Abstract
The β1 integrins play an important role in the modulation of cancer cell proliferation and tumor growth. We have previously shown that β1 integrins associate with insulin-like growth factor type 1 receptor (IGF-IR) and regulate IGF-stimulated prostate cancer cell proliferation. In the present study, we find that downregulation of β1 in prostate cancer cells inhibits IGF-IR and AKT activation. We also show that β1 downregulation in prostate cancer two- and three-dimensional (3-D) cell cultures significantly reduces expression of GLI1, a transcription factor known to be regulated by the IGF/AKT signaling pathway and to be a downstream effector of sonic hedgehog. Re-expression of GLI1 rescues the inhibitory effect of β1 downregulation on prostate cancer cell proliferation in 3-D cultures. We find that downregulation of β1 reduces surface expression of associated α integrin subunits, predominantly α5 and at a lower extent: α2, α3 and α4. Our results indicate that the β1/IGF-IR complex regulates expression of GLI1, which in turn promotes cancer cell proliferation in 3-D cultures.
Keywords: IGF-IR, GLI1, prostate cancer, β1 integrins, AKT
Introduction
Extracellular matrix (ECM) proteins and growth factors activate several signaling pathways via, respectively, integrins and growth factor receptors. The cross-talk between these pathways is known to regulate several physiological functions including cell adhesion, migration, proliferation and differentiation (Comoglio et al., 2003; Eliceiri, 2001).
Integrins are heterodimers consisting of α and β subunits. Currently, 24 heterodimers of the integrin family, consisting of 18 α and 8 β subunits, have been described (Alam et al., 2007; Hynes, 2002) and their ability to activate specific signaling pathways has been investigated (Alam et al., 2007). Integrin signaling plays a key role in the alteration of cellular growth and tumor progression through the regulation of gene expression, apoptosis, cell adhesion, proliferation and migration (Felding-Habermann, 2003). Several studies have reported the association between deregulation of integrin, ECM or growth factor expression with the progression of prostate cancer to an advanced stage (Culig et al., 2005; Goel et al., 2008). Among the β subunits, β1 is the predominant subunit expressed by prostatic epithelium. Five β1 variant subunits, β1A, β1B, β1C, β1C-2, and β1D, generated by alternative splicing, have been described. Two variants, β1C and β1A, are shown to be expressed in normal prostatic epithelium. β1A is upregulated in prostate cancer, whereas β1C is markedly downregulated in adenocarcinoma (Fornaro et al., 1999; Goel et al., 2008). We have shown upregulation of β1 expression in a mouse model designated TRAMP (transgenic adenocarcinoma of mouse prostate) (Goel et al., 2005). The findings that the expression of the β1A integrin variant is upregulated (Goel et al., 2005) and is necessary for cells' ability to form tumors in vivo (Goel et al., 2009b) pinpoint an important role of the β1A integrin during prostate cancer progression.
β1 integrins play an important role in disrupting the formation of normal acini structure. β1 inhibition causes formation of polarized acini of malignant human breast cancer cells mediated by anti-proliferative and pro-apoptotic signaling (Weaver et al., 1997). Previously, our group and others have shown a direct interaction between β1 integrins and IGF-IR (Goel et al., 2004; Tai et al., 2003). Downregulation of β1 blocks IGF-stimulated cell proliferation and transformation of prostate cancer cells (Goel et al., 2005), but the effect of β1 downregulation on activation of IGF-IR and tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1) has never been described. Expression of wild-type, but not kinase-inactive, IGF-IR in non-transformed breast epithelial cells (MCF-10A) causes formation of large abnormal structures (Irie et al., 2005). Over-expression of IGF-IR in the same cells also results in disruption of apical basal polarization (Yanochko and Eckhart, 2006). Recently, Kim et al. expressed a constitutively active form of IGF-IR in non-transformed MCF-10A cells and found larger and disrupted acini with protrusions (Kim et al., 2007). Similar to β1 inhibition, an IGF-IR blocker reduces cell proliferation, and resulted in the formation of hollow polarized lumen in MCF7 breast cancer cells (Litzenburger et al., 2009). All these studies point to a possible involvement of β1-IGF-IR complex in the regulation of cell proliferation in 3-D cultures.
The sonic hedgehog (SHH)/GLI signal transduction pathway controls a variety of developmental processes involved in embryogenesis. Besides embyrogenesis, aberrant activation of SHH pathways have been implicated in several malignancies like lung, pancreatic and prostate cancer (Kasper et al., 2006). The expression of SHH and GLI1, a downstream effector of SHH, is upregulated in human prostate cancer as compared to normal prostatic epithelia (Sanchez et al., 2004). Treatment with cyclopamine, a SHH inhibitor, blocks proliferation of prostate cancer cell lines (PC3, DU145 and 22RV1) as well as of primary prostate tumor cultures expressing GLI1 (Karhadkar et al., 2004; Sanchez et al., 2004). This inhibitory effect of cyclopamine is bypassed by over-expression of GLI1 (Karhadkar et al., 2004). Ectopic expression of GLI1 increases β1 levels along with increased proliferation and invasiveness in ovarian cancer cells and these effects are reverted by cyclopamine (Liao et al., 2009). These results suggest a possible interaction between β1 integrins and SHH pathway in cancer.
In the present study, we show that β1 downregulation reduces IGF1-stimulated tyrosine phosphorylation of IGF-IR, activation of AKT as well as expression of GLI1. We, then, demonstrate that β1 integrins regulate proliferation of prostate cancer cells in 3-D cultures in a GLI1-dependent manner.
Materials and Methods
Reagents and antibodies
Reagents used for this study include: lipofectamine 2000, oligofectamine (OLF, Invitrogen) and Matrigel (BD Bioscience). The following monoclonal antibodies (mAbs) were used: to human β1, clone-18 (BD Bioscience) and TS2/16 (ATCC); to α2, P1H5 (Life Technologies); to α3, P1B5; to α4, P4C2 (both were kindly provided by Dr. Elizabeth Wayner); to α5, P1D6 (Life Technologies); to α6, clone GoH3 (kindly provided by Dr. Arnoud Sonnenberg); to αv, L230 (ATCC); to mouse α5, 5H10 (BD Bioscience); to hemagglutinin, 12CA5 (ATCC) and to phosphotyrosine, PY20 (Santa Cruz). The following rabbit polyclonal Abs were used: to ERK1 (C-16, this Ab also cross-reacts with ERK2); to phospho-AKT (Ser 473); to AKT; to GLI1; to FAK; to IGF-IRβ; to IRS-1 (all these Abs were purchased from Santa Cruz) and to the cytoplasmic domains of αv or α5 (kindly provided by Dr. Erkki Ruoslahti). We also used rat IgG (Pierce) and mouse IgG (Sigma) as a control.
Cell lines and transfectants
PC3, DU145 and TRAMP-C2 cells were cultured as described (Goel et al., 2005; Goel et al., 2009b). PC3 and DU145 cells were stably transfected with plasmids containing either pEGFP (vector) or pEGFP-β1-shRNA using lipofectamine 2000 (Goel et al., 2009b). G418-resistant clones were pooled to generate populations. PC3 cells were stably transfected with pLKO-β6-shRNA (Open Biosystem, clone ID TRCN0000057704) and these stable transfectants were used as control cell lines (designated as PC3-cont-shRNA).
3-D cultures
PC3 and DU145 stable transfectants (PC3-β1-shRNA, PC3-vector, PC3-cont-shRNA, DU145-β1-shRNA and DU145-vector) were embedded in Matrigel as single cells and cultured for 12 days (Weaver et al., 1997). Colonies in 3-D cultures were observed under a phase contrast microscope and images were captured using an Olympus IX71 inverted microscope with IPLab V3.55 (Scanalytics, Inc). Colony size was measured using a grid by phase contrast microscopy. In some experiments, PC3-cont-shRNA and PC3-β1-shRNA cells were transiently transfected with either EGFP or GLI1-GFP (kindly provided by Dr. Junhao Mao) before embedding and culturing in Matrigel.
Embedded multicellular structures were scooped with the Matrigel included and placed into cold 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4 and fixed for 3 hours at room temperature. The samples were washed in the same buffer at 4°C for several days, then post-fixed in 1% osmium tetroxide in 0.1M cacodylate buffer, pH 7.4 at 4°C for 30 minutes, dehydrated in graded ethanols and embedded in Epon 812 with propylene oxide used as the transitional solvent (Underwood et al., 2006). Semi-thin sections were prepared and stained with 1% toluidine blue in 1% borax and imaged with a Zeiss Axioscope microscope. These images were used to count the number of cells per colony. A minimum of 20 cross-sections was used.
Immunoblotting (IB)
PC3-β1-shRNA, PC3-vector, DU145-β1-shRNA and DU145-vector transfectants were lysed and immunoblotted as previously described (Fornaro et al., 2000). Colonies of PC3-β1-shRNA and PC3-cont-shRNA transfectants cultured in Matrigel were isolated using PBS-EDTA as described (Weaver et al., 1997); cells were lysed and immunoblotted using Abs to GLI1, β1 or ERK.
TRAMP-C2 cells were transiently transfected with siRNA to either β1A or β1C (used as a control) or treated with OLF alone (Goel et al., 2005). Cells were lysed and immunoblotted as previously described (Fornaro et al., 2000).
PC3-cont-shRNA and PC3-β1-shRNA cells, transiently transfected with either EGFP or GLI1-GFP, were lysed 48 hours after transfection and IB was performed using Abs to GLI1 or ERK, as a loading control.
Immunoprecipitation (IP)
PC3-β1-shRNA or PC3-cont-shRNA transfectants were cultured in serum-free medium (SFM) for 24 hours. Cells were incubated in the presence or absence of IGF-1 (100 ng/ml) for 10 minutes. Cells were lysed and proteins were immunoprecipitated using Abs to IGF-IRβ or IRS-1 and protein A-Sepharose. Immunoprecipitated proteins were separated using 10% SDS-PAGE and immunoblotted using Abs to phosphotyrosine (p-Tyr), IGF-IRβ or IRS-1.
FACS analysis
PC3-β1-shRNA, PC3-cont-shRNA and PC3-vector transfectants were detached and analyzed by FACS using P1H5 (a-α2), P1B5 (a-α3), P4C2 (a-α4), P1D6 (a-α5), GoH3 (a-α6), L230 (a-αv), TS2/16 (a-β1), mIgG or 12CA5 (cont IgG). TRAMP-C2 cells were transiently transfected with siRNA to either β1A or β1C (used as a control). Cells were detached and FACS analysis was performed to detect surface expression of α6 (GoH3), α5 (5H10), or, as negative control, 12CA5 or rat IgG (Goel et al., 2009b).
GLI1 luciferase assay
PC3 parental, PC3-vector, PC3-cont-shRNA or PC3-β1-shRNA cells were transfected with pCMV-β-galactosidase (Dr. Michael Lu) and 8×3'GLI-BS pδ51LucII reporter construct [eight directly repeated copies of 3'GLI-BS into plasmid pδ=51LucII, kindly provided by Dr. Hirosh Sasaki (Sasaki et al., 1997)]. Cell lysates were prepared with the reporter lysis buffer and GLI luciferase activity was assayed using Luciferase reagent (Promega). β-galactosidase activity was assayed using the Galacto-Lite Plus reagent (Tropix).
Statistical analysis
Statistical analysis was conducted using the Student's t-test except in one instance, when colony size in 3-D cultures was determined and summarized in ordered categories of increasing size. In this case, the colony size was compared among different groups using a Chi-square test for linear trend with 1 degree of freedom.
All p-values were based on two-tailed tests.
Results
β1 integrins stimulate IGF-IR signaling and GLI1 expression
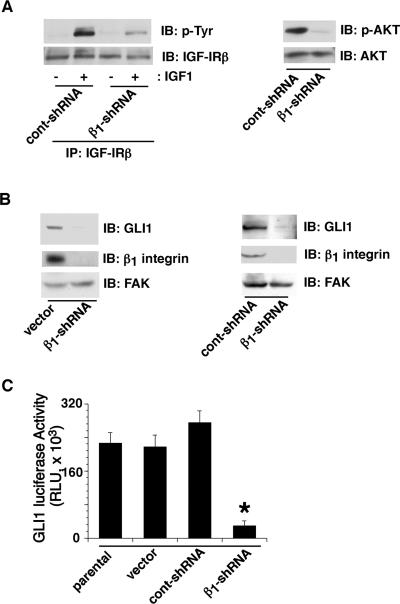
We have previously shown that β1 integrins regulate IGF-IR's mitogenic and transforming ability in prostate cancer cells (Goel et al., 2005). To study the effect of β1 downregulation on IGF-IR signaling pathway, we measured activation of IGF-IR and its downstream effectors, IRS-1 and AKT, in PC3 cells. β1 downregulation shows significant inhibition of IGF-1 dependent tyrosine phosphorylation of IGF-IR (Fig. 1 part A, left) and IRS-1 (data not shown) as well as serine phosphorylation of AKT (Fig. 1 part A, right). Since it has been shown that IGF-1 potentiates GLI activity induced by low levels of SHH and this is mediated by activation of PI 3-kinase/AKT pathway (Riobo et al., 2006), we analyzed the effect of β1 downregulation on expression and activity of GLI1. Our results show that transfection of β1 integrin shRNA reduces expression of GLI1 as compared to vector (Fig. 1 part B, left) or control shRNA (Fig. 1 part B, right) transfected cells, as evaluated by immunoblotting. As a consequence, GLI1 activity measured by reporter gene assay is also decreased in cells expressing β1 integrin shRNA (Fig. 1 part C). These data indicate that β1 downregulation reduces expression and activity of GLI1.
Fig. 1. β1 integrins promote IGF-IR activation and increase GLI1 expression.
A: PC3-β1-shRNA or PC3-cont-shRNA transfectants were serum starved and stimulated with or without IGF-1. Cell lysates were immunoprecipitated using Abs to IGF-IRβ and immunoblotted using Abs to phosphotyrosine (p-Tyr, PY20) or IGF-IRβ (left panels). In some experiments, cell lysates from IGF-1 treated PC3-β1-shRNA or PC3-cont-shRNA transfectants were immunoblotted using Abs to p-AKT or AKT (right panels). B: PC3-vector, PC3-β1-shRNA or PC3-cont-shRNA were detached, lysed and immunoblotted using Abs to GLI1, β1 (C-18) or FAK, as a loading control. C: PC3 parental, PC3-vector, PC3-cont-shRNA or PC3-β1-shRNA cells were transfected with pCMV-β-galactosidase and 8×3'GLI-BS pδ51LucII reporter construct. GLI reporter activity was analyzed by luciferase assay. The data were normalized using β-galactosidase activity. Data are expressed as means ±SEM (*p=0.0072).
Downregulation of β1 integrins by shRNA reduces cell proliferation in 3-D cultures
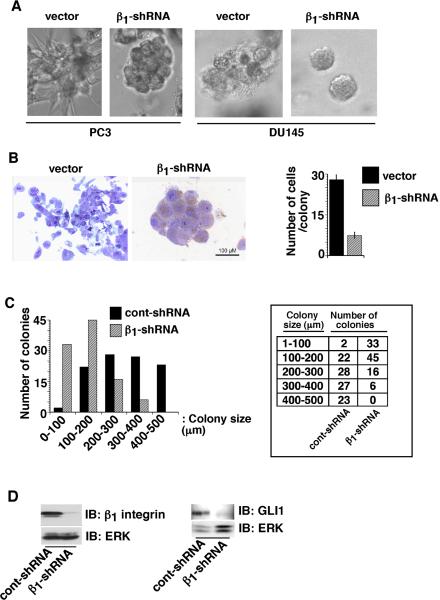
To study the role of β1 downregulation in 3-D cultures, we used PC3 and DU145 prostate cancer cells stably expressing either shRNA to β1 integrins (PC3-β1-shRNA) or vector (PC3-vector) alone. We performed immunoblotting to confirm the downregulation of β1 integrin protein levels in β1-shRNA expressing cells in monolayer cultures (data not shown). When cultured in Matrigel as shown in Figure 2, part A, vector-transfected PC3 cells still expressing β1 integrins are organized into large, loosely connected masses with an irregular morphology, whereas cells expressing β1-shRNA form smaller, more organized structures with a more glandular morphology. We obtained similar results using DU145 cells cultured in Matrigel (Fig. 2 part A). PC3-β1-shRNA 3-D cultures show increased cell-cell contacts (Fig. 2 part B, left) and contribute to a more normal morphology compared to cells expressing β1 integrins. We counted the number of cells in the multicellular structures formed by vector or PC3-β1-shRNA transfectants after 12 days in Matrigel cultures. There is a significantly reduced number of cells per structure upon β1 integrin downregulation (Fig. 2 part B, right). The colony dimensions are decreased (Fig. 2 part C) consistent with both the decreased cell number and the increased cell-cell contacts observed after β1 integrin downregulation. We confirmed by immunoblotting that β1 was downregulated in the 3-D cultures of PC3-β1-shRNA cells up to 12 days (Fig. 2 part D). We conclude that β1 integrin downregulation decreases cell proliferation and reverts PC3 prostate cancer cells towards a more normal phenotype in 3-D reconstituted basement membrane cultures.
Fig. 2. β1 integrins reduce cell proliferation in 3-D cultures.
A: PC3-vector, PC3-β1-shRNA, DU145-vector and DU145-β1-shRNA transfectants were cultured in Matrigel. Colonies in 3-D cultures were observed under a phase contrast microscope and images were captured. B: PC3-vector and PC3-β1-shRNA transfectants cultured in Matrigel were fixed and sections were stained with 1% toluidine blue (left panels). Number of cells in each colony was counted and shown as average with SEM (n=20, right panel, p<0.00001). C: PC3-cont-shRNA and PC3-β1-shRNA transfectants were cultured in Matrigel for 12 days. Colonies in 3-D cultures were observed and the size of each colony was measured using a phase contrast microscope. Chi-square for linear trend with 1 degree of freedom is 73.462 (p<0.00001). D: PC3-cont-shRNA and PC3-β1-shRNA transfectants were cultured in Matrigel for 12 days. Cells were isolated from Matrigel using PBS-EDTA and then lysed. Lysates were separated and immunoblotted using Abs to β1 (C-18), GLI1 or ERK, as a loading control.
GLI1 increases proliferation of β1-shRNA expressing PC3 cells in 3-D cultures
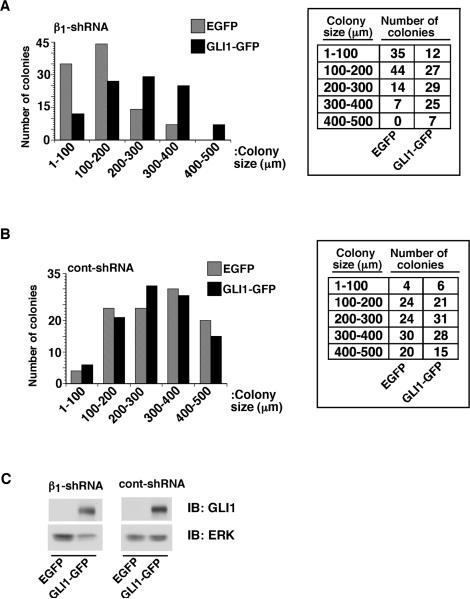
To study if GLI1 expression can rescue the inhibitory effect on cell proliferation in 3-D cultures due to β1 downregulation, PC3-cont-shRNA and PC3-β1-shRNA cells were transiently transfected with human GLI1. Expression of GLI1, but not of control DNA, causes increased colony size in PC3-β1-shRNA cells cultured in 3-D Matrigel (Fig. 3 part A). No significant change in colony size is observed in PC3-cont-shRNA cells in 3-D cultures upon expression of either GLI1 or of control DNA (Fig. 3 part B). We confirmed by immunoblotting that the transfected GLI1 is expressed up to 12 days (Fig. 3 part C). These results suggest that GLI1 mediates β1-dependent cell proliferation in 3-D cultures.
Fig. 3. GLI1 expression increases proliferation of PC3-β1-shRNA cells in 3-D cultures.
PC3-β1-shRNA (A) and PC3-cont-shRNA (B) cells were transfected with either EGFP or GLI1-GFP. Cells were cultured in Matrigel for 12 days. Colonies in 3-D cultures were observed and the size of each colony was measured using a phase contrast microscope. Expression of GLI1 shows statistically significant differences in colony sizes in PC3-β1-shRNA (Chi-square for linear trend with 1 degree of freedom is 36.181, p<0.00001), but not in PC3-cont-shRNA cells (Chi-square for linear trend with 1 degree of freedom is 0.608, p=0.44). C: PC3-β1-shRNA cells were transiently transfected with either EGFP or GLI1-GFP. Cells were lysed and immunoblotted using Abs to GLI1 or ERK, as a loading control.
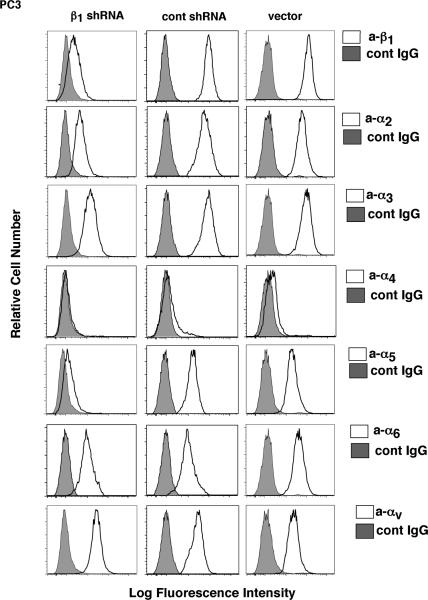
β1 integrin downregulation affects the expression of the associated α subunits
β1 integrins heterodimerize with several α subunits. To study the effect of β1 downregulation on expression of various α subunits known to associate with β1 integrins, we performed FACS using PC3 transfectants. As shown in Figure 4, β1 downregulation causes reduced expression of α2, α3, α4, and α5 subunits. The most significant effect is observed on the expression of α5 subunits, suggesting that α5 is a major partner for β1 in prostate cancer cells. In contrast, expression of α6 and αv integrin subunits is not changed. These results can be explained by the fact that PC3 cells express endogenous β3, β4 and β5 integrins (data not shown), which can heterodimerize with α6 and αv subunits. These observations were further confirmed by using TRAMP-C2 cells, a mouse prostate cancer cell line. In these cells, downregulation of β1 integrins reduces expression of α5 subunit, but not αv or α6 subunit (data not shown). These data indicate that the α5β1 is the predominant integrin heterodimer that regulates GLI1 expression and proliferation of prostate cancer cells in 3-D cultures.
Fig. 4. β1 integrin downregulation affects the expression of the associated α subunits.
PC3-β1-shRNA, PC3-cont-shRNA and PC3-vector transfectants were detached and analyzed by FACS using TS2/16 (a-β1), P1H5 (a-α2), P1B5 (a-α3), P4C2 (a-α4), P1D6 (a-α5), GoH3 (a-α6), L230 (a-αv), or 12CA5 (cont IgG).
Discussion
The novel finding described in this study is the discovery of a new function for β1 integrins in regulating the expression of GLI1 and, consequently, GLI-dependent cancer cell proliferation in 3-D cultures.
Several studies have shown that GLI1 expression can be regulated by either classical or non-classical pathways (Lauth and Toftgard, 2007). The classical pathway is mediated by binding of SHH to Patched, a transmembrane protein. Binding of SHH to Patched suppresses the inhibitory activity of Patched on Smoothened, as a consequence stimulates expression of GLI1 (Sanchez et al., 2005). Although an indirect involvement of β1 integrins in the classical pathway has been demonstrated by Blaess et al., who have shown that SHH binds laminin and promotes cell proliferation in a β1-dependent manner (Blaess et al., 2004), integrins have not been shown to directly regulate SHH expression or activity. While it is unlikely that the classical pathway mediates GLI1 regulation by β1 integrins observed in this study, it is likely that non-classical pathways mediated by PI 3-kinase/AKT, K-Ras/Raf/MEK/MAPK and TGF-β/Smad are involved. These pathways are known to potentiate SHH/GLI signaling (Lauth and Toftgard, 2007) and to be activated by integrins (Goel et al., 2009a; Munger et al., 1999). Among others, the IGF-1/AKT pathway is relevant to this study since it has been shown to increase GLI1 activity via increased expression of GLI2 (Riobo et al., 2006). Based on the results described in this study, we hypothesize that reduced PI 3-kinase/AKT activity due to deregulated IGF-IR signaling upon β1 downregulation may cause reduced expression of GLI2, and as a consequence, reduced expression of GLI1. This hypothesis remains to be investigated.
The present study shows that downregulation of β1 integrins in prostate cancer cells reduces colony size in 3-D Matrigel cultures and results in the formation of regular glandular structures, whereas irregularly-shaped structures are formed by control cells. We conclude that β1 integrins may promote a transformed phenotype in prostate epithelial cells. Indeed, our findings are relevant to prostate cancer, since the SHH pathway has been shown to regulate prostate cancer progression (Karhadkar et al., 2004; Stecca et al., 2005). Using a similar 3-D system, it has also been shown that inhibition of β1 causes phenotypic reversion of malignant human breast cancer cells via cell proliferation (Park et al., 2006; Park et al., 2008; Weaver et al., 1997; Zhang et al., 2009). Therefore, given the ability of GLI1 to rescue this effect, it is conceivable that GLI1 may regulate cell proliferation and phenotypic reversion in prostate as well as in breast cancer cells.
Our data suggest that the effect of β1 integrins on cell proliferation and GLI1 expression in 3-D cultures is mediated by signaling via the IGF-IR/AKT pathway. These findings are relevant to prostate cancer, since the IGF-IR/AKT pathway has been shown to regulate prostate cancer progression (Majumder and Sellers, 2005; Sachdev and Yee, 2007). Similarly, inhibition of IGF-IR has been reported to block proliferation of breast cancer cells in 3-D cultures (Litzenburger et al., 2009). Therefore, β1 integrins may regulate these aberrations in a wider set of carcinomas.
Finally, it is necessary to stress that the downregulation of β1 integrins reduces expression of associated α subunits, predominantly α5 and at a lower extent α2, α3 and α4; but not α6 and αv, which are also known to heterodimerize with β1 integrins. Among others, α5 is significantly inhibited by β1 shRNA, thus suggesting that α5β1 is the functionally predominant β1 integrin heterodimer in these cells. The data also indicate that other β integrin subunits do not play a crucial role in the novel pathway analyzed in this study.
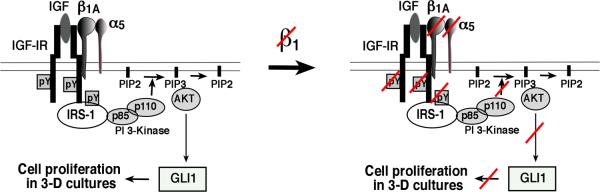
In summary, we describe a new mechanism of regulation of GLI1 expression, and consequently of cancer cell proliferation, by β1 integrins in 3-D cultures and propose that this pathway is mediated by IGF-IR and AKT (Fig. 5).
Fig. 5. β1/IGF-IR complex regulates prostate cancer cell proliferation in 3-D cultures through GLI1 expression.
A model for the interaction between β1 integrins and IGF-IR regulating cell proliferation of prostate cancer cells in 3-D cultures in an AKT/GLI1-dependent manner is shown.
Acknowledgements
We would like to thank Dr. Junhao Mao for critical discussion, Karen Imbalzano for technical assistance and Alana Calapai for help with the preparation of the manuscript.
Contract grant sponsor: National Institute of Health; Contract grant number: CA109874 (LRL), CA89720 (LRL) and PO1 CA82834 (LRL and JAN); Contract grant sponsor: American Cancer Society Institutional Research Grant; Contract grant number: IRG-93-033 (HLG).
Literature Cited
- Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- Blaess S, Graus-Porta D, Belvindrah R, Radakovits R, Pons S, Littlewood-Evans A, Senften M, Guo H, Li Y, Miner JH, Reichardt LF, Muller U. ®1-integrins are critical for cerebellar granule cell precursor proliferation. J Neurosci. 2004;24:3402–3412. doi: 10.1523/JNEUROSCI.5241-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP. Integrin and growth factor receptor crosstalk. Circ Res. 2001;89:1104–1110. doi: 10.1161/hh2401.101084. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Steger CA, Bennett AM, Wu JJ, Languino LR. Differential role of ®1C and ®1A integrin cytoplasmic variants in modulating focal adhesion kinase, protein kinase B/AKT, and Ras/Mitogen-activated protein kinase pathways. Mol Biol Cell. 2000;11:2235–2249. doi: 10.1091/mbc.11.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Tallini G, Zheng DQ, Flanagan WM, Manzotti M, Languino LR. p27(kip1) acts as a downstream effector of and is coexpressed with the ®1C integrin in prostatic adenocarcinoma. J Clin Invest. 1999;103:321–329. doi: 10.1172/JCI4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Alam N, Johnson IN, Languino LR. Integrin Signaling aberrations in Prostate Cancer. Am J Transl Res. 2009a;1:211–220. [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Breen M, Zhang J, Das I, Aznavoorian-Cheshire S, Greenberg NM, Elgavish A, Languino LR. ®1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65:6692–6700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- Goel HL, Fornaro M, Moro L, Teider N, Rhim JS, King M, Languino LR. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J Cell Biol. 2004;166:407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;3:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Moro L, Murphy-Ullrich JE, Hsieh CC, Wu CL, Jiang Z, Languino LR. ®1 integrin cytoplasmic variants differentially regulate expression of the antiangiogenic extracellular matrix protein thrombospondin 1. Cancer Res. 2009b;69:5374–5382. doi: 10.1158/0008-5472.CAN-09-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27:3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauth M, Toftgard R. Non-canonical activation of GLI transcription factors: implications for targeted anti-cancer therapy. Cell Cycle. 2007;6:2458–2463. doi: 10.4161/cc.6.20.4808. [DOI] [PubMed] [Google Scholar]
- Liao X, Siu MK, Au CW, Wong ES, Chan HY, Ip PP, Ngan HY, Cheung AN. Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30:131–140. doi: 10.1093/carcin/bgn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenburger BC, Kim HJ, Kuiatse I, Carboni JM, Attar RM, Gottardis MM, Fairchild CR, Lee AV. BMS-536924 reverses IGF-IR-induced transformation of mammary epithelial cells and causes growth inhibition and polarization of MCF7 cells. Clin Cancer Res. 2009;15:226–237. doi: 10.1158/1078-0432.CCR-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin 〈v®6 binds and activates latent TGF®1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. ®1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. ®1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr. Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Clement V, Ruiz i Altaba A. Therapeutic targeting of the Hedgehog-GLI pathway in prostate cancer. Cancer Res. 2005;65:2990–2992. doi: 10.1158/0008-5472.CAN-05-0439. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3® floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- Stecca B, Mas C, Ruiz i Altaba A. Interference with HH-GLI signaling inhibits prostate cancer. Trends Mol Med. 2005;11:199–203. doi: 10.1016/j.molmed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Tai YT, Podar K, Catley L, Tseng YH, Akiyama M, Shringarpure R, Burger R, Hideshima T, Chauhan D, Mitsiades N, Richardson P, Munshi NC, Kahn CR, Mitsiades C, Anderson KC. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of ®1-integrin and phosphatidylinositol 3'-kinase/AKT signaling. Cancer Res. 2003;63:5850–5858. [PubMed] [Google Scholar]
- Underwood JM, Imbalzano KM, Weaver VM, Fischer AH, Imbalzano AN, Nickerson JA. The ultrastructure of MCF-10A acini. J Cell Physiol. 2006;208:141–148. doi: 10.1002/jcp.20639. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in 3-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanochko GM, Eckhart W. Type I insulin-like growth factor receptor over-expression induces proliferation and anti-apoptotic signaling in a three-dimensional culture model of breast epithelial cells. Breast Cancer Res. 2006;8:R18. doi: 10.1186/bcr1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fournier MV, Ware JL, Bissell MJ, Yacoub A, Zehner ZE. Inhibition of vimentin or ®1 integrin reverts morphology of prostate tumor cells grown in laminin-rich extracellular matrix gels and reduces tumor growth in vivo. Mol Cancer Ther. 2009;8:499–508. doi: 10.1158/1535-7163.MCT-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]