Abstract
The nucleotide sequence of part of the late region of the polyoma virus genome was determined. It contains coding information for the major capsid protein VP1 and the C-terminal region of the minor proteins VP2 and VP3. In the sequence with the same polarity as late mRNA's, all coding frames are blocked by termination codons in a region around 48 units on the physical map. This is the region where the N-terminus of VP1 and the C-termini of VP2 and VP3 have been located (T. Hunter and W. Gibson, J. Virol. 28:240-253, 1978; S. G. Siddell and A. E. Smith, J. Virol. 27:427-431, 1978; Smith et al., Cell 9:481-487, 1976). There are two long uninterrupted coding frames in the late region of polyoma virus DNA. One lies at the 5′ end of the sequence and contains potential coding sequences for VP2 and VP3. The other contains 383 consecutive sense codons starting with the ATG at nucleotide position 1,218, extends from 47.5 to 25.8 units counterclockwise on the physical map, and is located where the VP1 gene has been mapped. The VP1 gene overlaps the genes for proteins VP2/VP3 by 32 nucleotides and uses a different coding frame. From the DNA sequence, the amino acid sequence of VP1 was predicted. The proposed VP1 sequence is in good agreement with other data, namely, with the partial N-terminal amino acid sequence and the total amino acid composition. The VP1 coding frame terminates with a TAA codon at 25.8 map units. This is followed by an AATAAA sequence, which may act as a processing signal for the viral late mRNA's. When both nucleotide and amino acid sequences are compared with their counterparts in the related simian virus 40, extensive homologies are found over the entire region of the two viral genomes. Maximum homology appears to occur in those regions which code for the C-termini of the VP1 proteins. The overlap region of VP1 with VP2/VP3 of polyoma virus is shorter by 90 nucleotides than is that of simian virus 40 and shows very limited homology with the simian virus 40 sequence. This leads to the suggestion that the overlap segments of both viruses have been freed from stringency imposed on drifting during evolution and that proteins VP2 and VP3 of polyoma virus may have been truncated by the appearance of a termination codon within the sequence.
Full text
PDF


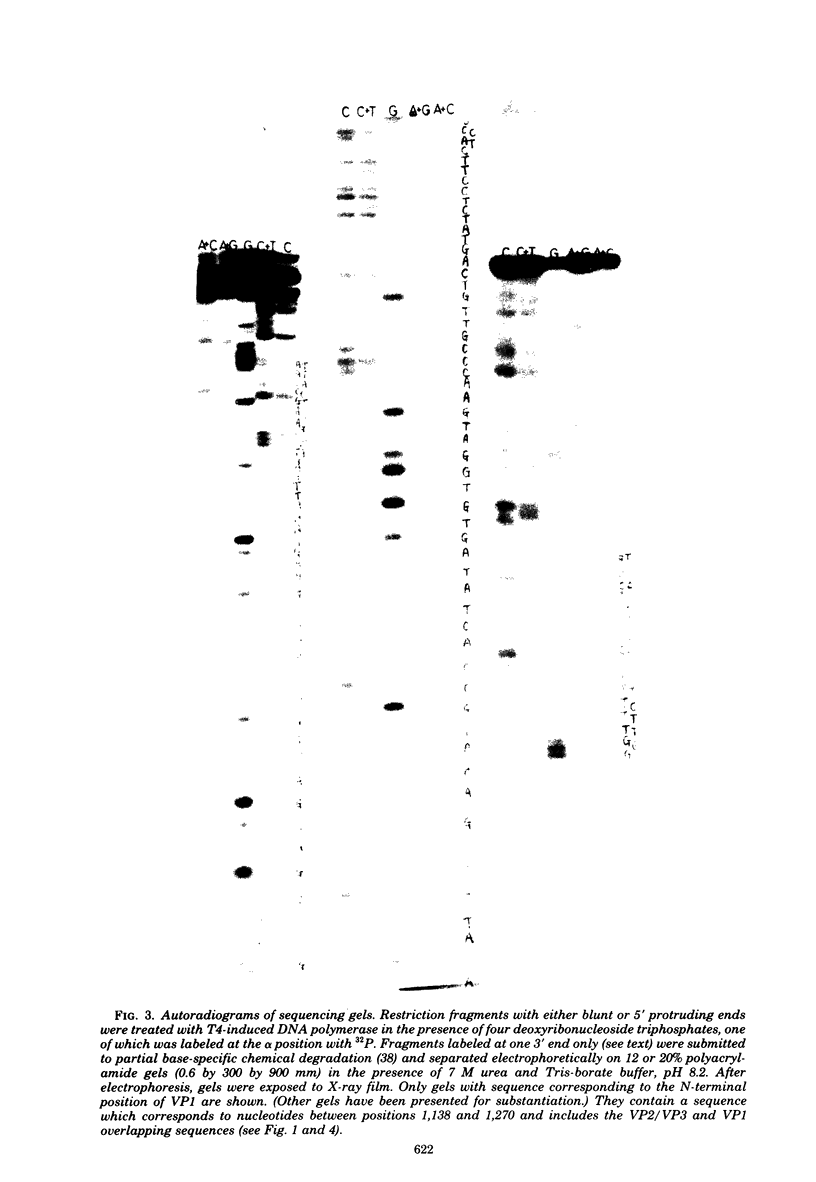


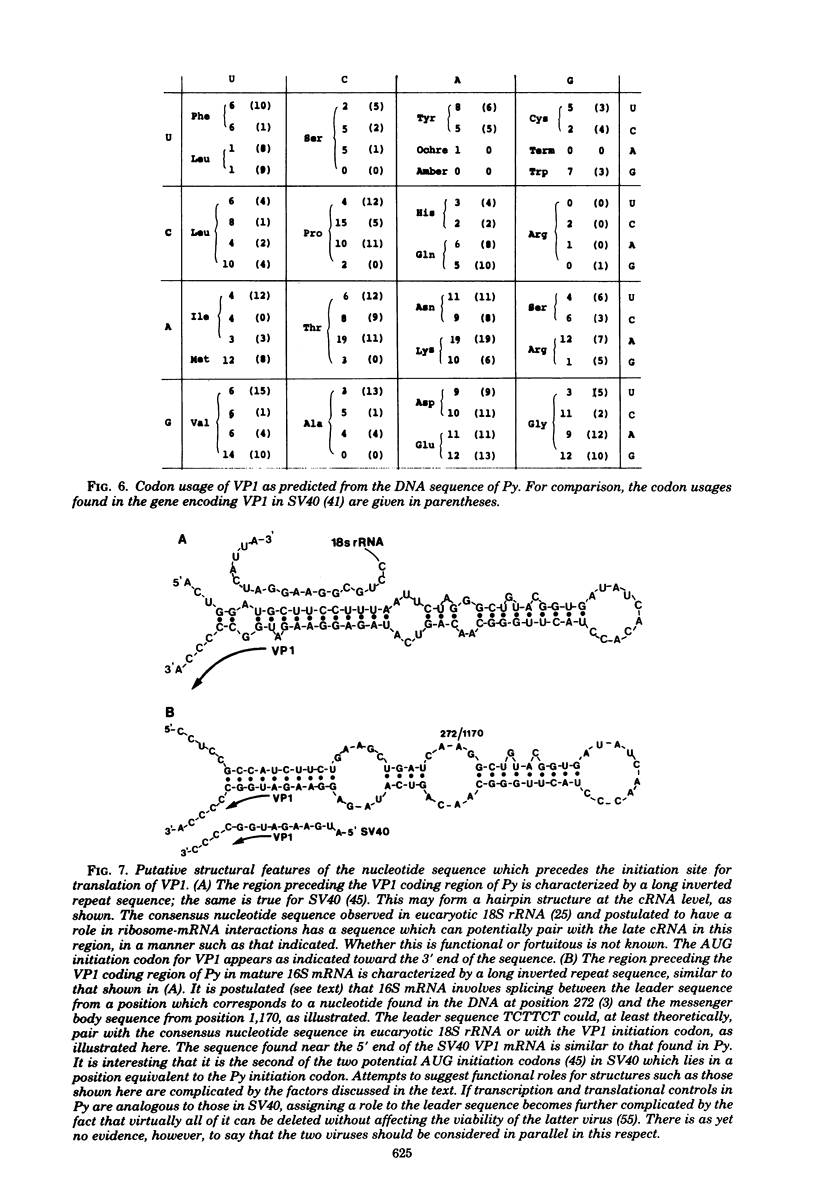

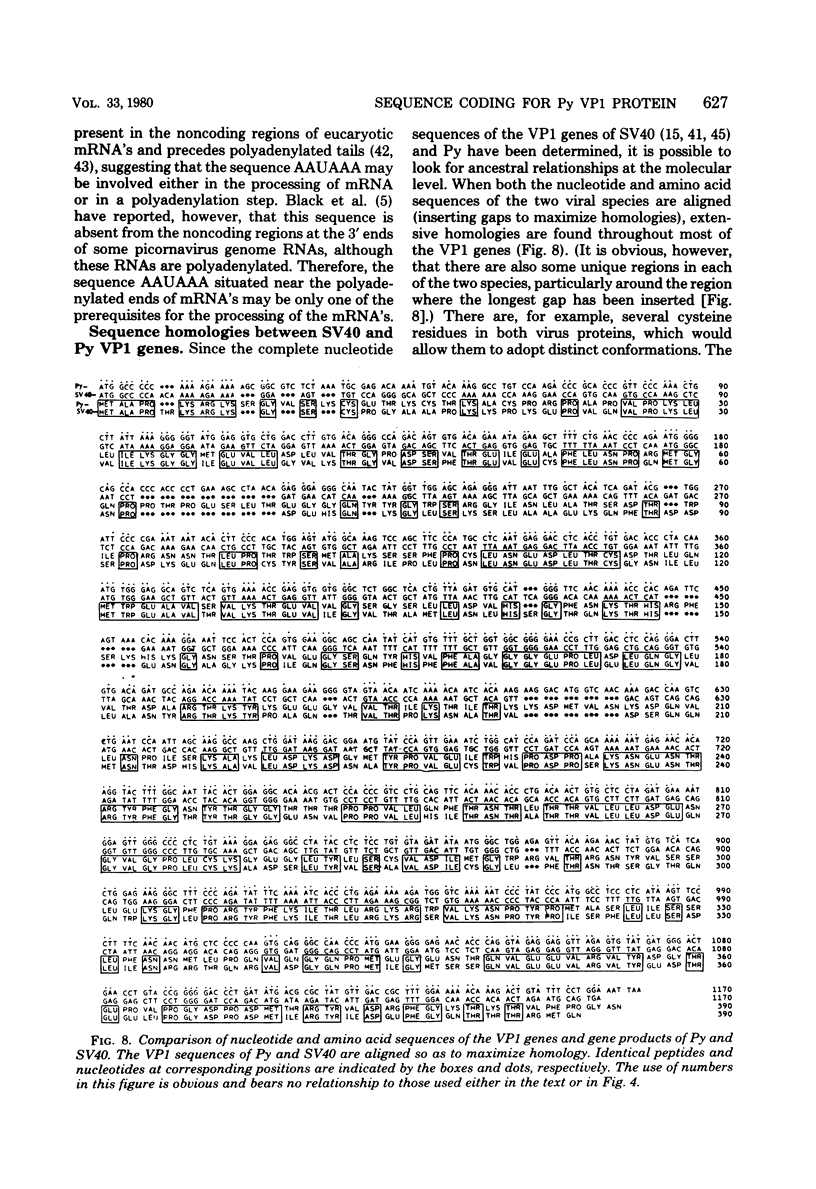



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H. Transcription during productive infection with polyoma virus and Simian virus 40. Cell. 1976 May;8(1):1–12. doi: 10.1016/0092-8674(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Soeda E., Walsh J. E., Smolar N., Griffin B. E. Polyoma virus DNA: Sequence from the late region that specifies the leader sequence for late mRNA and codes for VP2, VP3, and the N-terminus of VP1. J Virol. 1980 Feb;33(2):606–618. doi: 10.1128/jvi.33.2.606-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti E. Characterization of late polyoma mRNA. J Virol. 1974 Aug;14(2):249–260. doi: 10.1128/jvi.14.2.249-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., O'Malley B. W., Robertson M. A., Staden R., Tanaka Y., Brownlee G. G. Nucleotide sequence homology at 12 intron--exon junctions in the chick ovalbumin gene. Nature. 1978 Oct 12;275(5680):510–513. doi: 10.1038/275510a0. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R., Rogiers R., Van de Voorde A., Fiers W. Overlapping of the VP2-VP3 gene and the VP1 gene in the SV40 genome. Cell. 1977 Oct;12(2):529–538. doi: 10.1016/0092-8674(77)90129-5. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Brown D. D., Reeder R. H. Composition and structure of chromosomal and amplified ribosomal DNA's of Xenopus laevis. J Mol Biol. 1970 Jul 28;51(2):341–360. doi: 10.1016/0022-2836(70)90147-6. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J., Davis R. W. An electron microscopic method for studying and mapping the region of weak sequence homology between simian virus 40 and polyoma DNAs. J Mol Biol. 1975 May 15;94(2):135–149. doi: 10.1016/0022-2836(75)90073-x. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Fried M., Griffin B. E. Organization of the genomes of polyoma virus and SV40. Adv Cancer Res. 1977;24:67–113. doi: 10.1016/s0065-230x(08)61013-1. [DOI] [PubMed] [Google Scholar]
- Galibert F., Sedat J., Ziff E. Direct determination of DNA nucleotide sequences: structure of a fragment of bacteriophage phiX172 DNA. J Mol Biol. 1974 Aug 15;87(3):377–407. doi: 10.1016/0022-2836(74)90093-x. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Choudary P. V., Lebowitz P., Weissman S. M. The 5'-terminal leader sequence of late 16 S mRNA from cells infected with simian virus 40. J Biol Chem. 1978 May 25;253(10):3643–3647. [PubMed] [Google Scholar]
- Gibson W., Hunter T., Cogen B., Eckhart W. Altered virion proteins of a temperature-sensitive mutant of polyoma virus, ts59. Virology. 1977 Jul 1;80(1):21–41. doi: 10.1016/0042-6822(77)90378-6. [DOI] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Griffin B. E. Fine structure of polyoma virus DNA. J Mol Biol. 1977 Dec 5;117(2):447–471. doi: 10.1016/0022-2836(77)90137-1. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E., Maddock C. New classes of viable deletion mutants in the early region of polyoma virus. J Virol. 1979 Sep;31(3):645–656. doi: 10.1128/jvi.31.3.645-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Mellor A., Smith A. E., Waterfield M. D. Partial amino-terminal sequences of the polyoma nonhistone proteins VP1, VP2, and VP3 synthesized in vitro. J Virol. 1980 Feb;33(2):631–636. doi: 10.1128/jvi.33.2.631-636.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Waterfield M. D., Miller L. K., Fried M. Correlation between genetic loci and structural differences in the capsid proteins of polyoma virus plaque morphology mutants. Cell. 1977 Jun;11(2):331–338. doi: 10.1016/0092-8674(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Hunter T., Gibson W. Characterization of the mRNA's for the polyoma virus capsid proteins VP1, VP2, and VP3. J Virol. 1978 Oct;28(1):240–253. doi: 10.1128/jvi.28.1.240-253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUG A. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. II. COMMENTS ON OTHER WORK. J Mol Biol. 1965 Feb;11:424–431. doi: 10.1016/s0022-2836(65)80067-5. [DOI] [PubMed] [Google Scholar]
- Kamen R., Favaloro J., Parker J. Topography of the three late mRNA's of polyoma virus which encode the virion proteins. J Virol. 1980 Feb;33(2):637–651. doi: 10.1128/jvi.33.2.637-651.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Favaloro J., Parker J., Treisman R., Flavell A. J., Cowie A., Legon S. Structure of polyoma virus mRNAs synthesized in productively infected mouse cells. Differentiation. 1979;13(1):45–46. doi: 10.1111/j.1432-0436.1979.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Kamen R., Shure H. Topography of polyoma virus messenger RNA molecules. Cell. 1976 Mar;7(3):361–371. doi: 10.1016/0092-8674(76)90165-3. [DOI] [PubMed] [Google Scholar]
- Kimura M. Evolutionary rate at the molecular level. Nature. 1968 Feb 17;217(5129):624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of the genetic map of the polyoma genome. J Virol. 1976 Jun;18(3):824–832. doi: 10.1128/jvi.18.3.824-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T. Evolution of overlapping genes. Nature. 1978 Apr 6;272(5653):532–535. doi: 10.1038/272532a0. [DOI] [PubMed] [Google Scholar]
- Pan J., Reddy V. B., Thimmappaya B., Weissman S. M. Nucleotide sequence of the gene for the major structural protein of SV40 virus. Nucleic Acids Res. 1977 Aug;4(8):2539–2548. doi: 10.1093/nar/4.8.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. G., Fellner P., Black D. N., Rowlands D. J., Harris T. J., Brown F. 3'-Terminal nucleotide sequences in the genome RNA of picornaviruses. Nature. 1978 Nov 16;276(5685):298–301. doi: 10.1038/276298a0. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Merregaert J., Van Emmelo J., Fiers W. Sequence of 129 nucleotides at the 3'-terminus of encephalomyocarditis virus RNA. Eur J Biochem. 1978 Jul 3;87(3):551–561. doi: 10.1111/j.1432-1033.1978.tb12406.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Cheng C. C., Brownlee G. G. Sequence analysis of eukaryotic mRNA. Prog Nucleic Acid Res Mol Biol. 1976;19:123–134. doi: 10.1016/s0079-6603(08)60914-9. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rogiers R., van de Voorde A., Soeda E., Fiers W. Nucleotide sequence of the simian virus 40 Hind-K restriction fragment. Eur J Biochem. 1978 Apr;85(1):205–224. doi: 10.1111/j.1432-1033.1978.tb12229.x. [DOI] [PubMed] [Google Scholar]
- Russell G. J., Walker P. M., Elton R. A., Subak-Sharpe J. H. Doublet frequency analysis of fractionated vertebrate nuclear DNA. J Mol Biol. 1976 Nov;108(1):1–23. doi: 10.1016/s0022-2836(76)80090-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Smith A. E. Polyoma virus has three late mRNA's: one for each virion protein. J Virol. 1978 Aug;27(2):427–431. doi: 10.1128/jvi.27.2.427-431.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Kamen R., Mangel W. F., Shure H., Wheeler T. Location of the sequences coding for capsid proteins VP1 and VP2 on polyoma virus DNA. Cell. 1976 Nov;9(3):481–487. doi: 10.1016/0092-8674(76)90093-3. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Griffin B. E. Polyoma virus. The early region and its T-antigens. Nucleic Acids Res. 1979 Oct 25;7(4):839–857. doi: 10.1093/nar/7.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Soeda E., Kimura G., Miura K. Similarity of nucleotide sequences around the origin of DNA replication in mouse polyoma virus and simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):162–166. doi: 10.1073/pnas.75.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N. Segments of simian virus 40 DNA spanning most of the leader sequence of the major late viral messenger RNA are dispensable. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2556–2560. doi: 10.1073/pnas.76.6.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Deppert W. Intermolecular disulfide bonds: an important structural feature of the polyoma virus capsid. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):255–257. doi: 10.1101/sqb.1974.039.01.033. [DOI] [PubMed] [Google Scholar]