Abstract
Chronic wasting disease (CWD) and sheep scrapie can be transmitted via indirect environmental routes, and it is known that soil can serve as a reservoir of prion infectivity. Given the strong interaction between the prion protein (PrP) and soil, we hypothesized that binding to soil enhances prion resistance to enzymatic digestion, thereby facilitating prion longevity in the environment and providing protection from host degradation. We characterized the performance of a commercially available subtilisin enzyme, the Prionzyme, to degrade soil-bound and unbound CWD and HY TME PrP as a function of pH, temperature, and treatment time. The subtilisin enzyme effectively degraded PrP adsorbed to a wide range of soils and soil minerals below the limits of detection. Signal loss occurred rapidly at high pH (12.5) and within 7 d under conditions representative of the natural environment (pH 7.4, 22°C). We observed no apparent difference in enzyme effectiveness between bound and unbound CWD PrP. Our results show that although adsorbed prions do retain relative resistance to enzymatic digestion compared with other brain homogenate proteins, they can be effectively degraded when bound to soil. Our results also suggest a topical application of a subtilisin enzyme solution may be an effective decontamination method to limit disease transmission via environmental ‘hot spots’ of prion infectivity.
Introduction
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are fatal neurodegenerative diseases that include bovine spongiform encephalopathy (BSE or ‘mad cow’ disease), scrapie (sheep and goats), chronic wasting disease (CWD, deer, elk, and moose), and Creutzfeldt-Jakob disease (CJD, humans) (1, 2). Strong evidence indicates that the infectious agent of prion diseases is comprised solely of PrPSc (the prion), an abnormally-folded isoform of a normal cellular protein, PrPc (1, 3). The misfolded conformation of PrPSc conveys distinct biochemical properties, including resistance to proteolysis and inactivation techniques, increased hydrophobicity, and a propensity for aggregation (1, 4).
Infectious CWD and scrapie prions are shed from living hosts and present in mortalities and can remain infectious after years in the environment (5, 6). It has been demonstrated that the environment serves as a reservoir of CWD infectivity, facilitating a sustained incidence of CWD in free-ranging cervid populations and complicating efforts to eliminate disease in captive herds. Prions are known to adsorb to a wide range of soils and soil minerals and strongly resist desorption by detergent and chaotropic treatments (5, 7, 8). Given that prions bound to soil particles remain infectious through oral consumption (9, 10), the potential for CWD or scrapie transmission by exposure to contaminated soil exists since cervids and other ruminants are known to ingest and inhale soil (11, 12). Thus, soil and soil minerals may act as significant environmental reservoirs of infectivity.
It is highly desirable to develop practical, in situ methods for decontaminating soil and other environmental surfaces exposed to infectious prions. Incineration of prion-contaminated material is currently considered the most effective disposal method (13), and extended autoclaving coupled with chemical treatment is the most effective disinfection method (4). However, incineration or autoclaving of contaminated soil or farm infrastructure (paddocks, fences, barns) from CWD or scrapie-endemic environments is not practical. Bacterial enzymes which effectively degrade prions have been identified but are most effective at conditions that are infeasible in the field (high pH and temperature) (14, 15).
An enzyme-based treatment that is effective under environmentally-relevant conditions (pH near neutral, temperatures below 40°C) could be used to lower or eliminate infectivity at identified or presumed CWD and scrapie ‘hot spot’ ground surfaces in captive and wild settings. Pilon and colleagues recently evaluated the performance of a commercially available bacterial subtilisin enzyme against RML-strain mouse PrPSc under mild conditions (pH 7.9) (16). Complete loss of PrP western blot signal did not occur within 29 h at 20°C, and animal survival increased only 14% in bioassay. Here we evaluate a similar enzyme treatment, the ‘Prionzyme’ (Genencor), for its effectiveness against PrP bound to soil. Prionzyme treatment of infectious mouse brain homogenate (301v strain) for 30 min at pH 12 and 60°C led to a decrease in infectivity of at least 7-log and greater than 65% survival in bioassay following intracerebral inoculation (17). However, its effectiveness at lower temperatures and pH is unknown.
The resistance or susceptibility of PrP to enzymatic degradation conferred upon binding to soil has not been evaluated. We hypothesized that, given the strong interaction between prions and soil, binding to soil enhances prion resistance to enzymatic degradation, thereby facilitating prion longevity in the environment and providing protection from in vivo degradation once ingested or inhaled. Therefore, the objectives of this research were: 1) to determine any changes in prion susceptibility to enzymatic digestion upon binding to soil and 2) to evaluate the efficacy of using a commercially available enzyme to decontaminate soil samples. Prions were adsorbed to a range of soil minerals and whole soils and then exposed for up to 7 d to Prionzyme solutions at various pH and temperature conditions. Experiments were conducted using prion-infected brain homogenates from rodents (HY TME-infected hamsters) and CWD-infected elk, as we previously found differences in CWD and HY PrP adsorption and susceptibility to degradation (18, 19).
Materials and Methods
Tissue Sources
Brain tissue was collected from hamsters infected with the HY TME (hyper strain of transmissible mink encephalopathy (TME)) agent at terminal disease (20). Brain tissue from uninfected hamsters and an elk infected with CWD were also used (18). Brain tissue was homogenized to 10% (w/v) in Dulbecco’s phosphate-buffered saline (DPBS) without Ca++ or Mg++ (Mediatech, Herndon, VA) using strain-dedicated Tenbroeck tissue grinders (Kontes, Vineland, NJ).
PrP Adsorption
Gamma-irradiated fine white sand (Fisher Scientific, Pittsburgh, PA), Dickinson sandy loam soil (a Typic Hapludoll), Rinda silty clay loam soil (a Vertic Epiaqualf), and sodium bentonite clay (CETCO, Arlington Heights, IL, surface area = 22.3 m2/g) were used and have been previously characterized (7). Silicon dioxide powder (99% pure, particle size 0.5–10 μm, 80% between 1–5 μm, surface area = 6.1 m2/g, Sigma Aldrich, St. Louis, MO) and humic acid-coated silica gel particles (SiO2-HA, surface area = 209 m2/g) (21) were also used. To obtain soil-bound PrP, 10% brain homogenate was combined with soil in 1X DPBS and gently rotated at 24 rpm (Mini Labroller, Labnet, Edison, NJ) at 22°C. Incubation time, as well as soil, buffer, and homogenate:soil ratios were selected to achieve maximum or near-maximum PrP adsorption based on previously published results (7, 19) and are detailed in Table S1. Samples were removed after incubation and centrifuged at 100 g for 5 min. The supernatant was removed, and the pellets were washed 2 times with DPBS. The original supernatant, first wash, and final pellet were collected and stored at −80°C. Subsequent washes did not contain measurable PrP (7).
Enzyme Digestion assays
Soil samples were thawed, resuspended in DPBS adjusted to the desired pH using 1M sodium hydroxide (NaOH), and then aliquoted into individual 0.2 ml polypropylene tubes (Fisher Scientific). An aqueous enzyme solution commercially available as ‘Prionzyme M’ was obtained from Genencor/Danisco (Rochester, NY) and adjusted to the desired pH using 1M NaOH. The Prionzyme M is a proprietary serine protease derived from a Bacillus subtilis strain. The Prionzyme solution was obtained pre-buffered with proprietary amounts of propylene glycol and sodium formate. The Prionzyme, referred to hereafter in this paper as the ‘subtilisin’ or ‘subtilisin enzyme’, was added to the soil samples or brain homogenate at the desired dose and pH. Samples were briefly vortexed and then incubated at 22, 37, or 50°C without agitation for the desired time. Sample pH did not change significantly throughout incubations (data not shown). Samples were then frozen at −80°C until western blot analysis. All experiments were performed in triplicate except SiO2-HA, where n = 5.
Western Blotting
Desorption of PrP from soil prior to gel electrophoresis was accomplished by boiling soil samples in SDS-PAGE sample buffer (Lammeli buffer containing 2% SDS) at 100°C for 10 min. Sample volumes loaded into gels are shown in Table S1. All controls and unbound PrP sample volumes were 2.5 μl. Western blot/SDS-PAGE analysis was performed as described previously (18) without modification. Hamster samples were immunoblotted with mAb 3F4 (Chemicon, Temecula, CA, 1:10,000 dilution), which reacts with residues 110–113 (MKHM) in hamster PrP. Elk samples were immunoblotted with L42 (R-Biopharm, Marshall, MI, 1:1000), which reacts with residues 145–163 in elk PrP. Blots were developed with Pierce Supersignal West Femto maximum-sensitivity substrate and imaged on a Kodak 2000R imaging station (Kodak, Rochester, NY). The Prionzyme did not exhibit nonspecific binding to the primary or secondary antibodies used (data not shown). No soils exhibited nonspecific binding (data not shown).
Quantitative Analysis
Blot images were analyzed using Kodak 1D 4.0 software (Kodak, Rochester, NY), which output the net intensity of each blot (total darkness minus background). Net intensities of sample replicates (n= 3 or 5) were normalized as a percent of the average of four replicate untreated controls run on the same gel. There was no significant variance in control blot intensities with respect to brain homogenate pH for either HY TME or CWD-elk (see Figures S3A and S4A), so all samples were normalized to pH 7.4 controls.
Direct Detection Assay
A direct detection method described previously (7) was used without modification to quantify prion adsorption to sand and sandy loam soil. Further details of this method can be found in the Supporting Information.
Results
Enzymatic Digestion of Unbound Prions
Initial testing of the subtilisin ‘Prionzyme’ performance against PrP was conducted using HY TME brain homogenates at pH levels between 7 and 12.5 and temperatures of 22, 37, and 50°C using a 30 min incubation time. A 50% (v/v) Prionzyme dose did not yield higher degradation than a 10% dose (data not shown), so a 10% dose was used throughout this study (unless otherwise specified) for optimal digestion. Total protein (Coomassie blue) staining of subtilisin-treated HY samples indicates that it effectively degraded all detectable proteins present in the brain homogenate (Figure S1, lane 3). In addition, PrPc from uninfected hamster brain homogenate was completely degraded by the subtilisin within 30 min (Figure S2, lane 2).
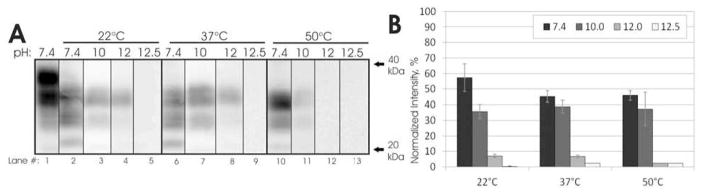
pH strongly influenced unbound PrP digestion. HY PrP degradation at pH 7.4, which is a physiologically-representative pH and within the range of potential soil pH values, was approximately 50% compared with controls for all three temperatures tested (Figure 1). HY PrP degradation increased to 65% at pH 10, and no PrP was detected at pH 12 (for 50°C) or pH 12.5 (for 22 and 37°C). Temperature had only minor effects on HY PrP degradation over the range tested. Results at pH 7.4 and 10 were very similar for all three temperatures (Figure 1). At pH 12, some signal remained at 22°C and 37°C, but no signal was present at 50°C. CWD-elk PrP digestion with the subtilisin enzyme exhibited a similar dependence on pH, and digestion of CWD PrP was more effective over the pH and temperature ranges evaluated when compared with HY results (Figure S3, panel A). Digestion was approximately 75%, 85%, and 100% at pH 7.4/22°C, pH 10.0/37°C, and pH 12.5/50°C, respectively.
Figure 1.
Subtilisin enzyme treatment of HY TME PrP at various temperatures and pH. Panel A: Representative blots of 2.5 μl of a 10% BH. All blots probed with mAb 3F4 after a 30 min treatment with a 10% Prionzyme dose. No samples treated with proteinase K. Panel B: Quantification of results from panel A. Blot intensities were normalized to a frozen, untreated pH 7.4 control (Panels A, lane 1). Error bars show ± the standard error
We also tested longer enzyme treatment times under environmentally-relevant conditions (pH 7.4, 22°C). HY PrP signal decreased through 48 h when treated with the subtilisin but substantial PrP remained after 7 d treatment (Figure S5, panel A). In contrast, while CWD PrP did not degrade further through 48 h compared to the 30 min treatment, complete loss of signal occurred after 7 d treatment (Figure 2A).
Figure 2.
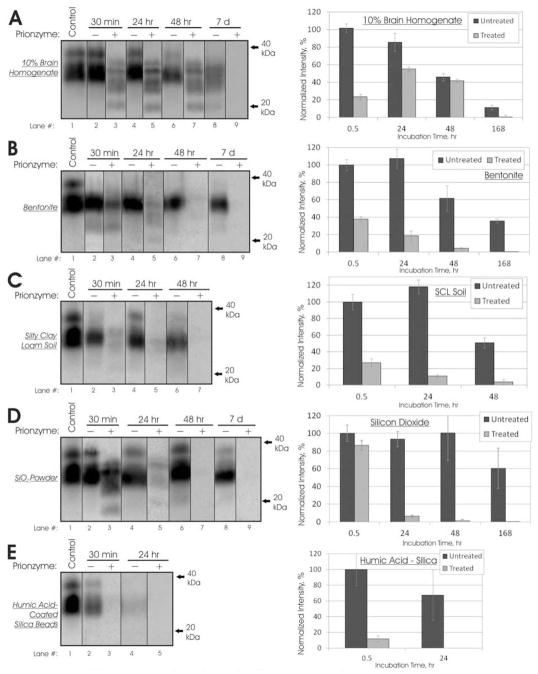
Subtilisin enzyme digestion of soil bound and unbound CWD-elk PrP at pH 7.4 and 22°C. Left column: Representative immunoblots of CWD-elk PrP after Prionzyme treatment for up to 7 days. Soil amounts imaged are shown in Table S1. All blots used mAb L42. Right column: Signal intensities of sample blots were normalized against 30 min untreated samples (lane 2), except in Panel A. Error bars show ± 1 standard error of the mean. Panel A: 10% Brain Homogenate. Results normalized against frozen pH 7.4 controls. Panel B: Bentonite clay. Panel C: Silty clay loam soil. Panel D: SiO2 Powder. Panel E: Humic acid coated silica beads (SiO2-HA).
Enzymatic Digestion of Soil-Bound Prions
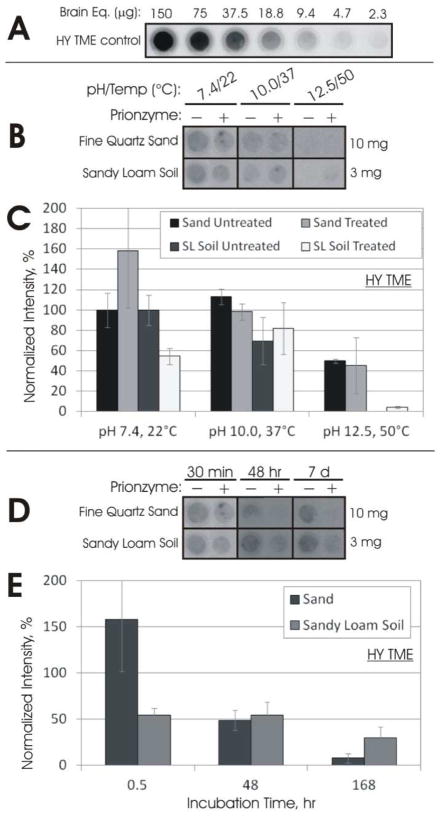
To evaluate PrPSc resistance to enzymatic digestion when bound to soil, we adsorbed CWD and HY PrP to a range of soils and soil minerals, including silicon dioxide (SiO2) powder, bentonite clay, silica gel particles coated with humic acid (SiO2-HA, a model sorbent representative of soil organic matter), silty clay loam soil, fine quartz sand, and a sandy loam soil. Adsorption was performed under the conditions described in Table S1, which were based on our previous characterization of prion protein adsorption to soil in the competitive brain homogenate matrix (7, 19). PrPc from uninfected hamster brain homogenate bound to all soils used was completely degraded by the subtilisin enzyme within 30 min at pH 7.4 and 22°C (Figure S2).
Enzyme treatment of CWD PrP bound to soils yielded similar results when compared against data obtained from treatment of unbound PrP over the three conditions tested (pH 7.4/22°C, pH 10.0/37°C, and pH 12.5/50°C). After a 30 minute treatment with a 10% subtilisin dose at pH 7.4/22°C, CWD PrP bound to soil was degraded between 60 and 85%, depending on the soil type (Figure S3). Degradation at pH 10.0/37°C was 45–95% and at pH 12.5/50°C was 85–100%. These results are similar to unbound PrP results (75%, 85%, and 100%, respectively, Figure S3, panel A).
Enzyme treatment of HY PrP bound to soil was less effective at pH 7.4/22°C and pH 10.0/37°C, where the treatment clearly truncated the PrP but did not reduce signal for any soil/mineral except silty clay loam soil (Figure S4). These results contrast with the unbound results (50% and 65% degradation, respectively, Figure S4, panel A). However, as with unbound PrP, bound HY PrP was completely degraded at pH 12.5/50°C for all soils except SiO2-HA.
Longer enzyme treatments of bound CWD PrP at pH 7.4/22°C led to faster reductions in PrP signal compared with unbound PrP. Complete loss of bound CWD PrP signal occurred after 24 h for SiO2-HA, 48 h for silty clay loam soil and SiO2 powder, and 7 d for pure bentonite clay (with near complete loss at 48 h) (Figure 2). In comparison, a large proportion of unbound CWD PrP remained after 48 h treatment but complete loss of signal occurred at 7 d (Figure 2A).
Bound HY PrP was more resistant at longer exposure times at pH 7.4/22°C. Treatment for 24 and 48 h did not reduce signals of PrP bound to bentonite clay, silty clay loam soil, or SiO2-HA. This is in contrast to unbound HY PrP, where PrP levels gradually decreased between exposure times of 30 min and 48 h. Complete loss of unbound HY PrP did not occur after 7 d whereas complete loss of bound HY PrP signal occurred after 7 d for all soils except SiO2-HA.
We compared subtilisin enzyme effectiveness at 10, 1, and 0.1% doses against CWD PrP bound to bentonite and SiO2 to determine sensitivity to enzyme concentration. For both minerals, there was no apparent difference in enzyme effectiveness between the 1% and 10% doses after 48 h treatment (Figure S6). The subtilisin treatment was somewhat less effective at the 0.1% dose. Increasing the dose from 10% to 50% did not improve degradation of bound or unbound PrP (data not shown).
We applied a direct detection method described previously (7) (see Supporting Information) to quantify HY PrP adsorption to fine quartz sand and sandy loam soil. This method directly detects PrP bound to soil and thus does not require desorption. A standard dilution curve is used to quantify PrP bound to soil (Figure 3A). Sand-bound PrP did not decrease after 30 min treatment at pH 7.4/22°C and pH 10.0/37°C (Figure 3B and 3C), similar to all other soil results except silty clay loam soil (Figure S4). Longer treatments at pH 7.4/22°C yielded a 50% decrease in PrP after 48 h and near-complete signal loss at 7 d (Figure 3D and E). Enzyme treatment of sandy loam soil was somewhat effective at pH 7.4/22°C but not effective at pH 10.0/37°C (Figure 3B and 3C). A 7 d treatment at pH 7.4/22°C did not completely digest sandy loam-bound HY PrP (30% remained), in contrast to the results from all soils tested by SDS desorption/western blot (Figure S5). For both sand and sandy loam soil, very low amounts of PrP were detected at pH 12.5/50°C in both controls and treated samples, which suggests that under these extreme conditions, most PrP desorbed or changed conformation such that the mAb 3F4 epitope was obscured.
Figure 3.
Subtilisin Enzyme Digestion of Bound HY TME PrP Quantified by Direct Detection of HY TME. All blots detected by mAb 3F4. A: Two-fold control dilution of HY brain homogenate used to quantify PrP bound to soil. B: Representative immunoblots of HY TME bound to fine quartz sand or sandy loam soil treated with 10% Prionzyme for 30 min. C: Quantified results from panel B. All samples were normalized to the untreated pH 7.4/22°C control. Error bars show ± the standard error. D: Representative immunoblots of bound HY TME treated at pH 7.4/22°C with 10% Prionzyme for up to 7 d. E: Quantified results from panel D. All samples were normalized to an untreated pH 7.4/22°C control incubated for the same time.
Discussion
Susceptibility of Unbound and Soil-Bound PrP to Enzymatic Digestion
Our results show that PrP binding to soil does not necessarily impact prion susceptibility to enzymatic digestion. Differences in subtilisin enzyme effectiveness between unbound and bound HY PrP were inconsistent. After a 30 min exposure period, bound HY PrP was more resistant to digestion than unbound PrP for 3 of 4 soil/minerals tested under three different pH/temperature conditions (Figure S4). After longer exposure periods, complete loss of signal occurred for 3 of 4 soil/minerals but similar behavior did not occur for unbound HY PrP (Figure S5). Results for CWD PrP were more consistent. After 30 min treatment under the three pH/temperature conditions, there was no noticeable difference between bound and unbound CWD PrP degradation (Figure S3). Longer treatments of bound CWD PrP under mild conditions led to faster reduction in PrP levels compared with unbound PrP (Figure 2). Thus, enzymatic digestion of CWD-elk PrP bound to soil is possible and may even be enhanced compared with unbound PrP.
Comparisons of bound and unbound PrP resistance to enzymatic digestion must be considered in light of the method used to quantify bound PrP. SDS-PAGE detection of PrP following desorption via boiling in SDS has PrP recovery efficiencies ranging from 13 to 72 % depending on soil and PrP type (HY or CWD, see Table S1). Thus, a proportion of bound PrP is not desorbed and detected. It is plausible that the observed loss of signal is due to the complete loss of desorbable PrP and that most non-desorbable PrP remains intact following enzyme treatment. Our direct detection data (Figure 3) indicates that complete or near-complete digestion of bound PrP can occur using the Prionzyme (as seen with the fine sand), but a significant amount of PrP bound to the sandy loam soil was resistant to digestion. Further studies into the relationship between PrP adsorption and resistance to enzymatic digestion are necessary. It is possible that if the non-desorbable fraction of bound PrP is also non-digestible, it may be differentially bound to the soil surface and possibly not infectious.
Although enzyme effectiveness varied with soil type, there does not appear to be significant, consistent trends across both CWD and HY PrP. Further experimentation and analysis of enzymatic digestion of adsorbed PrP may yield insights in PrP adsorption mechanisms as a function of soil type and prion species/strain but is beyond the limits of this paper. However, it is interesting to note that the untreated HY and CWD PrP signals for bentonite clay and for silty clay loam soil decreased over time to less than 35% at 7 d compared with the 30 min signal (Figures 2 and S5, Panels B and C). Although such a decrease could be due to degradation by factors other than the subtilisin enzyme, it was not seen in the SiO2 PrP signal. In addition, PrP adsorption to bentonite and silty clay loam does not decrease through at least 60 d (7). Thus, the decreased signal is likely due to less efficient desorption, which would imply changes in the PrP adsorption state over time.
Overall, HY PrP was more resistant to subtilisin digestion than CWD PrP in both bound and unbound states. Although we previously observed CWD PrP to be more resistant than HY PrP to degradation from endogenous proteases found in brain homogenate (18), the ‘Prionzyme’ bacterial subtilisin is likely dissimilar to the endogenous proteases responsible for the previously observed degradation. Differences between HY and CWD PrPSc conformation and aggregation (which are poorly understood) as well as differences in macromolecule structures contained in their respective brain homogenates may explain the observed difference in this study. In addition, we have previously observed differences in HY and CWD PrP adsorption (19), although the exact nature and extent of these differences is unclear. In this study, recovery of bound CWD PrP following SDS desorption was higher than HY PrP for all soils except SiO2-HA (Table S1), further suggesting differences in PrP adsorption between HY and CWD. Such differences could lead to increased HY PrP resistance to digestion relative to CWD PrP.
Implications for Environmental and Host Degradation of Soil-Bound PrPSc
Although we have shown bound PrPSc to be vulnerable to enzymatic digestion, bound and unbound PrPSc are more resistant to digestion than a great majority of other proteins present in brain homogenate. Bound and unbound PrPc was completely digested within 30 min at pH 7.4 and 22°C (Figure S2), whereas PrPSc remained after 30 min when unbound or bound to any of the soils tested (Figures S3 and S4). Total protein staining of samples also shows that Prionzyme treatment digests all detectable protein content whether bound or unbound to soil (Figure S1). Digestion with proteinase K yielded similar results (data not shown). Thus, it can be concluded that, compared to other proteins present in brain homogenate, PrPSc retains its relative resistance to enzymatic degradation upon binding to soil.
Digestion of unbound PrPSc under conditions relevant to environmental transmission has been previously demonstrated. Russo et al. used a birnessite manganese oxide treatment at pH 4 to eliminate HY immunoreactivity (22). Huang et al. found composting of scrapie-infected tissue reduced or completely eliminated PrPSc as detected by western blot (23). Infectivity was not determined in either study. Microbiological consortia taken from the rumen and colon of cattle could degrade PrPSc to undetectable levels within 20 hours under anaerobic conditions at 37°C, although significant infectivity remained (24, 25). A similar study using alimentary tract fluids from sheep found only trace amounts of PrPSc following incubation (26).
In this study, we present the first data showing digestion of PrPSc when bound to soil. Given that we have observed only minor differences in PrPSc resistance to digestion between bound and unbound states, it could be hypothesized that PrPSc binding to soil does not confer increased resistance to degradation in the environment or in an animal host. Alternately, we have shown PrPSc to be more resistant to digestion compared to the vast majority of other proteins when bound to soil. Thus, it remains important to determine what role, if any, binding to soil plays (with respect to increased resistance to degradation) in sustaining prion longevity in the environment and facilitating transmission via ingestion or inhalation.
Decontamination of Soil-Bound Prions with an Enzyme Solution
For all soils tested, there was a complete loss of PrP immunoreactivity by 7 days (Figure 2); therefore, the results of this study suggest that an enzyme treatment under environmentally-relevant conditions (neutral pH and 22°C) could be used as a decontamination method for soil exposed to CWD or other prion diseases. However, it is critical to note that prion infectivity does not absolutely correlate with PrPSc abundance as detected by western blot. A complete loss of PrP immunoreactivity does not always denote a complete loss of prion infectivity (14, 27, 28). Scherbel et al. observed little or no reduction in 263K infectivity after complete loss of immunoreactivity following digestion by rumen consortia (25). Johnson et al. found reduced but significant infectivity after elimination of HY immunoreactivity following ultraviolet-ozone treatment (28). Nevertheless, PrPSc amounts can be correlated to infectivity in many instances (29). As noted earlier, Prionzyme treatment of infectious 310v strain mouse brain homogenate led to a 7-log or greater decrease in infectivity in animal bioassay (17).
If significant reductions in infectivity upon enzyme treatment were observed in bioassay, it could be feasible to devise a field-scale treatment method consisting of a topical spray of a dilute subtilisin-containing solution to suspected prion ‘hot-spots’ in free-ranging populations and captive settings. Given that we did not agitate the soil samples during treatment, a field-scale method would potentially require minimal effort and ground disturbance. Our results also suggest that the treatment could be applied without raising the solution pH (except possibly in acidic soils) and would not need to be applied under high temperatures, although temperatures below 22°C may slow or decrease treatment effectiveness. Negative effects on soil biology due to an enzyme treatment would need to be evaluated before field-scale application.
Although no sensitive methods for detection of prions in the environment currently exist, it can be assumed that locations of concentrated prion infectivity could be formed at areas of communal activity where shedding of prions in saliva, urine, or feces occurs, such as wallows or mineral licks (5, 30). Animal mortality sites, where highly-infectious central nervous system matter would enter the environment, could also be hot spots (31). Complete elimination of CWD infectivity would not necessarily be required for a topical environmental enzyme treatment to be successful. Given the extremely small infectious dose of CWD, it may not be possible to eliminate infectivity completely in any one area, but substantial (orders of magnitude) reductions in PrPSc in environmental hot spots could lead to a significant reduction in indirect transmission of prion disease.
Supplementary Material
Table S1. PrP adsorption to soil and soil minerals. Supplemental Methods. Direct detection of bound PrP. Figure S1. Subtilisin enzyme digestion of bound and unbound Protein. Figure S2. Subtilisin enzyme digestion of bound and unbound PrPc. Figure S3. Digestion of bound and unbound CWD-elk PrP under varied pH and temperature. Figure S4. Digestion of bound and unbound HY TME PrP under varied pH and temperature. Figure S5. Digestion of bound and unbound HY TME PrP at pH 7.4 and 22°C. Figure S6. Effect of Prionzyme dose on digestion of bound CWD-elk PrP.
Acknowledgments
We thank Ronald Shikiya and Qi Yuan for technical assistance, Ken Clinkenbeard for the CWD-elk brain, and Scott Hygnstrom for helpful discussions. Humic acid-coated silica gel particles were kindly provided by Robert Bulman. This research was supported in part by the USDA/APHIS/Wildlife Services/National Wildlife Research Center, the UNL Research Council, the UNL Othmer and Milton Mohr Fellowships, and the National Center for Research Resources (P20 RR0115635-6 and C06 RR17417-01).
Literature Cited
- 1.Prusiner SB. An introduction to prion biology and diseases. In: Prusiner SB, editor. Prion biology and diseases. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2004. [Google Scholar]
- 2.Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi) J Wildlife Dis. 2007;43:309–314. doi: 10.7589/0090-3558-43.2.309. [DOI] [PubMed] [Google Scholar]
- 3.Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci US A. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DM. Inactivation of transmissible degenerative encephalopathy agents: A review. Vet J. 2000;159:10–17. doi: 10.1053/tvjl.1999.0406. [DOI] [PubMed] [Google Scholar]
- 5.Saunders SE, Bartelt-Hunt SL, Bartz JC. Prions in the environment: Occurrence, fate, and mitigation. Prion. 2008;2:162–169. doi: 10.4161/pri.2.4.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB. Asymptomatic deer excrete infectious prions in faeces. Nature. 2009;461:529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders SE, Bartz JC, Bartelt-Hunt SL. Prion protein adsorption to soil in a competitive matrix is slow and reduced. Environ Sci Technol. 2009;43:7728–7733. doi: 10.1021/es901385t. [DOI] [PubMed] [Google Scholar]
- 8.Leita L, Fornasier F, Nobili MD, Bertoli A, Genovesi S, Sequi P. Interactions of prion proteins with soil. Soil Biol Biochem. 2006;38:1638–1644. [Google Scholar]
- 9.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral transmissibility of prion disease is enhanced by binding of soil particles. PLoS Pathog. 2007;3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS ONE. 2007;3:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arthur WJ, Alldredge AW. Soil ingestion by mule deer in northcentral Colorado. Range Ecol Manage. 1979;32:67–71. [Google Scholar]
- 12.Beyer WN, Connor EE, Gerould S. Estimates of soil ingestion by wildlife. J Wildlife Manage. 1994;58:375–382. [Google Scholar]
- 13.Brown P, Rau EH, Lemieux P, Johnson BK, Bacote AE, Gajdusek DC. Infectivity studies of both ash and air emissions from simulated incineration of scrapie-contaminated tissues. Environ Sci Technol. 2004;38:6155–6160. doi: 10.1021/es040301z. [DOI] [PubMed] [Google Scholar]
- 14.McLeod AH, Murdoch H, Dickinson J, Dennis MJ, Hall GA, Buswell CM, Carr J, Taylor DM, Sutton JM, Raven ND. Proteolytic inactivation of the bovine spongiform encephalopathy agent. Biochem Biophys Res Com. 2004;317:1165–1170. doi: 10.1016/j.bbrc.2004.03.168. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka M, Miwa T, Horii H, Takata M, Yokoyama T, Nishizawa K, Watanabe M, Shinagawa M, Murayama Y. Characterization of a proteolytic enzyme derived from a Bacillus strain that effectively degrades prion protein. J Appl Microbiol. 2007;102:509–515. doi: 10.1111/j.1365-2672.2006.03080.x. [DOI] [PubMed] [Google Scholar]
- 16.Pilon JL, Nash PB, Arver T, Hoglund D, VerCauteren KC. Feasibility of infectious prion digestion using mild conditions and commercial subtilisin. J Virol Met. 2009;161:168–172. doi: 10.1016/j.jviromet.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Genencor/Danisco Prionzyme M: Product information. 2008
- 18.Saunders SE, Bartz JC, Telling GC, Bartelt-Hunt SL. Environmentally-relevant forms of the prion protein. Environ Sci Technol. 2008;42:6573–6579. doi: 10.1021/es800590k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders SE, Bartz JC, Bartelt-Hunt SL. Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environ Sci Technol. 2009;43:5242–5248. doi: 10.1021/es900502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartz JC, Kramer ML, Sheehan MH, Hutter JAL, Ayers JI, Bessen RA, Kincaid AE. Prion interference is due to a reduction in strain-specific PrPSc levels. J Virol. 2007;81:689–697. doi: 10.1128/JVI.01751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo G, Guczi J, Reiller P, Geckeis H, Bulman RA. Investigation of complexation of thorium by humic acid using chemically immobilized humic acid on silica gel. Radiochimica Acta. 2006;94:553–557. [Google Scholar]
- 22.Russo F, Johnson CJ, Johnson CJ, McKenzie D, Aiken JM, Pedersen JA. Pathogenic prion protein is degraded by a manganese oxide mineral found in soils. J Gen Virol. 2009;90:275–280. doi: 10.1099/vir.0.003251-0. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Spencer JL, Soutryine A, Guan J, Rendulich J, Balachandran A. Evidence of degradation of abnormal prion protein in tissues from sheep with scrapie during composting. Can J Vet Res. 2007;71:34–40. [PMC free article] [PubMed] [Google Scholar]
- 24.Scherbel C, Richner R, Groschup MH, Mueller-Hellwig S, Scherer S, Dietrich R, Maertlbauer E, Gareis M. Degradation of scrapie associated prion protein (PrPSc) by the gastrointestinal microbiota of cattle. Vet Res. 2006;37:695–703. doi: 10.1051/vetres:2006024. [DOI] [PubMed] [Google Scholar]
- 25.Scherbel C, Pichner R, Groschup MH, Mueller-Hellwig S, Scherer S, Dietrich R, Maertlbauer E, Gareis M. Infectivity of scrapie prion protein PrPSc following in vitro digestion with bovine gastrointestinal microbiota. Zoo Public Health. 2007;54:185–190. doi: 10.1111/j.1863-2378.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 26.Jeffrey M, González L, Espenes A, Press C, Martin S, Chaplin MJ, Davis L, Landsverk T, MacAldowie C, Eaton S, McGovern G. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol. 2006;209:4–14. doi: 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 27.Barron RM, Campbell SL, King D, Bellon A, Chapman KE, Williamson RA, Manson JC. High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPScin vivo. J Biol Chem. 2007;282:35878–35886. doi: 10.1074/jbc.M704329200. [DOI] [PubMed] [Google Scholar]
- 28.Johnson CJ, Gilbert P, McKenzie D, Pedersen JA, Aiken JM. Ultraviolet-ozone treatment reduces levels of disease-associated prion protein and prion infectivity. BMC Res Notes. 2009;2:121. doi: 10.1186/1756-0500-2-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bessen RA, Marsh RF. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VerCauteren KC, Burke PW, Phillips GE, Fischer JW, Seward NW, Wunder BA, Lavelle MJ. Elk use of wallows and potential chronic wasting disease transmission. J Wildlife Dis. 2007;43:784–788. doi: 10.7589/0090-3558-43.4.784. [DOI] [PubMed] [Google Scholar]
- 31.Jennelle CS, Samuel MD, Nolden CA, Berkley EA. Deer carcass decomposition and potential scavenger exposure to chronic wasting disease. J Wildlife Manage. 2009;73:655–662. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PrP adsorption to soil and soil minerals. Supplemental Methods. Direct detection of bound PrP. Figure S1. Subtilisin enzyme digestion of bound and unbound Protein. Figure S2. Subtilisin enzyme digestion of bound and unbound PrPc. Figure S3. Digestion of bound and unbound CWD-elk PrP under varied pH and temperature. Figure S4. Digestion of bound and unbound HY TME PrP under varied pH and temperature. Figure S5. Digestion of bound and unbound HY TME PrP at pH 7.4 and 22°C. Figure S6. Effect of Prionzyme dose on digestion of bound CWD-elk PrP.