Abstract
The tolerogenic nature of the liver allows daily exposure to gut-derived foreign Ags without causing inflammation, but it may facilitate persistent infection in the liver. NK cells play a central role in innate immunity, as well as in shaping the adaptive immune response. We hypothesized that the naive mouse liver maintains intrahepatic NK cells in a functionally hyporesponsive state. Compared with splenic NK cells, liver NK cells displayed a dampened IFN-γ response to IL-12/IL-18 stimulation. Importantly, the liver contains a significant population of functionally hyporesponsive NK cells that express high levels of the inhibitory receptor NKG2A and lack expression of MHC class I-binding Ly49 receptors. Adoptively transferred splenic NK cells that migrate to the liver displayed phenotypic and functional changes, suggesting that the liver environment modifies NK cell receptor expression and functional responsiveness. Notably, IL-10 is present at high levels within the liver, and in vivo blockade of IL-10R resulted in a decreased percentage of intrahepatic NKG2A+Ly49− NK cells. These data suggest that the liver environment regulates NK cell receptor expression and that IL-10 contributes to the regulation of liver NK cells, in part, by maintaining a greater percentage of the hyporesponsive NKG2A+Ly49− NK cells in the liver.
Natural killer cells play a central role in the innate immune response to intracellular pathogens and in shaping the adaptive response through their ability to directly lyse virally infected cells, secrete pro- and anti-inflammatory cytokines, and interact with and influence the maturation of dendritic cells (DCs) (1–3). NK cells develop from bone marrow-derived NK precursor cells that follow a stepwise acquisition of phenotypic markers, including early acquisition of NK1.1 and CD94/NKG2 receptors, followed by Ly49 receptors and CD49b (Dx5) (4–7). CD11b and CD43 also increase with maturation (4).
The liver is a unique organ that is exposed daily to foreign Ags derived from food and commensal flora that traffic from the gut (8, 9). The mechanisms underlying liver tolerance to such gut-derived Ags remain incompletely defined, but they may include a role for regulatory T cells, elimination of activated T cells, and the production of immunosuppressive cytokines, such as IL-10 (9–11). Certain pathogens may exploit the tolerogenic environment of the liver in attempts to avoid immune clearance and establish persistent infection. Indeed, the liver is the primary site of persistence for the chronic viral hepatitis agents hepatitis B virus (HBV) and hepatitis C virus (HCV) (12). Further elucidating the mechanisms contributing to liver tolerance will provide valuable insight into the design of novel therapeutic intervention strategies for chronic liver disease.
NK cells represent a large proportion of the lymphocyte population in the liver and might be involved in maintaining liver tolerance through their interactions with a variety of cell types and their ability to secrete pro- and anti-inflammatory cytokines (13–15). In a recent study, NK cells cocultured with hepatocytes were shown to alter the ability of DCs to prime CD4+ T cells, resulting in a regulatory T cell phenotype and function (16). Importantly, DC induction of the T cell regulatory phenotype was dependent on NKG2A engagement on NK cells during coculture with hepatocytes. Interestingly, NKG2A expression is reportedly increased on NK cells from patients with chronic HCV infection, suggesting a role for NKG2A in persistent viral infection (17, 18).
The immunosuppressive role of IL-10 has been well established (19, 20). With regard to IL-10 function in the liver, LPS treatment of Kupffer cells (KCs) leads to increased IL-10 production compared with polyinosinic:polycytidylic acid treatment, and increased IL-10 production by LPS treatment dampens the ability of the KCs to activate NK cells (21). In addition, NK–hepatic cell interactions via NKG2A-Qa-1b engagement can result in increased IL-10 and decreased IFN-γ production by NK cells, potentially leading to suboptimal T cell activation by DCs (16, 17).
We hypothesized that the local environment may influence liver NK cells by decreasing their ability to respond to stimulation, thus contributing to liver tolerance, as well as facilitating infection by liver-tropic pathogens. To determine the functional competence of liver NK cells, we examined the expression of various NK inhibitory and activating receptors. Our data show that the liver contains a prominent subset of NKG2A+ NK cells that lack Ly49 receptor expression. Importantly, this NKG2A+Ly49− NK cell subset is hyporesponsive to IL-12/IL-18 stimulation in the liver, and Ly49 expression appears sufficient to overcome this induced hyporesponsiveness. Adoptive transfer experiments suggest that the liver environment can modify NK cell receptor expression and responsiveness to cytokine stimulation and may preferentially retain immature NK cells within the liver. These data further establish a role for IL-10 in shaping intrahepatic NK cells, which are phenotypically distinct and functionally less responsive than those found within the spleen.
Materials and Methods
Mice
C57BL/6 female mice, 6–10 wk old, were obtained from Taconic Farms (Germantown, NY). Ly5.1 congenic female mice and MIP-1α–deficient (MIP-1α−/−) mice (both on C57BL/6 background and 6–10 wk old) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in a pathogen-free facility at the University of Virginia, Charlottesville. All mice were handled according to protocols approved by the University of Virginia Institutional Animal Care and Use Committee.
Cell preparation
Intrahepatic leukocytes were isolated from livers, as described previously. Briefly, the liver was perfused with PBS via the portal vein, followed by PBS plus 0.05% collagenase (Sigma-Aldrich, St. Louis, MO) and then washed with IMDM supplemented with 10% newborn calf serum. The liver sections were finely minced and further digested with PBS plus 0.05% collagenase at 37°C for 20 min. Hepatocytes were removed by centrifuging at 40 ×g for 4 min at 4°C. Mononuclear cells were purified on a 21% Nycodenz gradient after centrifugation at 1100 ×g for 20 min without braking. Splenocytes were prepared by mechanical disruption and isolation over a Ficoll gradient centrifugation at 1250 ×g for 20 min without braking. For preparation of purified NK cells, liver leukocytes and splenocytes were isolated as above, surfaced stained for CD3 and NK1.1, and sorted for CD3− NK1.1+ cells using a FACSVantage SE Turbo Sorter (BD Biosciences, San Jose, CA) with purities >98%.
Flow cytometry and intracellular staining
Cells were stained with Abs against CD3, CD27, CD11b, CD43, CD117, CD122, CD127, Dx5, Granzyme B, IFN-γ, IL-10, IL-10R, Ly5.1, Ly49C/I/F/H, Ly49G2, Ly49D, Ly49I, Ly49A, Ly49C/I, NK1.1, NKG2A, and NKG2D (all obtained from eBioscience, San Diego, CA, or BD Biosciences). Anti-Ly49H was kindly provided by Wayne Yokoyama (Washington University, St. Louis, MO). For intracellular cytokine staining, NK cells were stimulated with recombinant mouse IL-12 (100 ng/ml) and IL-18 (100 ng/ml) (both from R&D Systems, Minneapolis, MN) in the presence of monensin at a concentration of 1 × 106 cells/ml in IMDM containing 10% FBS at 37°C for 5 h. Cells were stained for intracellular IFN-γ using Cytofix/Cytoperm (BD Biosciences), according to the manufacturer’s instructions. Positive staining was determined using unstimulated cells. All samples were run on a FACSCanto (BD Biosciences) and analyzed using FlowJo software.
Adoptive transfer experiments
Liver and spleen leukocytes were obtained from Ly5.1 congenic mice, as described above. NK cells were enriched by negative selection on an autoMACS Separator using a NK Cell Isolation Kit (both from Miltenyi Biotec, Auburn, CA), according to the manufacturer’s instructions. Approximately 1 × 105 Ly5.1+ cells were transferred i.v. into wild-type (Ly5.2) C57BL/6 mice. For transfer of Ly49Hi and Ly49Lo/Neg NK cell subsets, splenocytes were isolated from wild-type C57BL/6 mice, as described above. Ly49Hi or Ly49Lo/Neg CD3− NK1.1+ cell subsets were sorted by positive selection on a FACSVantage SE Turbo Sorter (BD Biosciences) and transferred into naive Ly5.1 congenic hosts. Transferred cells were detected by flow cytometry in livers and spleens 48 h posttransfer. All samples were run on a FACSCanto and analyzed using FlowJo software.
Cytotoxicity assay
NK cells were purified from liver and spleen cell preparations, as described above. Purified liver or spleen NK cells were cocultured with CFSE-labeled YAC-1 target cells at E:T ratios of 30:1, 12:1, and 5:1 at 37°C for 4 h. CFSE labeling of YAC-1 cells was performed using the CellTrace CFSE Cell Proliferation Kit, according to the manufacturer’s instructions (Invitrogen, Eugene, OR). Following the coculture, dead target cells were detected by adding 5 μl of a 10-μM solution of TO-PRO-3 (Invitrogen, Eugene, OR) immediately before running the sample on a FACSCanto. Flow data were analyzed using FlowJo software. Dead target cells were defined as CFSE+TO-PRO-3+ cells within the CFSE+ cell gate. NK cytotoxicity was calculated as the percentage of target cell death in NK:target cell cocultures minus the spontaneous target cell death (YAC-1 cells cultured in the absence of NK cells).
Viral infection experiments
For analyzing the phenotypic and functional impact of viral infection on liver NK cell subsets, wild-type and MIP-1α−/− C57BL/6 mice were injected i.p. with 5 × 104 PFU murine CMV (MCMV). After 48 h, leukocytes were isolated from infected livers as well as from uninfected control mice. The surface expression of NK cell receptors and intracellular staining for IFN-γ were performed as described above.
ELISAs
Whole liver and spleen homogenates were obtained from naïve C57BL/6 female mice using a glass pestle. Cells and debris were pelleted at 1500 rpm for 6 min. Supernatants were carefully transferred to clean tubes and stored at −80°C. IL-10 levels were measured using a BD OptEIA mouse IL-10 ELISA kit (BD Biosciences), according to the manufacturer’s instructions. Because of significant background staining of liver homogenates, positive IL-10 levels were determined by subtracting background signal from wells containing everything except capture Ab.
In vivo IL-10R blockade experiments
For blocking experiments, 250 μg anti–IL-10R (clone 1B1.3a) or rat IgG1 was injected i.p. every 2 d for 6 d. Liver and spleen leukocytes were isolated, as described above, 2 d following the last injection. The surface expression of NK cell receptors and intracellular staining for IFN-γ were performed as described above.
Statistical analysis
Student t tests were used to evaluate the significance of the differences. A value of p < 0.05 was regarded as statistically significant.
Results
The liver contains a significant population of NKG2A+ NK cells that lack Ly49 expression
NK cells display cell surface inhibitory receptors for self-MHC class I molecules, including Ly49 and NKG2A. Inhibitory receptors are involved in NK tolerance and recognition of transformed or infected target cells. Because of their prominent presence in the liver (13,14), as well as their ability to secrete pro- and anti-inflammatory cytokines and influence the maturational state of DCs (15, 22), NK cells may play a central role in the tolerogenic nature of the liver. To examine their cell surface expression profile, we stained liver NK cells for various NK cell receptors. Fig. 1A is representative of the gating strategy used to define NK cells throughout this study. Compared with splenic NK cells, and in accord with previous work (23), liver NK cells expressed lower levels of all of the Ly49 receptors tested (Fig. 1B). In addition, liver NK cells expressed higher levels of the inhibitory receptor NKG2A. Similar to the spleen, NK cells isolated from the blood and lung also expressed higher levels of Ly49 receptors and lower levels of NKG2A than did liver NK cells (data not shown). Interestingly, liver NK cells expressed lower levels of the activating receptor NKG2D.
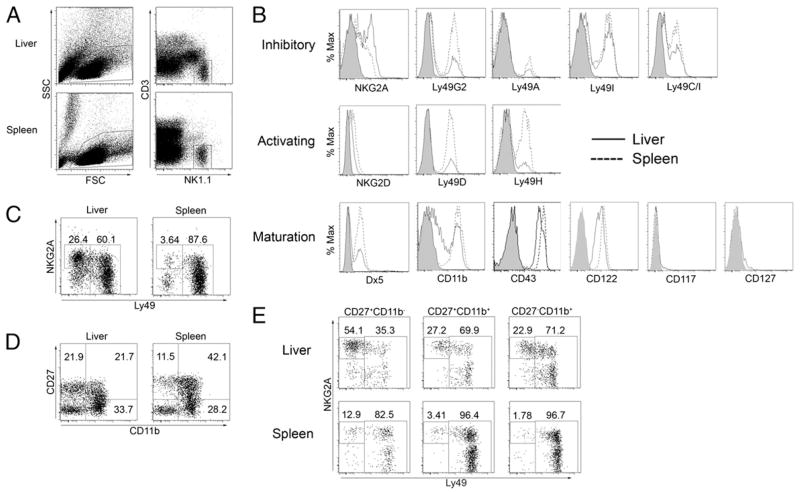
FIGURE 1.
NK cell receptor expression profile indicates liver contains a large proportion of immature NK cells. Liver and spleen tissue was harvested from 6- to 10-wk-old naive female C57BL/6 mice. Total leukocytes were isolated and stained for various NK cell receptors. A, NK cells are defined throughout this article as NK1.1+ cells lacking CD3 expression. B, Expression of various NK cell receptors on gated liver (solid line) and spleen (dashed line) NK cells. Shaded curves represent isotype control staining. C, Liver and spleen NK cell subsets according to surface expression of NKG2A and a mixture of Ly49 receptors (C/I/F/H/G2). D, Liver and spleen NK cell subsets gated according to surface expression of CD27 and CD11b. E, NKG2A/Ly49 expression on CD27+CD11b−, CD27+CD11b+, and CD27−CD11b+ subsets of liver and spleen NK cells. Data are representative of at least three independent experiments using at least three mice per group.
Developing NK cells were shown to sequentially express NKG2A, followed by Ly49 (4, 7); thus, the predominance of NKG2A expression and the relatively low expression of Ly49 receptors may reflect the presence of a population of less mature NK cells in the liver. Indeed, the presence of immature NK cells in the adult mouse liver has been reported (24). Our data confirm that a significant population of Dx5neg and CD11blow NK cells is found in the liver but not the spleen, lung, or blood. However, CD43 was expressed at high levels on NK cells from all organs tested (Fig. 1B and data not shown). Therefore, the liver may represent a distinct NK cell population consisting of a substantial subset of NKG2A+ NK cells that lack Ly49 receptor expression (Fig. 1C).
The markers CD27 and CD11b have been used to separate NK cells based on maturation. A recent report showed that CD27+CD11b− NK cells appear first after bone marrow reconstitution, followed by CD27+CD11b+ and then CD27− CD11b+ NK cells (25). Our analysis showed that the liver contains a significant population of CD27+ CD11b− NK cells, whereas spleen, lung, and blood NK cells are almost entirely the more mature CD27+CD11b+ and CD27− CD11b+ subsets (Fig. 1D and data not shown). Further analysis revealed that increased Ly49 receptor expression correlated with increased maturational state, as defined by CD27 and CD11b staining, in the liver and the spleen (Fig. 1E). However, in all subsets analyzed, fewer liver NK cells expressed Ly49 receptors, whereas a greater proportion expressed NKG2A. Taken together, the receptor expression profile of liver NK cells presented in this study supports that the liver contains a large population of NK cells that are phenotypically distinct compared with other NK cells present in the periphery.
Liver NKG2A+Ly49− NK cells are hyporesponsive to cytokine stimulation
Because recent studies have shown that the surface expression of inhibitory NK receptors for self-MHC class I ligands coincides with the acquisition of effector functions by individual NK cells, we next assessed liver NK cell subsets for effector activity (26, 27). IL-12 and -18 are important cytokines in driving proinflammatory responses to pathogens, as well as potent activators and inducers of cytokine production by NK cells (28–30). Following stimulation with IL-12 and IL-18, a smaller proportion of liver NK cells produced IFN-γ compared with spleen NK cells (Fig. 2A). Although the percentage of liver IFN-γ+ NK cells was not statistically different within a single experiment, the overall trend in multiple experiments consistently showed that a lower percentage of liver NK cells produced IFN-γ. In addition, the mean fluorescence intensity of intracellular IFN-γ staining was significantly lower in liver NK cells (Fig. 2B). Because mature NK cells express Ly49 receptors, we considered that the liver NK cell population might be less functionally competent as a result of the larger proportion of NK cells lacking Ly49 receptor expression. When NK cells were gated according to Ly49 receptor expression, we found that Ly49 receptor expression correlated with greater IFN-γ production in the liver and spleen (Fig. 2C). Thus, in accord with previous findings, liver and spleen NK cells expressing Ly49 receptors display functional competence (26, 27).
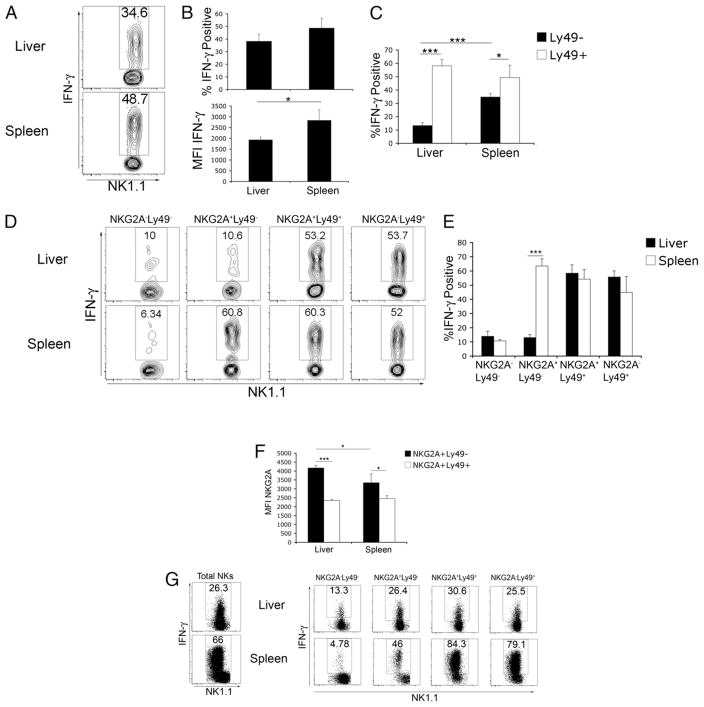
FIGURE 2.
Liver NKG2A+Ly49− NK cells are hyporesponsive to cytokine stimulation. A–E, Total female C57BL/6 liver or spleen leukocytes were stimulated with IL-12 and -18, followed by intracellular staining for IFN-γ. Plots are gated on CD3−NK1.1+ cells. A, IFN-γ production by total liver and spleen NK cells. B, Combined results from a single experiment showing the percentage of IFN-γ+ liver and spleen NK cells as well as mean fluorescent intensity of intracellular IFN-γ staining. C, Combined results from a single experiment of IFN-γ production by Ly49− and pan NK Ly49+ (C/I/F/H/G2) NK cells. D, IFN-γ production by NK cell subsets based on NKG2A and pan Ly49 expression. E, Combined results from a single experiment showing intracellular IFN-γ staining by NK cell subsets gated according to NKG2A and pan NK Ly49 expression. F, Combined results from a single experiment of mean fluorescent intensity of NKG2A staining on Ly49+ and Ly49− NK cells. G, Dot plots of intracellular IFN-γ staining in cell-sorted total NK cells and flow-gated NK cell subsets. Data in A–F are representative of three independent experiments containing three mice per experiment. Data in G are representative of two independent experiments containing NK cells sorted from at least six mice. *p < 0.05; ***p < 0.0005.
Because of the abundance of NKG2A receptors on intrahepatic NK cells, we next examined the effect of NKG2A expression on the functional capacity of liver and spleen NK cells. As expected, NK cells from either organ lacking expression of NKG2A and Ly49 receptors were relatively insensitive to cytokine stimulation, whereas NK cells expressing Ly49 receptors readily produced IFN-γ (Fig. 2D, 2E). Furthermore, NKG2A expression contributed little, if any, additional control to Ly49+ NK cells because NKG2A+Ly49+ and NKG2A−Ly49+ NK cells in the liver and spleen produced comparable IFN-γ. However, the production of IFN-γ by intrahepatic NKG2A+Ly49− NK cells was greatly reduced compared with spleen NKG2A+Ly49− NK cells (Fig. 2D, 2E). We further found that the level of NKG2A expression was significantly higher on NKG2A+Ly49− NK cells in the liver compared with all other NKG2A-expressing NK cells in the liver or spleen (Fig. 2F).
Several studies reported tolerogenic activity for various cell types within the liver, including DCs and KCs (21, 31–33). Within the stimulation conditions used in Fig. 2A–E, it is possible that additional intrahepatic cells may be dampening the ability of the liver NK cells to produce IFN-γ in response to IL-12/IL-18, or conversely, additional splenocytes enhancing the functional response of splenic NK cells. To assess the direct ability of liver NK cells to respond to cytokine stimulation, sorted liver and spleen NK cells were stimulated, as above. Unexpectedly, we found that sorted liver NK cells were dramatically less responsive to cytokine stimulation compared with spleen NK cells in all subsets analyzed (Fig. 2G). These results suggested that the liver environment is suppressing the functional responsiveness of all NK cell subsets and that cytokine stimulation alone is insufficient to overcome the suppression. However, in the context of additional liver leukocytes, cytokine stimulation did restore full functional competence in Ly49+ liver NK cells but not NKG2A+Ly49− liver NK cells.
NKG2A+Ly49− liver NK cells express high levels of granzyme B and produce IL-10
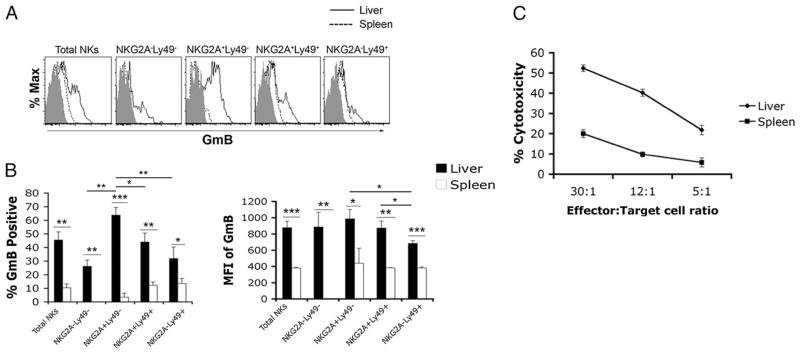
In addition to IFN-γ production, NK cell effector functions include cytotoxic activity and anti-inflammatory cytokine production. To extend the functional analysis of liver NK cells, we measured the intracellular levels of granzyme B in liver and spleen NK cells directly ex vivo, as well as their cytotoxic activity against the NK-susceptible YAC-1 cell line. Total liver NK cells contained elevated levels of granzyme B compared with spleen NK cells (Fig. 3A, 3B), which correlated with increased cytotoxic activity (Fig. 3C). Further analysis of NK cell subsets revealed that a greater percentage of NKG2A+ Ly49− liver NK cells stained positive for granzyme B compared with other liver NK cell subsets and spleen NK cells (Fig. 3A, 3B).
FIGURE 3.
Higher granzyme B levels in liver NK cells correlates with enhanced cytotoxic activity. Liver and spleen leukocytes were isolated from naive C57BL/6 mice as before. A, Representative dot plots showing intracellular levels of granzyme B in liver and spleen NK cells. B, Combined results from a single experiment of positive percentage and mean fluorescent intensity of granzyme B intracellular staining. C, Liver and spleen NK cells were purified from 8- to 12-wk-old naive female C57BL/6 mice. Purified NK cells were cocultured with CFSE-labeled YAC-1 target cells at an E:T ratio of 30:1, 12:1, or 5:1 at 37°C for 4 h. Following the coculture, dead cells were detected by TO-PRO-3 staining measured by FACS analysis. Dead target cells were defined as CFSE+TO-PRO-3+ cells. Cytotoxicity percent was calculated as the percentage of target cell death in NK:target cell cocultures minus the spontaneous target cell death (YAC-1 cells cultured in the absence of NK cells). Data in A and B are representative of at least two independent experiments containing three mice. Data in C are representative of two independent experiments using purified NK cells pooled from ≥12 mice. *p < 0.05; **p < 0.005; ***p < 0.0005.
Previous reports suggested that NK cells are able to produce IL-10 (34, 35). Because IL-10 may play a significant role in the tolerant environment of the liver, we analyzed the ability of liver NK cell subsets to produce IL-10 in response to cytokine stimulation. Although low levels of IL-10 staining were noted in all liver NK cell subsets, NKG2A+Ly49− NK cells seemed to be the major source of IL-10 within the liver NK cell population (Supplemental Fig. 1). In contrast, IL-10 staining in spleen NK cell subsets was negligible.
Liver environment suppresses functional capacity of NK cells
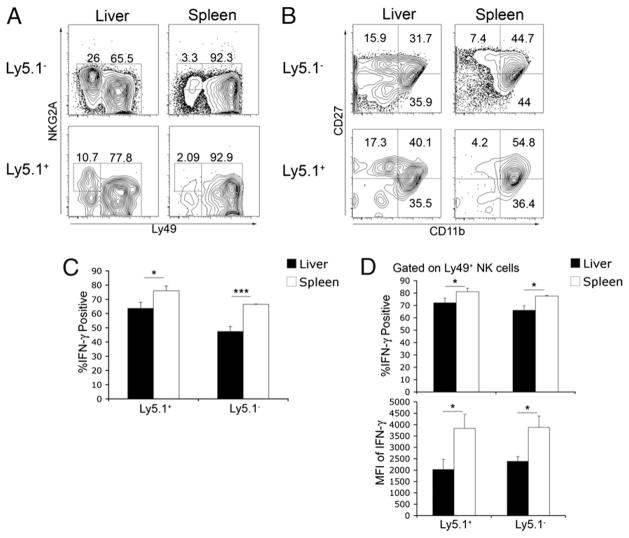
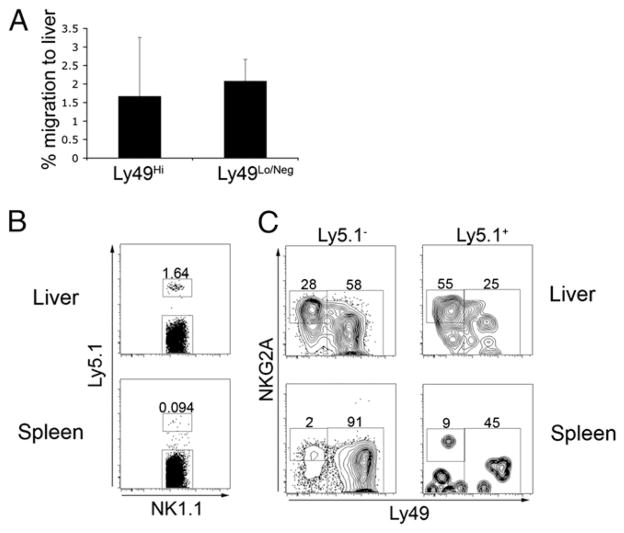
The receptor profile and lower functional responsiveness of the liver NK cell may be due to preferential retention/recruitment of a distinct subset of NK cells or, alternatively, the liver environment may modify NK cell receptor expression and functional responsiveness. To address this question, we performed adoptive transfer experiments using a CD45 (Ly5) mismatch. We examined the NK cell receptor expression on adoptively transferred spleen NK cells that had migrated to the liver or spleen 48 h posttransfer. Adoptively transferred spleen NK cells (Ly5.1+) that migrated to the liver showed a greater proportion of NKG2A+ cells that lacked Ly49 expression, whereas spleen NK cells that migrated back to the spleen were almost entirely Ly49+ (Fig. 4A). In addition, a greater proportion of spleen NK cells that migrated to the liver displayed the CD27+CD11b− phenotype (Fig. 4B). These data indicate that the liver can modify receptor expression on NK cells, potentially influencing their functional competence. Indeed, a lower proportion of spleen NK cells that migrated to the liver were IFN-γ+ following IL-12/IL-18 stimulation (Fig. 4C). In addition, when IFN-γ production was examined in Ly49-expressing NK cells, the percentage that was positive as well as the mean fluorescence intensity were lower in those NK cells that migrated to the liver compared with those that migrated to the spleen (Fig. 4D).
FIGURE 4.
Splenic NK cells that migrate to the liver display greater immature phenotype and decreased IFN-γ production. Splenic NK cells were isolated from 6- to10-wk-old naive female Ly5.1 congenic C57BL/6 mice. Approximately 6 × 105 Ly5.1+ NK cells were adoptively transferred i.v. into C57BL/6 mice (Ly5.2+). Forty-eight hours posttransfer, liver and spleen leukocytes were isolated and stained for CD3, NK1.1, Ly5.1, and various NK cell receptors. Plots represent data from CD3−NK1.1+-gated cells. A, Staining for NKG2A and Ly49 receptors on adoptively transferred (Ly5.1+) and endogenous (Ly5.1−) NK cells. B, Staining for CD27 and CD11b molecules on adoptively transferred (Ly5.1+) and endogenous (Ly5.1−) NK cells. C, Combined results of IFN-γ production by transferred (Ly5.1+) and endogenous (Ly5.1−) total NK cell populations. D, Combined results of transferred (Ly5.1+) and endogenous (Ly5.1−) Ly49+-gated NK cells. Data are representative of two independent experiments with two or three mice per group. *p <0.05; ***p <0.0005.
To further address the possibility of a preferential retention of a specific subset of NK cells within the liver, we sorted spleen NK cells into Ly49Hi and Ly49Lo/Neg subsets and transferred them into separate hosts. Regardless of Ly49 receptor expression, adoptively transferred spleen NK cells preferentially migrated back to the spleen (Fig. 5A). When liver NK cells were adoptively transferred, they displayed a preference to migrate back to the liver. Indeed, transferred liver NK cells were virtually undetectable in the spleen (Fig. 5B). Interestingly, the transferred cells that migrated back to the liver were enriched for the NKG2A+Ly49− subset of NK cells (Fig. 5C). Taken together, these data suggest that the naive liver environment is sufficient to dampen spleen NK cell IFN-γ production and that liver NKG2A+ NK cells preferentially migrate back to the liver following adoptive transfer.
FIGURE 5.
NK cells preferentially migrate back to the organ of isolation. Splenic or liver NK cells were isolated from naive female wild-type (Ly5.2) or Ly5.1 congenic, C57BL/6 mice, respectively, and were transferred i.v. into Ly5-mismatched hosts. Forty-eight hours posttransfer, liver and spleen leukocytes were isolated and stained for CD3, NK1.1, Ly5.1, and various NK cell receptors. Plots represent data from CD3−NK1.1+-gated cells. For adoptive transfer of Ly49Hi/Ly49Lo NK cells, Ly5.2+ splenic NK cells were sorted into Ly49Hi and Ly49Lo/Neg populations and adoptively transferred separately into Ly5.1 congenic C57BL/6 mice. A, The percentage of cells that migrated to the liver was calculated by dividing the absolute number of transferred cells that migrated to the liver by the absolute number of transferred cells that migrated to the spleen and liver ([liver absolute no./(spleen absolute no. + liver absolute no.)] ×100). Data are combined from two independent experiments. B, Ly5.1+ liver NK cells were adoptively transferred i.v. and detected in liver and spleen 48 h. posttransfer. C, NKG2A and pan Ly49 staining on transferred (Ly5.1+) and endogenous (Ly5.1−) NK cells. Data in B and C are representative of two independent experiments with two or three mice per group.
Although the naive liver environment is immunosuppressive, the liver is capable of clearing many pathogens, including lymphocytic choriomeningitis virus and MCMV. To determine the functional capacity of liver NK cells in response toviral infection, we measured the functional response of liver NK cell subsets following MCMV infection. Compared with naive mice, NKG2A+Ly49− liver NK cells from MCMV-infected mice were capable of producing IFN-γ with similar magnitude to that of Ly49+ liver NK cells (Supplemental Fig. 2). Because the recruitment of blood or spleen NK cells to the liver following viral infection may confound the interpretation of these results, we also analyzed liver NK cell function in MCMV-infected mice deficient in MIP-1α (MIP-1α−/−), which were shown to be impaired in the migration of NK cells to the liver following MCMV infection (36). Intracellular IFN-γ staining was similar in all liver NK cell subsets from MCMV-infected wild-type and MIP-1α−/− mice (Supplemental Fig. 2). Thus, although NKG2A+Ly49− liver NK cells from the naive liver respond poorly to cytokine stimulation, full functional competence can be achieved under certain conditions.
IL-10 modifies liver NK cell subset composition and functional responsiveness
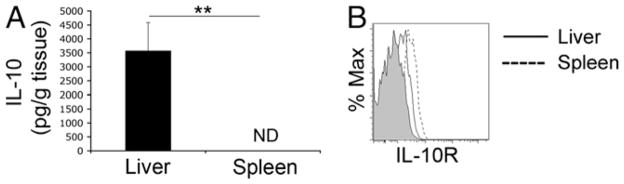
The immunosuppressive nature of IL-10 has been well documented in many facets of the immune response (20), and it may be critical to the tolerogenic nature of the liver (9, 21). Indeed, a recent report suggested that high levels of IL-10 produced by KCs in response to LPS may contribute to liver tolerance by dampening the effect of proinflammatory cytokines present in the liver (21). Fig. 6A shows that the liver contains a large amount of IL-10, compared with the spleen, as measured by ELISA. In addition, IL-10R is expressed on liver and spleen NK cells (Fig. 6B).
FIGURE 6.
Liver contains high levels of IL-10. A, Liver and spleen tissue was isolated from naive 6- to 10-wk-old C57BL/6 female mice and weighed prior to homogenization. Tissue was gently homogenized in Iscove’s media using a glass pestle. Cells and debris were pelleted by centrifugation, and supernatants were collected and stored at −80°C. IL-10 levels were measured in triplicate by ELISA. Cytokine levels represent positive signal above background (wells containing no capture Ab). **p <0.005. B, IL-10R expression on total liver (solid line) and spleen (dashed line) NK cells. Shaded graph represents isotype control staining. Data are representative of three independent experiments using three mice.
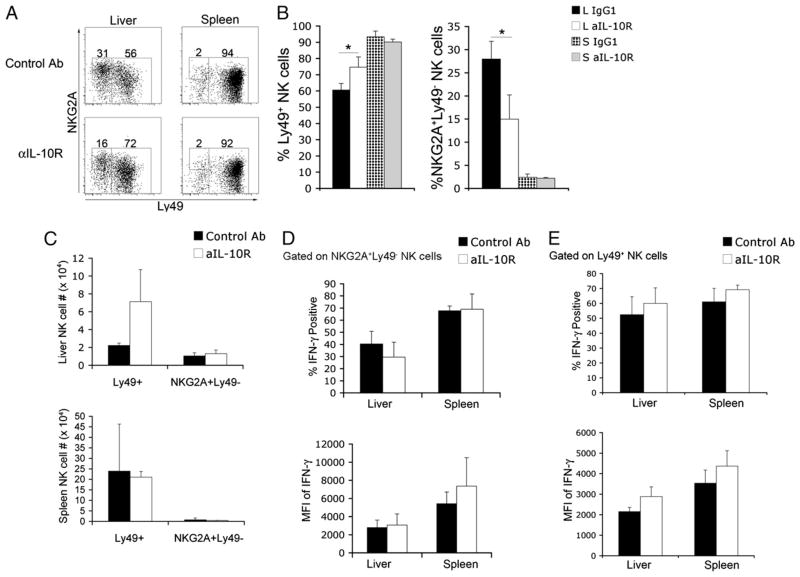
We hypothesized that IL-10 within the liver could be contributing to the lower functional capacity of liver NK cells. Administration of IL-10R–blocking Ab resulted in a significant increase in Ly49+ liver NK cells (Fig. 7A). This increase in the proportion of Ly49+ NK cells, and the accompanying decrease in the proportion of NKG2A+Ly49− NK cells, was specific to the liver compartment (Fig. 7B). In addition, there was no significant difference in the absolute number of NK cells between treatments (Fig. 7C). Surprisingly, IL-10R blockade did not significantly increase the production of IFN-γ by NKG2A+Ly49− or Ly49+ liver NK cells (Fig. 7D, 7E). Importantly, Ly49 expression on spleen NK cells did not seem to be affected by IL-10R blockade.
FIGURE 7.
IL-10R blockade shifts liver NK cell subsets to a greater proportion of Ly49-expressing NK cells. Six- to 10-wk-old female C57BL/6 mice were injected i.p. with 250 μg of IL-10R–blocking Ab or isotype control every 2 d over a 6-d period. Total leukocytes were isolated from liver and spleen tissue. A, Expression of NKG2A and Ly49 receptors on liver and spleen NK cells from control and anti-IL-10R–treated mice. B, Combined results from a single experiment showing NKG2A+Ly49− and Ly49+ NK cell subsets. *p <0.05. C, Combined results from a single experiment showing absolute numbers of Ly49+ and NKG2A+ Ly49− NK cells following IL-10R blockade or control Ab injection. Combined results of a single experiment displaying the percentage of IFN-γ+ NK cells and the mean fluorescence intensity of intracellular IFN-γ staining in liver and spleen NKG2A+Ly49− (D) and Ly49+ (E) NK cells from control and anti-IL-10R–treated mice. Data showing surface expression of NKG2A and Ly49 receptors are representative of three independent experiments, and intracellular IFN-γ staining is representative of two independent experiments using three mice per group.
Discussion
The normal liver is exposed to a daily barrage of gut-derived foreign Ags that must be absorbed and dealt with in a manner that does not elicit an inflammatory response (8, 9). Therefore, the liver is armed with a variety of immunetolerance mechanisms that may include the induction of regulatory T cells and the elimination of activated T cells (9). Recent reports also highlighted a role for DCs (31–33) and KCs in maintaining liver tolerance (21). Our findings suggest that an additional contribution to liver tolerance may include regulation of NK cells so that they are functionally less responsive.
In this report, we demonstrated that the liver contains a large proportion of NK cells that are less responsive to cytokine-inducing stimulation. Multiple factors may be contributing to lower IFN-γ production by liver NK cells, including a decreased proportion of Ly49-expressing NK cells. Indeed, Ly49 expression on NK cells correlated with greater IFN-γ production in liver and spleen NK cells. A reduced functional response among liver NK cells corresponds with a smaller proportion of NK cells expressing Ly49 receptors. Enhanced function by Ly49+ NK cells could reflect maturational differences, because the ability to secrete IFN-γ increases with NK maturation (37). However, sorted Ly49+ liver NK cells were less responsive to cytokine stimulation than were Ly49+ NK cells from the spleen, indicating that there are likely additional factors unique to the liver influencing the NK cell functional response.
NKG2A is a major inhibitory receptor expressed by liver NK cells; its high expression coincided with hyporesponsiveness observed in intrahepatic NKG2A+Ly49− NK cells, suggesting a significant role for NKG2A in regulating liver NK cell effector functions. Importantly, Ly49 expression appears sufficient to overcome this hyporesponsiveness, because there was no statistical difference in IFN-γ production between NKG2A+Ly49+ and NKG2A−Ly49+ NK cells. It is interesting to note that a majority of fetal NK cells were reported to express CD94/NKG2 molecules, while lacking surface expression of Ly49 receptors (6). Liver NKG2A+Ly49− NK cells, although less responsive to cytokine stimulation than Ly49-expressing NK cells, may have other functions within the liver. Intracellular staining for IL-10 indicated that a greater proportion of NKG2A+Ly49− liver NK cells produced IL-10 compared with other liver NK cell subsets or spleen NK cells (Supplemental Fig. 1). In addition, the analysis of intracellular granzyme B revealed that liver NK cells contained higher amounts compared with spleen NK cells and that this increased granzyme B level correlated with enhanced cytotoxicity against YAC-1 target cells (Fig. 3). This is most intriguing because of the observation that the weak immunostimulatory function associated with the liver DC population may be due, in part, to differences in subtype composition (31). Further analysis is required to determine whether the cytolytic potential and/or cytokine production of specific NK cell subsets in the liver may contribute to the shaping of the liver DC population. NK cell subset analysis of intracellular granzyme B levels indicated that NKG2A+Ly49− liver NK cells have a greater cytolytic potential (Fig. 3A). Interestingly, a recent report showed that the killing of immature DCs by human NK cells was mediated by a subset of NK cells that express NKG2A but lack killer Ig-like receptor (KIR) expression (38).
The adoptive transfer experiments of spleen NK cells reported herein suggest that the liver environment can modify NK cell functional responsiveness. Given the immunosuppressive functions of IL-10 and its high levels in the liver, determined by ELISA, IL-10 may play a critical role in maintaining liver tolerance. IL-10 was reported to inhibit the expression of the costimulatory molecules B7-1/B7-2 on DCs and macrophages, influencing their ability to optimally activate T cells (39, 40), whereas autocrine IL-10 signaling in DCs was shown to inhibit their migration to draining lymph nodes (41). IL-10 may also lead to lower NK cell activation through the inhibition of IL-12 production (42), and KC production of IL-10 was shown to directly dampen NK cell activation and decrease IFN-γ production (21). Our data suggest that IL-10 may also contribute to liver tolerance by decreasing the percentage of NK cells expressing Ly49 receptors within the liver. Indeed, blocking IL-10R led toanincreaseinLy49-expressingNKcells in the liver. Notably, we also observed a greater percentage of Ly49-expressing NK cells in the livers of IL-10–deficient mice compared with wild-type C57BL/6 mice (data not shown).
Although there existed a trend toward a greater percentage of IFN-γ–positive NK cells, as well as increased mean fluorescence intensity of IFN-γ staining, in the total liver NK cell population from anti-IL-10R–treated animals, the differences were not statistically significant. Therefore, although IL-10 contributes to the overall shaping of the liver NK cell population, additional regulatory factors likely influence IFN-γ production by liver NK cells in response to cytokine stimulation. Futhermore, the mechanism of IL-10 regulation of NK cells in the liver remains unclear. One possibility is that IL-10 directly regulates Ly49 expression through the inhibition of NF-κB activation, which was suggested to play a role in regulating Ly49 receptor expression (43, 44). Alternatively, IL-10R blockade may modify the chemokine profile of the liver, resulting in different NK cell subsets migrating to the liver (45). Indeed, although we favor a hyporesponsiveness induced by the liver environment, the results of our adoptive transfer experiments could be explained by a preferential migration of less functionally responsive NK cells. The potential role of differential chemokine production in the IL-10R blockade phenotype reported herein is under investigation.
Liver DCs and KCs are likely candidates for regulating the NK cell population. Recent studies highlighted receptor expression differences on liver DCs and KCs compared with spleen DCs and peritoneal macrophages, noting lower T cell activation capabilities by liver DCs and KCs (46, 47). Whether liver DCs and/or KCs contribute to the immature phenotype and lower functional response of liver NK cells reported herein is also under investigation.
These findings have important significance for liver tolerance as well as for the treatment of certain persistent pathogens, such as HBV and HCV, which may exploit the tolerogenic nature of the liver in evading the immune system and establishing chronic infection. HCV is particularly intriguing because of the high rate of persistence, estimated at 70–80% of infected individuals (12). In contrast, HBV is cleared by >95% of healthy adults (12). Several reports indicated an impairment of NK cells associated with chronic HCV infection (48–50), leading to a proposed model that targeting NK cells is central to HCV persistence in the liver (51). Host and viral factors may contribute to the impact of HCV infection on NK cell function. Indeed, the outcome of HCV infection was shown to be influenced by certain KIR/HLA-type combinations, because weaker inhibitory KIR/HLA combinations positively correlated with the resolution of infection (52). In addition, our unpublished observations suggest that infection with an HCV core protein-expressing adenovirus results in enhanced levels of NKG2A on liver NK cells compared with infection with a β-gal–expressing adenovirus (M.G. Lassen and Y.S. Hahn, unpublished observations). Importantly, increased NKG2A expression was reported on NK cells isolated from patients with chronic HCV infection (17, 18). Furthermore, NK cells isolated from livers infected with an HCV core protein-expressing adenovirus showed reduced IFN-γ production compared with NK cells from livers infected with a β-gal–expressing adenovirus (M.G. Lassen and Y.S. Hahn, unpublished observations). Lower NK production of IFN-γ could result in suboptimal DC maturation, leading to inefficient T cell activation. However, MCMV infection results in strong IFN-γ production by NKG2A+Ly49− and Ly49+ liver NK cells (Supplemental Fig. 2). This may reflect a differential role for NKG2A+Ly49− liver NK cells, depending on the nature of the pathogen. The salivary glands are the primary site of viral persistence in latent CMV infection. In contrast, the liver is the primary site of viral replication during chronic HCV infection. Certain persistent pathogens may have evolved immunoevasion mechanisms that target tolerogenic factors involved in regulating the liver NK cell population, including modulation of NKG2A expression on liver NK cells.
The data presented herein indicate an important contribution for IL-10 in regulating the functional capacity of the liver NK cell population, in part by decreasing the percentage of the more functionally responsive Ly49+ subset of NK cells. Further characterization and elucidation of the mechanism of IL-10 regulation of liver NK cells may increase our understanding of liver tolerance and may lead to the development of novel and improved therapeutic strategies for persistent liver pathogens.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Healths Grants DK063222 and 1U19AI083024-01 (to Y.S.H.) and Training Fellowship 5T32AI00749611.
We thank Susan Landes for her excellent technical assistance.
Abbreviations used in this paper
- DC
dendritic cell
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- KC
Kupffer cell
- KIR
killer Ig-like receptor
- MCMV
murine CMV
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 2.Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 3.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 5.Dorfman JR, Raulet DH. Acquisition of Ly49 receptor expression by developing natural killer cells. J Exp Med. 1998;187:609–618. doi: 10.1084/jem.187.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sivakumar PV, Gunturi A, Salcedo M, Schatzle JD, Lai WC, Kurepa Z, Pitcher L, Seaman MS, Lemonnier FA, Bennett M, et al. Cutting edge: expression of functional CD94/NKG2A inhibitory receptors on fetal NK1.1+Ly-49− cells: a possible mechanism of tolerance during NK cell development. J Immunol. 1999;162:6976–6980. [PubMed] [Google Scholar]
- 7.Williams NS, Kubota A, Bennett M, Kumar V, Takei F. Clonal analysis of NK cell development from bone marrow progenitors in vitro: orderly acquisition of receptor gene expression. Eur J Immunol. 2000;30:2074–2082. doi: 10.1002/1521-4141(200007)30:7<2074::AID-IMMU2074>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Adams DH, Eksteen B, Curbishley SM. Immunology of the gut and liver: a love/hate relationship. Gut. 2008;57:838–848. doi: 10.1136/gut.2007.122168. [DOI] [PubMed] [Google Scholar]
- 9.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 10.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26:512–517. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol. 2004;164:511–519. doi: 10.1016/S0002-9440(10)63141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 13.Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 14.Klugewitz K, Blumenthal-Barby F, Eulenburg K, Emoto M, Hamann A. The spectrum of lymphoid subsets preferentially recruited into the liver reflects that of resident populations. Immunol Lett. 2004;93:159–162. doi: 10.1016/j.imlet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Hamerman JA, Ogasawara K, Lanier LL. NK cells in innate immunity. Curr Opin Immunol. 2005;17:29–35. doi: 10.1016/j.coi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Jinushi M, Takehara T, Tatsumi T, Yamaguchi S, Sakamori R, Hiramatsu N, Kanto T, Ohkawa K, Hayashi N. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 18.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 21.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 23.Daniels KA, Devora G, Lai WC, O’Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, Yagita H, Kinoshita K, Okumura K, Smyth MJ. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–2089. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 28.Chaix J, Tessmer MS, Hoebe K, Fuséri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T, Kawakami K, Qureshi MH, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 31.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 32.Shu SA, Lian ZX, Chuang YH, Yang GX, Moritoki Y, Comstock SS, Zhong RQ, Ansari AA, Liu YJ, Gershwin ME. The role of CD11c+ hepatic dendritic cells in the induction of innate immune responses. Clin Exp Immunol. 2007;149:335–343. doi: 10.1111/j.1365-2249.2007.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008;112:3175–3185. doi: 10.1182/blood-2008-05-159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant LR, Yao ZJ, Hedrich CM, Wang F, Moorthy A, Wilson K, Ranatunga D, Bream JH. Stat4-dependent, T-bet-independent regulation of IL-10 in NK cells. Genes Immun. 2008;9:316–327. doi: 10.1038/gene.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maroof A, Beattie L, Zubairi S, Svensson M, Stager S, Kaye PM. Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity. 2008;29:295–305. doi: 10.1016/j.immuni.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar-Mather TP, Hamilton TA, Biron CA. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Invest. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vosshenrich CA, Samson-Villéger SI, Di Santo JP. Distinguishing features of developing natural killer cells. Curr Opin Immunol. 2005;17:151–158. doi: 10.1016/j.coi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–1666. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 39.Buelens C, Willems F, Delvaux A, Piérard G, Delville JP, Velu T, Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–2672. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 40.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 41.Demangel C, Bertolino P, Britton WJ. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002;32:994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- 43.Pascal V, Nathan NR, Claudio E, Siebenlist U, Anderson SK. NF-kappa B p50/p65 affects the frequency of Ly49 gene expression by NK cells. J Immunol. 2007;179:1751–1759. doi: 10.4049/jimmunol.179.3.1751. [DOI] [PubMed] [Google Scholar]
- 44.Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 inhibits pulmonary NF-kappaB activation and lung injury induced by hepatic ischemia-reperfusion. Am J Physiol. 1999;277:L919–L923. doi: 10.1152/ajplung.1999.277.5.L919. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Klintman D, Sato T, Hedlund G, Schramm R, Jeppsson B, Thorlacius H. Interleukin-10 mediates the protective effect of Linomide by reducing CXC chemokine production in endotoxin-induced liver injury. Br J Pharmacol. 2004;143:865–871. doi: 10.1038/sj.bjp.0706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 47.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corado J, Toro F, Rivera H, Bianco NE, Deibis L, De Sanctis JB. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109:451–457. doi: 10.1046/j.1365-2249.1997.4581355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 50.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 2006;12:363–372. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 52.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.