Abstract
We examined in tree shrews the effect of age on the development of, and recovery from, myopia induced with a negative lens. Starting at 11, 16, 24, 35 or 48 days after natural eye-opening (days of visual experience [VE]), juvenile tree shrews (n = 5 per group) wore a monocular −5 D lens for 11 days. A long-term lens-wear group (n=6) began treatment at 16 days of VE and wore the lens for 30 days. A young adult group (n = 5) began to wear a −5 D lens between 93 and 107 days of VE (mean ± SD, 100 ± 6 days of VE) and wore the lens for 29 to 54 days (mean ± SD, 41.8 ± 9.8 days). The recovery phase in all groups was started by discontinuing −5 D lens wear. Contralateral control eyes in the three youngest groups were compared with a group of age-matched normal eyes and showed a small (<1 D), transient myopic shift. The amount of myopia that developed during lens wear was measured as the difference between the treated and control eye refractions. After 11 days of lens wear, the induced myopia was similar for the four younger groups (near full compensation: 11 day, −5.1 ± 0.4 D; 16 day, −4.7 ± 0.3 D, 24 day, −4.9 ± 0.4 D; 35 day, −4.0 ± 0.02) and slightly less in the oldest juvenile group (48 day, −3.3 ± 0.5 D). The young adult animals developed −4.8 ± 0.3 D of myopia after a longer lens-wear period. The rate of compensation (D/day) was high in the 4 youngest groups and decreased in the 48-day and young adult groups. The refractions of the long-term lens-wear juvenile group remained stable after compensating for the −5 D lens. During recovery, all animals in the youngest group recovered fully (< 1 D residual myopia) within seven days. Examples of both rapid (< 10 days) and slow recovery (> 12 days) occurred in all age groups except the youngest. Every animal showed more rapid recovery (higher recovery slope) in the first 4 days than afterward. One animal showed extremely slow recovery. Based on the time-course of myopia development observed in the youngest age groups, the start of the susceptible period for negative lens wear is around 11 to 15 days after eye opening; the rate of compensation remains high until approximately 35 days of VE and then gradually declines. Compensation is stable with continued lens wear. The emmetropization mechanism, both for negative lens compensation and recovery, remains active into young adulthood. The time-course of recovery is more variable than that of compensation and seems to vary with age, with the amount of myopia (weakly) and with the individual animal.
Keywords: myopia, lens compensation, recovery, negative lenses, age
Introduction
Concave (negative-power) lenses have been used in many species of animals to study the normal emmetropization mechanism by producing lens-induced compensation (Schaeffel et al., 1988; Hung et al., 1995; Graham & Judge, 1999; Siegwart, Jr. & Norton, 1999; Shen & Sivak, 2007; Howlett & McFadden, 2009). In this paradigm, a negative lens is held in front of one eye using a goggle frame or other device. The lens shifts the focal plane away from the cornea, making the eye hyperopic in comparison with the fellow control eye. Within a period of a few days (tree shrews, chicks, fish) or weeks (monkeys) the vitreous chamber of the treated eye begins to elongate, moving the retina to the shifted focal plane, eliminating the induced hyperopia and restoring emmetropia, measured while the negative lens is in place (Schaeffel et al., 1988; Irving et al., 1991; Hung et al., 1995; Siegwart, Jr. & Norton, 1999; Shen & Sivak, 2007).
Form deprivation, whether achieved by placing a diffuser over an eye or by surgical eyelid closure, removes visual feedback provided by mid- and high spatial frequencies and also produces axial elongation and myopia (Sherman et al., 1977; Wiesel & Raviola, 1977; Wallman et al., 1978; Norton & Rada, 1995; Smith, III et al., 1999a).
After monocular negative lens treatment or form deprivation, if the negative lens or form deprivation is removed, the treated eye is myopic and is elongated relative to the contralateral control or normal eyes. In juvenile animals, recovery then occurs (Wallman & Adams, 1987; Norton, 1990; Qiao-Grider et al., 2004; McFadden et al., 2004; Troilo & Nickla, 2005). During recovery, the axial elongation rate of the treated eye slows below normal in the still-growing eye (Siegwart, Jr. & Norton, 2005; Moring et al., 2007). The optics of the treated (and control) eyes continue to mature, moving the focal plane away from the cornea and back to the retina so that, in most cases, the eye's refractive state and axial length eventually match those of the fellow control eye or normal eyes of the same age (Wallman & Adams, 1987; Siegwart, Jr. & Norton, 1998; Qiao-Grider et al., 2004; Troilo & Nickla, 2005).
The susceptible period for form deprivation-induced myopia has been examined by measuring the amount of myopia produced by a fixed period of deprivation applied at different ages (Wallman & Adams, 1987; Siegwart, Jr. & Norton, 1998; Smith, III et al., 1999a; Qiao-Grider et al., 2004). Generally animals are more susceptible when they are younger. However, it has been found that form deprivation is ineffective in tree shrews until approximately two weeks after eye opening (McBrien & Norton, 1992; Siegwart, Jr. & Norton, 1998). Susceptibility remains high in tree shrews until approximately 40 – 45 days after eye opening and then declines. In tree shrews, chicks, and monkeys form-deprivation myopia has also been induced in adolescent or adult animals with longer periods of form deprivation suggesting that the emmetropization mechanism remains active in adulthood (Siegwart, Jr. & Norton, 1998; Papastergiou et al., 1998; Smith, III et al., 1999a).
Although negative lens-wear and form deprivation are similar in that both produce an elongated, myopic eye, evidence has suggested that the two may act via somewhat different mechanisms in the retina (Schaeffel et al., 1994; Wildsoet & Wallman, 1995; Kee et al., 2001; Diether et al., 2007). Thus, the susceptible periods may differ in onset, duration or peak sensitivity. Susceptibility to negative lens-induced myopia has not been studied systematically in tree shrews.
Some authors have questioned whether animal models are good models for human myopia (Zadnik & Mutti, 1995). To the extent that negative lens-induced myopia may be thought of as a model for human myopia it is important to know the time-course of susceptibility to negative lens-produced hyperopia. Thus, one purpose of this study was to examine the amount of negative lens compensation produced by a period of lens wear in tree shrews at different ages, including young adult animals, and the rate at which this compensation develops. In addition, this study examined the rate at which recovery occurred as a function of age, which also had not been previously studied in detail.
Methods
The thirty-one treated juvenile tree shrews (Tupaia glis belangeri) and five young adults used in this study were raised by their mothers in our breeding colony on a 14 hr light/10 hr dark cycle as were 14 normal animals measured for comparison with the control eyes of the treated animals. During the study, the animals were housed individually in the animal colony in well lit cages (156 – 548 lux). This variation was due to the relationship of the cages to the overhead fluorescent light fixtures. All procedures in this study were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Tree shrew pups open their eyes about three weeks after birth. The first day both eyes are open is defined as day 1 of visual experience (VE). Tree shrews reach sexual maturity by approximately 4 months of age (about 100 days of VE).
Experimental Groups
There were seven experimental groups, summarized in Fig. 1, designed to overlap with those used to examine the susceptible period for form deprivation myopia in tree shrews (Siegwart, Jr. & Norton, 1998). Animals in each group wore a goggle frame with a monocular −5 D lens covering one eye and an open frame around the other, control eye. Across all groups, the treated eye was randomly assigned and was balanced between left and right eyes. Both sexes were represented, with 21 female and 15 male animals. All groups except one (35 days of VE, all female) had no more than 4 of one sex. To maximize genetic variability, the number of animals from the same parents was minimized within a group such that the animals in most groups were from different parents. There were 2 groups (11-22 and 48-60) with two pups from the same parents; in the 16-27 group there were two sets of siblings (the five pups were from three sets of parents).
Fig. 1.
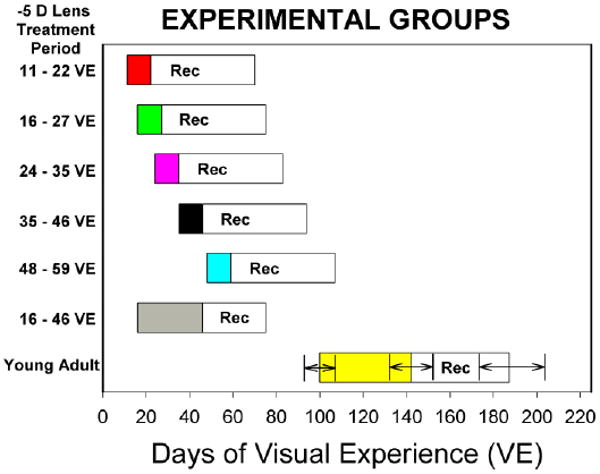
Experimental groups. Filled bars indicate the ages at which each group wore a monocular −5 D lens. “Rec” indicates the recovery period of unrestricted visual experience after lens wear was discontinued. Because the young adult group varied in the onset, length of lens wear, and length of the recovery period, the means and standard deviations are shown in the figure.
The first day of lens wear was designated as treatment day 1. In five groups (5 animals per group) that explored the effect of age on susceptibility and recovery in juvenile animals, lens wear began at 11, 16, 24, 35, or 48 days of VE. The lens was worn by the juvenile animals for 11 days, except for a sixth, long-term lens-wear group (n=6) that wore the −5 D lens for 30 days (from 16 to 46 days of VE) to examine whether eyes would maintain a stable compensation for a −5 D lens and whether being elongated for a longer period of time would affect recovery when compared with the group that wore the lens for 11 days and began recovery at the same age. A seventh group (n=5), comprised of young adult animals began lens wear between 93 and 107 days of VE (mean ± SD, 100 ± 6 days of VE). Animals in the young adult group wore the lens for 29 to 54 days (mean ± SD, 41.8 ± 9.8 days).
At the end of the treatment period, lens wear was discontinued. For the juvenile animals, the recovery period began at 22, 27, 35, 46 and 59 days of VE. The juvenile eyes were allowed to recover for 48 days (29 days for the long-term lens-wear group). For the young adults, recovery began at 136 to 148 days of VE (mean ± SD, 141.4 ± 4.4) and regular measurements continued for 30 to 64 days (mean ± SD, 44.6 ± 12.4 days), until the course of recovery was established. Animals that had not recovered by the end of the pre-planned recovery period were periodically measured until they recovered or until recovery could be projected based on the slope of the refractive differences.
Prior to monocular myopia induction, the refractive states of the two normal eyes are well correlated (Norton & McBrien, 1992; Smith, III et al., 1999b; Norton et al., 2006b); thus, it is reasonable to expect a fully recovered eye to return to very near the same refractive state of the contralateral eye. The depth of focus of the tree shrew eye was estimated by Norton & McBrien as ±1.2 D based on the formula of Green et al (1980). A more recent estimate was made by Dr. Ramkumar Ramamirtham (personal communication, 2009) based on wavefront measurements in tree shrew (Ramamirtham et al., 2003). He used the aberration coefficients (4 mm pupil size) measured on normal and control eyes of seven tree shrews. A Fourier transform of the aberrations provided a series of modulation transfer functions for a variety of defocus values. Using a spatial frequency (3.3 c/deg) near the acuity limit of tree shrews (Petry et al., 1984) the depth of defocus values were estimated based on 80% of the MTF curve. These typically indicated a depth of focus of ±0.37 D. Based on these estimates and on observed normal interocular differences (Norton et al., 2006a), the refractions of “fully recovered” eyes should be no more than 1 D different from the fellow control eyes.
To examine possible effects of lens wear on the untreated control eyes, values were compared to measurements on 14 normal animals, involved in other studies, whose noncycloplegic refraction was measured at various times during the period 11 to 45 days of VE. The measurement schedule varied across animals, so that on some days values from as few as three animals were available, while on other days measures from as many as 14 were available. On average, each day's measurement included 8.6 animals.
Procedures and Measurements
Pedestal Surgery
Each tree shrew was fitted with a dental acrylic pedestal to which the lens-containing goggle frame was later clipped. The pedestal also facilitated holding the animal in place for autorefractor measures. The pedestal was fitted under anesthesia as previously described (Siegwart & Norton, 1994). This occurred one day before lens wear began in the youngest two groups and three days before the start of lens wear in the older groups. A three day delay between pedestal installation and lens wear has been used in previous studies in this lab (Siegwart, Jr. & Norton, 2001; Siegwart, Jr. & Norton, 2002; Siegwart, Jr. & Norton, 2005). The shorter delay in the youngest animals was used so the animals were as old as possible at the time of pedestal installation because all animals were weaned after recovering from anesthesia and 21 days of VE is the normal weaning age. Nothing in the refractive development (rate of decrease from hyperopia) in the control eyes of the early-weaned animals seemed to differentiate them from pups that were weaned later.
Ocular Component Dimensions
While the animals were anesthetized for the pedestal installation, the ocular component dimensions (anterior segment, lens thickness, vitreous chamber depth, and axial length) were measured with A-scan ultrasonography (Norton & McBrien, 1992). This ensured that the right and left eyes were in the normal range and did not significantly differ from each other, as one eye would serve as a control.
Because of concerns that the anesthesia required for A-scan measurements might affect the recovery process, no A-scan measures were made in the juvenile animals at the start of recovery. However, based on numerous studies in tree shrews in which myopia was induced with a negative lens (Siegwart, Jr. & Norton, 1999; Siegwart, Jr. & Norton, 2005; Norton et al., 2006b; Moring et al., 2007) it is safe to assume that the induced myopia was due to enlargement of the vitreous chamber with no significant corneal differences and minimal lens thinning in the treated eye. This was confirmed in the young adult animals which, because of reduced concern about anesthesia affecting recovery, were measured at the start of recovery. The A-scan measures were repeated in all groups at the end of the recovery period to confirm that any remaining refractive differences between the treated and control eyes were correlated with differences in the axial length.
Refractive Measures
Awake noncycloplegic refractive measures were made daily for the first 11 lens-treatment days and then less frequently in the long-term (16 to 46 day) and young-adult treatment groups. Daily measures were also made for the first 10 days of recovery for the juvenile groups and then less frequently until the end of the recovery period. The young adult animals were measured less frequently during recovery, typically every fifth day. On treatment days 1, 6 and 12 (and intermittently thereafter for the long-term lens-wear group) all animals had additional refractive measures made with the −5 D lens in place. In the youngest juvenile group (11 days of VE start) and the young adults, with-the-lens measures were made whenever refraction was measured. This allowed determination of how similar to the control eye was the refractive state of the treated eye while wearing the −5 D lens. All measures except the final one (at the end of recovery) were made without cycloplegia because atropine treatment has been found to reduce the amount of induced myopia (McKanna & Casagrande, 1981). The cycloplegic measures were made at least one hour after instillation of two drops of 1% ophthalmic atropine sulfate (Bausch & Lomb, Tampa, FL).
Refractive measures were made in a darkened room with a Nidek ARK 700A autorefractor in the morning. The lighted target in the autorefractor was turned off to avoid presenting images to the eyes (which might trigger accommodation) while they were being measured. The eyes were aligned using the video monitor, such that the autorefractor was centered on the cornea and aimed on the pupillary axis. The animals rarely blinked or made large movements of their eyes. If they did, the measures were retaken. Previous studies that compared autorefractor measures with streak retinoscopy have found that both measure similar amounts of induced myopia (Norton et al., 2000; Norton et al., 2003). An additional benefit of the autorefractor measures is that they can be made on awake animals, while streak retinoscopy has required anesthesia, precluding daily measurements. The autorefractor measures were converted to the spherical equivalent refraction at the corneal plane.
Lens related Procedures
Lenses were cleaned twice daily, in the morning and afternoon with the animals in their nest box in a dimly lit room. If a lens became severely scratched (typically once in 11 days, more in 30 days) the lens was replaced while the animal was kept in the dark.
Data Analysis
Measures of refractive state and axial component dimensions were entered into Excel spreadsheets. For most measures, the difference between the treated eye and its fellow control eye was calculated (treated eye – control eye). One-way, factorial or repeated-measures analysis of variance (ANOVA, Statistica, StatSoft, Inc. Tulsa, OK) were used to examine differences across groups or to examine the development of myopia or of recovery. Paired t-tests also were used to compare measures made at more than one point on individual animals. Alpha was 0.05 for both tests.
Results
Control Eyes
As shown in Fig. 2, normal tree shrew eyes at 11 days of VE are still completing their descent from hyperopia toward emmetropia. Thus, the initial refractions in the youngest lens-wear group (11 days of VE) were significantly hyperopic compared to the other groups (one-way ANOVA, p < 0.05). Comparison of the control eye refractions in the three youngest groups to the normal eyes (Fig. 2) indicated that there was a small, transient, but statistically significant myopic shift in the control eyes during the time that the treated eyes wore the −5 D lens (factorial ANOVA, p < 0.05). As shown in Fig. 2B, this appeared after 1 day of lens wear in the group that began lens wear at 11 days of VE but was transient, disappearing after 3 days of lens wear. In the juvenile group treated from 16 to 27 days of VE, the control eye refractions were similar to the normals at the start of lens-wear by the other eye but, measured throughout the 11 day lens-wear period, were, on average, −0.7 ± 0.1 D lower than the normal eye refractions. The control eyes of the long-term lens-wear group (16 to 46 days of VE) showed a similar initial control-eye decrease during the first 10 days of lens wear but the control eye refractions returned to normal after about two weeks and therefore could serve as a reference for judging the amount of induced myopia and the extent to which the treated eyes recovered. The effect was small in all three groups, so the control eyes remained slightly hyperopic. In the older juvenile groups, the control eye refractions also were significantly lower than in the normal eyes. However, this difference was present in both eyes at the start of treatment, before lens wear began (larger symbols in Fig. 2B), suggesting that the lower refractions in these groups were group-related rather than treatment related.
Fig. 2.
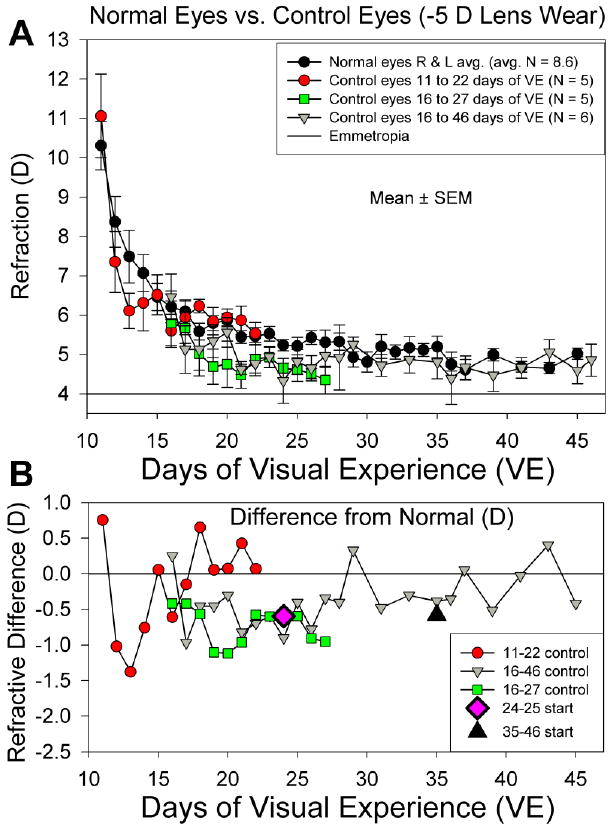
A. Normal- and control-eye refractive values (mean ± SEM) as a function of age. The control eyes from groups that began lens wear at 11 or 16 days of VE developed a small, transient myopic shift. Estimated emmetropia is an autorefractor value of +4 D due to the “small eye artifact” (Glickstein & Millodot, 1970; Ramamirtham et al., 2003; Norton et al., 2003). B. Average difference between the control-eye and normal-eye refractions for the three groups whose control eyes showed a decrease in refraction from normal. Starting differences (relative to normal eyes) for the 24- and 35-day control eyes are also shown.
Lens Compensation as a Function of Age
As shown in Fig. 3, the treated eyes in all groups responded to the −5 D lens so that the treated eyes of all groups were significantly myopic at the end of lens wear (repeated measures ANOVA, p < 0.05). Across all groups, the amount of myopia was unrelated to the starting refraction (regression, p > 0.05). Table 1 lists the amount by which the treated eyes were myopic compared to the control eyes at the end of lens treatment. This did not differ significantly amongst the four youngest groups, which either fully or nearly compensated for the lens. The amount of myopia was significantly less in the 48 – 59 day group than in the younger four groups (one-way ANOVA, p <0.05). The young adult animals, with a longer lens-wear period, compensated fully for the lens.
Fig. 3.
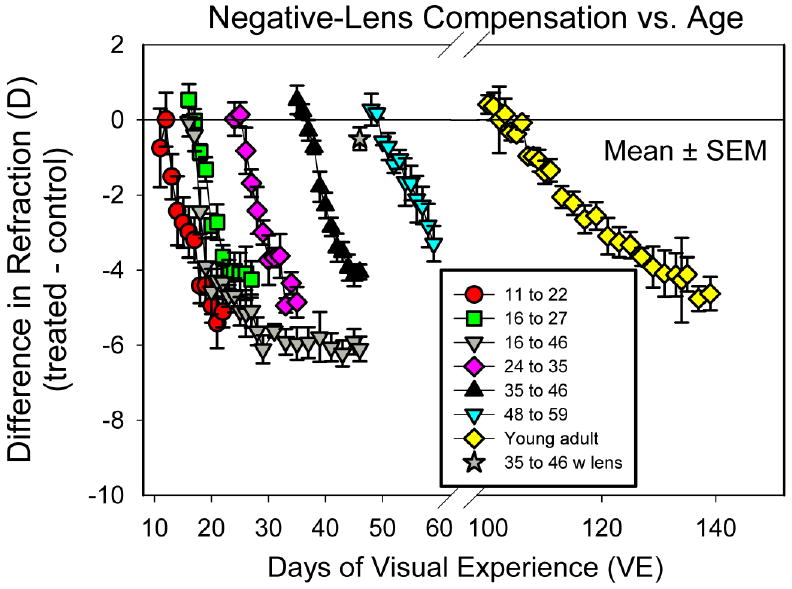
Development of myopia during −5 D lens wear. Group mean ± SEM values of the refractive difference between the −5 D lens-wearing eyes and control eyes in the five juvenile groups with 11 days of treatment, the juvenile group with 30 days of lens treatment and the young adult group. The star at 46 days of VE indicates the with-the-lens refractive difference of the treated eyes of the long-term lens-wear group at the end of treatment.
Table 1.
Measures of Relative Myopia and Compensation during −5 D Lens Wear
| Treatment Groups (Days of VE) | 11 to 22 | 16 to 27 | 16 to 46 After 30 days | 24 to 35 | 35 to 46 | 48 to 59 | Young adult |
|---|---|---|---|---|---|---|---|
| Myopia: (Treated eye – control eye) | −5.1 ± 0.4* | -4.7 ± 0.3* includes 16 to 46 (after 11 days) | −6.1 ± 0.3* | −4.9 ± 0.4* | −4.1 ± 0.2* | −3.3 ± 0.5*† | −4.8 ± 0.3* after 41.8 ± 9.8 days |
| Compensation: (Treated eye with lens– control eye) | 0.1 ± 0.5 | -0.6 ± 0.9 16 to 27 only | -0.5 ± 0.3 | −0.3 ± 0.4 | 1.7 ± 0.6‡ | 2.3 ± 0.4‡ | 0.3 ± 0.3 after 41.8 ± 9.8 days |
| Average slope first 4 days (D/day) | -0.90 ± 0.18 | -1.02 ± 0.14 includes 16 to 46 (after 11 days) | ---- | -0.67 ± 0.15 | -0.56 ± 0.15 | -0.38 ± 0.11# | -0.15 ± 0.07# |
| Average slope 11 days (D/day) | -0.48 ± 0.1 | -0.44 ± 0.05 includes 16 to 46 (after 11 days) | ---- | -0.48 ± 0.06 | -0.47 ± 0.03 | -0.30 ± 0.06 | -0.17 ± 0.04⫯ |
| Days of lens wear until treated eyes significantly myopic | 2 | 2 includes 16 to 46 (after 11 days) | ---- | 3 | 3 | 4 | 4 |
| Days until treated eyes >1 D myopic | Mean, 3 longest, 6 | mean 2.6 longest, 4 includes 16 to 46 (after 11 days) | ---- | Mean, 2.8 longest, 4 | Mean, 3.6 longest, 5 | Mean, 5.6 longest, 9 | Mean, 8.6 longest, 11 |
treated eyes were significantly myopic relative to fellow control eyes
the induced myopia was significantly less than in the other groups
the treated eyes, with-the-lens, were significantly different from the control eyes
the 4-day slope was significantly lower in the two oldest groups compared to the other groups
the young adult slope was significantly lower than the other groups
When the treated eye refractions were measured with the −5 D lens in place (Table 1), they were not significantly different from the control eyes except in the 35 – 46 and 48 – 59 day groups in which the with-the-lens refractions were significantly hyperopic in comparison with the control eyes (paired t-tests, p < 0.05).
Although the four youngest groups (starting at 11, 16, 24 and 35 days of VE) did not differ significantly in the amount of induced myopia, there were slight differences between them. Fig. 4 shows the mean refraction of the treated and control eyes in each group during the lens-wear period, along with the measured refractions of the individual animals. In the two youngest age groups (three groups with treatment starting at 11 and 16 days of VE, fig. 4A, 4B, 4C) the rate at which the treated eye refraction decreased appeared somewhat steeper in the first four days than subsequently. In the older groups, the slope was relatively linear throughout the lens-wearing period. In order to compare across groups with the same statistic, linear regressions were calculated to give the slope of the myopic changes (increase in myopia relative to the control eyes; D/day) for the first four days and for the entire 11-day period for all groups. Overall, the slopes were not related to the initial refraction in the treated eye, measured on treatment day 1, before treatment began (regression, p > 0.05). Table 1 also displays two additional measures of the rate of myopia development, the number of days of lens wear before the treated eyes in each group became significantly myopic compared with the control eyes and the length of time until the treated eyes were at least −1 D myopic.
Fig. 4.
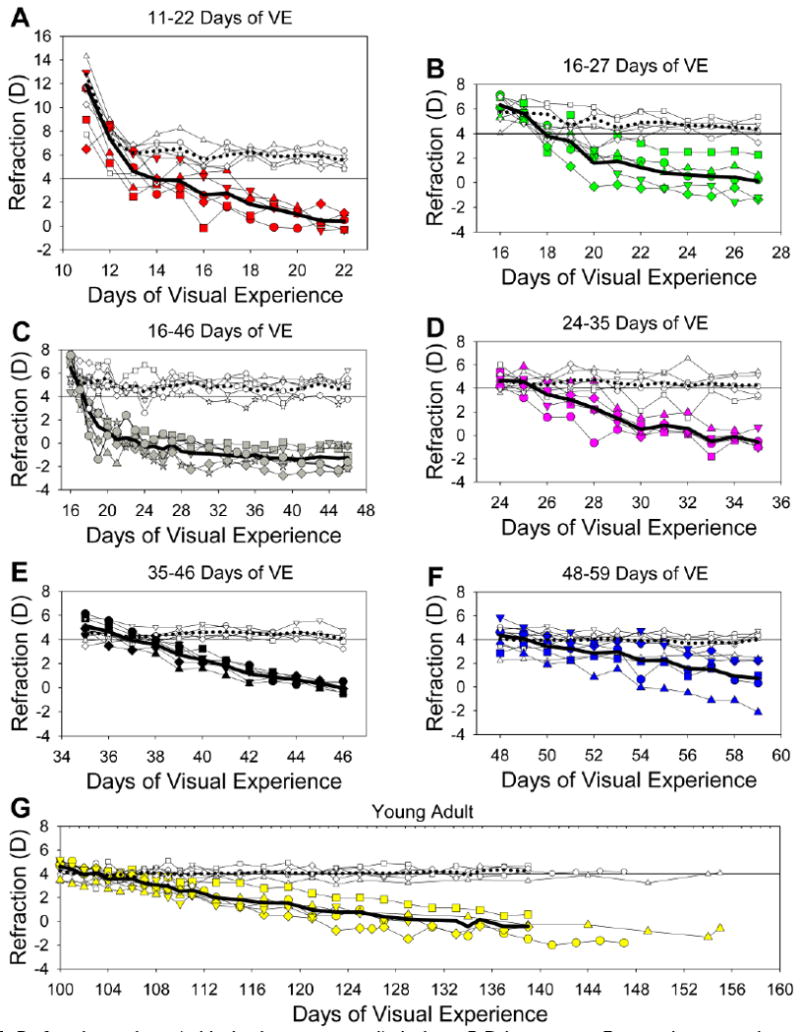
Refractive values (with the lens removed) during −5 D lens wear. For each group, the treated eye and control eye refractions are shown, along with the mean refractions. Filled symbols, treated eyes; open symbols, control eyes; thick solid line, treated eye average; thick dotted line, control eye average. The abscissa for the young adult animals has been expanded to reflect the longer period of lens wear. Starting day for all animals in the Young Adult group were set to 100 days of VE.
Based on these several measures, the animals that began minus lens compensation at 16 days of VE appeared to have a slightly stronger response to minus lens wear than did the other groups. For instance, the slope of myopia development in the first 4 days was the steepest (−1.02 D/day), the average delay before the eyes were −1 D myopic (2.6 days) was the shortest, and the delay until the treated eyes of the group were significantly myopic (2 days) all indicated a rapid, strong response to the minus lens when lens treatment began at 16 days of VE. The group that began treatment at 11 days of VE was very similar, but had a slightly lower overall (11-day) slope. With increasing starting age, the slopes gradually declined and the response latency increased, such that the slopes of the 48-day and young adult groups were significantly lower than those of the four younger groups (one-way ANOVA, p < 0.05). The slope of the myopia development in the young adult group over the first 11 days of their lens-wearing period did not differ significantly from the slope over the entire treatment period (paired t-test, p > 0.05.)
Long-term lens-wear group
As shown in Figs. 3 and 4C, the treated eyes in the long-term lens-wear group compensated for the −5 D lens and maintained full compensation until lens wear was discontinued after 30 days. The final myopia, measured after 30 days of treatment, was −6.1 ± 0.3 D relative to the control eyes. This is more than the −4.9 D amount expected from wearing the lens, after correcting for lens effectivity. However, the refractions of the treated eyes, while wearing the lens (4.0 ± 0.5 D), were not significantly different from the control eyes (4.5 ± 0.4 D) also measured while the treated eyes wore the lens (paired t-test, p > 0.05). The difference (0.5 ± 0.4 D) is indicated by the star in Fig. 3. The with-the-lens refraction also was not significantly myopic compared with age-matched normal eyes (unpaired t-test, p > 0.05). The amount of myopia did not change significantly from the 14th through the 30th day of lens wear (one-way ANOVAs examining both treated eye refraction and refractive differences, p > 0.05). Thus, continued negative lens wear produced a stable refractive change that neither progressed nor regressed over time once with-the-lens emmetropization had been achieved.
Response delay after onset of minus lens wear
As also shown in Table 1, in the groups that began minus lens wear at 11 or 16 days of VE, the treated eyes were significantly myopic, compared to their fellow control eyes, after 2 days of lens wear. In the next three age groups, the treated eyes were significantly myopic after 3 days and this delay was 7 days in the young adult group. The average length of treatment before individual animals achieved a 1 D myopic difference was similar in the 11, 16, 24 and 35-day groups, and increased in the 48 day and young adult animals. The longest individual delay in the 11 day group was 6 days, suggesting that susceptibility to minus lens wear did not begin in that animal until around 15 days of VE.
Amount of induced hyperopia
As shown in Fig. 4, the refractive state at the start of negative lens treatment was not the same across all groups; the 11 – 22 day group (Fig. 4A) was more hyperopic than the other groups. The −5 D lens increased the hyperopia by approximately 5 D in all groups. Thus, the initial with-the-lens hyperopia was greater in this group than in the other groups. However, as shown in Table 1, the initial and overall rates of myopia development did not differ across the four youngest groups. Thus, variations in the absolute amount of hyperopia across these ages did not translate into variations in the rate of myopia development.
Recovery
When the −5 D lens was removed, all of the animals experienced myopia in the treated eye. All groups except one initially had comparable amounts of induced myopia (Table 2). The exception was the group that began recovery at 59 days of VE, which had developed less myopia than the other groups (one-way ANOVA, p < 0.05, Fishers LSD). The differences between the recovering and control eyes (non-cycloplegic refractions) are plotted in Figs. 5 and 6. In all animals, this difference decreased during the first 4 days of recovery (first 7-10 days in the young adults). The slope of this initial recovery across age groups is shown in Table 2. The initial recovery slope was highest (fastest recovery) in the youngest group (recovery started at 22 days of VE) but did not differ between the other age groups (one-way ANOVA, p < 0.05). The initial recovery slope was unrelated to the amount of myopia initially experienced by the recovering eyes (Fig. 7A).
Table 2.
Measures of Recovery from −5 D Lens Compensation
| Recovery Started (Days of VE) | 22 | 27 | 35 | 46 | 46, after 30 days – 5 D wear | 59 | Young adult 141.4 ± 4.4 |
|---|---|---|---|---|---|---|---|
| Starting Myopia: Treated eye – control eye (D) (mean ± SEM) | −5.1 ± 0.4 | -4.3 ± 0.5 | −4.9 ± 0.4 | −4.1 ± 0.2 | −6.1 ± 0.3 | −3.3 ± 0.5* | −4.8 ± 0.3 |
| Initial slope (D/day) (mean ± SEM) | 1.2 ± 0.1† (N=5) | 0.7 ± 0.2 (N=5) | 0.4 ± 0.2 (N=5) | 0.4 ± 0.1 (N=5) | 0.5 ± 0.1 (N=6) | 0.5 ± 0.2 (N=5) | 0.3 ± 0.1 (N=5) |
| Later slope (D/day) (N=number of animals) (mean ± SEM) | All had recovered | 0.1 ± 0.04 (N=4) | 0.1 ± 0.03 (N=5) | 0.1 ± 0.01 (N=5) | 0.3 ± 0.1 (N=5) | 0.2 ± 0.1 (N=3) | 0.1 ± 0.02 (N=3) |
| Mean ± SEM Days to recovery (Tr. – cont diff. < 1 D) | 4.2 ± 0.6‡ (N=5) | 13.8 ± 5.1 (N=5) | 46.6 ± 14.8 (N=5) | 25.0 ± 5.7 (N=5) | 16.4 ± 4.6 (N=5) Excludes 201 days | 15.6 ± 6.9 (N=5) | 37± 13.9 (N=5) |
this group had less initial myopia than the other groups
this group had significantly higher initial recovery slopes than the other groups
this group recovered more quickly than the other groups
Fig. 5.
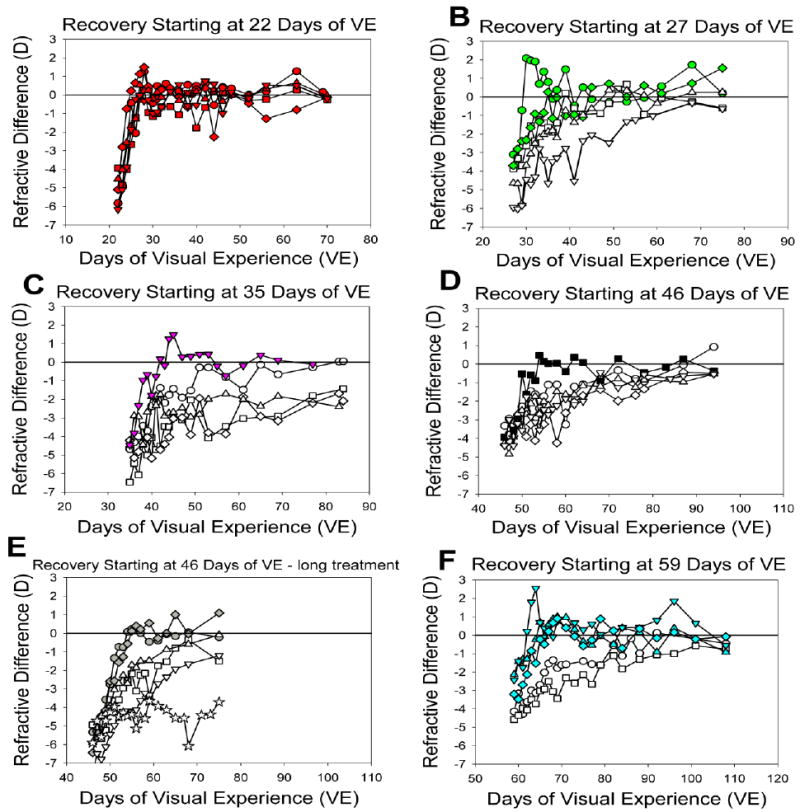
Recovery of the treated eyes of individual juvenile animals starting at different ages is shown as the non-cycloplegic difference in refraction of the recovering eye and its fellow control eye. Filled symbols indicate animals that recovered quickly (10 days or less). The scale on the abscissa is the same in all graphs to facilitate comparisons of the rate of recovery.
Fig. 6.
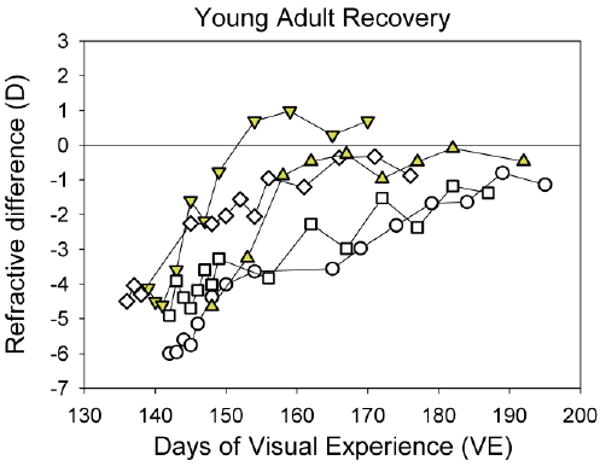
Recovery of young adult animals, showing the refractive difference between the recovering and fellow control eyes for each of the five animals. Filled symbols indicate animals that recovered quickly (less than 10 days).
Fig. 7.
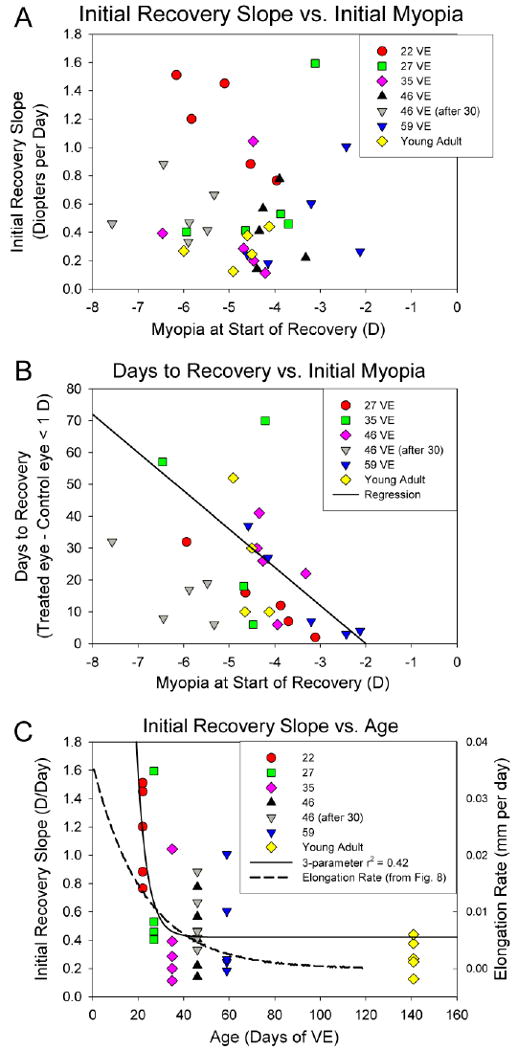
A. Initial recovery slope for all age groups as a function of the initial amount of myopia. This slope was unrelated to the amount of myopia that had developed. B. Excluding the youngest age group, the length of time to recover from the induced myopia (to < 1D) was weakly, but significantly related to the amount of myopia (r2 = 0.14, p < 0.05). The regression (solid line) includes the animal that was very slow to recover (projected recovery at 201 days), but that data point is not plotted on the graph. C. Initial recovery slope as a function of age. The solid line is a 3-parameter exponential function fitted to the initial recovery slopes. The dashed line is the daily rate of axial elongation calculated from the exponential function fitted to the normal axial lengths in Fig. 8.
Overall, the initial recovery slope was not significantly related to the initial (4-day) compensation slope (regression, p > 0.05). However, if the long-term lens-wear group was excluded, the regression was statistically significant (p < 0.049). Across groups, only the group that began recovery at 35 days of VE showed a significant correlation between the initial compensation slope and the initial recovery slope (higher compensation slope related to higher recovery slope).
All of the animals in the youngest group recovered from the induced myopia very rapidly so that the recovering eye was less than 1 D myopic, compared to its fellow control eye, after seven days. All of the older groups contained some animals that recovered relatively rapidly (3 to 10 days) and others that exhibited slower recovery (12 – 201 days, indicated by the open symbols in Figs. 5 & 6). For animals with more than two data points after the initial 4-day recovery, a later recovery slope was calculated using the non-cycloplegic differences in refraction up to, and including, the first data point where the difference was less than 1 D. These later recovery slopes are also shown in Table 2, along with the number of animals for which this slope could be calculated. For every animal with both an initial and a later recovery slope, the later slope was lower (less rapid recovery) than the initial slope and did not differ significantly across age groups (one-way ANOVA, p > 0.05). However, individual animals that had faster initial recovery slopes also had higher later recovery slopes (dependent t-test, p < 0.05). The later recovery slopes also were not related to the initial amount of myopia.
The recovering animals could be divided into a “rapid recovery” group (in which no later slope could be calculated) and a “slower recovery” group. The rapid recovery animals did not differ from the slower recovery animals in the amount of initial myopia (t-test, p > 0.05) but the initial recovery slopes of these animals were higher than those of the slower recovery animals.
During the pre-planned recovery period, most of the treated eyes recovered until their refractions were similar to those of their fellow control eyes (Fig. 5 and Fig. 6) and, once a treated eye had recovered, the refractive measures of both the recovered and control eyes remained stable near emmetropia, based on non-cycloplegic measures. The mean number of days to recovery (< 1 D residual myopia compared to the fellow control eye myopia) across all groups is listed in Table 2. This was weakly, but significantly, related to the initial amount of induced myopia (r2 = 0.14, P < 0.05); the animals with less initial myopia recovered more quickly (Fig 7B).
When cycloplegic autorefractor measures were made at the end of the recovery period, the recovering eye was less than 1 D myopic, relative to the fellow control eye, in 22 of the 25 animals that wore the −5 D lens for 11 days. Three animals in the group that began recovery at 35 days of VE recovered slowly and incompletely and had a residual myopia (>1 D) when pre-planned measurements were discontinued at 84 days of VE. Periodic noncycloplegic measures were made subsequently and all recovered eventually. For the long-term lens-wear group that began recovery at 46 days of VE, the pre-planned recovery period was shorter (29 days) than for the other groups. Four of the six animals in that group had recovered at that point. Periodic measures after that on the fifth animal showed the treated eye would have recovered to within 1 D of the control eye after 32 days of recovery if the recovery period had been the same length (48 days) as in the groups with 11 days of lens wear. A sixth animal showed extremely slow recovery and was projected to have recovered by 201 days and is not included in the Days to Recovery averages in Table 2. This animal's initial recovery slope (0.33 D/Day) was lower than all of the other animals that began recovery at this age. Overall, however, the initial recovery slopes of the long-term lens-wear group did not differ significantly from the other group that began recovery at the same age after only 11 days of lens wear, nor did the number of days to recover, including the very slow recovery animal (independent t-tests, p > 0.05).
The animals in the young adult group (Fig. 5) were all over 5 months of age at the start of recovery, past the point of sexual maturity of tree shrews. Despite this, the recovery slopes of this group were not significantly different from those of the juvenile groups.
Cycloplegic vs. non-cycloplegic refractive measures
As expected, in all groups, the cycloplegic refractive measures made at the end of the recovery period were more hyperopic than the noncycloplegic refractions for both the control eyes and the treated eyes due to removal of a small amount of accommodation by the cycloplegia (Norton et al., 2000). The overall change was 0.84 ± 0.04 D. Both eyes were affected similarly, so that the recovering eye vs. control eye differences did not differ significantly between the non-cycloplegic and cycloplegic measures (dependent t-test, p > 0.05), confirming that the non-cycloplegic refractive measures provided a valid estimate of the shifted refractive state of the recovering eyes relative to the fellow control eyes.
The high initial recovery slopes of the youngest group of animals, along with the decreased slope with increasing age, suggested that this measure, like many developmental factors, might decrease as a function of the log of age at the start of the recovery period. Shown in Fig. 7C is a 3-parameter exponential function (slope = y0+a*exp[-b*age] fitted to the initial recovery slope data using SigmaPlot 9.01 (Systat Software, Inc), where y0 = 0.4215, a = 101.0604 and b = 0.2233) provides a better fit (R2 of 0.42). Also shown is the normal rate of axial elongation (mm/day) calculated from the 3-parameter exponential function shown in Fig. 8. Although the normal growth rate declines with age, the function fitted to the initial recovery slope decreases much more steeply, suggesting that something in addition to normal growth may be involved in the more rapid decrease in the initial recovery slopes.
Fig. 8.
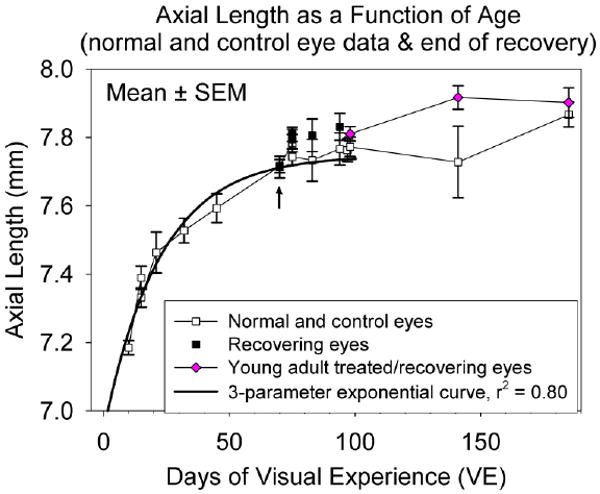
Axial length of eyes (group mean ± SEM) as a function of age and treatment. Open squares indicate normal eyes (right and left eyes averaged) at the time of pedestal installation and control eyes at the end of recovery. Filled squares indicate recovering eyes at the end of the recovery period. The arrow indicates the post-recovery treated-eye axial lengths of the youngest group, which obscure the control eye values. Diamonds indicate the treated eyes of the young adult group before treatment, after -5 D lens wear and at the end of the recovery period. Adult data are plotted at the mean time of pedestal installation, end of treatment and end of recovery. The solid line is a 3-parameter exponential function fitted to the data from the normal (pre-treatment) measurements.
Axial Length Measures
As expected, and as shown in Fig. 8, the tree shrew eyes grew larger as a function of age. The solid line is a 3-parameter exponential function (axial length = y0+a*(1-exp(-b*age)]) fitted using SigmaPlot to the pre-treatment (normal) axial length values of the individual animals in each group (right and left eyes averaged). The parameters of the curve were: y0=6.9426; a=0.8048; b=0.0444. From this exponential function, the daily rate of axial elongation (in mm/day) was calculated and plotted in Fig. 7C.
Axial component dimensions at the end of lens treatment were made only in the young adult group. In keeping with previous studies (Siegwart, Jr. & Norton, 1999; Norton et al., 2006b), the vitreous chamber in the young adult animals was 0.13 ± 0.02 mm deeper in the treated eyes than in the control eyes at the end of lens wear. At the end of the recovery period, this difference had decreased to −0.10 ± 0.11 mm. During recovery, the mean value of the recovering-eye axial length decreased slightly from the end-of-treatment value, but the change was not statistically significant (paired t-test, p > 0.05).The control eye axial length (7.87 ± 0.04 mm) at this age (approximately 200 days) was less than has been measured in mature adults (8.07 ± 0.03 mm) (Norton & McBrien, 1992).
Measurements made at the end of the juvenile-group pre-planned recovery periods were consonant with the refractive measures. For the animals that had recovered refractively, the difference in refraction between the recovering and control eyes was negligible (-0.03 ± 0.13 D) as was the difference in axial length (0.02 ± 0.01 mm). For the six animals that had not yet recovered at the time of this A-scan measure, the refractive difference was -1.99 ± 0.45 D and the axial length difference was 0.08 ± 0.02 mm, confirming that the axial lengths were not yet the same in those six animals. In the older groups where some animals did not recover fully, the recovering eyes of the group were longer than the control eyes (black squares above the open squares in Fig. 8). As expected from previous studies, the dimensions of the anterior segment and lens, were not significantly different between the recovering and control eyes across groups (one-way ANOVA, p > 0.05).
Discussion
Negative Lens Compensation
Compensation for a −5 D lens occurs reliably in tree shrews at all ages examined, including young adults. The amount of induced myopia was limited by the power of the −5 D lens, unlike the open-loop situation of form deprivation where larger amounts of myopia can occur after similar treatment periods (Siegwart, Jr. & Norton, 1998). Thus, the rate of lens compensation (slope in D/day) may provide a better index of susceptibility in this study. In the three youngest groups (treatment for 11-22, 16-27, and 24-35 days of VE) the 4-day slope was high and similar across groups. With increasing age beyond this, both the 4-day and 11-day slopes gradually decreased so that full compensation did not develop in the limited time allowed, but refractive changes toward compensation still occurred in all age groups. The young adult animals achieved full compensation, but required a longer lens-wear period.
The profile of susceptibility to lens-induced myopia is similar to that of form deprivation myopia in tree shrew, with an onset by 11 to 15 days of VE, a broad peak to at least 35 days of VE, a gradual decline in older juvenile animals, and continued susceptibility in early adulthood (Siegwart, Jr. & Norton, 1998). It appears that susceptibility to lens-induced myopia may begin to decline slightly earlier (around 35 days of VE) than does susceptibility to form deprivation-induced myopia (around 45 days). Similar results, a high early susceptibility and decreased but continuing susceptibility in young adults, have been found with form deprivation-induced myopia in chicks, tree shrews, and monkeys (Wallman & Adams, 1987; Siegwart, Jr. & Norton, 1998; Smith, III et al., 1999a).
In tree shrews, susceptibility to form deprivation has been estimated to begin approximately two weeks after the eyes open (around 15 days of VE) (McBrien & Norton, 1992; Siegwart, Jr. & Norton, 1998). The daily refractive measures in the youngest age group (starting at 11 days of VE) allowed a closer examination of the onset to negative-lens wear than was available in the form-deprivation studies. In this group, as in the others, there was a delay between the application of a negative lens and a detectable refractive response of the eyes. In the three youngest groups, the mean number of days before all eyes in the group had achieved 1 D of myopia ranged between 2.6 and 3 days, suggesting that time is needed for retinal “go” signals to not only reach the sclera but also produce sufficient elongation to be detected refractively. The similar delay in the youngest group suggests that the susceptible period to negative lens-induced hyperopia may already have begun when the lens was applied at 11 days of VE. In one animal of this group, however, it was 6 days before the treated eye achieved 1 D of myopia relative to the control eye. Assuming a 2-day delay to develop 1 D of myopia, the susceptible period may have begun around 15 days of VE in this animal.
The defocus imposed by the −5 D lens is a powerful stimulus for elongation over a range of ages and accurately guides eyes toward reestablishing emmetropia with the lens in place. As was shown in Table 1, the response of eyes to the −5 D lens is, indeed, a compensation response. When the lens was first put in place on treatment day 1, the eyes were suddenly hyperopic. After 11 days of continuously wearing the lens, the refractive state of all the eyes had shifted toward with-the-lens emmetropia. As shown by the long-term lens-wear group, once with-the-lens emmetropia is established, it is maintained by the emmetropization mechanism, neither progressing nor regressing over time.
Animal and Human Susceptibility
The present study, taken together with other animal studies, should lay to rest the concern, raised over a decade ago (Zadnik & Mutti, 1995), that animal models may not be applicable to human myopia because the emmetropization mechanism in animals operates during the infantile stage of ocular growth whereas human myopia mostly develops in the juvenile age range (generally after 6 years of age). As will be reviewed in the following sections, recent studies suggest there is a broad susceptible period in both humans and animals during which the emmetropization mechanism is functional and environmentally-produced refractive changes can occur.
Early visual guidance
Comparing, first, the similarity between humans and animals shortly after birth, it is well established by the present study and by other studies with negative and positive lenses in animal models that the visual environment guides the elongation rate of the eye in the early (infantile) period (Irving et al., 1991; Siegwart, Jr. & Norton, 1999; Smith, III & Hung, 1999; Metlapally & McBrien, 2008). Recent data now show that human infants also use the visual environment to control the axial growth of their eyes in the first months after birth as their refractions move from hyperopia to near emmetropia (Mutti et al., 2005). Infants also develop elongated eyes rapidly in cases of form deprivation caused by infantile cataract or serious ptosis (O'Leary & Millodot, 1979; Hoyt et al., 1981; Rabin et al., 1981), suggesting that the presence of images on the retina is necessary to prevent excessive elongation. Thus like the animal models, the human emmetropization mechanism is active soon after birth and appears to depend on the visual environment to achieve and maintain emmetropia.
Later visual guidance
At older ages, comparable to the stage in development that most juvenile-onset human myopia develops, the present study (using negative lenses), along with those that have found form-deprivation myopia in adult animals (Wallman & Adams, 1987; Siegwart, Jr. & Norton, 1998; Smith, III et al., 1999a), clearly show that the emmetropization mechanism remains active through the juvenile period and into young adulthood and uses the visual environment to guide the axial elongation of the eye.
Just as the visual environment affects the refractive state in animals, recent studies have also found that the visual environment affects myopia progression in human juveniles. The Correction of Myopia Evaluation Trial (COMET) compared myopia progression over a three-year period in children with an environmental manipulation (progressive addition lenses [PALs]) that may have reduced hyperopic defocus, with myopia progression in children wearing standard single-vision lenses. The children were 6 through 11 years at the start of the trial. PALs significantly slowed myopia progression (Gwiazda et al., 2003) suggesting that susceptibility to the visual environment extends throughout childhood. In addition, form deprivation from corneal opacification that developed in children after 5 years of age produced myopia and axial elongation (Meyer et al., 1999). Thus, studies in both animal models and in humans provide evidence that the visual environment plays a role in refractive development from shortly after birth up through puberty.
Recovery from lens-induced myopia
The recovery data may be summarized by four generalizations: 1) At most ages, overall recovery was slower than was compensation for a negative lens and showed greater variability; 2) Recovery from lens-induced myopia in tree shrews is complete and rapid in the youngest animals; 3) All animals recovered more rapidly during the first few days than afterwards; 4) Both the initial and later recovery slopes were unrelated to the amount of induced myopia, but declined as a function of age.
As shown in Fig. 3 and Table 1, the response to negative lens wear was rapid (0.3 to 0.5 D/day) and consistent, with low standard deviations for the 11-day slopes, even at older ages when the slope of the compensation decreased. In contrast (Table 2 and Figs. 5 & 6), except at the youngest age, the average later recovery slopes were lower than during compensation (generally 0.1 D/day) and the time course of recovery was variable. The exception was recovery in the youngest group (recovery starting at 22 days of VE). The animals in this group exhibited rapid and complete compensation with an average slope (1.2 D/day) that exceeded (but not significantly) that observed even during the first four days of negative lens compensation in this group (0.9 D/day) and was significantly higher than the rate of lens compensation in the groups that wore the lens starting at 16 and 24 days of VE (p < 0.05).
Excluding the youngest group, for which a later recovery slope could not be calculated because the eyes had completed their recovery, all recovering eyes showed faster recovery (higher slope) during the first days than they did subsequently, and the slope of the initial and the later recovery was unrelated to the amount of myopia. It was, however, related to the animals' age. Axial component measures made at the time the goggle-holding pedestal was installed provided age-related axial measures that confirmed previous results (Siegwart, Jr. & Norton, 1999) that the axial elongation rate is rapid in young animals and declines with age, at a rate approximated by the log of the age. The more rapid recovery in the youngest animals, when the axial elongation rate is high, may reflect a greater ability at this age to slow the rapid growth, which, when the growth rate is high, can have a substantial refractive impact.
A similar pattern of complete recovery in young tree shrews was found after form deprivation-induced myopia (Siegwart, Jr. & Norton, 1998) although the refractive state was not measured until 48 days after the onset of recovery. At this point, all of the youngest animals, in which recovery began at 19 or 27 days of VE, had recovered fully. McBrien et al. (2000) found that recovery from approximately 6 D of myopia, begun at 20 days of VE produced an average recovery of 16% in the treated eye was found after 24 hours. After 9 days, there was very little refractive difference between the two eyes. Thus, rapid and complete recovery from both lens-induced and form-deprivation-induced myopia occurs in very young tree shrews.
The pattern of variable recovery in older juvenile tree shrews was also found with form-deprivation myopia by Siegwart and Norton (1998). Some animals that began recovery after 33 days of VE recovered fully during the 48-day recovery period but others did not. Because refractions were not measured frequently during recovery, it was not appreciated that some of the animals that were fully recovered at the end of the 48 day period may have recovered more slowly and others more rapidly, as did animals in the present study.
Reduced rates of recovery from form deprivation in older animals have been found in other studies. In chicks, Wallman and Adams (1987) plotted recovery from form-deprivation myopia in individual birds measured after 2 weeks of unrestricted vision. Recovery in the oldest group was less complete than in the younger groups. Wildsoet and Schmid (2000) also found rapid and accurate recovery from form deprivation myopia in many chicks. However some birds showed incomplete recovery. Troilo and Nickla (2005) found that 6 of 12 marmosets with form deprivation-induced myopia demonstrated recovery, but the completeness of the recovery varied. Qiao-Grider et al. (2004) found recovery from form deprivation myopia in 18 monkeys, with 12 exhibiting complete recovery. They concluded that “the potential for recovery appears to depend on when unrestricted vision is restored, the severity of the deprivation-induced axial elongation, and possibly the method used to produce FDM.” Recovery from negative lens-induced myopia occurred in “many” of the macaque monkeys studied by Smith and Hung (1999), but recovery was complete in some and not in others. It thus appears that in other species, as in tree shrews, there is variation, particularly in older animals, in the rate at which animals recover and the completeness of recovery from negative lens-induced myopia as well as from form-deprivation myopia.
The juvenile long-term lens-wear group (treatment 16 to 46 days of VE) allowed comparison of recovery in animals that had remained fully compensated for over two weeks with ones that were just completing negative-lens compensation (the 35 to 46-day group). One possibility was that eyes that had remained elongated for a longer time period (though that elongation was not measured in this study) might not recover with the same time-course as ones that had a shorter “fully-compensated experience.” However (Fig. 5), the initial and the overall recovery slopes were neither significantly faster, nor slower, in the long-term lens-wear group than the 11-day treatment group although two animals in the long-term group did not recovery completely during the pre-planned recovery period.
Signals Controlling Eye Growth
The results of the present study have extended our understanding of the signals that control emmetropization in tree shrews, but still leave unanswered questions, particularly about the reasons for variability in the rate of recovery.
Lens compensation
Whenever in the juvenile or young adult period it was applied, the negative lens shifted the treated eyes in the hyperopic direction. After a delay of 2 – 3 days in the juvenile groups, the treated eyes began to reduce the induced hyperopia by increasing the axial length (inferred from previous studies, measured in the young adult group). By the end of the lens-wearing period, the younger animals had compensated for the lens, so that the treated-eye with-the-lens refractions were very close to those of the control eyes. That this lens-produced hyperopia produced refractive compensation at all ages implies that the emmetropization mechanism continuously monitors the eye's refractive state throughout the juvenile and young adult period. If an eye is hyperopic relative to the control eye, retinal signals must detect this and generate a “go” signal that is communicated to the sclera and results in refractive compensation to reduce the hyperopia.
That the long-term lens-wear group attained full compensation and remained at that level implies that the retinal “go” signal dissipates when the eye re-establishes with-the-lens emmetropia, so that the treated-eye refractions did not continue to change, but remained at an age-appropriate refractive state even though both the treated and control eyes continue to grow. Although the retinal “go” signal is in some sense satisfied when the treated eyes achieves this situation, there nonetheless must be a visual signal that maintains the eyes at refractive stability even though they must be (from other studies) elongated. Presumably, if a lens-wearing eye grew slightly less than needed to maintain emmetropia and became slightly hyperopic, the emmetropization mechanism would trigger increased elongation so as to re-establish emmetropia. That few individual animals appeared to overshoot with-the-lens emmetropia during lens wear may suggest that, although the emmetropization mechanism uses retinal signals to produce lens compensation, it is not easy for eyes to pass the emmetropization point; perhaps another factor, such as a scleral “eye-size factor” tends to resist the elongation process.
Recovery
When the negative lens-wear was discontinued, the eyes in all groups experienced myopia. It is also safe to assume that they were elongated, relative to control and normal eyes. Both of these factors – myopia and an “eye-size” or “shape” signal related to the eyes being elongated (van Alphen, 1961; Troilo & Wallman, 1991; Schaeffel & Howland, 1991; Nickla et al., 2005) – appear to play a role in the recovery process that continues until the refractive state and axial lengths are very similar in the recovered and control eyes.
That the refractive state provides a signal that initiates recovery seems clear from several facts: First, the eyes did not begin to recover until the negative lens was removed and the eyes experienced myopia, as shown by the stable with-the-lens emmetropia of the long-term lens-wear group. This group extends to lens-induced myopia the results of form deprivation studies (McBrien et al., 1999; Wildsoet & Schmid, 2000) that correcting myopia induced with form deprivation by use of negative lenses prevented the eyes from slowing their elongation rate and “recovering” from the induced myopia. Visual signals, the absence of myopia in these instances, over-rode the fact that the eyes were elongated. A second indication that a refractive-state signal is important is that all eyes moved toward recovery in the first days after the lens was removed; the changed (and myopic) refractive state precipitated the recovery. In contrast, tree shrews placed in darkness upon lens removal, preventing the myopic refractive state from being detected, did not recover (Norton et al., 2006a).
It is less clear whether the refractive state signal, by the time it reaches the sclera, is proportional to the amount of the myopia. The absence of a correlation between the amount of initial myopia and the initial rate of recovery may suggest that the refractive-error signal is one of direction more than it is one of magnitude. If the retinal “stop” signal is converted to a “slow down the elongation rate” signal at the sclera, the scleral slowing may simply continue as long as the eye is significantly myopic and dissipate as recovery is completed.
The evidence for an eye-size or shape factor in the present study is less direct because there were no measures of axial recovery comparable to the daily refractive recovery measures. A-scan measures did confirm that the young adult eyes became longer during compensation, that eyes that had not fully recovered were still elongated at the time of measurement, and that recovered eyes were the same length as their fellow control eyes. However, the end point of recovery was the same for the eye-size factor (equal lengths) as it was for the refractive signal (equal refractions) in this study.
Suggestions of a scleral component in the recovery come from several factors. The initial recovery slope was related to the age of the animals, with more rapid refractive recovery in younger, rapidly growing eyes and lower slope in older eyes whose normal elongation rate had slowed. This may have a structural basis related to the growth of the eyes. In a rapidly growing eye, it may be easier to slow the elongation rate than in an older eye where the growth rate is much slower. In addition, slowing the elongation rate by a fixed amount will have a larger optical effect in a smaller eye than it would in a larger eye. Frequent measurements of the axial component dimensions during recovery might help to understand the structural basis of the recovery rate.
Whatever the eventual time-course of recovery, all animals showed refractive recovery during the first four days. However, it appeared that the refractions started to recover more quickly (within 2 days) than they began to change during compensation (2 – 4 days). It would be surprising if, during recovery, the length of time it takes for retinal “stop” signals to be generated by the myopic refractive state and communicated to the sclera is longer than the length of time needed for hyperopic “go” signals to reach the sclera during compensation. It is thus possible that the sclera of the elongated recovering eyes is more readily responsive to the retinally-generated stop signals than is the normal sclera to the go signals.
Although both a myopic refractive state and an elongated eye may be important factors in recovery, even the two signals together do not reliably produce a rapid rate of recovery, as seen in some of the animals in the present study. It is not known what the differences are between animals that used eye-size and/or myopia to recover rapidly and fully and others that recovered slowly and/or incompletely. Because some animals in all age groups except the youngest showed slow recovery, age alone did not seem to be the controlling factor. It certainly appears that hyperopia produces a consistent increase in the elongation rate, producing myopia, while myopia combined with the eye-size factor, is less consistently able to slow the elongation rate. In eutherian mammals, which have an all-fibrous sclera, it may be more difficult to achieve a slowed elongation rate than it is for the vast majority of the animal kingdom that has an inner layer of scleral cartilage (Seko et al., 2008). Slowing axial elongation by slowing the growth of the cartilaginous sclera may be more consistently effective than slowing it by controlling the biomechanical property, visco-elasticity, of the fibrous sclera.
The genetics of individual animals may play a role in the ability of the retina to send the appropriate refractive signal and/or the sclera to respond appropriately to that signal. Of the 21 breeding pairs that provided pups for this study, 10 provided two or three pups. The initial recovery slope of pups from those parents did not appear to be strongly related to the parents (data not shown). However, there were too few animals from the same parents spread across groups to demonstrate whether the same parents consistently produced offspring with either rapid or slow recovery slopes.
Acknowledgments
Supported by National Eye Institute Grants RO1 EY05922 and P30 EY03039 (CORE). We thank Joel Robertson for technical assistance and Drs. Michael R. Frost and Hong Gao for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Diether S, Schaeffel F, Lambrou GN, Fritsch C, Trendelenburg AU. Effects of intravitreally and intraperitoneally injected atropine on two types of experimental myopia in chicken. Exp Eye Res. 2007;84:266–274. doi: 10.1016/j.exer.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vision Res. 1999;39:189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- Green DG, Powers MK, Banks MS. Depth of focus, eye size and visual acuity. Vision Res. 1980;20:827–835. doi: 10.1016/0042-6989(80)90063-2. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hoyt CS, Stone RD, Frommer C, Billson FA. Monocular axial myopia associated with neonatal eyelid closure in human infants. Am J Ophthalmol. 1981;91:197–200. doi: 10.1016/0002-9394(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optom Vis Sci. 1991;68:364–368. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42:575–583. [PubMed] [Google Scholar]
- McBrien NA, Gentle A, Cottriall C. Optical correction of induced axial myopia in the tree shrew: implications for emmetropization. Optom Vis Sci. 1999;76:419–427. doi: 10.1097/00006324-199906000-00022. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000;41:3713–3719. [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- McFadden S, Hawkins N, Howlett MHC. Recovery from experimentally induced myopia in the guinea pig. Exp Eye Res, 79, 92 2004 [Google Scholar]
- McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Doc Ophthalmol. 1981;28:187–192. [Google Scholar]
- Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8:1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- Meyer C, Mueller MF, Duncker GI, Meyer HJ. Experimental animal myopia models are applicable to human juvenile-onset myopia. Surv Ophthalmol, 44 Suppl. 1999;1:S93–102. doi: 10.1016/s0039-6257(99)00091-0. [DOI] [PubMed] [Google Scholar]
- Moring AG, Baker JR, Norton TT. Modulation of Glycosaminoglycan Levels in Tree Shrew Sclera during Lens-Induced Myopia Development and Recovery. Invest Ophthalmol Vis Sci. 2007;48:2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial Growth and Changes in Lenticular and Corneal Power during Emmetropization in Infants. Invest Ophthalmol Vis Sci. 2005;46:3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Sharda V, Troilo D. Temporal integration characteristics of the axial and choroidal responses to myopic defocus induced by prior form deprivation versus positive spectacle lens wear in chickens. Optom Vis Sci. 2005;82:318–327. doi: 10.1097/01.opx.0000159368.31481.de. [DOI] [PubMed] [Google Scholar]
- Norton TT. Experimental myopia in tree shrews. In: Bock G, Widdows K, editors. Myopia and the Control of Eye Growth. Chichester: Wiley; 1990. pp. 178–194. [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006a;47:4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006b;47:4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, German A, Robertson J, Wu W. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia [ARVO Abstract] Invest Ophthalmol Vis Sci, 41, S563 2000 [Google Scholar]
- Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80:623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DJ, Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979;35:1478–1479. doi: 10.1007/BF01962795. [DOI] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive shift in one-year-old chickens. Vision Res. 1998;38:1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- Petry HM, Fox R, Casagrande VA. Spatial contrast sensitivity of the tree shrew. Vision Res. 1984;24:1037–1042. doi: 10.1016/0042-6989(84)90080-4. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith, EL III. Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3361–3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- Rabin J, VanSluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981;20:561–564. [PubMed] [Google Scholar]
- Ramamirtham R, Norton TT, Siegwart JT, Roorda A. Wave aberrations of tree shrew eyes. Invest Ophthalmol Vis Sci, 44, E-abstract 1986 2003 [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- Seko Y, Azuma N, Takahashi Y, Makino H, Morito T, Muneta T, Matsumoto K, Saito H, Sekiya I, Umezawa A. Human sclera maintains common characteristics with cartilage throughout evolution. PLoS One. 2008;3:e3709. doi: 10.1371/journal.pone.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–4837. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Lab Animal Sci. 1994;44:292–294. [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Steady state mRNA levels in tree shrew sclera with form-deprivation myopia and during recovery. Invest Ophthalmol Vis Sci. 2001;42:1153–1159. [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43:2067–2075. [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999a;76:428–432. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic Physiol Opt. 1999b;19:90–102. [PubMed] [Google Scholar]
- Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005;46:1873–1881. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- van Alphen GWHM. On emmetropia and ametropia. Opt Acta (Lond) 1961;142(Suppl):1–92. [PubMed] [Google Scholar]
- Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Mutti DO. How applicable are animal myopia models to human juvenile onset myopia? Vision Res. 1995;35:1283–1288. doi: 10.1016/0042-6989(94)00234-d. [DOI] [PubMed] [Google Scholar]


