Abstract
Leptin and adiponectin play important physiological roles in regulating appetite, food intake, and energy balance and have pathophysiological roles in obesity and anorexia nervosa. To assess the relative contributions of day/night patterns in behaviors (sleep/wake cycle and food intake) and of the endogenous circadian pacemaker on observed day/night patterns of adipokines, in six healthy subjects we measured circulating leptin, soluble leptin receptor, adiponectin, glucose, and insulin levels throughout a constant routine protocol (38 h of wakefulness with constant posture, temperature, and dim light, as well as identical snacks every 2 h) and throughout sleep and fasting periods before and after the constant routine. There were significant endogenous circadian rhythms in leptin, glucose, and insulin, with peaks around the usual time of awakening. Sleep/fasting resulted in additional systematic decreases in leptin, glucose, and insulin, whereas wakefulness/food intake resulted in a systematic increase in leptin. Thus, the day/night pattern in leptin is likely caused by combined effects from the endogenous circadian pacemaker and day/night patterns in behaviors. Our data imply that alterations in the sleep/wake schedule would lead to an increased daily range in circulating leptin, with lowest leptin upon awakening, which, by influencing food intake and energy balance, could be implicated in the increased prevalence of obesity in the shift work population.
Leptin and adiponectin are adipocyte secreted hormones that regulate energy balance and metabolism, play important roles in the pathogenesis of obesity and the metabolic syndrome (1–4), and contribute to adverse cardiovascular consequences (5–7). There are day/night patterns in plasma leptin and adiponectin concentrations, with leptin rising (8–12) and adiponectin falling during the night (13). Considering the potential pathophysiological roles of these adipocytokines in the epidemic of obesity and associated disorders, it is important to fully understand the factors that control their day/night patterns of release. For instance, nocturnal eating syndrome and shift work are often associated with obesity and the metabolic syndrome (14–20). Although the mechanisms underlying these associations remain unknown, it is possible that disturbances in day/night adipokine patterns, induced by alterations in the sleep/wake and eating schedule, as occurs with jet lag and shift work, could, in turn, influence appetite, food intake, and energy balance in these populations and could have important implications for the increased obesity, diabetes, and cardiovascular disease in the shift work population (21–23). However, no prior studies have fully elucidated the causes underlying the day/night patterns of adipocytokine concentrations, and no study has directly assessed the relative contributions to this pattern from the endogenous body clock [the circadian pacemaker located in the suprachiasmatic nuclei of the hypothalamus (24)] and day/night patterns in behaviors (such as the sleep/wake cycle and food intake).
Thus, the aim of the current experiment was to test the hypothesis that endogenous circadian factors influence circulating adipokine concentrations and glucose in humans. A secondary aim was to determine the magnitude of any such circadian effect in relation to independent behavioral effects induced by day/night patterns in behaviors (the sleep/wake cycle and food intake). To overcome many of the limitations of previous studies, we made measurements throughout a protocol that permitted separation of endogenous circadian effects from systematic influences related to sleep or fasting and wakefulness or food intake by studying subjects: first, during sleep and fasting; second, throughout maintained wakefulness for 38 h with constant posture, constant temperature, and constant dim light, with identical snacks every 2 h; and third, throughout a period of sleep and fasting after this constant routine protocol (25). We used samples drawn every hour to measure concentrations of leptin, soluble leptin receptor (sOB-R) [representing the main leptin binding activity in serum, which indirectly determines the amount of biologically active free leptin at the hypothalamus (26–27)], adiponectin, glucose, and insulin in hourly blood samples taken throughout the entire protocol.
Subjects and Methods
Subjects
We studied six adult male subjects, whose mean age was 30.7 yr (range, 22–46) and whose mean body mass index (BMI) was 25.2 kg.m−2 (range, 23.0–26.6). Subjects were healthy, with no significant medical disorders, as assessed by history, physical examination, overnight polysomnography, psychological examination, pulmonary function tests, a 12-lead ECG, and routine blood and urine chemistry. The study was approved by the local Human Subjects internal review board. Subjects provided written informed consent before participation.
Protocol
To ensure a stable circadian baseline before entry into the laboratory, subjects were asked to establish a regular sleep-wake schedule, with their habitual bedtimes and waketimes varying by no more than 1 h each day for at least 2 wk before the laboratory study. This schedule was verified by time-logged telephone calls of bedtimes and waketimes for 2 wk as well as activity monitoring for 3–7 d (wrist-worn Actigraph; Ambulatory Monitoring, Ardsley, NY). For 1 wk before the study and throughout the study, subjects abstained from caffeinated drinks, smoking, and any medication. Each subject was then studied in an individual laboratory room isolated from external time cues. As part of a separate aim assessing circadian cardiopulmonary rhythmicity, noninvasive cardiopulmonary measurements were made every 2–4 h, including for 2–9 d before the current study, which enabled further laboratory acclimatization and a relatively steady state. To assess endogenous circadian effects, we used a 38-h constant routine protocol involving constant wakefulness, posture (semirecumbent), temperature (~23 ± 2 C), and dim light (<8 lux), with identical snacks every 2 h as previously described (25). Subjects were also monitored during sleep before the constant routine, and during a period of recovery sleep immediately after the constant routine (each sleep opportunity was 10 h). The constant routine protocol is designed to unmask underlying circadian rhythms as it reduces or eliminates other physiological influences from the environment, varied behaviors, large meals, and sleep. Upon awakening on the morning of the constant routine, subjects remained in bed and awake in a semirecumbent position for 38 h. Only minor postural changes and no vigorous activities were permitted. Experimenters were present in the laboratory throughout the constant routine to ensure that the subjects remained awake. A bottle was used to collect urine, and a bedpan was used for any bowel movements. A normal diet, consisting of three meals plus a snack, was provided on the day before the constant routine; however, to avoid the effects of large and varied meals on adipocyte physiology during the constant routine, small identical snacks were given every 2 h. The overall calories per 24 h, including during baseline, were calculated for each subject using the Harris-Benedict formula with an activity factor of 1.3. Each meal or snack was comprised of 25% fat, 50% carbohydrate, and 25% protein. The subjects also received up to 3.5 liters of fluids per 24-h period.
Measurements
Recordings of two electroencephalography channels, two electrooculography channels, and a submental electromyogram (Vitaport recorder; TEMEC, Kerkrade, The Netherlands) were made to enable scoring of sleep stages in the baseline and recovery sleep periods. Core body temperature was recorded throughout using a rectal temperature sensor (Yellow Springs Instrument Co., Inc., Yellow Springs, OH) to provide a marker of the endogenous circadian pacemaker.
Blood was sampled hourly during both wakefulness and sleep via an indwelling 18-gauge catheter in a forearm vein, and samples were taken from an adjacent room via a 12-foot small lumen extension catheter. Catheter patency was maintained between samples by infusing 0.45% saline with 10,000 IU/liter heparin at a rate of 20 ml/h. Blood samples were transferred to vacutainer tubes, centrifuged at −4 C, and the resulting plasma was stored in polystyrene tubes at −20 C until the assays were performed. Plasma adiponectin levels were measured using a RIA from Diagnostics Systems Laboratory (DSL, Webster, TX) with a sensitivity of 2 ng/ml and intraassay coefficient of variation (CV) of 6.6%. Plasma leptin levels were measured using a RIA (DSL) with a sensitivity of 0.5 ng/ml and intraassay CV of 8.3%. Plasma sOB-R levels were measured using a commercially available ELISA (DSL) with a sensitivity of 0.8 ng/ml and intraassay CV between 5.5 and 6.6%. Plasma insulin was assayed using a commercially available paramagnetic-particle chemiluminescent immunoassay (Beckman Access B; Diamond Diagnostics, Holliston, MA) with a sensitivity of 0.03 μIU/ml and an average intraassay CV of 3.1–5.6%. Plasma glucose was assayed using a commercially available assay (ACE glucose reagent; Alfa Wassermann, West Caldwell, NJ) with a sensitivity of 1 mg/dl and an average intraassay CV less than 2%.
Analysis of circadian rhythmicity and behavioral influences on glucose and hormones
All temperature, hormonal and glucose data collected in the first 5 h of the constant routine were excluded from analysis to avoid the effects of altered posture and sleep-wake transition. Each subject’s phase and period of the core body temperature circadian rhythm were estimated by nonlinear least-squares regression (28). Using the estimated core body temperature minimum (CBTmin) as an arbitrary phase marker (0 degrees), all data were then assigned a specific circadian phase from 0–359 degrees depending upon the time lag between the collected data and CBTmin as well as the duration of each individual’s estimated circadian period. The average circadian period for the subjects in this study was 24.6 h (SEM = 0.2 h).
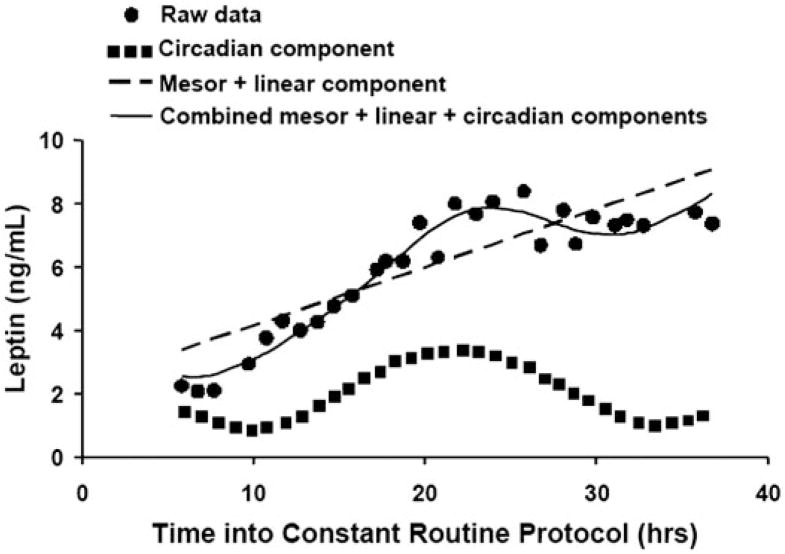
For the individuals’ data, the circadian phases and amplitudes of the hormonal and glucose variables were estimated by cosinor analysis (29). This technique uses multiple linear regression to calculate the mesor (the average level around which other fluctuations occur), the amplitude and phase of any circadian rhythm (relative to CBTmin), and any underlying linear trend (e.g. due to the effects of prolonged wakefulness or meals). The cosinor analysis also incorporates a circadian harmonic term (~12 h cosine), which provides better data fitting of rhythms that are not precisely cosinor in shape (because the summation of circadian plus harmonic cosine waveforms with different amplitudes and phases permits an infinite number of shapes). A graphical example of the cosinor analysis is shown in Fig. 1.
Fig. 1.
Example of cosinor analysis of circulating leptin data in one subject. Raw data are plotted from hourly blood samples. From the cosinor analysis, the thin dashed line depicts the mesor plus linear trend, the dotted line depicts the circadian component, and the solid line depicts the combined cosinor model (mesor + linear + circadian components). In this example, the combined cosinor analysis provided a very good fit to the data (r2 = 0.95). The range of r2 among subjects was 0.88–0.95.
The focus of this study is on the average changes within individuals over time rather than differences between individuals. To calculate the group mean circadian rhythmicity of the hormonal and glucose data independent from behavioral factors, it was necessary to subtract each individual’s linear trend and to express the data in normalized units (i.e. the percentage difference from the mean). Then, individuals’ data were aligned with respect to their subject-specific CBTmin, and cosinor analysis was performed to estimate the circadian amplitude and phase (relative to CBTmin). This group analysis excluded a linear trend term (this had already been subtracted from each subject’s data).
To calculate the group mean behavioral influences on the hormonal and glucose data (i.e. the effects of prolonged wakefulness or meals) independent from circadian rhythmicity, it was necessary to subtract each individual’s estimated circadian rhythm from the data and to express the data as the percentage difference from the mean. Then individuals’ data were aligned with respect to the sleep/wake schedule (instead of the circadian phase), and linear regression was performed to estimate the rate of change throughout prolonged wakefulness. Separate analogous linear regression analyses were performed to estimate the independent effects of sleep/fasting on the hormones and glucose after subtracting the circadian components. Because circadian rhythmicity was calculated during the constant routine, this last analysis relied upon our assumption that the circadian component could be extrapolated from the constant routine data to the sleep periods on either side of the constant routine.
Results
Circadian effects (independent from behavioral effects)
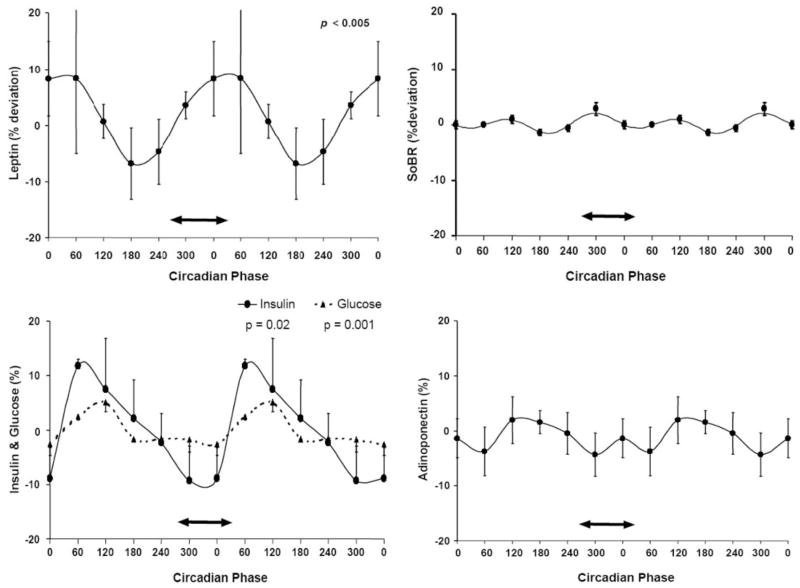
The cosinor analyses of leptin accounted for between 88 and 95% of the variability in individuals’ data (i.e. r2 for the cosinor analyses ranged from 0.88–0.95). Four of the six subjects had a significant circadian rhythm in circulating leptin (P < 0.05). The peak-to-trough amplitudes of the fundamental circadian rhythms averaged 1.4 ng/ml, equivalent to a daily fluctuation of 21% around the leptin mesor of 6.5 ng/ml. For the group, the circadian variation in circulating leptin was highly significant (P < 0.0001), with a peak-to-trough value, over the 24 h, of 16% of the mesor. The average circadian waveform is shown in Fig. 2 (upper left). Although all of these measurements were made during continued wakefulness, it can be seen that the endogenous circadian system caused a steady increase in leptin across the time that corresponds to the habitual sleep period in this group of subjects (with a peak just after the usual time of awakening, and a trough in the afternoon). Thus, the independent circadian profile is considerably different (i.e. ~8 h phase delayed) from the regular day/night leptin profiles when behaviors and meals are uncontrolled (see Discussion).
Fig. 2.
Group mean circadian variations in circulating leptin, glucose and insulin and lack of detectable circadian variations in sOB-R and adiponectin. Circadian phases are double plotted for better visualization of any rhythmicity. The ordinates are all the same scale and expressed as percentage deviation from the respective mean after subtraction of any linear trends in the individuals’ data. The mean and SE values of the data are shown segregated into 60° bins (~4 h). The solid lines describe the cosinor models. Significant circadian rhythms are indicated by P values where applicable. Although these data were collected during wakefulness, the group average habitual sleep period when living outside the laboratory is depicted by a double-headed arrow on each plot. There were significant circadian rhythms in circulating leptin, glucose, and insulin (left panels). The circadian system caused a steady increase in leptin across the usual sleep period and increases in both insulin and glucose around the time of habitual awakening. In contrast, there was no detectable circadian rhythm in either circulating sOB-R or adiponectin levels (right panels).
There was much less variability within subjects in the concentration of sOB-R. None of the subjects had a significant circadian rhythm in sOB-R, and there was no group mean circadian rhythm although a very small amplitude, approximately 12 h harmonic, was detected (P = 0.04), presumably representing a small ultradian rhythm in sOB-R as shown in Fig. 2 (top right). Because the circadian fluctuation in sOB-R was less than the fluctuation in total leptin, this implies that the biologically active free leptin had a circadian variation. Adiponectin concentrations were relatively stable, with only one subject showing an endogenous circadian rhythm, and there was no group mean circadian rhythm in adiponectin (Fig. 2, lower right).
There were significant mean group circadian variations in both glucose and insulin (Fig. 2, lower left). The peak-to-trough amplitude of the circadian rhythms was approximately 22% for insulin (P = 0.02) and 10% for glucose (P = 0.001). Although the circadian amplitude for glucose was smaller, it was highly significant due to the relative stability of this variable. Both the insulin and glucose levels were highest in the morning, with a substantial peak in the circadian component of insulin around the time of usual awakening (although all of these measurements were made during continued wakefulness and with regular small snacks).
Effect of wakefulness/meals (independent from circadian effects)
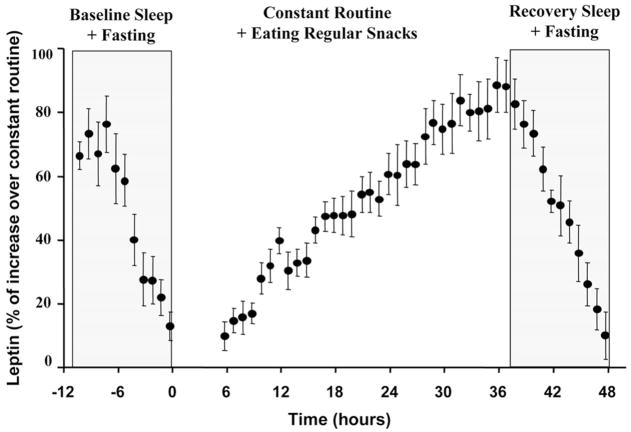
Independent of any circadian rhythm influences, all six subjects had a significant linear increase in leptin throughout the constant routine (i.e. during prolonged wakefulness with regular food intake). The average increase was equivalent to 3.33 ng/ml over the usual period of 16 h of continuous wakefulness. This represents an independent behavior-related daytime fluctuation of 51% around the leptin mesor of 6.5 ng/ml (Fig. 3). As leptin was increasing, prolonged wakefulness was associated with a significant group mean decrease in sOB-R equivalent to 3.2 ng/ml per 16 h of continuous wakefulness (i.e. 19% variability around the sOB-R mesor of 16.3 ng/ml). The fluctuation in sOB-R was opposite to the fluctuation in total leptin, implying that the biologically active free leptin had an even larger increase attributable to the prolonged wakefulness/eating period.
Fig. 3.
Group mean behavioral influences upon circulating leptin. The abscissa represents time before, during, and after the constant routine. To account for differences among subjects, the ordinate is expressed in normalized units as the percentage of the individual’s maximal leptin across the protocol. All data collected in the first 5 h of the constant routine were excluded from analysis to avoid the effects of altered posture and sleep-wake transition. Any circadian rhythmicity in the individual data were subtracted before group analysis. The mean and SE values of the data are shown. There was a significant decrease in leptin across each sleep period (when subjects also were fasting), and there was a significant increase in leptin during prolonged wakefulness (when subjects were receiving identical small snacks every 2 h).
There was a very small, but significant, decline in group mean adiponectin during wakefulness equivalent to 0.18 μg/ml over the usual period of 16 h of continuous wake-fulness (i.e. 4% decrease around the adiponectin mesor of 4.05 μg/ml). However, this is presumably of little physiological consequence because only one of the six subjects had a significant linear decline in adiponectin across the constant routine. Similarly, there were no statistically significant changes in group mean glucose or insulin attributable to prolonged wakefulness/meals.
Effect of sleep/fasting (independent from circadian effects)
In contrast to the increased circulating leptin during wake-fulness, each subject had a significant decrease in leptin across each sleep period (when subjects were also fasting), both before the constant routine and during recovery sleep. The mean decrease was −0.6 ng/ml per hour of sleep/fasting (range, −0.34 to −1.10 ng/ml per hour). This is equivalent to 4.8 ng/ml over the usual period of 8 h of sleep, representing an independent sleep/fasting-related fluctuation of 74% around the leptin mesor of 6.5 ng/ml (Fig. 3). Notably, the rate of fall during sleep/fasting was much greater than the rate of increase during wakefulness/eating (mean, +0.21 mg/ml per hour, see above).
The rate of fall of circulating leptin during sleep/fasting was almost identical in the baseline period and during recovery sleep (Fig. 3), despite improvements in average sleep efficiency (5.5% absolute increase; P < 0.01, paired t test), decreased sleep onset latency (13 min; not significant), and an average increase in nonrapid-eye-movement sleep stages III and IV (6.1% absolute increase; P < 0.05). The similar rate of decline despite altered sleep suggests that fasting rather than sleep per se causes the linear decrease in circulating leptin.
There were no independent sleep-related changes in sOB-R or adiponectin, but there were independent sleep-related decreases in both glucose and insulin. The mean reduction in circulating insulin attributable to sleep/fasting was −0.45 μIU/ml per hour, equivalent to −3.6 μIU/ml over the usual sleep period of 8 h (representing an independent sleep/fasting-related reduction in insulin of 36% around the mesor of 10.1 μIU/ml). The mean reduction in circulating glucose was −0.65 mg/dl per hour, equivalent to −5.2 mg/dl over the usual sleep period of 8 h (reduction in glucose of 5% around the mesor of 103.4 mg/dl).
Discussion
Although our prior work has suggested that nocturnal elevations of leptin are probably due to more than a circadian influence, and that sleep per se may also play a role (30), to our knowledge, this study is the first to directly assess the independent influences of the endogenous circadian pacemaker and various physiological behaviors on adipocyte and glucose physiology using a validated circadian protocol. We found significant endogenous circadian rhythms in leptin, glucose, and insulin, with peaks around the usual time of awakening, but no circadian rhythms in sOB-R or adiponectin. In addition, we detected systematic decreases in leptin, glucose, and insulin attributable to the effects of sleep/fasting, and a systematic increase in leptin attributable to wake-fulness/food intake. Because adipokines play important physiological and pathophysiological roles in obesity and the metabolic syndrome (31), these findings may provide potentially important implications in situations when physiological behaviors and the circadian system become misaligned, such as with jet lag, shift work, or clinical conditions such as the nocturnal eating syndrome (32–34).
Almost all previous studies quantifying the day/night pattern in adipokine, glucose, or insulin concentrations allowed sleep/fasting to occur at night and wakefulness/food intake during the day, making it impossible to determine whether the changes seen across the day and night could be due to the pattern of food intake over the 24-h period or the endogenous circadian system. Leptin levels have a robust 24-h rhythm (8–12), with leptin increasing monotonically from the morning through the afternoon and evening and peaking in the early part of the nocturnal sleep period. Shifting the time of both sleep and meals by 12 h also shifted the pattern of circulating leptin by the same period (35), but circadian rhythms may have also been changing during the protocol independent from food intake because there was no control of behaviors or light exposure during the wakeful-ness episodes. Use of continuous enteral administration and shifting of the sleep schedule by 8 h reveals possible independent circadian rhythm and sleep effects on circulating leptin (36). However, there were limitations in this study including an inability to assess the entire circadian cycle effect, the likely altered sleep quality after an acute sleep schedule shift, and the fact that enteral administration of nutrients may alter hormone levels by bypassing any important neural or endocrine signals from the stomach and gut (e.g. ghrelin) (32).
In the current study, we separated the potential circadian and behavioral influences and found that the endogenous circadian profile of circulating leptin is considerably different (i.e. approximately 8 h phase delayed) from that seen in the previously published, not fully controlled studies. Importantly, we found that, after subtracting the circadian effect, sleep/fasting was associated with a monotonic decline in leptin, and wakefulness/eating was associated with a monotonic increase in leptin. Therefore, these data suggest that the previously documented day/night pattern in leptin is caused by the combined effects of the endogenous circadian pacemaker and the day/night patterns in behaviors, with the latter having the greater effect across the 24-h cycle.
There is growing evidence of an endogenous circadian rhythm controlling circulating glucose (37). For example, observational studies in humans indicate that there is a day/night pattern in circulating glucose and insulin, with highest levels in the morning (38). Similar to leptin, studies on the effect of sleep on insulin and glucose regulation in healthy subjects (33) suggest the existence of independent circadian and sleep/fasting-related influences on glucose regulation. In a circadian study similar to the current study, but with constant glucose infusion, it was found that the first part of the sleep period was associated with a sharp increase in circulating glucose (due to a decrease in glucose tolerance that almost entirely counteracts any fasting-related drop in glucose), followed by a slight decline in the second half of an 8-h sleep period and fasting. When sleeping during the daytime, there is also an immediate increase in circulating glucose followed by a reduction over time (33–34, 39). Our data extend the observations from these relatively uncontrolled or observational studies by assessing these effects independently through subtracting the endogenous circadian components and examining the residual relationships between behaviors and circulating glucose and insulin. We found that sleep/fasting causes significant systematic reductions in both glucose and insulin, which is consistent with data from the second half of the sleep periods in these earlier studies. We show that healthy humans do have an endogenous circadian rhythm of glucose and insulin, with a peak at the usual time of awakening, at a time similar to that of the circadian-related peak in leptin (Fig. 2). Thus, there appears to be a circadian correlation between insulin and leptin (at least for the times of peak insulin and leptin) (Fig. 2), consistent with the previously detected correlation in longer studies of chronic diurnal fasting (40). Whether the associations found herein are due to a synchronous pacemaker effect or due to the physiologic feedback loop reciprocally regulating insulin and leptin levels remains to be demonstrated by interventional studies.
Results in our overweight, but nonobese, subjects in the current study (average BMI, 25 kg/m2) indicated a lack of a significant circadian rhythm of adiponectin. We recently reported that circulating adiponectin concentrations in healthy lean men (average BMI, 22.8 kg/m2) exhibited a day/night variation, with a significant decline at night, reaching a minimum in the early morning (13). In contrast, a similar study in obese subjects (average BMI, 57.5 kg/m2) failed to demonstrate a similar day/night variation, suggesting that important differences exist between lean vs. obese subjects (41). These differences among studies suggest that the day/night pattern in adiponectin (13) in lean individuals is mainly attributable to day/night patterns in behaviors (sleep/fasting and wakefulness/meals) rather than the endogenous circadian cycle and that this effect can be observed in obese individuals.
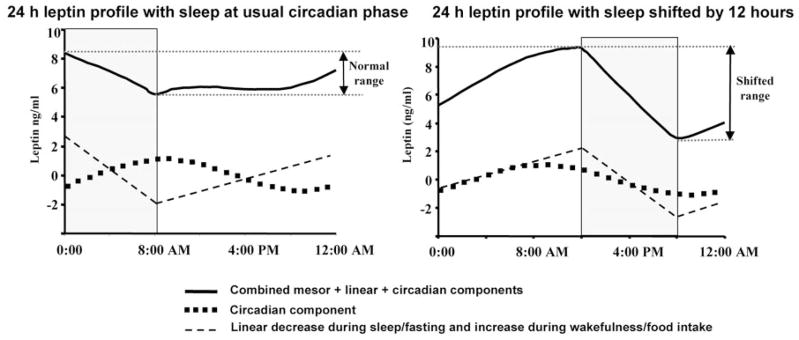
Shift work and travel to different time zones are increasingly prevalent in our society. There is also abundant evidence that shift work leads to obesity (15–20) and associated comorbidities, including insulin-resistance, the metabolic syndrome, and adverse cardiovascular events (21–23). The causes of shift-work-related obesity remain unknown, but increased calorie intake and/or decreased energy expenditure are important contributors (42). Based on the data presented herein, we hypothesize that changes of the day/night patterns of circulating leptin could be implicated in the pathogenesis of shift-work-related obesity. More specifically, summing the three factors that influence circulating leptin tested in the current study (i.e. the circadian system, wake-fulness/meals, and sleep/fasting), we have estimated how the usual day/night pattern of circulating leptin is likely to be altered by adjustments in the sleep/wake schedule (Fig. 4). With sleep occurring at the usual phase of the circadian rhythm (left panel of Fig. 4), circadian and behavioral fluctuations are almost out of phase and cancel each other to a large extent: leptin reaches a peak during the usual sleep period, declines throughout the rest of the night, begins to increase throughout the morning, reaches a plateau for approximately 8 h during the daytime, and then rises again in the 3 h before the usual sleep time. This profile is similar to published 24-h leptin profiles in protocols that permit sleep at night. In contrast, the daily leptin profile is likely to change quite considerably when altering the sleep/wake schedule relative to the circadian timing. When sleep occurs at an unusual phase of the circadian rhythm (i.e. shifted by 12 h) as may occur during shift work, the circadian and behavioral fluctuations of leptin would likely be in phase (Fig. 4). This would result in a fluctuation in circulating leptin of more than double the normal 24-h fluctuation. Moreover, in this model, the person would awaken with a very low level of leptin, which would be expected to increase appetite and alter neuroendocrine function at this time (4, 43). Note, these profiles are based on our data in which identical sized snacks are taken equispaced throughout the waking period. Such profiles may change if subjects have three or four larger meals during wakefulness. Thus, whether or not this simulated model reflects exactly the biology of night shift work in the real world remains to be examined by performing experiments in subjects working on a night shift schedule.
Fig. 4.
Adjusting sleep/wake schedule relative to the endogenous circadian phase increases the magnitude of the 24-h leptin profile. Left panel, Group mean 24-h circulating leptin profile with sleep occurring at the usual phase of the circadian rhythm; right panel, the same data but with sleep occurring at an unusual phase of the circadian rhythm (i.e. shifted by 12 h) as may occur during shift work. The sleep periods are shown by gray shaded blocks. The abscissa represents time of day. In each panel the thin dashed line depicts the group mean linear decrease during sleep/fasting and the linear increase during wakefulness/food intake, and the dotted line depicts the group mean circadian rhythmicity. The circadian time is the same in each panel, with the peak leptin attributable to the circadian system occurring around 0900 h (~3 h after the CBTmin). The top line in each panel represents circulating leptin (mesor plus circadian plus behavioral effects). When sleep occurs at an unusual phase of the body clock (right panel), the circadian and behavioral fluctuations are in phase, and the resultant circulating leptin has a larger 24-h fluctuation.
Although common obesity is associated with hyperleptinemia in humans (44–46), subjects with leptin receptor mutation(s), as well as hypoleptinemic subjects with either congenital or acquired leptin deficiency, are also obese (47–48). Moreover, it has been suggested that even heterozygotes for mutations of the leptin gene (approximately 10% of the population) also become obese due to relative leptin deficiency (47–48). We have recently shown that either short-term induced hypoleptinemia due to fasting, or long-term hypoleptinemia in conditions in which caloric expenditure exceeds caloric intake, is associated with dysregulation of the central mechanisms regulating neuroendocrine function and energy homeostasis (4, 43). In addition, leptin administration is also effective in regulating insulin resistance in hypoleptinemic states, including both congenital leptin deficiency and acquired states of leptin deficiency (1, 31, 49). Similar to other hormones with pulsatile and day/night patterns of secretion, these observations raise the possibility that the lower daily nadir in circulating leptin/free leptin levels (27, 41) may be of relevance to metabolism, neuroendocrine function, and development of obesity in the shiftwork population; but this remains to be directly shown by interventional studies.
Limitations of the study include the fact that our separation of circadian and behavioral effects relies upon the assumption that these effects are additive, as suggested by the statistical analyses. This needs to be directly studied by future experiments such as a forced desynchrony study where the circadian and behavior rhythms are experimentally uncoupled (50). We were also unable to assess independent effects of meals from wakefulness, or independent effects of fasting from sleep, but overwhelming evidence from this study and other studies suggests that fasting and meals have greater effects when compared with sleep and wakefulness effects. Our findings are quite different from other recent research showing that experimentally restricted sleep (4 h per night for 6 nights) lowered both glucose tolerance (51) and the 24-h profile of leptin (52), raising the possibility that incremental sleep restriction may have different effects from acute sleep deprivation. Thus, further experiments, possibly of longer duration, are needed to sort this out.
In summary, we found significant endogenous circadian rhythms in leptin, glucose, and insulin, with peaks around the usual time of awakening, but no circadian rhythms in sOB-R or adiponectin. In addition, we detected systematic decreases in leptin, glucose, and insulin attributable to the effects of sleep/fasting, and a systematic increase in leptin attributable to wakefulness/food intake. Our data imply that alterations in the sleep/wake schedule can lead to an increased peak-to-trough daily swing in the physiologically active circulating free leptin, which may influence food intake and energy balance and could thus be implicated in the increased obesity and cardiovascular disease in the shift work population (15). Further studies are needed to assess whether the 24-h leptin profile in response to three meals/d would be different; but, because the circadian peak in leptin coincides with the usual time of awakening in the absence of shift work (left panel of Fig. 4), altering the sleep schedule in either direction and by any amount is expected to always cause a lower trough level of leptin and hypoleptinemia upon awakening. The important clinical relevance of these fluctuations and the need for further interventional studies in the shift work population is further supported by similar observations in patients with nocturnal eating disorders who have an attenuated nocturnal rise in plasma leptin when compared with weight matched controls, suggesting that their nocturnal eating could be a result of the absence of a nocturnal anorexic signal provided by leptin (14).
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL64815 and K24 HL76446 (to S.A.S.), General Clinical Research Center Grant MO1 RR02635 (to Brigham and Women’s Hospital), and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 57875 (to C.S.M.).
Abbreviations
- BMI
Body mass index
- CBTmin
core body temperature minimum
- CV
coefficient of variation
- sOB-R
soluble leptin receptor
References
- 1.Coleman RA, Herrmann TS. Nutritional regulation of leptin in humans. Diabetologia. 1999;42:639–646. doi: 10.1007/s001250051210. [DOI] [PubMed] [Google Scholar]
- 2.Cupples WA. Peptides that regulate food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1370–R1374. doi: 10.1152/ajpregu.00129.2003. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum M, Leibel RL. The role of leptin in human physiology. N Engl J Med. 1999;341:913–915. doi: 10.1056/NEJM199909163411211. [DOI] [PubMed] [Google Scholar]
- 4.Welt CK, Chan JL. Recombinant human leptin administration in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 5.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bio-active substances. Ann NY Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 7.Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- 8.Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes. 1999;48:334–341. doi: 10.2337/diabetes.48.2.334. [DOI] [PubMed] [Google Scholar]
- 9.Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab. 2001;86:90–96. doi: 10.1210/jcem.86.1.7136. [DOI] [PubMed] [Google Scholar]
- 10.Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Negro PP, Mulla A, Veldhuis JD, Cearnal L, Flier JS, Gold PW. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab. 1998;83:4140–4147. doi: 10.1210/jcem.83.11.5291. [DOI] [PubMed] [Google Scholar]
- 11.Saad MF, Riad-Gabriel MG, Khan A, Sharma A, Michael R, Jinagouda SD, Boyadjian R, Steil GM. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83:453–459. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 12.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 14.Birketvedt GS, Florholmen J, Sundsfjord J, Osterud B, Dinges D, Bilker W, Stunkard A. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA. 1999;282:657–663. doi: 10.1001/jama.282.7.657. [DOI] [PubMed] [Google Scholar]
- 15.Ishizaki M, Morikawa Y, Nakagawa H, Honda R, Kawakami N, Haratani T, Kobayashi F, Araki S, Yamada Y. The influence of work characteristics on body mass index and waist to hip ratio in Japanese employees. Ind Health. 2004;42:41–49. doi: 10.2486/indhealth.42.41. [DOI] [PubMed] [Google Scholar]
- 16.Di Lorenzo L, De Pergola G, Zocchetti C, L’Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, Soleo L. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27:1353–1358. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 17.Lowden A, Holmback U, Akerstedt T, Forslund A, Forslund J, Lennernas M. Time of day type of food–relation to mood and hunger during 24 hours of constant conditions. J Hum Ergol (Tokyo) 2001;30:381–386. [PubMed] [Google Scholar]
- 18.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76:424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 19.Parkes KR. Shift work and age as interactive predictors of body mass index among offshore workers. Scand J Work Environ Health. 2002;28:64–71. doi: 10.5271/sjweh.648. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutsson A. Shift work and coronary heart disease. Scand J Soc Med Suppl. 1989;44:1–36. [PubMed] [Google Scholar]
- 22.Nakamura K, Shimai S, Kikuchi S, Tominaga K, Takahashi H, Tanaka M, Nakano S, Motohashi Y, Nakadaira H, Yamamoto M. Shift work and risk factors for coronary heart disease in Japanese blue-collar workers: serum lipids and anthropometric characteristics. Occup Med (Lond) 1997;47:142–146. doi: 10.1093/occmed/47.3.142. [DOI] [PubMed] [Google Scholar]
- 23.Scheen AJ. Clinical study of the month. Does chronic sleep deprivation predispose to metabolic syndrome? Rev Med Liege. 1999;54:898–900. [PubMed] [Google Scholar]
- 24.Schwartz WJ. Suprachiasmatic nucleus. Curr Biol. 2002;12:R644. doi: 10.1016/s0960-9822(02)01155-7. [DOI] [PubMed] [Google Scholar]
- 25.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 26.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 27.Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–2112. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- 28.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 29.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- 30.Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, Meier-Ewert HK, Dinges DF, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 32.Druce MR, Small CJ, Bloom SR. Minireview: gut peptides regulating satiety. Endocrinology. 2004;145:2660–2665. doi: 10.1210/en.2004-0089. [DOI] [PubMed] [Google Scholar]
- 33.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 34.Simon C. Ultradian pulsatility of plasma glucose and insulin secretion rate: circadian and sleep modulation. Horm Res. 1998;49:185–190. doi: 10.1159/000023169. [DOI] [PubMed] [Google Scholar]
- 35.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 37.La Fleur SE. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol. 2003;15:315–322. doi: 10.1046/j.1365-2826.2003.01019.x. [DOI] [PubMed] [Google Scholar]
- 38.Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, Lolli C, Campbell P, Brunetti P, Gerich JE. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes. 1984;33:1150–1153. doi: 10.2337/diab.33.12.1150. [DOI] [PubMed] [Google Scholar]
- 39.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kassab SE, Abdul-Ghaffar T, Nagalla DS, Sachdeva U, Nayar U. Serum leptin and insulin levels during chronic diurnal fasting. Asia Pac J Clin Nutr. 2003;12:483–487. [PubMed] [Google Scholar]
- 41.Calvani M, Scarfone A, Granato L, Mora EV, Nanni G, Castagneto M, Greco AV, Manco M, Mingrone G. Restoration of adiponectin pulsatility in severely obese subjects after weight loss. Diabetes. 2004;53:939–947. doi: 10.2337/diabetes.53.4.939. [DOI] [PubMed] [Google Scholar]
- 42.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–523. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 43.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valle M, Gascon F, Martos R, Bermudo F, Ceballos P, Suanes A. Relationship between high plasma leptin concentrations and metabolic syndrome in obese pre-pubertal children. Int J Obes Relat Metab Disord. 2003;27:13–18. doi: 10.1038/sj.ijo.0802154. [DOI] [PubMed] [Google Scholar]
- 45.Bowen H, Mitchell TD, Harris RB. Method of leptin dosing, strain, and group housing influence leptin sensitivity in high-fat-fed weanling mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R87–R100. doi: 10.1152/ajpregu.00431.2002. [DOI] [PubMed] [Google Scholar]
- 46.Cupples WA. Addressing leptin resistance. Am J Physiol Regul Integr Comp Physiol. 2003;284:R86. doi: 10.1152/ajpregu.00629.2002. [DOI] [PubMed] [Google Scholar]
- 47.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 48.Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, Jebb SA, Lip GY, O’Rahilly S. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–35. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 49.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 50.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 51.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 52.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on physiological rhythms. Rev Neurol (Paris) 2003;159:6S11–6S20. [PubMed] [Google Scholar]