Abstract
Goals of Work
Despite advances in allogeneic hematopoietic stem cell transplantation (HSCT), post-transplant complications are common and patients’ symptom experience has not been well documented.
Purpose
To characterize the symptom experience of adult patients pre-transplantation and days 0, 30 and 100 after allogeneic HSCT.
Methods
Data from 76 participants enrolled in a prospective Health-Related Quality of Life (HRQL) study were used. Symptom occurrence, distress, and clusters were determined based on the 11 symptoms of the Symptom Distress Scale (SDS).
Results
Participants were on average 40 years old (SD ± 13.5). The majority (54%) received reduced intensity conditioning. Prevalent symptoms included fatigue (68%) and worry (68%) at baseline; appetite change (88%) at day 0; and fatigue at days 30 (90%) and 100 (81%). Participants reported the following symptoms as severely distressing: worry (16%) [baseline], insomnia (32%) [Day 0], appetite change (22%) [Day 30] and fatigue (11%) [Day 100]. The total SDS score was highest at day 0 (M = 26.6 ± 7.6) when the highest number of symptoms were reported [Mdn = 8 (1 - 11)]. Symptoms formed clusters comprised of fatigue, appearance change, and worry at baseline, and fatigue, insomnia and bowel changes at days 0 and 30. Compared to those with low symptom distress, participants with moderate/severe symptom distress reported poorer HRQL.
Conclusion
Allogeneic HSCT patients present for transplantation with low symptom distress yet experience multiple symptoms and high symptom distress after HSCT conditioning. Understanding the symptom experience of allogeneic HSCT patients can guide management strategies and improve HRQL.
Keywords: Complications, Cancer, Recovery, Clinical Assessment, Symptom Burden
Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) is an intense and potentially curative treatment for many hematologic diseases. The treatment involves intensive radiotherapy and/or chemotherapy to achieve the immunosuppression necessary to permit subsequent engraftment of healthy hematopoietic stem cells from a related or unrelated donor. This preparative regimen together with the effects of acute and chronic graft versus host disease (GVHD) and the immunosuppressants used to limit GVHD are associated with a wide range of toxicities [1-3]. These include pancytopenia, infection, bleeding, mucositis, weight loss or weight gain, esophagitis, nausea, vomiting, diarrhea, anorexia, skin rash, graft failure, pain, mood disturbances and changes in body image and appearance. The symptom experience, in combination with biobehavioral factors unique to the patient and family, can result in a range of responses to treatment, including anxiety, depression, hopelessness, functional morbidity, and even premature withdrawal from treatment and/or nonadherence [4,5]. Reducing the burden of symptoms is therefore an important treatment goal. To this end, an understanding of the symptom experience to inform the development of interventions is essential.
The process of allogeneic HSCT can be divided into several phases. The conditioning or preparatory stage begins 7-10 days prior to the infusion of stem cells. During this phase, the patient receives intensive radiotherapy and/or high dose chemotherapy. After the preparative regimen and stem cell infusion, patients are typically hospitalized for 2-4 weeks while they recover their blood counts and are closely monitored for infection and other acute complications such as GVHD. Once blood counts have recovered and acute toxicities resolved, patients are discharged to the local community, returning to the outpatient clinic at the transplant center 2-3 times per week for evaluation. During this time, patients and their caregivers are responsible for self-management of treatment side effects and a complex array of medications, including immunosuppressants. If major complications are largely resolved, patients are able to return to their home community approximately 100 days after transplantation.
Few prior studies have examined the symptom experience in the early post-transplant period through the first 100 days of recovery. These studies have been composed of small mixed autologous and allogeneic samples, and have reported only limited symptom data derived from health related quality of life (HRQL) measures [6-14]. Moreover, there has been no systematic study of the symptom experience, including symptom clusters, in allogeneic HSCT recipients during the process of hospital discharge and transition to the community. An improved understanding of the symptom experience in allogeneic HSCT recipients through each of these important transitions is needed and would be valuable for planning interventions to reduce the burdensome consequences of uncontrolled symptoms.
A symptom may be defined as a subjective experience reflecting changes in the biopsychosocial functioning, sensations or cognition of an individual [15]. The patient’s experience of symptoms is multidimensional and includes physiologic sensations and interpretive processes through which patients make meaning of their symptoms and decide how to respond to them. Several authors suggest that dimensions of the symptom experience include symptom occurrence, frequency/duration, quality/intensity, interference with function, distress and bother [15-21].
Although research has often focused on single symptoms, it is also recognized that several symptoms may be experienced concurrently. The simultaneous occurrence of symptoms, termed symptom clusters, may have a deleterious effect on health outcomes such as functional status and quality of life [22-25]. Symptom cluster work is still in a preliminary stage of conceptual and methodological development and consensus on the precise definition of a symptom cluster and the optimal statistical methods for identifying symptom clusters has not been reached. Dodd et al. [24] have defined a symptom cluster as three or more concurrent symptoms (e.g. pain, insomnia and fatigue) that are related to each other. To date studies have explored the identification of symptom clusters in patients with lung cancer, breast cancer, and mixed solid tumors [25-29]. Efforts to strengthen the definition of a symptom cluster and to develop more sophisticated analytic strategies to identify clusters and explore their impact on health outcomes is ongoing [22, 25, 30-33].
The aim of this study was to examine the symptom experience of adult patients at four time points during early recovery following allogeneic HSCT. The study was guided by the following research questions. At baseline and days 0, 30 and 100 following allogeneic HSCT: (1) what symptoms are most prevalent? (2) what symptoms are most distressing? (3) what symptoms form the most prevalent symptom cluster? (4) what is the relationship between symptom distress and selected demographic and clinical characteristics? We also explored the association of symptom distress with HRQL at 30 and 100 days following allogeneic HSCT.
Methods
Design and Participants
This study used data collected as part of a prospective, longitudinal study of HRQL in adult patients with hematologic disorders undergoing their first allogeneic HSCT from a histocompatible leukocyte antigen-identical family donor. The study was conducted at the National Institutes of Health Clinical Center between October 1999 and September 2002. After institutional review board approval, data were gathered from patients who were literate in English or Spanish. Study procedures were completed in the patient’s primary language.
Symptom Distress Scale (SDS) and Medical Outcomes Short Form 36 Health Survey (SF-36 version 1) data were gathered at the following four time points pre-transplant and during the early transplant recovery period: 1) baseline (before transplant conditioning commenced) and 2) days 0, 30 and 100 after allogeneic HSCT. Of the 78 participants who completed baseline questionnaires, 2 participants withdrew prior to day 30 owing to language difficulties and were excluded from the analysis. Therefore, 76 participants comprised the sample for this analysis. Patient acuity and death occurring across the study period accounted for the attrition pattern. This attrition pattern reflects the treatment intensity of allogeneic HSCT, and has been previously reported, together with the primary findings of this study [34].
Measures
Symptom Experience
The SDS was used to capture symptom occurrence and symptom distress, and to provide data for symptom cluster analysis. The SDS is a 13 item self-report questionnaire designed to measure the degree of distress associated with 11 symptoms: nausea, appetite change, insomnia, pain, fatigue, bowel changes, concentration, appearance, worry (outlook), breathing, and cough [35]. For each symptom, participants rate “how they have been feeling lately” on a Likert scale with 1 indicating no problem with the symptom and 5 indicating the maximum amount of problem. For two of the symptoms, pain and nausea, participants also rate the frequency with which they experience these symptoms.
Symptom occurrence was defined in terms of symptom prevalence and symptom count. Symptom prevalence was defined by a participants’ rating of ≥ 2 for any of the 11 symptoms on the SDS. Therefore, the symptom was considered as present for participants who rated a symptom as 2. To characterize the occurrence of multiple symptoms, a symptom count score was derived from the total number of symptoms indicated by each participant as present.
Symptom distress was defined by participant rating of the degree of distress for each SDS symptom and total SDS score. The degree of distress associated with each symptom on the SDS was indicated as mild (item score of 2), moderate (item score of 3) or severe (item score of 4 or 5). A total symptom distress score was derived from the SDS by summing participants’ ratings of the 13 symptom items. The total SDS score range from 13 to 65 with higher scores indicating a greater degree of distress. Clinical interpretive guidelines as described by the author of the measure suggest a total SDS score less than 25 indicates low symptom distress, 25 to 32 indicates moderate distress, and 33 and above indicates severe distress [35]. As a measure of overall symptom distress, the SDS has demonstrated evidence of reliability and validity in a wide variety of patients with cancer including those with hematological diseases, patients with other chronic illnesses [35] and Spanish-speaking study samples [14, 35-39].
A Symptom cluster was defined as the simultaneous occurrence of at least 3 of the 11 symptoms as measured by the SDS. Symptoms were considered to form a cluster if they were at least moderately (rs > 0.30) and significantly (p<0.01) related to one another, and simultaneously were independent of other SDS symptom(s) (rs ≤ 0.30).
Health Related Quality of Life
The Physical Component (PCS) and Mental Component (MCS) summary scores of the SF-36 served as measures of HRQL. The SF-36 is a generic health profile instrument that assesses the most important health concepts relevant to an individual’s functioning and wellbeing [40]. The 36 items on the SF-36 are divided into 8 multi-item subscales that evaluate the following different dimensions of health: physical function, role-physical, role-emotional, social functioning, bodily pain, mental health, vitality, and general health perceptions.
Using normed-based scoring methods, the PCS and MCS are derived by weighting each subscale score with the appropriate physical or mental factor score coefficients from the 1998 United States general population (GP) and then aggregating the weighted scores across subscales [41]. PCS and MCS scores above 50 are interpreted as above the mean of the GP. A higher PCS represents minimal to no physical limitations, bodily pain, and role activities due to physical health, and favorable evaluations of health [41]. A higher MCS suggests a more positive affect with minimal to no psychological distress or limitations in social/role activities due to emotional problems, and high energy levels.
The psychometric properties of the SF-36 have been evaluated extensively and support its reliability and validity as a measure of functional health and well-being in patients with chronic illness including cancer [41] and those undergoing allogeneic HCST [34]. The SF-36 is acceptable to Spanish-speaking populations and has demonstrated strong psychometric properties in international clinical trials [42, 43].
Analysis
Univariate descriptive analyses were applied to delineate a demographic and clinical profile of participants and characterize their symptom experience as derived from the SDS at each time point. The symptom reported by the highest percentage of participants was considered the most prevalent. The symptom rated by the highest percentage of participants as severe (item score 4 or 5) was identified as most distressing. In addition the total number of symptoms (symptom count) and a total SDS score were calculated. Symptom clusters were identified by examining Spearman Rho correlation coefficients between each of the 11 SDS symptoms at each time point. As previously defined, a symptom cluster formed when at least 3 symptoms were at least moderately (rs > 0.30) and significantly (p < 0.01) related to one another and simultaneously independent of other symptom(s) (rs ≤ 0.30). The symptom cluster reported by the highest percentage of participants was considered the most prevalent.
SDS interpretive guidelines [35] were used to create the following clinically meaningful symptom distress groups: low (SDS total score < 25) and moderate/severe (SDS total score ≥ 25). Relationships between symptom distress groups and selected baseline demographic and clinical characteristics (age, gender, marital status, education level, conditioning intensity, and disease status) and HRQL outcomes (PCS and MCS) were examined using Student t-tests or Chi Square analyses. The level of significance was set at a two-sided α = 0.05.
Results
The demographic and clinical characteristics of the sample at baseline (N = 76) are presented in Table 1. Participants were primarily male (67%) with a median age of 37.5 years (range 18-71 years). Forty-one (54%) participants received reduced intensity conditioning (RIC) and 35 (46%) participants received myeloablative conditioning (MC) prior to allogeneic HSCT.
Table 1.
Baseline Demographic and Clinical Characteristics (N = 76)
| Characteristics | n | % |
|---|---|---|
| Gender | ||
| Male | 51 | 67 |
| Female | 25 | 33 |
| Age: Mean ± Standard deviation | 40.2 ± 13.5 | |
| Median (range) | 37.5 (18-71) | |
| Marital Status | ||
| Married | 48 | 63 |
| Not Married | 28 | 37 |
| Ethnicity | ||
| Caucasian | 35 | 46 |
| Hispanic | 23 | 30 |
| Asian | 7 | 9 |
| Black | 5 | 7 |
| Other | 6 | 8 |
| Education | ||
| High School or less | 28 | 37 |
| Some College/Trade | 17 | 22 |
| College/ Post Graduate | 31 | 42 |
| Performance Status (Eastern Cooperative Oncology Group) | ||
| Grade 0 | 52 | 68 |
| Grade 1 | 22 | 29 |
| Grade 2 | 2 | 3 |
| Conditioning Intensity | ||
| Reduced Intensity | 41 | 54 |
| Myeloablative | 35 | 46 |
| Disease Category | ||
| Acute leukemia | 13 | 17 |
| Chronic leukemia | 29 | 38 |
| Lymphoma/Multiple Myeloma | 22 | 29 |
| Myelodysplastic Syndrome | 9 | 12 |
| Non-hematological malignancy | 3 | 4 |
| Disease Status | ||
| Remission/Stable | 50 | 66 |
| Progressive | 26 | 34 |
Symptom Experience
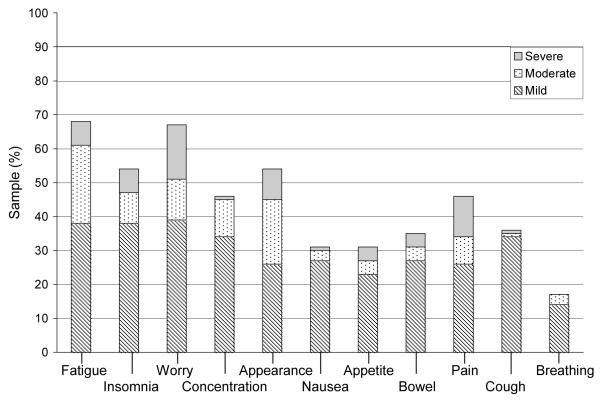
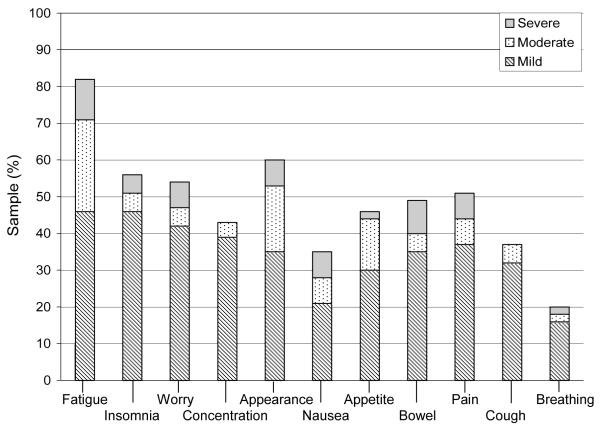
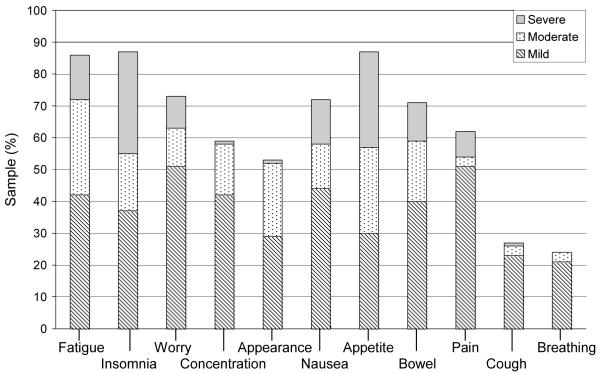
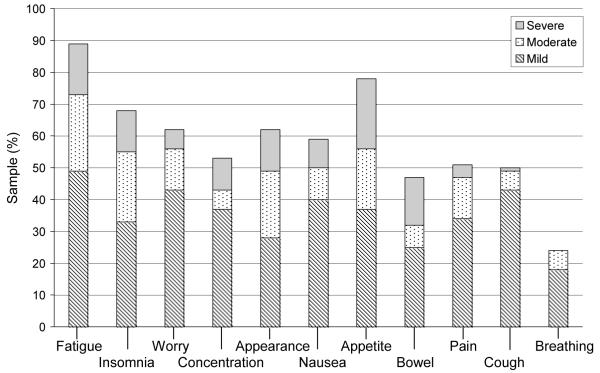
Symptom prevalence reported by the participants at each time point is shown in Figures 1 through 4. Across study points fatigue, worry and appetite change were the most prevalent. At baseline, fatigue and worry were equally prevalent in 50 (68%) participants. At day 0, appetite change was reported by 64 (88%) participants followed by fatigue and insomnia equally prevalent in 63 (86%) participants. Fatigue was reported at both days 30 and 100, by 60 (90%) and 46 (81%) participants, respectively. Trouble breathing was the least prevalent symptom (present in 14% to 22% of participants) across study time points. Participants reported the greatest number of symptoms at day 0 [Mdn = 8 (1 - 11)] and day 30 [Mdn = 7 (0 - 11)]. By day 100, the number of symptoms was identical to baseline [Mdn = 5 (0 - 11)].
Figure 1.
Baseline symptom prevalence and distress
Note: n = 74, incomplete survey responses n = 2;
Baseline symptom count: Median 5 (range 0-11); Symptom distress (mean±SD) score: 21.2 ± 5.9.
Figure 4.
Day 100 symptom prevalence and distress
Note: n = 57; Day 100 symptom count: Median 5 (range 0-11); Symptom distress (mean ±SD) score: 21.6 ± 6.2.
The symptoms reported as most distressing varied across study time points (Figures 1 – 4). At baseline, 12 (16%) participants reported worry as the most distressing symptom. On day 0, 23 participants (32%) reported insomnia as most distressing, while at day 30, appetite change was most distressing (n = 15; 22%). Days 0 and 30 were also associated with the highest total SDS mean score (M = 26.6 ± 7.6; M = 24.9 ± 7.3 respectively). At day 100, 6 (11%) participants reported fatigue as the most distressing symptom. The total SDS mean scores at baseline and day 100 were comparable (M = 21.2 ± 5.9, M = 21.6 ± 6.2, respectively).
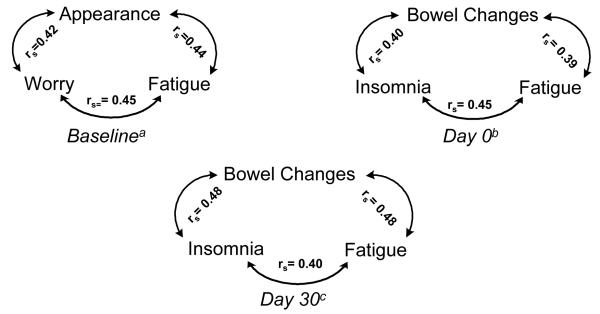
Symptom clusters were identified at baseline and days 0 and 30 (Figure 5). No symptom cluster was found at day 100. At baseline, the most prevalent symptom cluster consisted of fatigue, worry and appearance (n = 30; 41%). At days 0 and 30, the most prevalent symptom cluster was identical and consisted of insomnia, bowel change and fatigue (n = 46, 63%; n = 34, 51%, respectively). The demographic and clinical characteristics of participants who reported experiencing the three symptoms that defined the most prevalent symptom clusters are contrasted in Table 2 with those participants who did not report the most prevalent symptom cluster. Participants experiencing the symptom cluster at each time point consistently reported a higher total SDS mean score and greater total number of symptoms compared with those who did not experience the most prevalent cluster.
Figure 5.
Relationships (rs) Between Symptoms in the Most Prevalent Symptom Clusters at Baseline and Days 0 and 30 after Allogeneic HSCT
Note: rs = Spearman Rho Correlation
an= 74, bn = 73, cn = 67
Table 2.
Symptom Cluster Sample Characteristics at Baseline and Days 0 and 30 Following Allogeneic HSCT.
| Baselinea |
Day 0b |
Day 30c |
||||
|---|---|---|---|---|---|---|
| Cluster Symptoms: Fatigue, Worry, Appearance |
Cluster Symptoms: Fatigue, Insomnia, Bowel Change |
Cluster Symptoms: Fatigue, Insomnia, Bowel Change |
||||
| Cluster Symptoms Co-Occur |
Cluster Symptoms Do Not Co-Occur |
Cluster Symptoms Co-Occur |
Cluster Symptoms Do Not Co-Occur |
Cluster Symptoms Co-Occur |
Cluster Symptoms Do Not Co-Occur |
|
| n(%) | 30 (40.5) | 44 (59.5) | 46 (63.0) | 27 (37.0) | 34 (50.7) | 33 (49.3) |
|
Symptom Experience
| ||||||
| Total SDS | ||||||
| M (SD) | 25.9 (5.4) | 18.1 (3.8) | 30.0 (6.2) | 20.9 (6.1) | 29.9 (6.5) | 19.8 (3.6) |
|
Symptom
Count |
||||||
| Median (range) | 7.0 (4-11) | 2.0 (0-8) | 9.0 (5-11) | 4.0 (1-7) | 8.0 (6-11) | 5.0 (0-9) |
|
| ||||||
|
Characteristics
| ||||||
| Age | ||||||
| Median (range) | 36.5 (21-71) | 39.5 (18-68) | 36.0 (18-71) | 38.0 (21-66) | 43.5 (18-71) | 34.0 (20-66) |
| Male n (%) | 20 (66.7) | 29 (65.9) | 27 (58.7) | 21 (77.8) | 25 (73.5) | 21 (63.6) |
| RIC n (%) | 19 (63.3) | 22 (50.0) | 22 (47.8) | 17 (63.0) | 19 (55.9) | 18 (54.5) |
| ECOG n (%) | ||||||
| 0 – 1 | 29 (96.6) | 43 (97.7) | 34 (73.9) | 25 (92.6) | 22 (64.7) | 29 (87.8) |
| ≥ 2 | 1 (3.3) | 1 (2.3) | 10 (21.7) | 1 (3.7) | 10 (29.3) | 2 (6.1) |
| A-GVHD n (%) | — | — | — | — | 11 (32.4) | 4 (12.1) |
Note: SDS = Symptom Distress Score (range 13-65, higher scores greater distress), Symptom count (range 1-11) SD = Standard Deviation, RIC = Reduced Intensity Conditioning, ECOG = Eastern Cooperative Oncology Group performance status, A-GVHD = Acute Graft Versus Host Disease; ≥ Grade 2. No Symptom cluster identified for Day 100.
n = 74,
n = 73,
n = 67
Relationships with Symptom Distress Groups
Across study time points, few significant relationships existed between demographic and clinical characteristics, and symptom distress groups (Table 3). At baseline, participants who reported moderate/severe symptom distress were more likely to be preparing to receive a reduced intensity conditioning regimen as compared to participants reporting low symptom distress who were more likely to be preparing for a myeloablative conditioning regimen (X2 (1, n = 74) = 7.51, p = 0.006). At day 0, those reporting moderate/severe symptom distress were significantly younger than those participants who reported low symptom distress (t(71) = 2.02, p = 0.047). At day 30, participants reporting moderate/severe symptom distress were comparable relative to baseline disease risk status, whereas those reporting low symptom distress who were more likely to have been in remission or have had stable disease at the time of allogeneic HSCT (X2 (1, n = 67) = 4.32 (1), p = 0.038).
Table 3.
Significant Relationships between Demographic and Clinical Variables and Symptom Distress Groups.
| Symptom Distress Groups | Baseline |
Day 0 |
Day 30 |
||||
|---|---|---|---|---|---|---|---|
| (n = 74) | (n = 73) | (n = 67) | |||||
| <25a | ≥25b | <25a | ≥25b | <25a | ≥25b | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| 56 (76) | 18 (24) | 31 (42) | 42 (58) | 38 (57) | 29 (43) | ||
| SDS: Mean (SD) | 18.4 (3.2) | 29.9 (3.1) | 19.7 (3.4) | 31.7 (5.4) | 19.7 (3.0) | 31.7 (5.5) | |
|
| |||||||
|
Characteristics
| |||||||
| Transplant type | |||||||
| MC | 30 (55) | 3 (16) | 12 (39) | 22 (52) | 16 (42) | 14 (48) | |
| RIC | 26 (46) | 15 (83) | 19 (61) | 20 (48) | 22 (58) | 15 (52) | |
| p-value | 0.006* | 0.247 | 0.615 | ||||
| Baseline Disease Status | |||||||
| Remission/Stable | 39 (70) | 10 (55) | 21 (68) | 28 (66) | 30 (79) | 16 (55) | |
| Progressive | 17 (30) | 8 (44) | 10 (32) | 14 (33) | 8 (21) | 13 (45) | |
| p-value | 0.272 | 0.923 | 0.038* | ||||
| Age: Mean ± SD | 39.9 (13.5) | 41.1 (14.6) | 43.2 (12.1) | 36.9 (13.7) | 38.0 (13.7) | 42.7 (13.1) | |
| p-value | 0.737 | 0.047* | 0.167 | ||||
MC Myeloablative Conditioning, RIC Reduced Intensity Conditioning, SDS Symptom Distress Score
p < 0.05
Low symptom distress
Moderate or severe symptom distress
The PCS and MCS mean scores at days 30 and 100 for both symptom distress groups were consistently below the 1998 GP norms except for participants with low symptom distress at day 100 (Table 4). At days 30 and 100, participants with moderate/severe symptom distress reported significantly lower PCS (t = 3.88 (65), p < 0.001 versus t = 2.71 (55), p = 0.009) and MCS (t = 2.09 (65), p = 0.04 versus t = 2.64 (55), p = 0.011) mean scores compared to participants with low symptom distress.
Table 4.
Health-Related Quality of Life Outcomes in Symptom Distress Groups at Days 30 and 100 Following Allogeneic HSCT
| Symptom Distress Groups |
Day 30 | Day 100 | |||
|---|---|---|---|---|---|
| <25a | ≥25b | <25a | ≥25b | ||
| n = 38 | n = 29 | n = 45 | n = 12 | ||
| PCS | 40.3 (6.4) | 33.8 (7.3) | 42.7 (8.5) | 35.4 (7.6) | |
| p-value | <0.001* | 0.009* | |||
| MCS | 45.2 (7.9) | 40.6 (10.0) | 50.0 (8.2) | 42.3 (11.8) | |
| p-value | 0.040* | 0.011* | |||
PCS Physical component summary score, MCS Mental component summary score
p < 0.05
Low symptom distress
Moderate or severe symptom distress
Discussion
Although patients who have undergone an allogeneic HSCT may have a challenging year of recovery [44], data suggest that their symptom distress and quality of life returns to baseline values [34, 45, 46]. This first year of recovery represents an opportunity for the healthcare team to improve the clinical care of patients undergoing allogeneic HSCT by helping them anticipate their symptom experience and employ management strategies designed to diminish consequences of burdensome symptoms.
This study focused explicitly on symptom experience during the early post-transplant period in patients undergoing allogeneic HSCT, many of whom received reduced intensity conditioning. The results of this study indicate allogeneic HSCT patients present with low symptom distress yet experience multiple symptoms and high symptom distress at days 0 and 30 after transplant conditioning. By day 100, when patients are preparing to return to their home community, the experience is characterized by few symptoms and a low level of distress. Although this improvement is notable, a subset of patients continues to experience a high level of symptom distress that was associated with an inferior quality of life.
The results of this study support previous research that suggests that in patients preparing for allogeneic HSCT overall the symptom burden is generally low (few symptoms, low symptom distress) [4, 7, 9, 10, 47], while emotional distress is prominent [4, 6, 7, 48]. A high level of worry was the most prevalent and distressing symptom reported by participants in this study prior to allogeneic HSCT, and at least half of the participants reported worry as a concern during the early transplant period. The emotional distress reported by allogeneic HSCT patients is higher than that of a normative sample [46] and has been associated with various negative consequences [4, 49-52]. Little is known about the factors contributing to emotional distress in patients following transplant. A recent study however suggests that decrements in cognitive function are associated with emotional distress and fatigue [53] and the extent to which such decrements contribute to the experience of worry deserves exploration in this patient population. Therefore, despite suggestions that emotional distress should be considered the “sixth vital sign” [54], and evidence that screening for distress is feasible in HSCT patients [4], measures for the clinical evaluation of emotional distress have received only limited study in this population [4, 55].
Poor sleep quality [7, 9, 10] and fatigue [7, 9, 13, 56] also contribute substantially to the symptom experience of patients during their allogeneic HSCT trajectory, particularly during early recovery. In support of previous studies, both fatigue and insomnia were widely prevalent across all time points with over one-third of participants experiencing moderate or severe levels of distress with these two symptoms at days 0 and 30. Poor sleep quality can result from frequent awakenings and from the administration of medications that interfere with sleep quality and cause disruption to usual sleep patterns [57-59]. In addition, delirium which is characterized by sleep-wake cycle disturbances may contribute to higher levels of fatigue in patients undergoing autologous and allogeneic HSCT [60]. Although a recent review concludes that a strong interrelationship may exist between cancer-related fatigue and sleep disorders [61], the prevalence and correlates of fatigue and poor sleep quality in patients undergoing HSCT have not been systematically examined, and such studies are fundamental to the development of effective interventions to improve these symptoms. The multifactorial etiology of fatigue [62, 63] underscores the importance of tailoring interventions for fatigue according to the phase of recovery. Further research is also needed to evaluate in HSCT recipients the effectiveness of fatigue interventions shown to have efficacy in other patient populations [64].
Consistent with prior studies [7, 9, 10], participants in our sample reported the highest level of symptom occurrence and symptom distress immediately following the conditioning regimen. In addition to high levels of fatigue and insomnia, the symptom experience during this phase reflects gastrointestinal (GI) toxicities characteristic of the early post-transplant period including nausea [7, 9,13], anorexia [7, 9, 13, 56], xerostomia [7, 13], taste changes [7] and diarrhea [7, 9, 10]. Although well described, GI complications have been reported as the most debilitating side effect of HSCT [65], in some cases delaying hospital discharge [66, 67] and contributing to hospital readmission following HSCT [68]. In addition, the relationship between poor nutritional status and clinical outcomes such as fever [69] and GVHD severity [70] are being explored. Various management strategies exist [71]; however, the findings of this study support a conclusion that more research is needed to reduce the burden of gastrointestinal symptoms in allogeneic HSCT patients and understand the impact on clinical outcomes such as functional status, nutritional status, and length of stay.
It is plausible that had more comprehensive symptom measures been selected and applied in this population [60, 72-74] additional symptoms, than those reported in this study, may have emerged as prevalent. As a secondary analysis, this study was constrained by the use of an instrument that evaluates only two aspects of the symptom experience: symptom occurrence and symptom distress. Other important dimensions such as frequency/duration, quality/intensity, or interference with function could not be examined.
Clinical and demographic predictors of patients who are likely to experience higher levels of symptom distress during allogeneic HSCT are not consistent at each time point during early recovery. In this study, participants preparing to undergo reduced intensity conditioning, those who are younger, and those with progressive disease at baseline reported higher levels of symptom distress at varying time points following allogeneic HSCT. These patient characteristics may be used to identify transplant recipients who may derive particular benefit from systematic assessment of the symptom experience and the provision of tailored interventions. Inconsistent associations between demographic and clinical variables and symptom distress have also been documented in older women with cancer [75] and in those undergoing reduced intensity conditioning [14]. Though little is known about the risk factors for symptom distress across the transplant recovery period, a study by Prieto et al. [12] noted that older age and high levels of symptom distress at the time of the stem cell infusion were associated with higher levels of distressing symptoms at the time of hospital discharge. Further research is needed to expand our understanding of factors that influence the level of symptom distress following allogeneic HSCT and those that might mediate the relationship with clinical and demographic variables such as psychosocial characteristics or comorbidities.
Approximately 3 months (day 100) following allogeneic HSCT, patients experience a turning point towards the chronic phase of transplant recovery and prepare to transition from the transplant center to their home community. The symptom experience at this time corresponds to the improvement of treatment related toxicities and physical recovery that have been documented in previous studies [4, 47].
This is the first study to describe the occurrence of symptom clusters in allogeneic HSCT recipients. The identification of a cluster comprised of fatigue, worry and appearance change prior to transplant has not been previously described. Recent studies reveal that fear of disease relapse [76], poor body image [77] and psychological distress [78] predict higher levels of fatigue in cancer survivors. However, a more complete description of the prevalence and correlates of this three symptom cluster in patients preparing for allogeneic HSCT is indicated. Our observation that fatigue, insomnia and bowel changes co-occur at Day 0 and at Day 30 also require more evaluation to confirm one or more unifying etiologies. Several other reports describe gastrointestinal symptoms, together with fatigue, and sleep disturbance in the early post-transplant period [9]. The effects of the pre-transplant conditioning regimen together with the nadir of blood counts, de-conditioning, sedating medications, and other physiologic and psychological events [60, 69] in the early post-transplant period may account for this clustering, and have been described in mixed allogeneic and autologous recipient populations [72]. However, by Day 30 acute GVHD surfaces as an additional explanatory factor for the continued presence of this specific cluster [79].
Acute GVHD produces multiple gastrointestinal symptoms, including diarrhea, and is accompanied by plasma cytokine aberrations [69, 80, 81] that are associated with both fatigue and sleep disturbance [82, 83]. Moreover, acute GVHD is typically managed with high doses of corticosteroids [84], which alone and together with high volume diarrhea can disrupt sleep patterns. The extent of symptom distress that occurs at Day 30 when fatigue, insomnia and bowel changes co-occur underscores the need for interventions to address the specific symptoms in this cluster and to explore the consequences of these symptoms including functional decline, fluid and electrolyte imbalances, nutritional compromise and infection.
Our small sample size precluded the use of more advanced statistical techniques to delineate symptom clusters. Nonetheless, the systematic method applied did allow for the identification of multiple symptom clusters. Of these, we chose to emphasize the most prevalent cluster at each time point in an effort to better address the clinical relevance of the related symptoms. In general, methods to analyze symptom clusters continue to evolve with a focus on large samples. However, consideration of how the method applied in this study might benefit studies with small sample sizes is warranted in future symptom cluster research.
The evidence base supporting our understanding of the symptom experience for recipients of allogeneic HSCT is still developing, and little is known about the symptom experience of patients as they continue their recovery and self management at a distance from the transplant center. Our results suggest that symptom distress persists through Day 100 following HSCT, and underscore the urgent need to improve our understanding of the symptom experience and its consequences in this population particularly as it relates to symptoms such as worry, sleep disturbance, fatigue and gastrointestinal complications. The development and testing of symptom management interventions remain underdeveloped components of the evidence base that supports the care of patients undergoing HSCT. Higher levels of symptoms distress have been predictive of higher levels of symptom distress during recovery [4], poor quality of life [13], poor general health [47], greater emotional distress [8], and overall mortality [85]. Since higher levels of symptom distress have deleterious consequences for the process of psychological and physical recovery from HSCT, advances in the management of symptom distress in the patient during and following allogeneic HSCT represent a significant opportunity to improve clinical outcomes and reduce suffering, for this patient population.
Figure 2.
Day 0 symptom prevalence and distress
Note: n = 73; Day 0 symptom count: Median 8 (range 1-11); Symptom distress (mean ±SD) score: 26.6 ± 7.6.
Figure 3.
Day 30 symptom prevalence and distress
Note: n = 67; Day 30 symptom count: Median 7 (range 0-11); Symptom distress (mean ±SD) score: 24.9 ± 7.3.
Acknowledgments
Olena Prachenko, Nancy Kline Leidy, Karen Soeken, John Barrett, Richard Childs, Michael Bishop, Georgie Cusack, Helen Mayberry, Priscilla Rivera, Clare Hastings, Gwenyth Wallen, and the clinical and research BMT teams at the Clinical Center, NIH.
References
- 1.Moya R, Espigado I, Parody R, Carmona M, Marquez F, De Blas JM. Evaluation of readmissions in hematopoietic stem cell transplant recipients. Transplant Proc. 2006;38(8):2591–2592. doi: 10.1016/j.transproceed.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos EB, Jakubowski AA. Novel approaches in allogeneic stem cell transplantation. Curr Oncol Rep. 2006;8(5):325–336. doi: 10.1007/s11912-006-0054-0. [DOI] [PubMed] [Google Scholar]
- 3.Tabbara IA, Zimmerman K, Morgan C, Nahleh Z. Allogeneic hematopoietic stem cell transplantation: complications and results. Arch Intern Med. 2002;162(14):1558–1566. doi: 10.1001/archinte.162.14.1558. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Loberiza FR, Antin JH, Kirkpatrick T, Prokop L, Alyea EP, et al. Routine screening for psychosocial distress following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(1):77–83. doi: 10.1038/sj.bmt.1704709. [DOI] [PubMed] [Google Scholar]
- 5.Heinonen H, Volin L, Zevon MA, Uutela A, Barrick C, Ruutu T. Stress among allogeneic bone marrow transplantation patients. Patient Educ Couns. 2005;56(1):62–71. doi: 10.1016/j.pec.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Larsen J, Nordstrom G, Bjorkstrand B, Ljungman P, Gardulf A. Symptom distress, functional status and health-related quality of life before high-dose chemotherapy with stem-cell transplantation. Eur J Cancer Care (Engl) 2003;12(1):71–80. doi: 10.1046/j.1365-2354.2003.00315.x. [DOI] [PubMed] [Google Scholar]
- 7.Larsen J, Nordstrom G, Ljungman P, Gardulf A. Symptom Occurrence, Symptom Intensity, and Symptom Distress in Patients Undergoing High-dose Chemotherapy with Stem-cell Transplantation. Cancer Nurs. 2004;27(1):55–64. doi: 10.1097/00002820-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Fife BL, Huster GA, Cornetta KG, Kennedy VN, Akard LP, Broun ER. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18(7):1539–1549. doi: 10.1200/JCO.2000.18.7.1539. [DOI] [PubMed] [Google Scholar]
- 9.Hacker ED, Ferrans C, Verlen E, Ravandi F, Van Besien K, Gelms J, et al. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncol Nurs Forum. 2006;33(3):614–624. doi: 10.1188/06.ONF.614-624. [DOI] [PubMed] [Google Scholar]
- 10.Hacker ED, Ferrans CE. Quality of life immediately after peripheral blood stem cell transplantation. Cancer Nurs. 2003;26(4):312–322. doi: 10.1097/00002820-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hjemstad MJ, Loge JH, Evensen SA, Kvaloy SO, Fayers PM, Kaasa S. The course of anxiety and depression during the first year after allogeneic or autologous stem cell transplantation. Bone Marrow Transplant. 1999;24:1219–1228. doi: 10.1038/sj.bmt.1702046. [DOI] [PubMed] [Google Scholar]
- 12.Prieto JM, Atala J, Blanch J, Carreras E, Rovira M, Cirera E, et al. Psychometric study of quality of life instruments used during hospitalization for stem cell transplantation. J Psychosom Res. 2004;57(2):201–211. doi: 10.1016/j.jpsychores.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Zittoun R, Achard S, Ruszniewski M. Assessment of quality of life during intensive chemotherapy or bone marrow transplantation. Psychooncology. 1999;8(1):64–73. doi: 10.1002/(SICI)1099-1611(199901/02)8:1<64::AID-PON337>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Diez-Campelo M, Perez-Simon JA, Gonzalez-Porras JR, Garcia-Cecilia JM, Salinero M, Caballero MD, et al. Quality of life assessment in patients undergoing reduced intensity conditioning allogeneic as compared to autologous transplantation: results of a prospective study. Bone Marrow Transplant. 2004;34(8):729–738. doi: 10.1038/sj.bmt.1704646. [DOI] [PubMed] [Google Scholar]
- 15.Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 16.McClement SE, Woodgate RL, Degner L. Symptom distress in adult patients with cancer. Cancer Nurs. 1997;20(4):236–243. doi: 10.1097/00002820-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Tishelman C, Degner LF, Mueller B. Measuring symptom distress in patients with lung cancer. Cancer Nurs. 2000;23(2):82–90. doi: 10.1097/00002820-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Tishelman C, Degner LF, Rudman A, Bertilsson K, Bond R, Broberger E, et al. Symptoms in patients with lung carcinoma: distinguishing distress from intensity. Cancer. 2005;104(9):2013–2021. doi: 10.1002/cncr.21398. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong TS. Symptoms experience: a concept analysis. Oncol Nurs Forum. 2003;30(4):601–606. doi: 10.1188/03.ONF.601-606. [DOI] [PubMed] [Google Scholar]
- 20.Posey AD. Symptom perception: a concept exploration. Nursing forum. 2006;41(3):113–124. doi: 10.1111/j.1744-6198.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- 21.Goodell TT, Nail LM. Operationalizing symptom distress in adults with cancer: a literature synthesis. Oncol Nurs Forum. 2005;32(2):E42–E47. doi: 10.1188/05.ONF.E42-E47. [DOI] [PubMed] [Google Scholar]
- 22.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31(1):85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: a pilot study. Oncol Nurs Forum. 2000;27(9):1443–1448. [PubMed] [Google Scholar]
- 24.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- 25.Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33(5):E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 26.Gift AG, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004;31(2):202–212. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- 27.Walsh D, Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer. 2006;14(8):831–836. doi: 10.1007/s00520-005-0899-z. [DOI] [PubMed] [Google Scholar]
- 28.Chen ML, Tseng HC. Symptom clusters in cancer patients. Support Care Cancer. 2006;14(8):825–830. doi: 10.1007/s00520-006-0019-8. [DOI] [PubMed] [Google Scholar]
- 29.Bender CM, Ergun FS, Rosenzweig MQ, Cohen SM, Sereika SM. Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nurs. 2005;28(3):219–225. doi: 10.1097/00002820-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–284. doi: 10.1097/00002820-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Barsevick AM, Dudley WN, Beck SL. Cancer-related fatigue, depressive symptoms, and functional status: a mediation model. Nurs Res. 2006;55(5):366–372. doi: 10.1097/00006199-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Fox SW, Lyon DE. Symptom clusters and quality of life in survivors of lung cancer. Oncol Nurs Forum. 2006;33(5):931–936. doi: 10.1188/06.ONF.931-936. [DOI] [PubMed] [Google Scholar]
- 33.Parker KP, Kimble LP, Dunbar SB, Clark PC. Symptom interactions as mechanisms underlying symptom pairs and clusters. J Nurs Scholarsh. 2005;37(3):209–215. doi: 10.1111/j.1547-5069.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 34.Bevans MF, Marden S, Leidy NK, Soeken K, Cusack G, Rivera P, et al. Health-related quality of life in patients receiving redcued-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:101–109. doi: 10.1038/sj.bmt.1705406. [DOI] [PubMed] [Google Scholar]
- 35.McCorkle R, Cooley ME, Shea JA. The user’s manual for the Symptom Distress Scale. Instrument Manual. 1994 [Google Scholar]
- 36.Lawrence CC, Gilbert CJ, Peters WP. Evaluation of symptom distress in a bone marrow transplant outpatient environment. Ann Pharmacother. 1996;30(9):941–945. doi: 10.1177/106002809603000904. [DOI] [PubMed] [Google Scholar]
- 37.Cooley ME, McCorkle L, Knafl GJ, Rimar J, Barbieri MJ, Davies M, et al. Comparison of health-related quality of life questionnaires in ambulatory oncology. Qual Life Res. 2005;14(5):1239–1249. doi: 10.1007/s11136-004-5534-9. [DOI] [PubMed] [Google Scholar]
- 38.Winer EP, Lindley C, Hardee M, Sawyer WT, Brunatti C, Borstelmann NA, et al. Quality of life in patients surviving at least 12 months following high dose chemotherapy with autologous bone marrow support. Psychooncology. 1999;8(2):167–176. doi: 10.1002/(SICI)1099-1611(199903/04)8:2<167::AID-PON354>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Krupski TL, Sonn G, Kwan L, Maliski S, Fink A, Litwin M. Ethnic variation in health-related quality of life among low-income men with prostate cancer. Ethn Dis. 2005;15:461–468. [PubMed] [Google Scholar]
- 40.Ware J, Snow K, Kosinski M, Gandel B. SF-36 Health Survey. Manual and Interpretation Guide. The Health Institute, New England Medical Center; Boston: 1997. [Google Scholar]
- 41.Ware JE, Jr, Kosinski M. SF-36 Physical and Mental Health summary scales: A manual for users of version 1. 2nd edition Quality Metric Inc.; Lincoln: 2001. [Google Scholar]
- 42.Dapueto JJ, Servente L, Francolino C, Hahn EA. Determinants of quality of life in patients with cancer: A South American study. Cancer. 2005;103(5):1072–1081. doi: 10.1002/cncr.20870. [DOI] [PubMed] [Google Scholar]
- 43.Vilagut G, Ferrer M, Rajmil L, Rebollo P, Permanyer-Miralda G, Quintana JM, et al. El Custionario de Salud SF-36 espanol: una decada de experiencia y nuevos desarrollos [The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments.] Gaceta sanitaria. 2005;19(2):135–150. doi: 10.1157/13074369. [DOI] [PubMed] [Google Scholar]
- 44.Kopp M, Schweigkofler H, Holzner B, Nachbaur D, Niederwieser D, Fleischhacker WW, et al. Time after bone marrow transplantation as an important variable for quality of life: results of a cross-sectional investigation using two different instruments for quality-of-life assessment. Ann Hematol. 1998;77(1-2):27–32. doi: 10.1007/s002770050407. [DOI] [PubMed] [Google Scholar]
- 45.Hjermstad M, Holte H, Evensen S, Fayers P, Kaasa S. Do patients who are treated with stem cell transplantation have a health-related quality of life comparable to the general population after 1 year? Bone Marrow Transplant. 1999;24(8):911–918. doi: 10.1038/sj.bmt.1701998. [DOI] [PubMed] [Google Scholar]
- 46.Syrjala KL, Chapko MK, Vitaliano PP, Cummings C, Sullivan KM. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant. 1993;11(4):319–327. [PubMed] [Google Scholar]
- 47.Larsen J, Nordstrom G, Ljungman P, Gardulf A. Factors associated with poor general health after stem-cell transplantation. Support Care Cancer. 2007;15:849–857. doi: 10.1007/s00520-006-0200-0. [DOI] [PubMed] [Google Scholar]
- 48.Trask PC, Paterson A, Riba M, Brines B, Griffith K, Parker P, et al. Assessment of psychological distress in prospective bone marrow transplant patients. Bone Marrow Transplant. 2002;29(11):917–925. doi: 10.1038/sj.bmt.1703557. [DOI] [PubMed] [Google Scholar]
- 49.Andorsky DJ, Loberiza, Lee SJ. Pre-transplantation physical and mental functioning is strongly associated with self-reported recovery from stem cell transplantation. Bone Marrow Transplant. 2006;37(9):889–895. doi: 10.1038/sj.bmt.1705347. [DOI] [PubMed] [Google Scholar]
- 50.Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38(4):255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- 51.Prieto JM, Blanch J, Atala J, Carreras E, Rovira M, Cirera E, et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol. 2002;20(7):1907–1917. doi: 10.1200/JCO.2002.07.101. [DOI] [PubMed] [Google Scholar]
- 52.Molassiotis A. Further evaluation of a scale to screen for risk of emotional difficulties in bone marrow transplant recipients. J Adv Nurs. 1999;29(4):922–927. doi: 10.1046/j.1365-2648.1999.00979.x. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoetic stem cell transplant patients. J Pain Symptom Manage. 2007;33(1):13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Bultz BD. Emotional distress: the sixth vital sign--future directions in cancer care. Psychooncology. 2006;15(2):93–95. doi: 10.1002/pon.1022. [DOI] [PubMed] [Google Scholar]
- 55.Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow transplant patients. Psychooncology. 2006;15(7):604–612. doi: 10.1002/pon.993. [DOI] [PubMed] [Google Scholar]
- 56.Hacker ED. Quantitative measurement of quality of life in adult patients undergoing bone marrow transplant or peripheral blood stem cell transplant: a decade in review. Oncol Nurs Forum. 2003;30(4):613–629. doi: 10.1188/03.onf.613-631. [DOI] [PubMed] [Google Scholar]
- 57.Berger AM, Parker KP, Young-McCaughan S, Mallory GA, Barsevick AM, Beck SL, et al. Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum. 2005;32(6):E98–E126. doi: 10.1188/05.ONF.E98-E126. [DOI] [PubMed] [Google Scholar]
- 58.Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33(1):E18–E26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 59.Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, McCarthy K, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007;34(2):393–402. doi: 10.1188/07.ONF.393-402. [DOI] [PubMed] [Google Scholar]
- 60.Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J Clin Oncol. 2007;25(10):1223–1231. doi: 10.1200/JCO.2006.07.9079. [DOI] [PubMed] [Google Scholar]
- 61.Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(Suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 62.Prieto JM, Blanch J, Atala J, Carreras E, Rovira M, Cirera E, et al. Clinical factors associated with fatigue in haematologic cancer patients receiving stem-cell transplantation 275. Eur J Cancer. 2006;42(12):1749–1755. doi: 10.1016/j.ejca.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell SA, Beck SL, Hood LE, Moore K, Tanner ER. Putting evidence into practice: evidence-based interventions for fatigue during and following cancer and its treatment. Clin J Oncol Nurs. 2007;11(1):99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- 65.Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ. Patient reports of complications of bone marrow transplantation. Support Care Cancer. 2000;8(1):33–39. doi: 10.1007/s005209900095. [DOI] [PubMed] [Google Scholar]
- 66.Cetkovsky P, Skopek P, Schutzova M. Causative factors for prolonged hospitalization beyond the point of engraftment in patients after autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2000;26(8):877–880. doi: 10.1038/sj.bmt.1702632. [DOI] [PubMed] [Google Scholar]
- 67.Sheean PM, Braunschweig C, Rich E. The incidence of hyperglycemia in hematopoietic stem cell transplant recipients receiving total parenteral nutrition: a pilot study. J Am Diet Assoc. 2004;104(9):1352–1360. doi: 10.1016/j.jada.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 68.Grant M, Cooke L, Bhatia S, Forman SJ. Discharge and unscheduled readmissions of adult patients undergoing hematopoietic stem cell transplantation: Implications for developing nursing interventions. Oncol Nurs Forum. 2005;32(1):E1–E8. doi: 10.1188/05.onf.e1-e8. [DOI] [PubMed] [Google Scholar]
- 69.Malone FR, Leisenring WM, Storer BE, Lawler R, Stern JM, Aker SN, et al. Prolonged anorexia and elevated plasma cytokine levels following myeloablative allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2007;40:765–772. doi: 10.1038/sj.bmt.1705816. [DOI] [PubMed] [Google Scholar]
- 70.Mattsson J, Westin S, Edlund S, Remberger M. Poor oral nutrition after allogeneic stem cell transplantation correlates significantly with severe graft-versus-host disease. Bone Marrow Transplant. 2006;38(9):629–633. doi: 10.1038/sj.bmt.1705493. [DOI] [PubMed] [Google Scholar]
- 71.Warnick E, Ezzone S, Schmit-Pokorny K. Principles, practice, and nursing insights. Jones and Bartlett Publishers; Sudbury, Massachusetts: 2007. Gastrointestinal EffectsBlood and marrow stem cell transplantation; pp. 207–243. [Google Scholar]
- 72.Anderson KO, Giralt SA, Mendoza TR, Brown JO, Neumann JL, Mobley GM, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplant. 2007;39:759–766. doi: 10.1038/sj.bmt.1705664. [DOI] [PubMed] [Google Scholar]
- 73.Hoekstra J, Vernooij-Dassen MJFJ, de Vos R, Bindels PJE. The added value of assessing the ‘most troublesome’ symptom among patients with cancer in the palliative phase. Patient Educ Couns. 2007;65(2):223–229. doi: 10.1016/j.pec.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Schulz-Kindermann F, Mehnert A, Scherwath A, Schirmer L, Schleimer B, Zander AR, et al. Cognitive function in the acute course of allogeneic hematopoietic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;39(12):789–799. doi: 10.1038/sj.bmt.1705663. [DOI] [PubMed] [Google Scholar]
- 75.Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. The influence of symptoms, age, comorbidity and cancer site on physical functioning and mental health of geriatric women patients. Women Health. 1999;29(3):1–12. doi: 10.1300/J013v29n03_01. [DOI] [PubMed] [Google Scholar]
- 76.Gielissen MFM, Schattenberg AVM, Verhagen CAHH, Rinkes MJ, Bremmers MEJ, Bleijenberg G. Experience of severe fatigue in long-term survivors of stem cell transplantation. Bone Marrow Transplant. 2007;39(10):595–603. doi: 10.1038/sj.bmt.1705624. [DOI] [PubMed] [Google Scholar]
- 77.Liavaag AH, Drum A, Fossa SD, Trope C, Dahl AA. Controlled study of fatigue, quality of life, and somatic and mental morbidity in epithelial ovarian cancer survivors: How lucky are the lucky ones? J Clin Oncol. 2007;25(15):2049–2056. doi: 10.1200/JCO.2006.09.1769. [DOI] [PubMed] [Google Scholar]
- 78.Young KE, White CA. The prevalence and moderators of fatigue in people who have been successfully treated for cancer. J Psychosom Res. 2006;60(1):29–38. doi: 10.1016/j.jpsychores.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 79.Wu D, Hockenbery DM, Brentnall TA, Baehr PH, Ponec RJ, Kuver R, et al. Persistent nausea and anorexia after marrow transplantation: A prospective study of 78 patients. Transplantation. 1998;66(10):1319–1324. doi: 10.1097/00007890-199811270-00010. [DOI] [PubMed] [Google Scholar]
- 80.Fowler DH, Foley J, Hou JWS, Odom J, Castro K, Steinberg SM, et al. Clinical “cytokine storm” as revealed by monocyte intracellular flow cytometry: correlation of tumor necrosis factor with severe gut graft-versus-host disease. Clin Gastroenterol Hepatol. 2004;2(3):237–245. doi: 10.1016/s1542-3565(04)00011-4. [DOI] [PubMed] [Google Scholar]
- 81.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: Pathobiology and management. Exp Hematol. 2001;29(3):259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 82.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11(5):1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 83.Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug Etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs. 2006;8(2):157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- 84.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis. 2007;2(1):35. doi: 10.1186/1750-1172-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Molassiotis A, Van Den Akker OB, Milligan DW, Goldman JM. Symptom distress, coping style and biological variables as predictors of survival after bone marrow transplantation 76. J Psychosom Res. 1997;42(3):275–285. doi: 10.1016/s0022-3999(96)00298-x. [DOI] [PubMed] [Google Scholar]