Abstract
Genetic polymorphisms described for a number of enzymes involved in the metabolism of tobacco carcinogens and alcohol have been linked to increase cancer risk. Racial disparities in cancer between Whites and populations of African descent are well documented. In addition to differences in access to health care, both environment and genetic factors and their interaction may contribute to the increased cancer risk in minority populations. We reviewed the literature to identify case-control studies that included subjects of African descent. Meta analyses investigating the association of genetic polymorphisms in tobacco metabolic genes and cancer were performed. While several genes and cancers have been studied, only one or two studies per gene for each cancer site have been published, with the exception of breast (CYP1A1 and CYP1B1), lung (GSTM1, CYP1A1, and NQO1) and prostate (CYP3A4 A293G and CYP17). Marginal statistically significant associations were observed for CYP3A4 A293G and CYP17 5'UTR polymorphisms and prostate cancer. Our findings support the need for additional genetic association studies of breast, prostate and lung cancers that include a larger number of minority participants. Since incidence and mortality rates for these cancers rank highest among populations of African descent concentrated research in these areas are warranted.
Keywords: cancer, Africans, African-Americans, genetic susceptibility
INTRODUCTION
Phase I and Phase II metabolic genes, encode important enzymes in the metabolism of tobacco carcinogens. Phase I enzymes are responsible for converting chemicals into compounds that bind mainly with DNA, thus being genotoxic. An example of phase I enzymes is the Cytochrome P-450(CYP) family which play a major role in tobacco carcinogen activation. Phase II enzymes are involved in the cellular detoxification of many carcinogens. The Glutathione S-transferases (GSTM1, GSTP, GSTT1) is an example of a phase II enzyme. Substrates for GSTs include acetaldehyde, an alcohol metabolite, and several intermediate metabolites of polyaromatic hydrocarbons (PAHs) found in tobacco smoke. Genetic polymorphisms have been described for a number of enzymes involved in the metabolism of tobacco carcinogens and alcohol, and many of these polymorphisms have been linked to phenotypic differences in enzyme activity or expression 1,2. Differences in the metabolic activation and detoxification pathways of these metabolic genes are likely to be a major source of inter-individual variation in cancer susceptibility. However, in the absence of the main exposure (such as tobacco smoke), the contribution of these genetic factors is likely relevant. Genetic polymorphisms modulate the association observed between exposure (such as tobacco smoke) and cancer. Therefore, gene-environment interactions must be considered when evaluating the associations between exposures and diseases.
Racial disparities in cancer risk between Whites and populations of African descent have been well documented in the US. Between 1975 and 2006, the age-adjusted incidence rates for all cancer sites combined were 466.6/100,000 for Whites and 505.9/100,000 for African-Americans 3. In addition to differences in access to health care, it is likely that both environment and genetic factors and their interaction contribute to the increased cancer risk observed in minority populations. However, few studies addressing gene-environment interactions have been conducted. For a number of cancer types, case-control studies have reported that polymorphisms in tobacco metabolic genes are associated with cancer risk 4-6 but investigations of these associations according to ethnic background are limited. Some case-control studies include smaller numbers of African-American subjects compared to Caucasians, therefore are unable to report meaningful results for African-Americans due to lack of statistical power. For example, a previous pooled analysis of case-control studies evaluating the association of GSTM1 and CYP1A1 polymorphisms in oral and paharyngeal cancer reported no overall association, although the odds ratio in 294 African-Americas and Africans cases combined was almost 2.07. We have systematically reviewed the literature to identify all case-control studies that have included subjects of African ancestry and provided a summary of these existing studies. We have also performed meta analyses investigating the association of genetic polymorphisms in tobacco metabolic genes and cancer risk in populations of African descent.
METHODS
Selection Criteria
A Medline literature search for case-control and nested case-control studies published between 1966 and October 5, 2009 on the association of metabolic gene polymorphisms and any cancer in populations of African descent was conducted using the search term: (african-american OR african) AND (gene OR polymorphism) AND cancer. Only studies published in English, French, Spanish or Italian and reporting genotypes for incident cases and controls using PCR methods were included in this review. Genes included were both Phase I and Phase II metabolic genes. The search yielded 80 original articles. Studies that did not provide race-specific genotype data by cases and controls were excluded from the analysis. Overlap of study subjects was evaluated by comparing sources of data described in the published methods and through cross-referencing using the Genetic Susceptibility to Environmental Carcinogens (GSEC) database (www.gsec.net)8. In the case of multiple publications reporting overlapping data the more inclusive study was retained. An additional search was performed with each gene polymorphism identified from the first search along with alternative names AND (variant OR polymorphism) AND cancer. This search yielded 19 additional studies.
Of the 99 publications, three were excluded because data on subjects of African descent was combined with those of mixed ancestry 9-11; three publications presented data that overlapped with more inclusive publications from the same authors 12-14; 7 did not provide race-specific genotype data 15-21; three provided allele frequencies only 22-24 one did not report genotype data for the controls 25; two studies reported only on haplotypes 26,27; and one was a methylation study and did not report on gene polymorphisms 28. After exclusions, 79 studies remained for consideration in this review and meta-analysis (see Table 1, Supplemental Digital Content 1, which describes the publications included in this study).
Statistical analysis
Study-specific crude odds ratios and 95% confidence intervals were re-calculated to assess the association of each metabolic gene polymorphism and cancer, based on the reported genotype data by cases and controls. Meta-analytical techniques were applied for all metabolic gene polymorphisms reported in three or more studies using inverse-variance weighting to calculate the fixed and DerSimonian & Laird weighting to calculate random effects summary estimates 29. Random effects summary estimates were reported only when between–study heterogeneity was observed. Heterogeneity was evaluated using a Q-statistic, with significance considered at p< 0.10 30, and I2 metric and 95% confidence interval to measure the percent variation in the odds ratio due to heterogeneity 31,32. I2 values that are 50% or higher indicate large between-study heterogeneity while values of 25%–50% indicate moderate between-study heterogeneity. In the absence of statistical heterogeneity, the summary or meta odds ratio (OR) and corresponding 95% confidence interval (CI) was reported based on the fixed effects model; when heterogeneity was observed, the results from the random effects model were reported. Corresponding forest plots were generated for a visual representation of all meta-analyses. The overall estimate and confidence interval reported in the figures represent the meta OR and CI. The Harbord test 33 was used to test for small-study effects, with significance considered at p < 0.10. The definition of race was self-reported in the studies included in this analysis, and African-American populations have various degrees of admixture. In an attempt to create homogeneous groups for analysis, differences in genotype frequencies between African-Americans and other African control populations were tested for the genes which were included in the meta analysis. Tests on the equality of proportions were performed for each group and summary odds ratios were reported separately. In addition, stratified analyses according to geographic areas were performed where possible. All statistical analyses were carried out using STATA SE, version 10 software (StataCorp LP, College Station, TX).
RESULTS
We have summarized the studies that have reported data for various polymorphisms in populations of African descent and grouped according to cancer type (see Table 1, Supplemental Digital Content 1, which describes the publications included in this study). For breast cancer, 20 polymorphisms have been studied but for 16 of the polymorphisms (79%) only one or two studies were conducted. There were three studies on CYP1A1 Ile/Val, four studies on CYP1B1 V432L and five studies each on CYP1A1 Msp1 and CYP1A1 African-American specific polymorphisms. For lung cancer, 18 polymorphisms have been studied, but for 14 of the polymorphisms (78%) only one or two studies were published. There were seven studies on CYP1A1 Msp1, four studies each on GSTM1 deletion and NQO1 (C609T) and three studies on CYP1A1 (AA-specific) polymorphisms. For prostate cancer, 27 polymorphisms have been studied, but for 25 of them (95%) only one or two studies have reported data. There were four studies on CYP3A4 (A293G) and_five studies on CYP17 (5'UTR). For all other cancers (bladder, pancreas, kidney, head & neck, colon, brain, ovarian, esophagus and leukemia) there was one or two publications for each polymorphism.
When looking at the genes for which only one or two studies reported data, there was no significant association between the polymorphism and the corresponding cancer, except for one study of GSTT deletion and prostate cancer showing an OR of 0.5 (95% Confidence Interval [CI] = 0.8-0.88)34; a single study of CYP1B1 (R48G) (OR: 2.22; 95% CI = 1.01-5.09), CYP17 (rs17115144) (OR: 3.92; 95% CI = 1.04-14.43), CYP19 (rs11636639) (OR: 2.36; 95% CI = 1.10-5.05), CYP19 (rs3751592) (OR: 2.88; 95% CI = 1.39-6.03) and CYP27B1 (OR: 0.29; 95% CI = 0.08-0.88) and prostate cancer35; one study of CYP3A43 (P340A) and prostate cancer (OR: 3.54; 95% CI = 1.36-9.94)36; one study of CYP17 (5'UTR) and lung cancer (OR: 3.03; 95% CI = 1.19-8.17)37 and one study of ADH2*3 (R370C) as well as ALDH2*2 (Q487K) polymorphisms and esophageal cancer (OR: 2.19; 95% CI = 1.23-3.90 and OR: 9.26; 95% CI = 1.16-419.64, respectively)38.
Meta analysis
GSTM1 deletion
For all cancers, seventeen publications reported data on populations of African descent, for a total of 1,437 cancer cases and 2,026 controls. A meta estimate of cancer risk with the GSTM1 deletion was only possible for lung cancer since four studies were conducted (497 cases and 624 controls). The source of controls was for the most part the healthy population (3/4 studies) and all of the subjects were African-American. The meta OR was 1.26 (95% CI: 0.96-1.65). There was no statistical evidence of heterogeneity among the four studies on lung cancer (Q: 3.47; p = 0.324; I 2 = 14%, 95% CI: 0-87); nor small-study effect (p = 0.548).
CYP1A1 Msp1
For all cancers, there were 14 studies on CYP1A1 Msp1 polymorphism and cancer in populations of African descent, for a total of 1,782 cases and 2,213 controls. Seven studies were conducted on lung cancer (960 cases and 1,189 controls) and five on breast cancer (763 cases and 864 controls).
For lung cancer, four out of the seven studies (57%) involved controls from the healthy population. Five included African-American subjects 37,39-42 and two included subjects from Brazil 43,44. There was no significant difference in the frequency of the CYP1A1 Msp1 polymorphism between African-Americans and Brazilians (42.5% vs. 43.7%, p = 0.847). The odds ratios for the CYP1A1 Msp1 variant in the two Brazilian studies were both increased but not significant and there was no overall association between the CYP1A1 Msp1 homozygous variant and lung cancer in African-Americans (meta OR: 0.93; 95% CI: 0.62-1.40). There was moderate heterogeneity between the African-American studies (Q statistic: 6.19, p: 0.186; I 2 = 35.0%, 95% CI: 0-76); and no evidence of small-study effect (p = 0.943). There was also no overall association between the CYP1A1 Msp1 heterozygotes and lung cancer (meta OR: 1.00; 95% CI: 0.82-1.21), with no evidence of heterogeneity between studies (Q statistic: 3.48; p = 0.481; I2 = 0.0%, 95% CI: 0-79), or of small-study effect (p = 0.379).
For breast cancer, four of the five studies involved African-American subjects and controls from the healthy population (543 cases and 646 controls)45-48. The fifth study involved a Nigerian population with hospital controls (220 cases and 218 controls)49. There was no significant difference in the frequency of the CYP1A1 Msp1 polymorphism between African-Americans and Africans from Nigeria (41.8% vs. 40.37%, p = 0.711). The meta OR for the CYP1A1 Msp1 homozygous variant in African-Americans (meta OR: 1.19; 95% CI: 0.71-1.99) was compared to the odds ratio for the single African study (OR: 0.91; 95% CI: 0.41-2.00) and neither was statistically significant. There was also no association between the CYP1A1 Msp1 heterozygous variant with breast cancer in African-Americans (meta OR: 0.95; 95% CI: 0.74-1.22) nor in the single African study (meta OR: 0.94; 95% CI: 0.61-1.44). For African-Americans, there was evidence of large between-study heterogeneity for both the homozygous (Q statistic: 7.42; p = 0.060; I2 = 60%, 95% CI: 0-87) and heterozygous (Q statistic: 5.02; p = 0.170; I2 = 40%, 95% CI: 0-80) variants; but no evidence of small-study effect for either (p = 0.626; p = 0.884).
CYP1A1 Ile/Val
For all cancers combined, nine studies included separate results on populations of African descent, for a total of 1,022 cases and 1,692 controls. A meta estimate of cancer risk with the CYP1A1 Ile/Val was only possible for breast cancer since four studies reported data for a total of 554 cases and 906 controls. The other cancers studied included ovarian, lung, pancreas and head & neck cancers. All four breast cancer studies included African-Americans and the source of controls was the healthy population. No significant association was reported in any individual study. A meta estimate was only possible for the association of CYP1A1 variant (Ile/Val and Val/Val allele carriers combined) and breast cancer (meta OR: 0.83; 95% CI: 0.50-1.37). There was no evidence of between study heterogeneity (Q statistic: 3.42; p = 0.332; I2 = 12%, 95% CI: 0-87); nor small-study effect (p = 0.121).
CYP1A1 African-American specific
There were 9 studies on the African specific polymorphism (1,123 cases and 1,624 controls), for lung, breast and ovarian cancer. Four studies reported data for breast cancer and three studies reported data for lung cancer.
For breast cancer four of the five studies included African-American populations (542 cases and 645 controls)45-48 and the fourth included a Nigerian population (229 cases and 227 controls)49. The source of controls was the healthy population for all except for the Nigerian study. There was a significant difference in the frequency of this polymorphism between African-Americans and Africans from Nigeria (15.8% vs. 24.2%, p = 0.005). The summary estimate was calculated for the association of CYP1A1 (AA-specific) variant (wt/var and var/var allele carriers combined) for African-Americans (meta OR: 1.14; 95% CI: 0.83-1.57) and was similar compared to the odds ratio reported for Nigerians (OR: 0.94; 95% CI = 0.60-1.48).
For lung cancer the three studies included African-American populations (319 cases and 626 controls)41,41,41,50,51,51,51 and all of the controls were from healthy populations. The summary estimate was also calculated for the association of CYP1A1 (AA-specific) variant (wt/var and var/var allele carriers combined) (meta OR: 1.00; 95% CI: 0.70-1.45). There was no evidence of heterogeneity among studies for lung (Q statistic: 1.65; p = 0.438; I2 = 0.0%, 95% CI: 0-90) and there was moderate heterogeneity between the African-American breast cancer studies (Q statistic: 5.61; p = 0.132; I2 = 47%, 95% CI: 0-82). There was no evidence of small-study effect for neither lung (p = 0.294) nor breast (p = 0.985).
CYP1B1 V432L
For all cancers combined six studies reported data separately for populations of African descent. Four were breast cancer studies and three of the four studies involved hospital controls. There were three breast cancer studies that included African-Americans (293 cases and 325 controls)48,52,53 and the fourth included a Nigerian population (228 cases and 226 controls)54. There was a significant difference in the frequency of this polymorphism between African-Americans and Africans from Nigeria (47.1% vs. 19.5%, p<0.0001). Neither the African-American studies nor the African study reported an association between the homozygote or heterozygote polymorphisms and breast cancer (data not shown). There was moderate heterogeneity between the African-American studies and no evidence of small-study effect (data not shown).
NQO1 (C609T)
Four studies on lung cancer and the NQO1 polymorphism in African populations were published and three of the four included healthy controls as comparison group (358 cases and 375 controls). All of the studies included African-Americans. No association with lung cancer was reported for the NQO1 (C609T) variant (CT + TT), (meta OR: 0.91; 95% CI = 0.67-1.23) and there was no evidence of heterogeneity among the studies (Q statistic: 0.68; p = 0.878; I2 = 0.0%, 95% CI: 0-85) nor small-study effect (p = 0.762).
CYP3A4 (A293G)
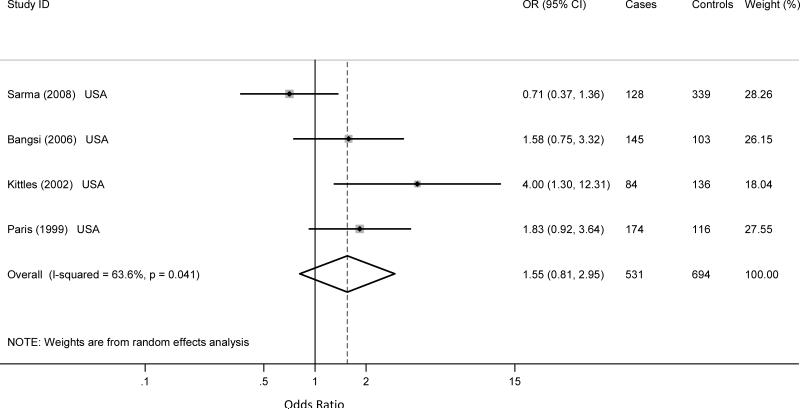
Four of the six studies involving the A293G polymorphism of the CYP3A4 gene were conducted on prostate cancer, for a total of 608 prostate cases and 776 controls. The other two studies were on breast cancer. There is a consensus among studies on the association between the polymorphism and prostate cancer in populations of African descent. Three of the four prostate cancer studies included African-American55-57 populations only while one included both African-American and Nigerian populations 58. There were 531 African-American cases and 694 controls; 77 cases and 82 controls that were Nigerian. There was a significant difference in the frequency of the CYP3A4 (A293G) polymorphism (GG) between African-Americans and Africans from Nigeria (41.2% vs. 74.4%, p<0.0001). While there was no association of CYP3A4 A293G homozygote variant and prostate cancer in Nigerians (OR: 0.3; 95% CI = 0.01-3.63), or in African-Americans the meta odds ratio was almost two-fold (meta OR: 1.55; 95% CI = 0.81-2.95) for African-Americans (Figure 1) but there was large between-study heterogeneity (Q statistic: 8.24; p = 0.041; I2 = 64.0%, 95% CI: 0-88); and no small-study effect (p = 0.231). Similarly, there was no association A293G heterozygous variants and prostate cancer for the Nigerian (OR: 0.38; 95% CI = 0.01-5.32) or the African-Americans (MetaOR: 1.26; 95% CI: 0.72-2.20) studies. There was large heterogeneity between the African-American studies (Q statistic: 6.63; p = 0.085; I2 = 55%, 95% CI: 0-85); and evidence of small-study effect (p = 0.05).
Figure 1.
Published case control studies show a significant association of the CYP3A4 (A293G) homozygous variant and prostate cancer in African-Americans. The shaded boxes represent the study-specific odds ratios, horizontal lines represent the confidence intervals; the size of the rectangles depict how each study is weighted in the analysis and the diamond represents the meta OR and its width represents the CI for the meta OR.
CYP17 (5'UTR)
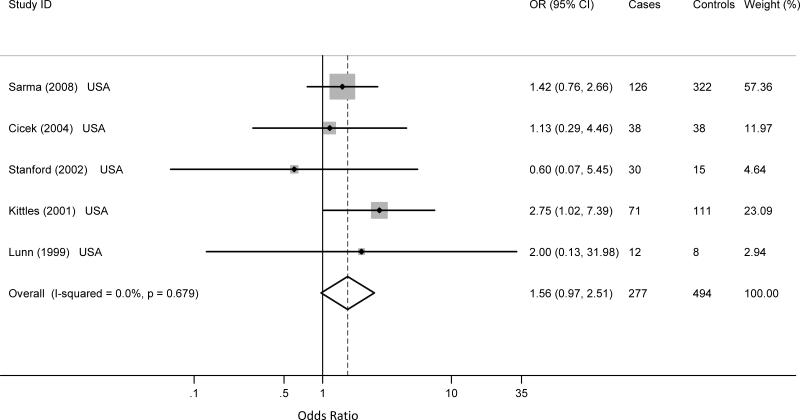
For all cancers combined, eight studies reported data for the CYP17 5'UTR polymorphism (595 cases and 839 controls). Five studies were conducted on prostate cancer, for a total of 277 cases and 494 controls 55,59-62. Two studies were on breast cancer48,63 and one study on lung cancer37.
All of the prostate cancer studies included African-American populations. The homozygous variant polymorphism was marginally associated with prostate cancer (meta OR: 1.56; 95% CI: 0.97 - 2.51), without evidence of heterogeneity among studies (Q statistics: 2.31; p = 0.679; I2 = 0.0%, 95% CI: 0-79); nor small-study effect (p = 0.759) (Figure 2). There was no statistically significant association between the heterozygous variant and prostate cancer (meta OR: 1.35; 95% CI: 0.65 – 2.80), although considerable between study heterogeneity was present (Q statistics: 13.39; p = 0.010; I2 = 70%, 95% CI: 24-88). There was no evidence of small-study effect (p = 0.276).
Figure 2.
Published case control studies show a marginal significant association of the CYP17 5'UTR homozygous variant and prostate cancer in African-Americans. The shaded boxes represent the study-specific odds ratios, horizontal lines represent the confidence intervals; the size of the rectangles depict how each study is weighted in the analysis and the diamond represents the meta OR and its width represents the CI for the meta OR.
DISCUSSION
This review of the literature indicates that while there is a wealth of studies on genetic polymorphisms and cancer risk, studies on populations of African descent are few. This observation is in contrast with the fact that for decades underrepresented minorities have shown higher mortality rates than most other ethnic groups from cancer 64,65. Despite the fact that studies have shown that genetic susceptibility to tobacco carcinogens increases individual cancer risk 66, information on individual susceptibility to tobacco and gene-environment interaction are lacking in subjects of African descent, mostly because they are underrepresented in current research studies. Therefore it is not yet possible to determine whether as association exists between a given genetic polymorphism and cancer risk in populations of African descent, and the degree of interaction between such polymorphisms and environmental exposure.
In the United States, studies have investigated reasons for the under representation of African-Americans in medical research. Shavers-Hornaday et al. reviewed the literature and reported that the reasons for underrepresentation of African-Americans in research may be due to participant barriers such as distrust; poor access; quality and utilization of health care; lack of knowledge about clinical trials; language and culture; as well as investigator barriers such as, failure to actively recruit participants due to pre-existing beliefs regarding the ability to recruit and retain participants; small number of minority research investigators; limited relationships between minority health care providers and fears of how research results will be interpreted 67. Nevertheless, the successful recruitment and participation of African-Americans have been accomplished by some investigators, although these studies are few. Efforts to increase the number of represented minority participants in research studies are warranted.
We have shown that for each gene, there are several different cancer types that were studied. The majority these include breast, prostate and lung cancers which are reported to be among the top four cancers with the highest incidence and mortality rates in African-Americans 3. Although several genes have been studied for various cancers, only one or two studies per gene for each cancer site have been published, with the exception of breast (CYP1A1 and CYP1B1), lung (GSTM1, CYP1A1, and NQO1) and prostate (CYP3A4 A293G and CYP17). For the breast cancer studies that reported data for African-Americans and Africans, the frequencies of the CYP1A1 African-American specific and CYP1B1 V432L variants in the controls were significantly different between the two populations. Neither of the two populations showed statistically significant associations of these genes and breast cancer. It is possible that the difference in gene variant frequencies between the two African-descent populations might be attributed to admixture among African-Americans, or to linkage disequilibrium with other relevant genetic polymorphisms suggesting that further studies in African descent populations are needed.
For prostate cancer a marginal association was observed for CYP3A4 A293G and CYP17 and prostate cancer in African-Americans while an inverse relationship was reported for CYP3A4 A293G in the single Nigerian study. Our findings suggest that the differences in prostate cancer risk for CYP3A4 A293G between African-Americans and Africans and the contribution of admixture in African-Americans needs further investigation. Studies of CYP3A4 A293G and prostate cancer in Caucasians report a marginally statistically significant increased odds ratio 56,58 ; two studies on CYP17 in Caucasians show no association59,62, one study reported an increased odds ratio61. Due to the small number of case-control studies in our meta analyses, the reproducibility of the reported results for each cancer/gene association could not be evaluated, which made it difficult to interpret the reported results.
The CYP17 gene encodes an enzyme which functions at key points in steroid hormone biosynthesis and metabolism pathways 68, and the polymorphism in the 5'-UTR is thought to affect hormone levels. High levels of androgens have been considered as risk factors for prostate cancer 69. However, the relationship between the CYP17 variant and increased hormone levels is inconclusive 70. In 2003, a meta analysis on the association between the CYP17 variant and prostate cancer was published and included 10 studies conducted in Europe, Asia and the United States (2,404 prostate cancer cases and 2755 controls) 71. The report showed that the overall contribution of the CYP17 5'UTR polymorphism to prostate cancer risk was not evident, however there were distinct differences based on ethnicity (European descent, OR: 1.04; 95% CI, 0.92–1.18; Asian descent OR: 1.06; 95% CI, 0.66–1.71; African descent OR: 1.56; 95% CI, 1.07–2.28). There were three studies in this meta analysis, 113 cases and 134 controls. While our meta analysis includes much larger population (5 studies, 277 cases and 494 controls), the findings from the earlier study was consistent with our findings and suggests a need for large-scale investigation of the association of the gene variant and prostate cancer risk in males of African descent. The gene product of CYP3A4 is involved in the oxidation of a large range of substrates including therapeutic drugs, steroids, fatty acids, and xenobiotics and similar to CYP17, also plays a role in androgen metabolism 72. It is biologically plausible that both these genetic polymorphisms may play a significant role in prostate cancer risk. Further, larger studies of the association of these gene variants and prostate cancer in populations of African descent are warranted.
Overall our findings support the need for larger studies of CYP1A1 Msp1 and breast cancer as well as CYP17 and CYP3A4 A293G and prostate cancer in African descent populations. The development of large-scale, population-based databases that document genetic variation in tobacco-related genes among case and control subjects that represent populations of African ancestry would serve as an important resource for cancer-control and prevention programs. Our findings support the need for additional genetic association studies of breast, prostate and lung cancers that include larger number of minority participants and a better definition of African ancestry. Specifically, there is a need to concentrate research on these three major cancers, since incidence and mortality ranks highest among populations of African descent. Furthermore, specific research focus is needed on genes with a sound hypothesis related to cancer risk in subjects of African descent. Our review of the literature reveals that the majority of studies have been conducted in the United States but still very few involve African or other populations of African ancestry such as African-Caribbean. This may be due to the limited financial and infrastructural resources available to conduct these studies. Nevertheless, it is clear that efforts to increase the number of studies in African descent populations outside of the US are warranted.
Supplementary Material
List of Supplemental Digital Content
Supplemental Digital Content 1: Table that describes the publications reporting on metabolic gene polymorphisms in populations of African descent. pdf
ACKNOWLEDGEMENTS
This work was supported in part by Grant Number KL2 RR024154-03 from the National Center for Research Resources (NCRR) 73, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research 74. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. This work was also supported in part by grant number R13CA130596A to CR and P20CA132385-01 to ET.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Daly AK, Cholerton S, Armstrong M, Idle JR. Genotyping for polymorphisms in xenobiotic metabolism as a predictor of disease susceptibility. Environ.Health Perspect. 1994;102:55–61. doi: 10.1289/ehp.94102s955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thier R, Bruning T, Roos PH, et al. Markers of genetic susceptibility in human environmental hygiene and toxicology: the role of selected CYP, NAT and GST genes. Int.J.Hyg.Environ.Health. 2003;206:149–71. doi: 10.1078/1438-4639-00209. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; Bethesda, MD: 2009. [Google Scholar]
- 4.Cote ML, Chen W, Smith DW, et al. Meta- and Pooled Analysis of GSTP1 Polymorphism and Lung Cancer: A HuGE-GSEC Review. American Journal of Epidemiology. 2009;169:802–14. doi: 10.1093/aje/kwn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langevin SM, Lin D, Matsuo K, et al. Review and pooled analysis of studies on MTHFR C677T polymorphism and esophageal cancer. Toxicology Letters. 2009;184:73–80. doi: 10.1016/j.toxlet.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paracchini V, Raimondi S, Gram IT, et al. Meta- and Pooled Analyses of the Cytochrome P-450 1B1 Val432Leu Polymorphism and Breast Cancer: A HuGE-GSEC Review. American Journal of Epidemiology. 2007;165:115–25. doi: 10.1093/aje/kwj365. [DOI] [PubMed] [Google Scholar]
- 7.Varela-Lema L, Taioli E, Ruano-Ravina A, et al. Meta-analysis and pooled analysis of GSTM1 and CYP1A1 polymorphisms and oral and pharyngeal cancers: a HuGE-GSEC review. Genet.Med. 2008;10:369–84. doi: 10.1097/GIM.0b013e3181770196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaspari L, Marinelli D, Taioli E. International collaborative study on genetic susceptibility to environmental carcinogens (GSEC): an update. Int J Hyg Environ Health. 2001;204:39–42. doi: 10.1078/1438-4639-00070. [DOI] [PubMed] [Google Scholar]
- 9.Hatagima A, Costa ECB, Marques CFS, Koifman RJ, Boffetta P, Koifman S. Glutathione S-transferase polymorphisms and oral cancer: A case-control study in Rio de Janeiro, Brazil. Oral Oncology. 2008;44:200–7. doi: 10.1016/j.oraloncology.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Honma HN, De Capitani EM, Perroud J, et al. Influence of p53 codon 72 exon 4, GSTM1, GSTT1 and GSTP1*B polymorphisms in lung cancer risk in a Brazilian population. Lung Cancer. 2008;61:152–62. doi: 10.1016/j.lungcan.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Dandara C, Parker MI. Association of cytochrome P450 2E1 genetic polymorphisms with squamous cell carcinoma of the oesophagus. Clin.Chem.Lab Med. 2005;43:370–5. doi: 10.1515/CCLM.2005.067. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Millikan RC, Bell DA, et al. Polychlorinated biphenyls, cytochrome P450 1A1 (CYP1A1) polymorphisms, and breast cancer risk among African American women and white women in North Carolina: a population-based case-control study. Breast Cancer Res. 2005;7:R12–R18. doi: 10.1186/bcr941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taioli E, Trachman J, Chen X, Toniolo P, Garte SJ. A CYP1A1 Restriction Fragment Length Polymorphism Is Associated with Breast Cancer in African-American Women. Cancer Research. 1995;55:3757–8. [PubMed] [Google Scholar]
- 14.Wenzlaff AS, Cote ML, Bock CH, et al. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26:2207–12. doi: 10.1093/carcin/bgi191. [DOI] [PubMed] [Google Scholar]
- 15.Feigelson HS, McKean-Cowdin R, Coetzee GA, Stram DO, Kolonel LN, Henderson BE. Building a Multigenic Model of Breast Cancer Susceptibility: CYP17 and HSD17B1 Are Two Important Candidates. Cancer Research. 2001;61:785–9. [PubMed] [Google Scholar]
- 16.Kato S, Shields PG, Caporaso NE, et al. Cytochrome P450IIE1 Genetic Polymorphisms, Racial Variation, and Lung Cancer Risk. Cancer Research. 1992;52:6712–5. [PubMed] [Google Scholar]
- 17.Kato S, Shields PG, Caporaso NE, et al. Analysis of cytochrome P450 2E1 genetic polymorphisms in relation to human lung cancer. Cancer Epidemiology Biomarkers Prevention. 1994;3:515–8. [PubMed] [Google Scholar]
- 18.Le Marchand L, Wilkens LR, Kolonel LN, Henderson BE. The MTHFR C677T Polymorphism and Colorectal Cancer: The Multiethnic Cohort Study. Cancer Epidemiology Biomarkers Prevention. 2005;14:1198–203. doi: 10.1158/1055-9965.EPI-04-0840. [DOI] [PubMed] [Google Scholar]
- 19.Le Marchand L, Donlon T, Kolonel LN, Henderson BE, Wilkens LR. Estrogen Metabolism-Related Genes and Breast Cancer Risk: The Multiethnic Cohort Study. Cancer Epidemiology Biomarkers Prevention. 2005;14:1998–2003. doi: 10.1158/1055-9965.EPI-05-0076. [DOI] [PubMed] [Google Scholar]
- 20.Yuan JM, Lu SC, Van Den BD, et al. Genetic polymorphisms in the methylenetetrahydrofolate reductase and thymidylate synthase genes and risk of hepatocellular carcinoma. Hepatology. 2007;46:749–58. doi: 10.1002/hep.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Chen L, Elahi A, Lazarus P, Tockman MS. Genetic analysis of microsomal epoxide hydrolase gene and its association with lung cancer risk. Eur.J Cancer Prev. 2005;14:223–30. doi: 10.1097/00008469-200506000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins GA, Cramer SD, Zheng SL, et al. Sequence variants in the human 25-hydroxyvitamin D3 1-alpha-hydroxylase (CYP27B1) gene are not associated with prostate cancer risk. Prostate. 2002;53:175–8. doi: 10.1002/pros.10144. [DOI] [PubMed] [Google Scholar]
- 23.Rao GG, Kurien A, Gossett D, Griffith WF, Coleman RL, Muller CY. A case-control study of methylenetetrahydrofolate reductase polymorphisms in cervical carcinogenesis. Gynecologic Oncology. 2006;101:250–4. doi: 10.1016/j.ygyno.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Wilborn TW, Lang NP, Smith M, Meleth S, Falany CN. Association of SULT2A1 allelic variants with plasma adrenal androgens and prostate cancer in African American men. The Journal of Steroid Biochemistry and Molecular Biology. 2006;99:209–14. doi: 10.1016/j.jsbmb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Zhu K, Hunter S, Payne-Wilks K, et al. Potential differences in breast cancer risk factors based on CYP1A1 MspI and African-American-specific genotypes. Ethn.Dis. 2006;16:207–15. [PubMed] [Google Scholar]
- 26.Haiman CA, Stram DO, Pike MC, et al. A comprehensive haplotype analysis of CYP19 and breast cancer risk: the Multiethnic Cohort. Human Molecular Genetics. 2003;12:2679–92. doi: 10.1093/hmg/ddg294. [DOI] [PubMed] [Google Scholar]
- 27.Setiawan VW, Cheng I, Stram DO, et al. A Systematic Assessment of Common Genetic Variation in CYP11A and Risk of Breast Cancer. Cancer Research. 2006;66:12019–25. doi: 10.1158/0008-5472.CAN-06-1101. [DOI] [PubMed] [Google Scholar]
- 28.Hoque MO, Feng Q, Toure P, et al. Detection of Aberrant Methylation of Four Genes in Plasma DNA for the Detection of Breast Cancer. Journal of Clinical Oncology. 2006;24:4262–9. doi: 10.1200/JCO.2005.01.3516. [DOI] [PubMed] [Google Scholar]
- 29.Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd.ed. BMJ; London, UK: 2001. [Google Scholar]
- 30.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat.Med. 1991;10:1665–77. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–6. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat.Med. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 34.Mallick S, Romana M, Blanchet P, Multigner L. GSTM1 and GSTT1 polymorphisms and the risk of prostate cancer in a Caribbean population of African descent. Urology. 2007;69:1165–9. doi: 10.1016/j.urology.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Beuten J, Gelfond JA, Franke JL, et al. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiology Biomarkers Prevention. 2009;18:1869–80. doi: 10.1158/1055-9965.EPI-09-0076. [DOI] [PubMed] [Google Scholar]
- 36.Stone A, Ratnasinghe LD, Emerson GL, et al. CYP3A43 Pro340Ala Polymorphism and Prostate Cancer Risk in African Americans and Caucasians. Cancer Epidemiology Biomarkers Prevention. 2005;14:1257–61. doi: 10.1158/1055-9965.EPI-04-0534. [DOI] [PubMed] [Google Scholar]
- 37.Cote ML, Yoo W, Wenzlaff AS, et al. Tobacco and estrogen metabolic polymorphisms and risk of non-small cell lung cancer in women. Carcinogenesis. 2009;30:626–35. doi: 10.1093/carcin/bgp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li DP, Dandara C, Walther G, Parker MI. Genetic polymorphisms of alcohol metabolising enzymes: their role in susceptibility to oesophageal cancer. Clin Chem Lab Med. 2008;46:323–8. doi: 10.1515/CCLM.2008.073. [DOI] [PubMed] [Google Scholar]
- 39.Ishibe N, Wiencke JK, Zuo ZF, McMillan A, Spitz M, Kelsey KT. Susceptibility to lung cancer in light smokers associated with CYP1A1 polymorphisms in Mexican- and African-Americans. Cancer Epidemiology Biomarkers Prevention. 1997;6:1075–80. [PubMed] [Google Scholar]
- 40.Shields PG, Caporaso NE, Falk RT, et al. Lung cancer, race, and a CYP1A1 genetic polymorphism. Cancer Epidemiology Biomarkers Prevention. 1993;2:481–5. [PubMed] [Google Scholar]
- 41.Taioli E, Ford J, Trachman J, Li Y, Demopoulos R, Garte S. Lung cancer risk and CYP1A1 genotype in African Americans. Carcinogenesis. 1998;19:813–7. doi: 10.1093/carcin/19.5.813. [DOI] [PubMed] [Google Scholar]
- 42.Wrensch MR, Miike R, Sison JD, et al. CYP1A1 variants and smoking-related lung cancer in San Francisco Bay area Latinos and African Americans. Int J Cancer. 2005;113:141–7. doi: 10.1002/ijc.20537. [DOI] [PubMed] [Google Scholar]
- 43.Sugimura H, Suzuki I, Hamada GS, et al. Cytochrome P-450 lA1 genotype in lung cancer patients and controls in Rio de Janeiro, Brazil. Cancer Epidemiology Biomarkers Prevention. 1994;3:145–8. [PubMed] [Google Scholar]
- 44.Honma HN, De Capitani EM, Barbeiro AS, Costa DB, Morcillo A, Zambon L. Polymorphism of the CYP1A1*2A gene and susceptibility to lung cancer in a Brazilian population. J Bras.Pneumol. 2009;35:767–72. doi: 10.1590/s1806-37132009000800008. [DOI] [PubMed] [Google Scholar]
- 45.Bailey LR, Roodi N, Verrier CS, Yee CJ, Dupont WD, Parl FF. Breast Cancer and CYPIA1, GSTM1, and GSTT1 Polymorphisms: Evidence of a Lack of Association in Caucasians and African Americans. Cancer Research. 1998;58:65–70. [PubMed] [Google Scholar]
- 46.Li Y, Millikan RC, Bell DA, et al. Cigarette smoking, cytochrome P4501A1 polymorphisms, and breast cancer among African-American and white women. Breast Cancer Res. 2004;6:R460–R473. doi: 10.1186/bcr814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taioli E, Bradlow HL, Garbers SV, et al. Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detect.Prev. 1999;23:232–7. doi: 10.1046/j.1525-1500.1999.09912.x. [DOI] [PubMed] [Google Scholar]
- 48.Kato I, Cichon M, Yee CL, Land S, Korczak JF. African American-preponderant single nucleotide polymorphisms (SNPs) and risk of breast cancer. Cancer Epidemiol. 2009;33:24–30. doi: 10.1016/j.canep.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okobia M, Bunker C, Zmuda J, et al. Cytochrome P4501A1 genetic polymorphisms and breast cancer risk in Nigerian women. Breast Cancer Res Treat. 2005;94:285–93. doi: 10.1007/s10549-005-9022-x. [DOI] [PubMed] [Google Scholar]
- 50.Kelsey KT, Wiencke JK, Spitz MR. A race-specific genetic polymorphism in the CYP1A1 gene is not associated with lung cancer in African Americans. Carcinogenesis. 1994;15:1121–4. doi: 10.1093/carcin/15.6.1121. [DOI] [PubMed] [Google Scholar]
- 51.London SJ, Daly AK, Fairbrother KS, et al. Lung Cancer Risk in African-Americans in Relation to a Race-specific CYP1A1 Polymorphism. Cancer Research. 1995;55:6035–7. [PubMed] [Google Scholar]
- 52.Bailey LR, Roodi N, Dupont WD, Parl FF. Association of Cytochrome P450 1B1 (CYP1B1) Polymorphism with Steroid Receptor Status in Breast Cancer. Cancer Research. 1998;58:5038–41. [PubMed] [Google Scholar]
- 53.Van Emburgh BO, Hu JJ, Levine EA, et al. Polymorphisms in CYP1B1, GSTM1, GSTT1 and GSTP1, and susceptibility to breast cancer. Oncol Rep. 2008;19:1311–21. [PMC free article] [PubMed] [Google Scholar]
- 54.Okobia MN, Bunker CH, Garte SJ, et al. Cytochrome P450 1B1 Val432Leu polymorphism and breast cancer risk in Nigerian women: a case control study. Infect.Agent.Cancer. 2009;4(Suppl 1):S12. doi: 10.1186/1750-9378-4-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarma AV, Dunn RL, Lange LA, et al. Genetic polymorphisms in CYP17, CYP3A4, CYP19A1, SRD5A2, IGF-1, and IGFBP-3 and prostate cancer risk in African-American men: the Flint Men's Health Study. Prostate. 2008;68:296–305. doi: 10.1002/pros.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bangsi D, Zhou J, Sun Y, et al. Impact of a genetic variant in CYP3A4 on risk and clinical presentation of prostate cancer among white and African-American men. Urol.Oncol. 2006;24:21–7. doi: 10.1016/j.urolonc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Paris PL, Kupelian PA, Hall JM, et al. Association between a CYP3A4 Genetic Variant and Clinical Presentation in African-American Prostate Cancer Patients. Cancer Epidemiology Biomarkers Prevention. 1999;8:901–5. [PubMed] [Google Scholar]
- 58.Kittles RA, Chen W, Panguluri RK, et al. CYP3A4-V and prostate cancer in African Americans: causal or confounding association because of population stratification? Hum Genet. 2002;110:553–60. doi: 10.1007/s00439-002-0731-5. [DOI] [PubMed] [Google Scholar]
- 59.Stanford JL, Noonan EA, Iwasaki L, et al. A polymorphism in the CYP17 gene and risk of prostate cancer. Cancer Epidemiology Biomarkers Prevention. 2002;11:243–7. [PubMed] [Google Scholar]
- 60.Kittles RA, Panguluri RK, Chen W, et al. Cyp17 promoter variant associated with prostate cancer aggressiveness in African Americans. Cancer Epidemiology Biomarkers Prevention. 2001;10:943–7. [PubMed] [Google Scholar]
- 61.Lunn RM, Bell DA, Mohler JL, Taylor JA. Prostate cancer risk and polymorphism in 17 hydroxylase (CYP17) and steroid reductase (SRD5A2). Carcinogenesis. 1999;20:1727–31. doi: 10.1093/carcin/20.9.1727. [DOI] [PubMed] [Google Scholar]
- 62.Cicek MS, Conti DV, Curran A, et al. Association of prostate cancer risk and aggressiveness to androgen pathway genes: SRD5A2, CYP17, and the AR. Prostate. 2004;59:69–76. doi: 10.1002/pros.10358. [DOI] [PubMed] [Google Scholar]
- 63.Weston A, Pan CF, Bleiweiss IJ, et al. CYP17 genotype and breast cancer risk. Cancer Epidemiology Biomarkers Prevention. 1998;7:941–4. [PubMed] [Google Scholar]
- 64.DeLancey JO, Thun MJ, Jemal A, Ward EM. Recent Trends in Black-White Disparities in Cancer Mortality. Cancer Epidemiology Biomarkers Prevention. 2008;17:2908–12. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 65.Piffath TA, Whiteman MK, Flaws JA, Fix AD, Bush TL. Ethnic Differences in Cancer Mortality Trends in the US, 1950-1992. Ethnicity & Health. 2001;6:105–19. doi: 10.1080/13557850120068432. [DOI] [PubMed] [Google Scholar]
- 66.Taioli E. Gene-environment interaction in tobacco-related cancers. Carcinogenesis. 2008;29:1467–74. doi: 10.1093/carcin/bgn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shavers-Hornaday VL, Lynch CF. Why are African Americans under-represented in medical.. Ethnicity & Health. 1997;2:31. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- 68.Picado-Leonard J, Miller WL. Cloning and sequence of the human gene for P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA. 1987;6:439–48. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- 69.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective Study of Sex Hormone Levels and Risk of Prostate Cancer. JNCI Journal of the National Cancer Institute. 1996;88:1118–26. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 70.Sharp L, Cardy AH, Cotton SC, Little J. CYP17 Gene Polymorphisms: Prevalence and Associations with Hormone Levels and Related Factors. A HuGE Review. American Journal of Epidemiology. 2004;160:729–40. doi: 10.1093/aje/kwh287. [DOI] [PubMed] [Google Scholar]
- 71.Ntais C, Polycarpou A, Ioannidis JPA. Association of the CYP17 Gene Polymorphism with the Risk of Prostate Cancer: A Meta-Analysis. Cancer Epidemiology Biomarkers Prevention. 2003;12:120–6. [PubMed] [Google Scholar]
- 72.Keshava C, McCanlies EC, Weston A. CYP3A4 polymorphisms--potential risk factors for breast and prostate cancer: a HuGE review. Am.J.Epidemiol. 2004;160:825–41. doi: 10.1093/aje/kwh294. [DOI] [PubMed] [Google Scholar]
- 73.National Institutes of Health National Institutes of Health, National Center for Research Resources . 2007 [Google Scholar]
- 74.NIH Roadmap for Medical Research NIH Roadmap for Medical Research, Re-engineering the Clinical Research Enterprise . 2007 [Google Scholar]
- 75.Rebbeck TR, Troxel AB, Walker AH, et al. Pairwise Combinations of Estrogen Metabolism Genotypes in Postmenopausal Breast Cancer Etiology. Cancer Epidemiology Biomarkers Prevention. 2007;16:444–50. doi: 10.1158/1055-9965.EPI-06-0800. [DOI] [PubMed] [Google Scholar]
- 76.Millikan RC. NAT1*10 and NAT1*11 Polymorphisms and Breast Cancer Risk. Cancer Epidemiology Biomarkers Prevention. 2000;9:217–9. [PubMed] [Google Scholar]
- 77.Le Marchand L, Haiman CA, Wilkens LR, Kolonel LN, Henderson BE. MTHFR Polymorphisms, Diet, HRT, and Breast Cancer Risk: The Multiethnic Cohort Study. Cancer Epidemiology Biomarkers Prevention. 2004;13:2071–7. [PubMed] [Google Scholar]
- 78.Millikan RC, Pittman GS, Tse CK, et al. Catechol-O-methyltransferase and breast cancer risk. Carcinogenesis. 1998;19:1943–7. doi: 10.1093/carcin/19.11.1943. [DOI] [PubMed] [Google Scholar]
- 79.Agalliu I, Langeberg WJ, Lampe JW, Salinas CA, Stanford JL. Glutathione S-transferase M1, T1, and P1 polymorphisms and prostate cancer risk in middle-aged men. Prostate. 2006;66:146–56. doi: 10.1002/pros.20305. [DOI] [PubMed] [Google Scholar]
- 80.Rybicki BA, Neslund-Dudas C, Nock NL, et al. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with the GSTP1 Ile105Val polymorphism. Cancer Detect Prev. 2006;30:412–22. doi: 10.1016/j.cdp.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ning B, Wang C, Morel F, et al. Human glutathione S-transferase A2 polymorphisms: variant expression, distribution in prostate cancer cases/controls and a novel form. Pharmacogenetics. 2004;14:35–44. doi: 10.1097/00008571-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Plummer SJ, Conti DV, Paris PL, Curran AP, Casey G, Witte JS. CYP3A4 and CYP3A5 genotypes, haplotypes, and risk of prostate cancer. Cancer Epidemiology Biomarkers Prevention. 2003;12:928–32. [PubMed] [Google Scholar]
- 83.Hooker S, Bonilla C, Akereyeni F, Ahaghotu C, Kittles RA. NAT2 and NER genetic variants and sporadic prostate cancer susceptibility in African Americans. Prostate Cancer Prostatic.Dis. 2008;11:349–56. doi: 10.1038/sj.pcan.4501027. [DOI] [PubMed] [Google Scholar]
- 84.Singal R, Ferdinand L, Das PM, Reis IM, Schlesselman JJ. Polymorphisms in the methylenetetrahydrofolate reductase gene and prostate cancer risk. Int J Oncol. 2004;25:1465–71. [PubMed] [Google Scholar]
- 85.Nowell S, Ratnasinghe DL, Ambrosone CB, et al. Association of SULT1A1 phenotype and genotype with prostate cancer risk in African-Americans and Caucasians. Cancer Epidemiology Biomarkers Prevention. 2004;13:270–6. doi: 10.1158/1055-9965.epi-03-0047. [DOI] [PubMed] [Google Scholar]
- 86.Ford JG, Li Y, O'Sullivan MM, et al. Glutathione S-transferase M1 polymorphism and lung cancer risk in African-Americans. Carcinogenesis. 2000;21:1971–5. doi: 10.1093/carcin/21.11.1971. [DOI] [PubMed] [Google Scholar]
- 87.Kelsey KT, Spitz MR, Zuo ZF, Wiencke JK. Polymorphisms in the glutathione S-transferase class mu and theta genes interact and increase susceptibility to lung cancer in minority populations (Texas, United States). Cancer Causes Control. 1997;8:554–9. doi: 10.1023/a:1018434027502. [DOI] [PubMed] [Google Scholar]
- 88.London SJ, Daly AK, Cooper J, Navidi WC, Carpenter CL, Idle JR. Polymorphism of Glutathione S-Transferase M1 and Lung Cancer Risk Among African-Americans and Caucasians in Los Angeles County, California. JNCI Journal of the National Cancer Institute. 1995;87:1246–53. doi: 10.1093/jnci/87.16.1246. [DOI] [PubMed] [Google Scholar]
- 89.Wrensch M, Kelsey KT, Liu M, et al. Glutathione-S-transferase variants and adult glioma. Cancer Epidemiology Biomarkers Prevention. 2004;13:461–7. [PubMed] [Google Scholar]
- 90.Cote ML, Wenzlaff AS, Bock CH, et al. Combinations of cytochrome P-450 genotypes and risk of early-onset lung cancer in Caucasians and African Americans: a population-based study. Lung Cancer. 2007;55:255–62. doi: 10.1016/j.lungcan.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.London SJ, Daly AK, Leathart JB, Navidi WC, Idle JR. Lung cancer risk in relation to the CYP2C9*1/CYP2C9*2 genetic polymorphism among African-Americans and Caucasians in Los Angeles County, California. Pharmacogenetics. 1996;6:527–33. doi: 10.1097/00008571-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 92.London SJ, Daly AK, Leathart JB, Navidi WC, Carpenter CC, Idle JR. Genetic polymorphism of CYP2D6 and lung cancer risk in African-Americans and Caucasians in Los Angeles County. Carcinogenesis. 1997;18:1203–14. doi: 10.1093/carcin/18.6.1203. [DOI] [PubMed] [Google Scholar]
- 93.Wu X, Shi H, Jiang H, et al. Associations between cytochrome P4502E1 genotype, mutagen sensitivity, cigarette smoking and susceptibility to lung cancer. Carcinogenesis. 1997;18:967–73. doi: 10.1093/carcin/18.5.967. [DOI] [PubMed] [Google Scholar]
- 94.London SJ, Daly AK, Cooper J, et al. Lung cancer risk in relation to the CYP2E1 Rsa I genetic polymorphism among African-Americans and Caucasians in Los Angeles County. Pharmacogenetics. 1996;6:151–8. doi: 10.1097/00008571-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 95.London SJ, Smart J, Daly AK. Lung cancer risk in relation to genetic polymorphisms of microsomal epoxide hydrolase among African-Americans and Caucasians in Los Angeles County. Lung Cancer. 2000;28:147–55. doi: 10.1016/s0169-5002(99)00130-0. [DOI] [PubMed] [Google Scholar]
- 96.Wu X, Gwyn K, Amos CI, Makan N, Hong WK, Spitz MR. The association of microsomal epoxide hydrolase polymorphisms and lung cancer risk in African-Americans and Mexican-Americans. Carcinogenesis. 2001;22:923–8. doi: 10.1093/carcin/22.6.923. [DOI] [PubMed] [Google Scholar]
- 97.Bock CH, Wenzlaff AS, Cote ML, Land SJ, Schwartz AG. NQO1 T allele associated with decreased risk of later age at diagnosis lung cancer among never smokers: results from a population-based study. Carcinogenesis. 2005;26:381–6. doi: 10.1093/carcin/bgh314. [DOI] [PubMed] [Google Scholar]
- 98.Saldivar SJ, Wang Y, Zhao H, et al. An association between a NQO1 genetic polymorphism and risk of lung cancer. Mutat Res. 2005;582:71–8. doi: 10.1016/j.mrgentox.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 99.Wiencke JK, Spitz MR, McMillan A, Kelsey KT. Lung cancer in Mexican-Americans and African-Americans is associated with the wild-type genotype of the NAD(P)H: quinone oxidoreductase polymorphism. Cancer Epidemiology Biomarkers Prevention. 1997;6:87–92. [PubMed] [Google Scholar]
- 100.London SJ, Lehman TA, Taylor JA. Myeloperoxidase genetic polymorphism and lung cancer risk. Cancer Res. 1997;57:5001–3. [PubMed] [Google Scholar]
- 101.Kirk GD, Turner PC, Gong Y, et al. Hepatocellular Carcinoma and Polymorphisms in Carcinogen-Metabolizing and DNA Repair Enzymes in a Population with Aflatoxin Exposure and Hepatitis B Virus Endemicity. Cancer Epidemiology Biomarkers Prevention. 2005;14:373–9. doi: 10.1158/1055-9965.EPI-04-0161. [DOI] [PubMed] [Google Scholar]
- 102.Tiemersma EW, Omer RE, Bunschoten A, et al. Role of Genetic Polymorphism of Glutathione-S-Transferase T1 and Microsomal Epoxide Hydrolase in Aflatoxin-associated Hepatocellular Carcinoma. Cancer Epidemiology Biomarkers Prevention. 2001;10:785–91. [PubMed] [Google Scholar]
- 103.Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW. Genetic Risk and Carcinogen Exposure: a Common Inherited Defect of the Carcinogen-Metabolism Gene Glutathione S-Transferase M1 (GSTM1) That Increases Susceptibility to Bladder Cancer. JNCI Journal of the National Cancer Institute. 1993;85:1159–64. doi: 10.1093/jnci/85.14.1159. [DOI] [PubMed] [Google Scholar]
- 104.Taylor JA, Umbach DM, Stephens E, et al. The Role of N-Acetylation Polymorphisms in Smoking-associated Bladder Cancer: Evidence of a Gene-Gene-Exposure Three-Way Interaction. Cancer Research. 1998;58:3603–10. [PubMed] [Google Scholar]
- 105.Duell EJ, Holly EA, Bracci PM, Liu M, Wiencke JK, Kelsey KT. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J Natl Cancer Inst. 2002;94:297–306. doi: 10.1093/jnci/94.4.297. [DOI] [PubMed] [Google Scholar]
- 106.Li D, Ahmed M, Li Y, et al. 5,10-Methylenetetrahydrofolate reductase polymorphisms and the risk of pancreatic cancer. Cancer Epidemiology Biomarkers Prevention. 2005;14:1470–6. doi: 10.1158/1055-9965.EPI-04-0894. [DOI] [PubMed] [Google Scholar]
- 107.Sweeney C, Farrow DC, Schwartz SM, Eaton DL, Checkoway H, Vaughan TL. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma: a case-control study. Cancer Epidemiology Biomarkers Prevention. 2000;9:449–54. [PubMed] [Google Scholar]
- 108.Olshan AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiology Biomarkers Prevention. 2000;9:185–91. [PubMed] [Google Scholar]
- 109.Park JY, Schantz SP, Stern JC, Kaur T, Lazarus P. Association between glutathione S-transferase pi genetic polymorphisms and oral cancer risk. Pharmacogenetics. 1999;9:497–504. [PubMed] [Google Scholar]
- 110.Liu S, Park JY, Schantz SP, Stern JC, Lazarus P. Elucidation of CYP2E1 5' regulatory RsaI/Pstl allelic variants and their role in risk for oral cancer. Oral Oncol. 2001;37:437–45. doi: 10.1016/s1368-8375(00)00099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park JY, Stimson PS, Lazarus P. Epoxide hydrolase genotype and orolaryngeal cancer risk: interaction with GSTM1 genotype. Oral Oncol. 2003;39:483–90. doi: 10.1016/s1368-8375(03)00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olshan AF, Weissler MC, Watson MA, Bell DA. Risk of head and neck cancer and the alcohol dehydrogenase 3 genotype. Carcinogenesis. 2001;22:57–61. doi: 10.1093/carcin/22.1.57. [DOI] [PubMed] [Google Scholar]
- 113.Huang K, Sandler RS, Millikan RC, Schroeder JC, North KE, Hu J. GSTM1 and GSTT1 polymorphisms, cigarette smoking, and risk of colon cancer: a population-based case-control study in North Carolina (United States). Cancer Causes Control. 2006;17:385–94. doi: 10.1007/s10552-005-0424-1. [DOI] [PubMed] [Google Scholar]
- 114.Butler LM, Millikan RC, Sinha R, et al. Modification by N-acetyltransferase 1 genotype on the association between dietary heterocyclic amines and colon cancer in a multiethnic study. Mutat.Res. 2008;638:162–74. doi: 10.1016/j.mrfmmm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keku T, Millikan R, Worley K, et al. 5,10-Methylenetetrahydrofolate Reductase Codon 677 and 1298 Polymorphisms and Colon Cancer in African Americans and Whites. Cancer Epidemiology Biomarkers Prevention. 2002;11:1611–21. [PubMed] [Google Scholar]
- 116.Holt SK, Rossing MA, Malone KE, Schwartz SM, Weiss NS, Chen C. Ovarian Cancer Risk and Polymorphisms Involved in Estrogen Catabolism. Cancer Epidemiology Biomarkers Prevention. 2007;16:481–9. doi: 10.1158/1055-9965.EPI-06-0831. [DOI] [PubMed] [Google Scholar]
- 117.Sellers TA, Schildkraut JM, Pankratz VS, et al. Estrogen bioactivation, genetic polymorphisms, and ovarian cancer. Cancer Epidemiology Biomarkers Prevention. 2005;14:2536–43. doi: 10.1158/1055-9965.EPI-05-0142. [DOI] [PubMed] [Google Scholar]
- 118.Chen CL, Liu Q, Pui CH, et al. Higher Frequency of Glutathione S-Transferase Deletions in Black Children With Acute Lymphoblastic Leukemia. Blood. 1997;89:1701–7. [PubMed] [Google Scholar]
- 119.Dandara C, Ballo R, Parker MI. CYP3A5 genotypes and risk of oesophageal cancer in two South African populations. Cancer Lett. 2005;225:275–82. doi: 10.1016/j.canlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 120.Dandara C, Li DP, Walther G, Parker MI. Gene-environment interaction: the role of SULT1A1 and CYP3A5 polymorphisms as risk modifiers for squamous cell carcinoma of the oesophagus. Carcinogenesis. 2006;27:791–7. doi: 10.1093/carcin/bgi257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Supplemental Digital Content
Supplemental Digital Content 1: Table that describes the publications reporting on metabolic gene polymorphisms in populations of African descent. pdf