.05). To determine whether P. gingivalis induced apoptosis through a caspase-dependent mechanism the pancaspase inhibitor, Z-VAD-fmk, was used. TNF-α stimulated apoptosis in fibroblasts was significantly inhibited by Z-VAD-fmk (P < 0.05) (Fig. 3B). In contrast, the pancaspase inhibitor had no effect on P. gingivalis-induced apoptosis (P > 0.05). We also examined the effect of a pan-caspase inhibitor on P. gingivalis (moi 100:1) stimulated apoptosis over a 24 h period and found that it did not reduce P. gingivalis-induced apoptosis (data not shown). Consistent to our result Urnowey and colleagues (2006) showed little to no increase in caspase-3 and -7 at 24 h of bacterial infection. The impact of P. gingivalis on caspase mediated apoptosis was investigated further. In a pilot study human gingival fibroblasts did not undergo apoptosis when exposed to P. gingivalis for 45 min followed by thorough rinsing (data not shown). To determine whether P. gingivalis modulated TNF-α-induced apoptosis cells were preincubated with P. gingivalis for 45 min, rinsed thoroughly and then incubated with TNF-α or a second incubation with P. gingivalis. Cells were assayed for caspase-3/7 activity or apoptosis. Pretreatment with P. gingivalis significantly inhibited TNF-α-induced caspase-3/7 activity (Fig. 4A). When apoptosis was measured, pretreatment of cells with P. gingivalis substantially reduced TNF-α-induced apoptosis but had no effect on the capacity of P. gingivalis itself to induce apoptosis (Fig. 4B). These results therefore indicate that P. gingivalis inhibits TNF-α-induced apoptosis by downregulating caspase-3 activity.
Fig. 3.
P. gingivalis-induced fibroblast apoptosis is caspase-independent.
A. Human gingival fibroblasts were incubated with P. gingivalis or TNF and caspase-3 mRNA levels were determined by real-time PCR.
B. Cells were preincubated with or without a pan-caspase inhibitor (Panc) for 1.5 h, rinsed thoroughly and then incubated with P. gingivalis or TNF for 3 h. Apoptosis was measured by ELISA as before. Each value represents the mean of three separate experiments ± SEM. The single asterisk indicates significant increase and the double asterisks indicate significant decrease (P < 0.05).
Fig. 4.
Pretreatment of fibroblasts with P. gingivalis downregulates caspase-3 activity and TNF-induced apoptosis. Cells were preincubated with P. gingivalis for 45 min and rinsed with PBS containing antibiotic. Following rinses cells were incubated in serum free media containing P. gingivalis (moi 300:1) or TNF (20 ng ml−1) for additional 3 h.
A. Caspase-3 activity was measured.
B. Apoptosis was measured by ELISA. Each value represents the mean of three separate experiments ± SEM. The single asterisk indicates significant increase and the double asterisks indicate significant decrease (P < 0.05).
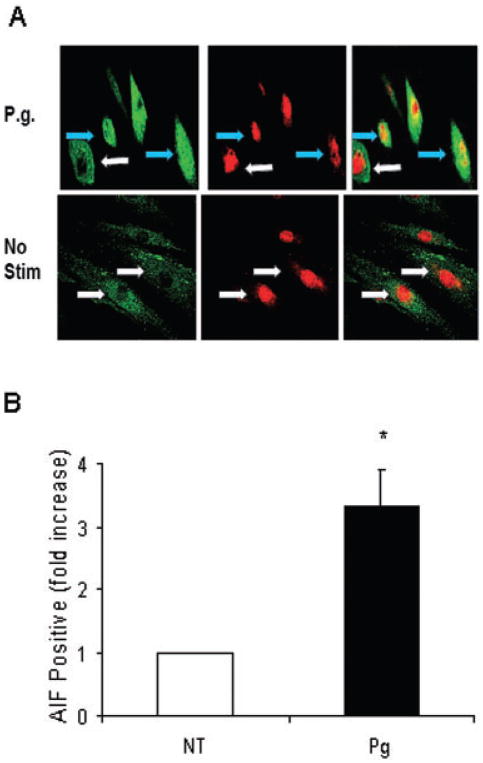
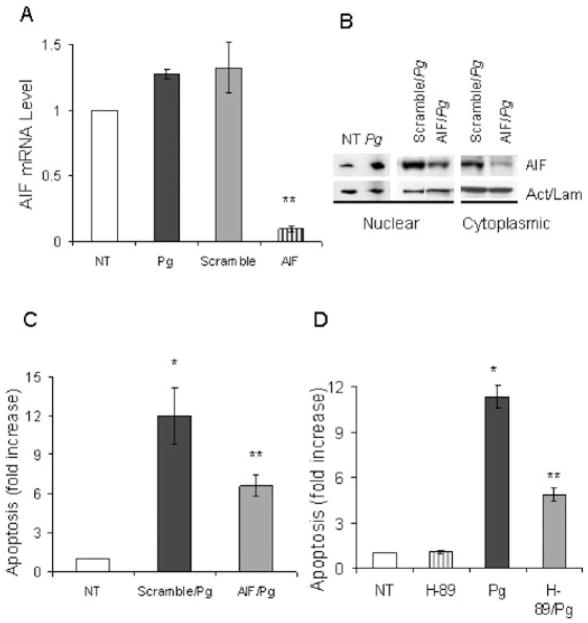
In order to investigate caspase-independent apoptosis experiments were performed focusing on AIF (Susin et al., 2000). When AIF is activated it translocates to the nucleus (Cande et al., 2002). Confocal microscopy of cells to assess AIF nuclear translocation was carried out using an anti-AIF antibody (Fig. 5A). The number of cells clearly demonstrating translocation of AIF to the nucleus increased threefold when compared with the unstimulated cells (Fig. 5B). siRNA studies were then carried out to functionally examine the role of AIF in P. gingivalis-induced apoptosis. AIF mRNA levels were reduced by approximately 90% by AIF siRNA compared with scrambled siRNA demonstrating effective silencing (Fig. 6A). This result was further confirmed using Western Blot analysis. AIF siRNA significantly reduced the presence of AIF in the cytoplasm as well as in the nucleus as compared with cells transfected with scrambled siRNA (Fig. 6B). In addition, P. gingivalis increased the level of AIF in the nuclear compartment compared with unstimulated cells, agreeing with data obtained by confocal microscopy in Fig. 5B. When the effect on apoptosis was measured AIF silencing significantly reduced P. gingivalis stimulated apoptosis by almost half (P < 0.05) (Fig. 6C). Furthermore, a protein kinase A inhibitor (H-89) shown to inhibit AIF activation (Park et al., 2005) also reduced P. gingivalis-induced apoptosis by approximately 50% (P < 0.05) (Fig. 6D). H-89 is non-toxic and does not stimulate apoptosis of fibroblasts at the concentration used.
Fig. 5.
P. gingivalis Induces Nuclear Translocation of AIF.
A. Human gingival fibroblasts treated with or without P. gingivalis were immunostained with antibody to AIF using an FITC (green) detection system and counterstained with propidium iodide (red) to identify the nuclear compartment. Yellow stain in the upper right panel identifies cells with AIF in the nuclear compartment. Blue arrows indicate nuclear AIF and the absence of AIF in the nucleus is designated by white arrows.
B. Cells positive for AIF nuclear translocation were quantified.
Fig. 6.
Silencing AIF gene reduces P. gingivalis-induced fibroblast apoptosis. Human gingival fibroblasts plated at 70% confluency were preincubated with AIF-siRNA (AIF) or scrambled-siRNA (Scramble) for 24 h. Cells were left for additional 24 h with regular media and incubated with P. gingivalis for 3 h.
A. AIF mRNA levels were measured by real-time PCR.
B. Nuclear and cytoplasmic proteins extracted from cells described in A. Blots were incubated with antibody to AIF stripped and then incubated with antibody against actin for cytoplasmic or lamin B for nuclear proteins.
C. Apoptosis was measured by ELISA.
D. Human gingival fibroblasts were preincubated with H-89 for 1 h, washed, and incubated with bacteria for 3 h. Apoptosis was measured by ELISA. Each value represents the mean of three separate experiments ± SEM. The single asterisk indicates significant increase and the double asterisks indicate significant decrease (P < 0.05).
Discussion
The impact of P. gingivalis on cell death has been controversial. Several investigators have reported that it induces cell death through gingipain activity (Johansson and Kalfas, 1998; Wang et al., 1999; Sheets et al., 2005) while others reported that gingipain proteases do not play a significant role (Morioka et al., 1993; Harris et al., 2002). It has also been reported that P. gingivalis reduces apoptosis in epithelial cells (Nakhjiri et al., 2001). One possible explanation for the different findings is that in many of the reports gingipain-induced apoptosis was not compared with that induced by intact P. gingivalis. Studies presented here clarify a mechanism through which P. gingivalis induces apoptosis. While high concentrations of P. gingivalis stimulate necrosis in vivo (Liu et al., 2004) we found that the bacterium directly induced relatively little necrosis in fibroblasts in vitro. Thus, the principal mechanism by which P. gingivalis induced cell death was through apoptosis when an infective and not a toxic number of bacteria were incubated with the cells. Moreover, the pro-apoptotic effect of P. gingivalis was separated from its proteolytic activity in time-course and dose–response experiments and by findings that protease inhibitors blocked cell rounding while having only a small impact on apoptosis compared with viable P. gingivalis. This was established using both the ELISA assay for cytoplasmic histone-associated DNA and the TUNEL assay.
The studies establish for the first time that P. gingivalis does not induce fibroblast apoptosis through the well-characterized caspase-dependent apoptotic pathways. This is based on findings that P. gingivalis-induced apoptosis was not affected by a pan-caspase inhibitor, Z-VAD-fmk, while this inhibitor blocked apoptosis induced by TNF-α. Consistent with this observation, P. gingivalis downregulated caspase-3 activity. This is physiologically significant because pretreatment of cells with P. gingivalis followed by rinsing and incubation with TNF-α blocked TNF-α stimulated apoptosis. However, pretreatment with P. gingivalis did not inhibit apoptosis induced by a second incubation with P. gingivalis largely because it does not use a caspase-3-dependent pathway.
Although it has been proposed that P. gingivalis gingipains promote fibroblast apoptosis through activation of caspase-3 (Sheets et al., 2005) we found no evidence that P. gingivalis induced caspase-3 activity measured with a sensitive luminescent kit, but rather, downregulated it. In addition we examined the effect of a pan-caspase inhibitor on P. gingivalis stimulated apoptosis over a 24 h period and found that it did not reduce P. gingivalis-induced apoptosis (data not shown). In contrast, long-term exposure of cells to Pg-proteases will cause extensive cell detachment and promote apoptosis due to loss of attachment. Since incubation with P. gingivalis from 3 to 24 h induced apoptosis under conditions where caspase-3 activity was blocked, it is unlikely that indirect mechanisms through protease-induced detachment play a prominent role in fibroblast apoptosis stimulated by intact P. gingivalis. Urnowey and colleagues (2006) demonstrated that P. gingivalis at early infection- (< 6 h) induced expression of antiapoptotic proteins in fibroblasts. Similarly, Nakhjiri et al. (2001) showed an increase in expression of antiapoptotic protein bcl-2 when epithelial cells were treated with P. gingivalis for 24 h, which in turn reduced apoptosis stimulated by camptothecin. Consistent with these studies pretreatment of fibroblasts with P. gingivalis reduced TNF-α stimulated apoptosis that can be explained in part, by the downregulation of caspase-3. Thus, P. gingivalis has a unique ability to suppress caspase-3-dependent apoptosis while at the same time exerting a pro-apoptotic effect through an alternative pathway. To investigate a caspase-independent pathway we examined AIF. Incubation of fibroblasts with P. gingivalis at an infective moi stimulated AIF translocation to the nucleus when measured by both confocal microscopy and immunoblot analysis. When AIF was downregulated by AIF siRNA, P. gingivalis-induced cell death was reduced by almost 50%. A second approach using a PKA inhibitor (H-89) that inhibits AIF activation (Park et al., 2005) reduced P. gingivalis-induced cell death by approximately 50%. This finding is consistent with a non-caspase-dependent mechanism reported for Pneumococcus-induced neuronal cell apoptosis (Braun et al., 2001). Thus, P. gingivalis may use a similar pathway to induce fibroblast cell death. However, it should be noted that not all apoptosis was inhibited by AIF siRNA or PKA inhibitor indicating that there likely to be another non-caspase-dependent pathway involved.
Experimental procedures
Cell culture conditions
Human gingival fibroblasts (HGF-1) were purchased from the American Type Culture Collection, ATCC (Manassas, VA). The cells were grown in fibroblast growth medium (FGM2) supplemented with 10% fetal bovine serum (Cambrex-Research Products, Pittsburgh, PA). For most assays cells from passages 5–10 were seeded in 24-well plates at 90% confluency (approximately 70 000 cells/well) overnight prior to starting experiments.
Porphyromonas gingivalis growth conditions
Porphyromonas gingivalis W83 was purchased from the American Type Culture Collection (ATCC). The bacteria were grown in a liquid broth of brain heart infusion, BHI, supplemented with hemin and menadione (Becton Dickinson, Sparks, MD) in an anaerobic chamber with GasPack containing 10% CO2, 10% H2 and 80% N2 (Becton Dickinson) for 1 day at 37°C. For each assay bacterial numbers were established by the number of colony-forming units of P. gingivalis serially diluted on blood agar plates (BD, Franklin Lake, NJ). In some experiments proteins released by P. gingivalis were tested for their effects on cell rounding and apoptosis. Culture supernatant from P. gingivalis W83 was filtered through 0.22 μM membrane, precipitated in ammonium sulphate (75% saturation), and chilled in −20°C for 30 min. Precipitated proteins were collected by centrifugation at 10 000 g for 20 min, and resuspended in phosphate-buffered saline (PBS). Protease activity was determined using N-benzoyl-DL-arginine-p-nitroanilide (BApNA) and N-acetyl-lysine-p-nitroanilide (AcKpNA) (Sigma-Aldrich, St. Louis, MO) as described by (Rangarajan et al., 1997).
Apoptosis and necrosis assays
Apoptosis was measured in human gingival fibroblasts using 24 well plates incubated in serum free media. In most experiments cells were incubated with P. gingivalis (moi 300:1). In some experiments cells were incubated with TNF-α (20 ng ml−1) (R and D systems) plus cycloheximide (5 μg ml−1), proteolytic extract (25 units ml−1), or arginine and lysine specific endopeptidases (25 units ml−1) (Geno Tech, St. Louis, MO). To evaluate the role of P. gingivalis proteases, inhibitors of Arg-gingipain or Lys-gingipain consisting of leupeptin (100 μM) or cathepsin B inhibitor II (100 μM) were added to the cells along with bacteria. It has been reported that leupeptin inhibits P. gingivalis RgpA/B but not Kgp (Houle et al., 2003; Andrian et al., 2004). Cathepsin B inhibitor II has been used to inhibit Kgp protease of P. gingivalis but does not inhibit RgpA/B (Houle et al., 2003). In order to block caspase activity human gingival fibroblasts were pretreated with the pancaspase inhibitor Z-VAD-fmk (100 nM) (EMD Biosciences) for 1.5 h before treatments with P. gingivalis or TNF-α. Cells were also preincubated with H-89 (50 μM) (EMD Biosciences) a selective inhibitor of protein kinase A (Chijiwa et al., 1990; Muniz et al., 1996; Muniz et al., 1997) before P. gingivalis treatment. Apoptosis of fibroblasts was assessed by ELISA measuring cytoplasmic histone-associated-DNA fragments (Roche Applied Science, Indianapolis, IN) as described (Alikhani et al., 2005). Necrosis was measured by lactose dehydrogenase (LDH) released into cell culture media following centrifugation at 400 g for 10 min using a CytoTox 96 cytotoxicity assay kit (Promega, Madison, WI). In some experiments images were captured using an inverted light microscope equipped with digital camera. All experiment described were carried out a minimum of three times with similar results.
Caspase-3 measurements
Human gingival fibroblasts grown in 6-well plates were treated with P. gingivalis or TNF-α as above and total RNA was extracted using an RNAeasy kit (Qiagen, Valencia, CA). The RNA was converted to cDNA with MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA) and caspase-3 mRNA levels were measured by real-time PCR using Taqman primers and probes (Applied Biosystems). mRNA levels are normalized to GAPDH mRNA and calculated relative to the unstimulated cells as by the manufacturer’s guideline. To assay for caspase-3 enzyme activity a CaspGlo-3/7 Assay kit was used (Promega). Following the manufacturer’s instruction, cells grown in 24-well plates and incubated with P. gingivalis for the indicated amount of time. In some experiments cells were preincubated with P. gingivalis for 45 min, rinsed thoroughly with PBS containing antibiotic and then stimulated with TNF-α (20 ng ml−1). Cells were lysed and caspase-3 activity was measured in each well by luminescence assay. A BCA assay (Bio-Rad) was performed to ensure that an equal amount of cell protein was present in each well.
TUNEL assay
Human gingival fibroblasts with or without bacterial treatments were washed and fixed in 4% paraformaldehyde in PBS for 30 min at 4°C and washed three times with PBS. Cells were stained by an in situ terminal dUTP nick-end labelling (TUNEL) assay by means of a TACS 2 TdT-Blue Label kit purchased from Trevigen (Gaithersburg, MD). Cells showing apoptotic stain were counted using inverted microscope.
Immunofluorescence study
Human gingival fibroblasts with or without P. gingivalis treatment were fixed using 4% paraformaldehyde in PBS for 30 min at 4°C and washed three times with PBS. For the detection of AIF translocation, cells were permeablized with 1% Triton X-100 in PBS for 5 min at room temperature and incubated for 1 h with rabbit anti-AIF polyclonal antibody (BD Biosciences). Cells were washed three times as before and incubated with FITC conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) for additional 1 h and then counterstained with propidium iodide. Fluorescence staining was detected using a confocal laser microscope (Axiovert, Carl Zeiss, Thornwood, NY) and positive cells showing nuclear translocation of AIF were counted. This experiment was performed three times with similar results.
siRNA study
To evaluate the role of AIF we used small interfering RNA (siRNA) that blocks the expression of this gene. Silencing aif gene expression in human gingival fibroblasts was achieved by using siRNA purchased from (Qiagen). This experiment was performed for the most part following the manufacturer’s guideline. Briefly, cells grown in 6- or 24-well plates were transfected with AIF or scrambled siRNA using HiPerFect transfection reagent (Qiagen) for 24 h when they were approximately 70% confluent. Cells were then rinsed and incubated in standard cell culture medium for an additional 24 h. Cells were then incubated with P. gingivalis after which they were assayed for AIF mRNA level or for presence of AIF in the nucleus using immunoblotting.
Immunoblots
Human gingival fibroblasts incubated with or without P. gingivalis were lysed for extraction of nuclear proteins using a nuclear extraction kit (Pierce, Rockford, IL). Briefly, 10 μg of nuclear extracts were separated on SDS-PAGE (12% polyacrylamide) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Invitrogen). The membrane was blocked with skim milk and washed before incubating with specific antibodies. The primary antibodies used in immunoblotting were rabbit antiserum against AIF (Pharmingen) and goat antiserum against actin, a cytoplasmic protein marker or lamin-B, a nuclear protein marker (Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibodies were donkey anti-rabbit or anti-goat conjugated with horseradish per-oxidase (HRP) (Santa Cruz Biotechnology). Immunoblots were developed using enhanced chemiluminescence reagents (Santa Cruz). When necessary, the blots were stripped with a Western blot stripping reagent (Pierce) for a second use.
Statistics
Statistical significance was determined by one way analysis of variant (ANOVA). Unless otherwise stated the values from three separate experiments were combined to obtain the mean and standard error of the mean. Significant differences were established at P < 0.05.
Acknowledgments
We would like to thank Alicia Ruff for help in preparing this manuscript and Dr. J. Potempa for helpful discussions. This work was funded by a grant from the NIDCR, DE07559.
References
- Alikhani M, Alikhani Z, Graves D. FOXO1 functions as a master switch that regulates gene expression necessary for TNF-induced fibroblast apoptosis. J Biol Chem. 2005;280:12096–12102. doi: 10.1074/jbc.M412171200. [DOI] [PubMed] [Google Scholar]
- Andrian E, Grenier D, Rouabhia M. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect Immun. 2004;72:4689–4698. doi: 10.1128/IAI.72.8.4689-4698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, Pathak SK, Bhattacharyya A, Pathak S, Basu J, Kundu M. The secreted peptidyl prolyl cis, trans-isomerase HP0175 of Helicobacter pylori induces apoptosis of gastric epithelial cells in a TLR4- and apoptosis signal-regulating kinase 1-dependent manner. J Immunol. 2005;174:5672–5680. doi: 10.4049/jimmunol.174.9.5672. [DOI] [PubMed] [Google Scholar]
- Braun JS, Novak R, Murray PJ, Eischen CM, Susin SA, Kroemer G, et al. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J Infect Dis. 2001;184:1300–1309. doi: 10.1086/324013. [DOI] [PubMed] [Google Scholar]
- Brozovic S, Sahoo R, Barve S, Shiba H, Uriarte S, Blumberg RS, Kinane DF. Porphyromonas gingivalis enhances FasL expression via upregulation of NFkappaB-mediated gene transcription and induces apoptotic cell death in human gingival epithelial cells. Microbiology. 2006;152:797–806. doi: 10.1099/mic.0.28472-0. [DOI] [PubMed] [Google Scholar]
- Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- Chen Z, Casiano CA, Fletcher HM. Protease-active extracellular protein preparations from Porphyromonas gingivalis W83 induce N-cadherin proteolysis, loss of cell adhesion, and apoptosis in human epithelial cells. J Periodontol. 2001;72:641–650. doi: 10.1902/jop.2001.72.5.641. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Graves DT, Jiang Y, Genco C. Bacterial virulence factors host response and impact on systemic health. Curr Op Inf Dis. 2000;13:227–232. doi: 10.1097/00001432-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Graves D, Oskoui M, Volejnikova S, Naguib G, Cai S, Desta T, et al. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J Dent Res. 2001;80:1875–1879. doi: 10.1177/00220345010800100301. [DOI] [PubMed] [Google Scholar]
- Graves D, Liu R, Alikhani M, Al-Mashat H, Trackman P. Diabetes-enhanced inflammation and apoptosis – impact on periodontal pathology. J Dent Res. 2006;85f:15–21. doi: 10.1177/154405910608500103. [DOI] [PubMed] [Google Scholar]
- Green D. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Harris JI, Russell RR, Curtis MA, Aduse-Opoku J, Taylor JJ. Molecular mediators of Porphyromonas gingivalis-induced T-cell apoptosis. Oral Microbiol Immunol. 2002;17:224–230. doi: 10.1034/j.1399-302x.2002.170404.x. [DOI] [PubMed] [Google Scholar]
- Houle MA, Grenier D, Plamondon P, Nakayama K. The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol Lett. 2003;221:181–185. doi: 10.1016/S0378-1097(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Imamura T, Travis J, Potempa J. The biphasic virulence activities of gingipains: activation and inactivation of host proteins. Curr Protein Pept Sci. 2003;4:443–450. doi: 10.2174/1389203033487027. [DOI] [PubMed] [Google Scholar]
- Johansson A, Kalfas S. Characterization of the proteinase-dependent cytotoxicity of Porphyromonas gigivalis. European J Oral Sci. 1998;106:863–871. doi: 10.1046/j.0909-8836.1998.eos106405.x. [DOI] [PubMed] [Google Scholar]
- Koulouri O, Lapping D, Radvar M, Kinane D. Cell division, synthetic capacity and apoptosis in periodontal lesions analyzed by in situ hybridization an immunohistochemistry. J Clin Periodotology. 1999;26:552–559. doi: 10.1034/j.1600-051x.1999.260810.x. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Ochiai K, Fukushima K. Volatile fatty acid, metabolic by-product of periodontopathic bacteria, induces apoptosis in WEHI 231 and RAJI B lymphoma cells and splenic B cells. Infect Immun. 1998;66:2587–2594. doi: 10.1128/iai.66.6.2587-2594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R, Jenkinson H. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Bossy-Wetzel E. Dueling activities of AIF in cell death versus survival: DNA binding and redox activity. Cell. 2002;111:147–150. doi: 10.1016/s0092-8674(02)01046-2. [DOI] [PubMed] [Google Scholar]
- Liu R, Desta T, He H, Graves D. Diabetes alters the response to bacteria by enhancing fibroblast apoptosis. Endocrinology. 2004;145:2997–3003. doi: 10.1210/en.2003-1601. [DOI] [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, Behl Y, Graves DT. Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol. 2006;168:757–764. doi: 10.2353/ajpath.2006.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka M, Hinode D, Nagata A, Hayashi H, Ichimiya S, Ueda M, et al. Cytotoxicity of Porphyromonas gingivalis toward cultured human gingival fibroblasts. Oral Microbiol Immunol. 1993;8:203–207. doi: 10.1111/j.1399-302x.1993.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Muniz M, Alonso M, Hidalgo J, Velasco A. A regulatory role for cAMP-dependent protein kinase in protein traffic along the exocytic route. J Biol Chem. 1996;271:30935–30941. doi: 10.1074/jbc.271.48.30935. [DOI] [PubMed] [Google Scholar]
- Muniz M, Martin ME, Hidalgo J, Velasco A. Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci USA. 1997;94:14461–14466. doi: 10.1073/pnas.94.26.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, Chung WO, Watanabe K, El-Sabaeny A, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- O’Brien-Simpson NM, Pathirana RD, Paolini RA, Chen YY, Veith PD, Tam V, et al. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J Immunol. 2005;175:3980–3989. doi: 10.4049/jimmunol.175.6.3980. [DOI] [PubMed] [Google Scholar]
- Park SY, Cho SJ, Kwon HC, Lee KR, Rhee DK, Pyo S. Caspase-independent cell death by allicin in human epithelial carcinoma cells: involvement of PKA. Cancer Lett. 2005;224:123–132. doi: 10.1016/j.canlet.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Rangarajan M, Smith SJ, US, Curtis MA. Biochemical characterization of the arginine-specific proteases of Porphyromonas gingivalis W50 suggests a common precursor. Biochem J. 1997;323:701–709. doi: 10.1042/bj3230701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets SM, Potempa J, Travis J, Casiano CA, Fletcher HM. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect Immun. 2005;73:1543–1552. doi: 10.1128/IAI.73.3.1543-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordet O, Khan QA, Pommier Y. Apoptotic topoisomerase I–DNA complexes induced by oxygen radicals and mitochondrial dysfunction. Cell Cycle. 2004;3:1095–1097. [PubMed] [Google Scholar]
- Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, et al. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000;192:571–580. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii R, Kadowaki T, Baba A, Tsukuba T, Yamamoto K. A functional virulence complex composed of gingipains, adhesins, and lipopolysaccharide shows high affinity to host cells and matrix proteins and escapes recognition by host immune systems. Infect Immun. 2005;73:883–893. doi: 10.1128/IAI.73.2.883-893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LR, Henson A, Reed JC, Salsbury FR, Thorburn A. Direct binding of Fas-associated death domain (FADD) to the tumor necrosis factor-related apoptosis-inducing ligand receptor DR5 is regulated by the death effector domain of FADD. J Biol Chem. 2004;279:32780–32785. doi: 10.1074/jbc.M401680200. [DOI] [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Tonetti M, Cortellini D, Lang N. In situ detection of apoptosis at sites of chronic bacterially induced inflammation in human gingival. Infection Immunity. 1998;66:5190–5195. doi: 10.1128/iai.66.11.5190-5195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnowey S, Ansai T, Bitko V, Nakayama K, Takehara T, Barik S. Temporal activation of anti- and pro-apoptotic factors in human gingival fibroblasts infected with the periodontal pathogen, Porphyromonas gingivalis: potential role of bacterial proteases in host signalling. BMC Microbiol. 2006;6:26. doi: 10.1186/1471-2180-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang P, Shirasu S, Shinohara M, Daito M, Oido M, Kowashi Y, Ohura K. Induction of apoptosis in human gingival fibroblasts by a Porphyromonas gingivalis protease preparation. Arch Oral Biol. 1999;44:337–342. doi: 10.1016/s0003-9969(99)00002-3. [DOI] [PubMed] [Google Scholar]
- Zappa U, Reinking-Zappa M, Graf H, Chase D. Cell populations associated with active probing attachment loss. J Periodontology. 1992;63:748–752. doi: 10.1902/jop.1992.63.9.748. [DOI] [PubMed] [Google Scholar]