Abstract
OBJECTIVE
Obstructive sleep apnea syndrome in children is associated with significant morbidity. Continuous positive airway pressure (CPAP) treats obstructive apnea in children, but is impeded by low adherence. We, therefore, sought to assess the effect of warm humidified air delivered through an open nasal cannula (treatment with nasal insufflation [TNI]) on obstructive sleep apnea in children with and without adenotonsillectomy.
METHODS
Twelve participants (age: 10 ± 1 years; BMI: 35 ± 14 kg/m2), with obstructive apnea-hypopnea syndrome ranging from mild to severe (2–36 events per hour) were administered 20 L/min of air through a nasal cannula. Standard sleep architecture, sleep-disordered breathing, and arousal indexes were assessed at baseline, on TNI, and on CPAP. Additional measures of the percentage of time with inspiratory flow limitation, respiratory rate, and inspiratory duty cycle were assessed at baseline and on TNI.
RESULTS
TNI reduced the amount of inspiratory flow limitation, which led to a decrease in respiratory rate and inspiratory duty cycle. TNI improved oxygen stores and decreased arousals, which decreased the occurrence of obstructive apnea from 11 ± 3 to 5 ± 2 events per hour (P < .01). In the majority of children, the reduction in the apnea-hypopnea index on TNI was comparable to that on CPAP.
CONCLUSIONS
TNI offers an alternative to therapy to CPAP in children with mild-to-severe sleep apnea. Additional studies will be needed to determine the efficacy of this novel form of therapy.
Keywords: pediatric obstructive sleep apnea, treatment of sleep apnea, TNI
Obstructive sleep apnea (OSA) in children is attributed to upper airway collapse1–3 that is associated with intermittent hypoxemia, neurocognitive dysfunction,4–8 and cardiovascular morbidity.9–12 Moreover, recent data suggest that milder degrees of obstructive sleep-disordered breathing are associated with neurobehavioral deficits,13,14 highlighting the social and medical burdens of sleep-disordered breathing in children.
Treatment of sleep apnea in children includes both medical15 and surgical options.16 Adenotonsillectomy is the treatment of choice for the presence of adenoid and tonsillar hypertrophy with OSA. For children who are not suitable candidates for surgery, refuse adenotonsillectomy, or have residual sleep apnea after surgical intervention, continuous positive airway pressure (CPAP)17 is the most effective treatment option. CPAP, however, is encumbered by suboptimal adherence,18 leaving a large number of children untreated. Therefore, alternative therapeutic strategies to CPAP are required to treat OSA in children more effectively. Recently, we demonstrated that air delivered at a high flow rate through a nasal cannula (treatment with nasal insufflation [TNI]) alleviated upper airway obstruction in adults with OSA.19 Children with upper airway obstruction during sleep, however, differ markedly with regard to the distribution of obstructive events. They have obstructive apneas predominantly during rapid eye movement (REM) compared with nonrapid eye movement (NREM) sleep.20 In contrast to adults, children commonly exhibit periods of prolonged stable partial upper airway obstruction during sleep, including during NREM sleep.21 Thus, whereas the rate of obstructive events per hour of sleep (apnea-hypopnea index [AHI]) allows for quantification of changes in upper airway obstruction for REM sleep, other measures are needed to assess upper airway obstruction during NREM sleep. It has been demonstrated previously that the inspiratory time relative to the duration of the respiratory cycle, the inspiratory duty cycle, increases linearly with the degree of upper airway obstruction.22–24 Therefore, we determined the effect of TNI on upper airway obstruction in children by assessing both the AHI and the inspiratory duty cycle. We hypothesized that TNI would alleviate upper airway obstruction during both REM and NREM sleep and that, in a significant proportion of children, improvements in the AHI would be similar to CPAP.
PATIENTS AND METHODS
Study Population
Children 5 to 15 years of age were recruited consecutively from the Johns Hopkins Pediatric Sleep Disorders Center if they had OSA and were recommended treatment with CPAP. Patients were excluded if they had a nocturnal oxygen requirement or other serious medical conditions. Informed consent was obtained from 1 parent or guardian, assent was obtained from the children, and the Johns Hopkins Medicine Institutional Review Board approved the protocol.
Protocols
Each participant underwent 2 over-night polysomnograms, 1 with TNI at 20 L/min and 1 night off TNI, performed in random order. For children who had participated in a clinical CPAP titration study before enrollment, that study was analyzed to compare the AHI between TNI and CPAP at the prescribed nasal pressure level. Children continued home use of CPAP while enrolled in the study.
Study Materials
Polysomnography
Sleep studies were performed with Somnologica (Embla, Broomfield, CO). Signals included electroencephalograms (leads C3-A2, C4-A1, and O1-A2), left and right electrooculograms, submental electromyogram, tibial electromyogram, electrocardiogram, and oxyhemoglobin saturation(Masimo, Irvine, CA). End-tidal CO2 (Novametrix, Murrysville, PA) was acquired from all of the participants during the baseline night, but the signal could not be obtained during the treatment night because of interference from TNI. Trans-cutaneous CO2 measurement (TCM3, Radiometer Kopenhagen, Copenhagen, Denmark) was acquired in 5 participants during the baseline and in 3 of those subjects during the treatment night with TNI. Airflow measurement was acquired with a nasal cannula (Salter Labs, Arvin, CA) connected to a differential pressure transducer (Pro-Tech, Mukilteo, WA). Respiratory effort was assessed with thoracic and abdominal inductive plethysmography (Embla), and body position was monitored via infrared video camera.
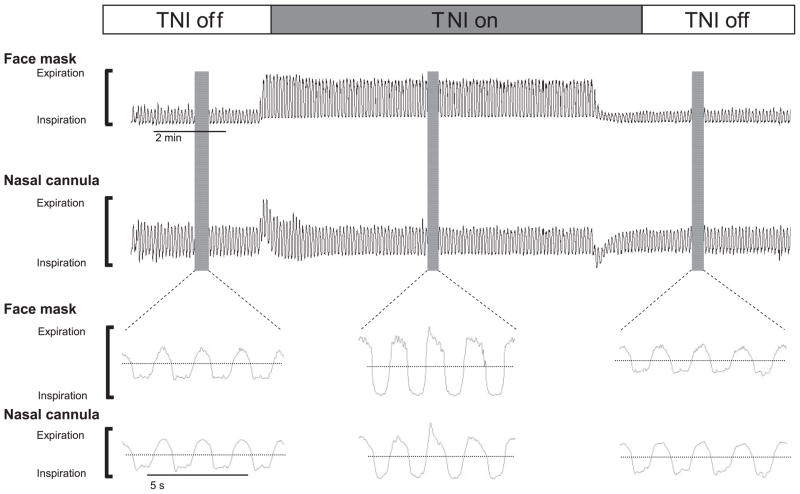
To ensure that the airflow signals acquired from the nasal cannula were not affected by TNI, preliminary studies were conducted comparing the qualitative airflow signals from a nasal cannula with those acquired concurrently from a nasal mask attached to a pneumotachograph while TNI was turned on and off during sleep. The contour and amplitude of the airflow signal acquired from the nasal cannula were consistently similar to those of the nasal mask when TNI was turned on and off, indicating that TNI did not interfere with our ability to detect inspiratory flow limitation (see Fig 1).
FIGURE 1.
Qualitative airflow signals from a nasal mask attached to a pneumotachograph compared with those acquired concurrently from a nasal cannula attached to a pressure transducer during TNI trials are depicted. Changes in baseline airflow in the nasal cannula at the onset and offset of TNI are attributed to an alternating current-coupled signal with a long time constant.
Nasal Insufflation (TNI)
An air compressor (TNI Medical GmbH, Heilbronn, Germany) delivered a constant flow rate at the level of the nasal prongs of the TNI cannula at a maximum of 20 L/min. A heater and humidifier regulated the temperature and humidity. A heated wire incorporated into the lumen of the nasal cannula maintained a temperature of 30°C to 33°C and a relative humidity of ~80% at the nasal outlet (Fig 2).
FIGURE 2.
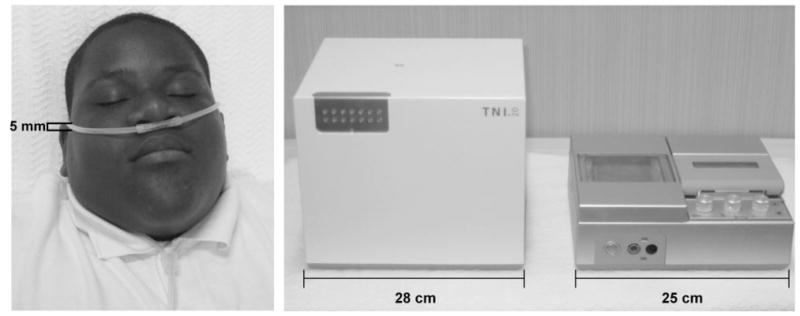
One study participant wearing the TNI cannula (left) and the TNI device is depicted (right). Cannula length is 1800.0 mm, outer diameter 5.0-mm cannula and nasal prongs, and inner diameter 3.4 mm. The weight of the TNI device including the compressor is ~10 kg.
Analysis
Sleep and Respiratory Events
Standard polysomnographic scoring techniques were used to stage sleep, arousals, and respiratory events.25 An AHI was calculated for obstructive and central respiratory events per hour of sleep separately for each individual for the entire night and for NREM and REM sleep. Because the primary outcome was effect on upper airway obstruction, AHI data pertain specifically to obstructive events. If REM sleep time was <20 minutes on either the baseline or TNI treatment night, the participant was excluded from the analysis of REM AHI.
Respiratory Pattern
Children with sleep-disordered breathing commonly exhibit apneic events during REM as compared with NREM sleep.20 Thus, upper airway obstruction during NREM sleep was also assessed by the following: percentage of time with flow-limited breathing, inspiratory duty cycle (length of the inspiratory time divided by length of the respiratory cycle), and respiratory rate for the baseline and TNI-treatment nights. Inspiratory flow limitation was assessed by visual inspection, as described previously,26–29 and quantified as the percentage of NREM sleep time that it was exhibited. A custom computer program that randomly generated two 3-minute samples per hour of NREM sleep determined breaths analyzed. All of the breaths in each sample were included in the analysis irrespective of the presence or absence of inspiratory flow limitation.
Statistical Analysis
Data are reported as the means ± SEMs. The Wilcoxon sign-rank test was performed (Stata 8, Stata Corp, College Station, TX) to compare differences in sleep architecture, arousal indexes, oxyhemoglobin saturation, and measures of sleep-disordered breathing between the baseline and TNI-treatment nights. P values of <.05 were considered significant.
RESULTS
Participant Characteristics
Twelve otherwise healthy children with sleep apnea aged 10 ± 2 years were enrolled. The majority of children were boys and obese (Table 1). Self-reported CPAP use was for <4 hours per night and <5 days per week in half of the children. Although these adherence rates are consistent with previous studies of CPAP use,18 most children in this study were ineffectively treated.
TABLE 1.
Patient Anthropometrics and Characteristics
| Patient ID No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Mean | SEM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthropometrics | ||||||||||||||
| Gender | F | M | M | M | M | M | M | M | M | F | F | F | 8 M/4 F | — |
| Age, y | 8 | 13 | 10 | 7 | 9 | 11 | 9 | 10 | 10 | 14 | 14 | 12 | 10 | 1 |
| Height, cm | 142 | 159 | 137 | 127 | 125 | 153 | 141 | 159 | 161 | 165 | 161 | 177 | 145 | 5 |
| Weight, kg | 53 | 72 | 75 | 22 | 25 | 92 | 54 | 134 | 50 | 129 | 116 | 132 | 75 | 12 |
| BMI, kg/m2 | 26 | 28 | 40 | 14 | 16 | 36 | 27 | 53 | 19 | 47 | 45 | 42 | 35 | 4 |
| BMI z score | 2.2 | 1.9 | 2.7 | − 1.5 | −0.1 | 2.7 | 2.2 | 2.9 | 1.1 | 2.8 | 2.8 | 1.5 | 1.6 | 0.4 |
| Previous treatment | ||||||||||||||
| CPAP, cm H2O | 11 | 8 | 7 | NA | 7 | 8 | 10 | 5 | 7 | 20 | 5 | NA | 9 | 1 |
| Adenotonsillectomy | + | + | + | − | + | − | + | − | − | + | + | + | 8+/4− | — |
| Disordered breathing indexes | ||||||||||||||
| AHI total, events per hour | 2 | 2 | 2 | 3 | 5 | 8 | 9 | 9 | 17 | 20 | 22 | 36 | 11 | 3 |
| SPO2 nadir, % | 96 | 90 | 92 | 95 | 87 | 90 | 88 | 84 | 94 | 79 | 83 | 68 | 87 | 2 |
| Peak CO2, mm Hg | 53 | 51 | 50 | 54 | 60 | 53 | 59 | 58 | 55 | 54 | 56 | 63 | 56 | 1 |
| % TST CO2 >50 mm Hg | 1 | 0.1 | 0 | 3 | 52 | 1 | 37 | 5 | 1 | 7 | 7 | 56 | 14 | 6 |
| Daytime symptoms | H, IC, BD | DS, H | H, IC | H, IC, BD | DS, H | None | H, IC | DS, BD | H, IC, BD | DS | DS | H, BD | — | — |
SPO2 nadir indicates oxyhemoglobin saturation; CO2 indicates end-tidal CO2; % TST CO2 >50 mm Hg, percentage of total sleep time that CO2 was >50 mm Hg; DS, daytime sleepiness; H, hyperactivity; IC, impaired concentration; BD, behavior disorder; M, male; F, female; —, no data; NA, not applicable.
Polysomnographic Responses to TNI
Sleep-Disordered Breathing Events
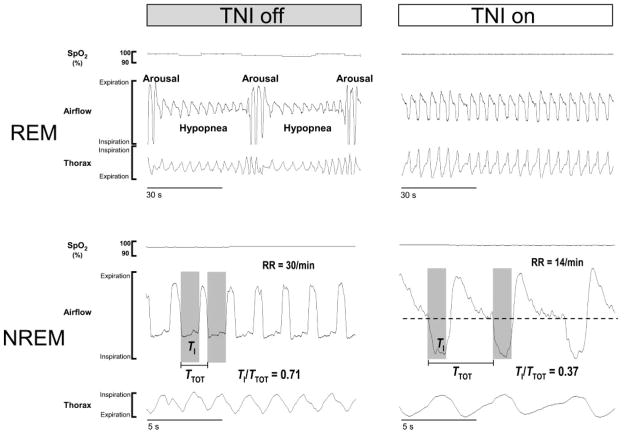
In Fig 3, the effect of TNI on sleep-disordered breathing is illustrated in 1 child (child No. 7). In this child, obstructive hypopneas were observed primarily during REM sleep without TNI treatment (Fig 2, top left). Each hypopnea was characterized by decreased inspiratory airflow and a small reduction in oxyhemoglobin saturation and was terminated by a cortical arousal from sleep. The last 4 breaths of each hypopnea had a flattened inspiratory flow contour with increasing inspiratory effort, indicating that these events were obstructive hypopneas. In contrast, on TNI (Fig 2, top right), hypopneas were abolished, leading to a stabilization of sleep and oxyhemoglobin saturation (Table 2).
FIGURE 3.
Two hypoponeas during REM sleep at baseline are shown (top left), which are abolished on TNI (top right). During NREM sleep, the same child exhibited inspiratory flow limitation characterized by plateauing of the inspiratory contour (bottom left), which was alleviated with TNI (bottom right). EOG indicates electrooculogram; EEG, electroencephalogram; SpO2, oxyhemoglobin saturation. RR indicates respiratory rate.
TABLE 2.
Sleep Efficiency and Stage Data Are Presented as the Percentage of Time/Total Sleep Time
| Variable | Baseline, Mean ± SEM | TNI, Mean ± SEM | P |
|---|---|---|---|
| Sleep architecture | |||
| Total sleep time, min | 296.6 ± 14.3 | 284.9 ± 16.3 | .07 |
| Sleep efficiency, % | 91.3 ± 2.7 | 86.2 ± 4.3 | .04 |
| Stage N1, % | 3.2 ± 0.8 | 5.2 ± 1.4 | .2 |
| Stage N2, % | 48.1 ± 3.1 | 51.1 ± 2.9 | .4 |
| Stage N3, % | 21.3 ± 3.7 | 20.9 ± 2.6 | .9 |
| Stage R, % | 16.2 ± 3.1 | 14.7 ± 2.2 | .7 |
| Arousal indexes, events per hour of sleep | |||
| Total | 13.9 ± 1.6 | 10.1 ± 1.7 | .05 |
| Respiratory | 8.0 ± 1.9 | 4.1 ± 1.5 | .02 |
| Spontaneous | 5.9 ± 0.6 | 5.9 ± 0.8 | .8 |
| Oxyhemoglobin saturation | |||
| Average during sleep, % | 98.0 ± 1.0 | 98.0 ± 1.0 | .7 |
| Average desaturation, % | 5.0 ± 1.0 | 3.0 ± 1.0 | .02 |
| Desaturation nadir, % | 88.0 ± 2.0 | 93.0 ± 1.0 | .01 |
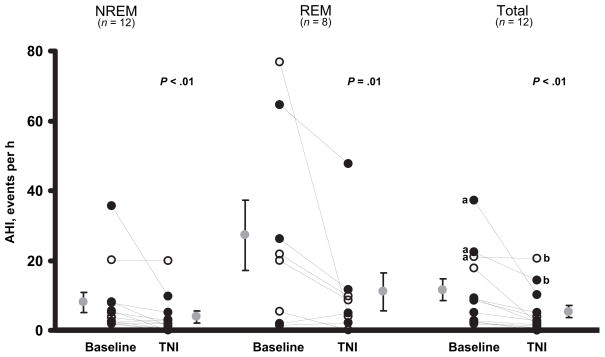
Similar responses were observed in the total AHI during the baseline compared with treatment with TNI, respectively (11 ± 3 vs 5 ± 2 events per hour) in nearly all of the children (Fig 4, right). Subanalyses were performed separately for REM and NREM sleep to assess the effect of sleep stage on the response to TNI. Four participants were excluded from the REM analysis because they did not have sufficient REM sleep time during the TNI treatment night, leaving 8 participants (boy: girl ratio: 7:1). During TNI treatment, the AHI was markedly reduced during both NREM from 8 ± 3 to 4 ± 2 events per hour (Fig 4, left) and REM sleep from 26 ± 10 to 11 ± 5 events per hour (Fig 4, middle). Central apnea rates were minimal (0.1 ± 0.1 and 0.3 ± 0.2 events per hour) during the baseline and TNI study nights, respectively. The effect of body position on the response was also assessed. During the baseline compared with the treatment night with TNI, respectively, the mean percentages of sleep time in the supine (78.0% ± 8.4% vs 79.0% ± 9.5%; P = .5) and side or prone body positions (22.0% ± 8.4% vs 21.0% ± 9.5%; P = .5) were comparable, but there were differences in 4 participants. Analysis of the AHI in these 4 children was performed while they were in a supine position only, and a greater decline in the AHI was observed with TNI treatment, indicating that changes in body position did not account for the reduction in the AHI observed on TNI treatment. Of note, polysomnographic responses were comparable in children with and without adenotonsillectomy (Fig 4).
FIGURE 4.
The AHIs are displayed for the baseline compared with the TNI-treatment night during NREM (left), REM (middle), and for the entire night (right). Data presented are means ± SEMs. a Participants with residual sleep apnea on TNI. bParticipants with suboptimal AHI responses on TNI compared with CPAP. ◦Children without adenotonsillectomy.
Sleep
Between the baseline and treatment with TNI nights, total sleep time and sleep stage distribution were similar, but we observed a slight decrease in sleep efficiency. The total and respiratory arousal indexes also decreased during the night of treatment with TNI, but the spontaneous arousal index was similar (Table 2).
Comparison With CPAP
Ten of the 12 children had undergone CPAP titration before this study. The mean prescribed nasal pressure was 9 ± 4 cm H2O (Table 1). The AHI on TNI treatment compared with CPAP treatment was 5 ± 2 vs 1 ± 1 events per hour (P = .08). As compared with CPAP, 2 children had suboptimal responses on TNI treatment (participant No. 10 and No. 11; Fig 4, denoted by b). Both of these children had severe sleep apnea (AHI >20 events per hour); 1 required a CPAP pressure of 20 cm H2O to alleviate upper airway obstruction and the other did not tolerate either the CPAP mask or the TNI device, which resulted in significant sleep disruption on both study nights. In the remaining children (n = 8), the AHI on TNI treatment compared with CPAP treatment was 2 ± 1 vs 1 ± 1 event per hour, indicating that, for the majority of children, treatment with TNI was comparable clinically to CPAP treatment.
Respiratory Pattern Responses to TNI
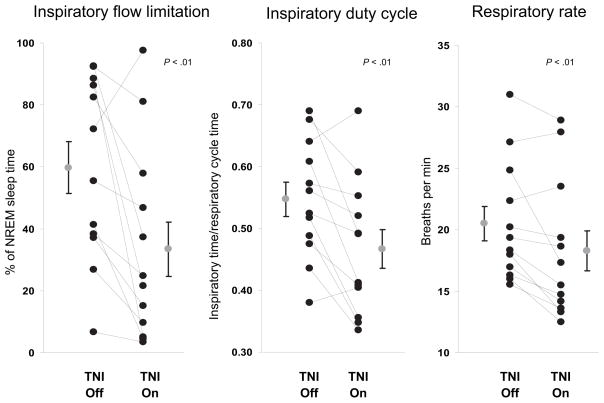
Sleep-disordered breathing in our study population was generally characterized by apneic events during REM sleep and prolonged periods of steady-state in spiratory flow limitation during NREM sleep. During the baseline study off of TNI treatment, the periods of inspiratory flow limitation were associated with elevations in respiratory rate and inspiratory duty cycle (Fig 2, bottom left). TNI reduced the amount of inspiratory flow limitation, which resulted in a decreased respiratory rate and inspiratory duty cycle (Fig 2, bottom right). Mean data for all of the participants during NREM sleep demonstrated that TNI reduced the amount of inspiratory flow limitation, which was associated with a decrease in both the inspiratory duty cycle and the respiratory rate (Fig 5).
FIGURE 5.
Graphic representations of the respiratory pattern during NREM sleep for the baseline compared with TNI: percentage of NREM sleep time with inspiratory flow limitation (left); inspiratory duty cycle, which is the inspiratory time/duration of the respiratory cycle (middle); and respiratory rate (right). Data presented are means ± SEMs.
DISCUSSION
There were 3 major findings in our study of TNI. First, in a group of predominantly obese children with and without adenotonsillectomy, TNI treated sleep apnea across a wide spectrum of disease severity. Second, in the majority of children, the reduction in the AHI with TNI was comparable to CPAP. Third, during NREM sleep, all of the children had prolonged periods of inspiratory flow limitation that were associated with an increase drespiratory rate and inspiratory duty cycle, all of which decreased with TNI. Our data suggest that TNI may offer an alternative to CPAP in some children for whom standard treatment approaches are not successful.
Previously, we demonstrated that TNI treated all of the adult participants with mild sleep apnea and approximately half of the adult participants with moderate and severe sleep apnea.19 Children in the current study were equally distributed across a spectrum of disease severity (mild AHI: >2 and ≤5 events per hour, n = 4; moderate AHI: >5 and ≤10 events per hour, n = 4; and severe AHI: >10 events per hour, n = 4). TNI reduced the AHI during both NREM and REM sleep, but this effect depended on the disease severity. In the 8 children with mild-to-moderate sleep apnea, TNI decreased the AHI consistently, with a mean reduction from 6 ± 3 to 2 ± 1 events per hour. In contrast, in the 4 children with severe sleep apnea, TNI had an inconsistent response: in 1 child, the AHI was unchanged, in 2 children the AHI decreased by 72% and 36% but the effect was suboptimal, and the fourth child had a marked reduction in the AHI from 17 to 2 events per hour. The 3 children whose AHI remained above 10 events per hour (Fig 4) were unique in the following aspects: they were all girls, markedly obese, and were in Tanner stage 2 to 3 as compared with the other children, who were generally younger and in Tanner stage 1. Thus, TNI might offer a treatment option for children with mild-to-moderate OSA and in selected children with severe OSA.
OSA is the result of increased upper airway collapsibility during sleep, as reflected in the critical closing pressure.2,3,30–33 Previously, we demonstrated that TNI primarily acts by slightly increasing pharyngeal pressure19 that was particularly effective in adults with minimal increases in critical closing pressure manifested clinically by snoring and hypopneas.34 Similarly, TNI alleviated inspiratory flow limitation during NREM sleep (Fig 4). The marked reduction in apneic events during REM sleep, however, was greater than anticipated. This finding suggests that TNI might have increased pharyngeal pressure more in children than adults because of the relatively larger size of the nasal cannula compared with the size of the nares. Alternatively, the slight increase in pharyngeal pressure might have increased lung volume to a greater degree in children resulting from higher chest wall and lung compliance,35 particularly during REM sleep, when the chest wall musculature is hypotonic.36 Increases in lung volume might have improved both oxygen stores and upper airway patency.37–40 Finally, it is also possible that insufflation of air might have stimulated upper airway neuromuscular responses, thereby improving upper airway patency.41 Regardless, the improvements in flow limitation, respiratory rate, and inspiratory duty cycle suggest that TNI increased inspiratory tidal volumes through increased inspiratory airflow. Moreover, the improvement in the AHI with TNI suggests that the increases in inspiratory airflow and tidal volumes were sufficient to prevent hypoxia or arousals, which has significant implications for the management of sleep-disordered breathing in children.
There are several advantages of TNI. First, the patient interface is a nasal cannula that is less cumbersome than a nasal mask and should be better tolerated by children during sleep. All of the participants readily agreed to sleep with a TNI device, and only 2 participants described mild discomfort once the TNI device began to deliver air. One complaint was temperature related and was easily adjusted to the participant’s preference; 1 participant intermittently removed the cannula during the course of the night but was unable to effectively verbalize her complaint. Second, TNI delivers heated and humidified air at the level of the nares, which avoids nasal dryness and irritation. Third, for the majority of children, the response to TNI was comparable to CPAP. Taken together, if improved comfort with TNI leads to increased adherence to treatment, TNI might ultimately be a more effective treatment option than CPAP, even in children with suboptimal responses. To assess this hypothesis, however, adherence with TNI needs to be assessed in the home setting. Fourth, the use of CPAP in children carries concern for the potential of compression of boney facial structures. TNI is an open system that is not dependent on a tightly sealed nasal mask obviating concerns of facial compression.
There were several limitations in our study. First, carbon dioxide levels were evaluated during the baseline study, but the high airflow rate of TNI eliminated the end-tidal CO2 measurement during the treatment night. The loss of a consistent CO2 measurement limited our ability to assess the effect of TNI on ventilation during the treatment night. Second, the sample size was limited, and the effect of TNI on severe sleep apnea is not completely understood. The spectrum of patients with regard to disease severity and previous adenotonsillectomy, however, was diverse and likely representative of patients who would require treatment for sleep apnea. Third, the total sleep times during both nights were limited because of testing conditions, which included an early awakening time. Additional evaluation of the effect of TNI on sleep time, sleep latency, and sleep architecture should be assessed in future studies. Fourth, the assessment of respiratory pattern changes during NREM sleep with TNI was measured with a random sample of breaths. It is possible that the changes observed in the respiratory pattern on TNI might have been more accurately characterized if the assessment was expanded to include all breaths during NREM sleep.
CONCLUSIONS
Adenotonsillectomy continues to be the treatment of choice for children with sleep apnea.16,42,43 The reduction in the AHI with TNI was comparable in children with and without adenotonsillectomy, indicating that TNI is a treatment option for children awaiting adenotonsillectomy and for those with residual sleep apnea after adenotonsillectomy (Fig 4). Moreover, there is significant controversy regarding which children with milder degrees of sleep-disordered breathing might benefit from treatment.15,44 The effect of TNI on sleep-disordered breathing indicates that TNI might provide an alternative to surgery and, as compared with CPAP, might be a more readily accepted treatment option.
The minimally intrusive nasal interface of TNI may improve adherence to treatment in children and may ultimately prove more effective in managing the long-term morbidity and mortality of sleep apnea. Additional studies will be required to extend these findings to additional pediatric populations, including younger children and infants and those children with neuromuscular or craniofacial disorders, to determine the ultimate role for TNI in the management of sleep-disordered breathing in children.
WHAT’S KNOWN ON THIS SUBJECT
Adenotonsillectomy is the treatment for children with sleep apnea. For children whom surgery is not recommended or have residual sleep apnea after surgery CPAP is recommended. CPAP, however, is encumbered by poor adherence, leaving a large number of children untreated.
WHAT THIS STUDY ADDS
We present a novel treatment for sleep apnea in children. Our data suggest that TNI may offer an alternative to CPAP in some children. The minimally intrusive interface of TNI may improve adherence to treatment.
Acknowledgments
This study was funded by National Heart, Lung, and Blood Institute grants HL-72126, HL-50381, HL-37379, and HL-077137 and National Health and Medical Research Council grant 353705.
ABBREVIATIONS
- OSA
obstructive sleep apnea
- CPAP
continuous positive airway pressure
- TNI
treatment with nasal insufflation
- REM
rapid eye movement
- NREM
nonrapid eye movement
- AHI
apnea-hypopnea index
Footnotes
FINANCIAL DISCLOSURE: Dr Schneider received consulting fees from TNI medical in 2006 and is entitled to royalty payments on the future sales of products described in this article. Under a separate licensing agreement between Dr Schneider and TNI medical and Johns Hopkins University, Dr Schneider is entitled to a share of royalty received by the university on sales of products described in this article. The terms of this agreement are being managed by Johns Hopkins University in accordance with its conflict of interest policies. Dr Schneider was not involved in the analysis of the study data, and data collected under this study were masked with respect to patient identifiers and clinical outcomes to Dr Schneider. The nonconflicted faculty members were responsible for all of the data analysis. Funding for the study described in this article was partially provided by TNI medical.
References
- 1.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anesthesia and sleep. Lancet. 2002;359(9313):1207–1209. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64(4):535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 3.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64(2):789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 4.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24(3):313–320. doi: 10.1093/sleep/24.3.313. [DOI] [PubMed] [Google Scholar]
- 5.Owens J, Opipari L, Nobile C, Spirito A. Sleep and daytime behavior in children with obstructive sleep apnea and behavioral sleep disorders. Pediatrics. 1998;102(5):1178–1184. doi: 10.1542/peds.102.5.1178. [DOI] [PubMed] [Google Scholar]
- 6.Weissbluth M, Davis A, Poncher J, Reiff J. Signs of airway obstruction during sleep and behavioral, developmental and academic problems. J Dev Behav Pediar. 1983;4(2):119–121. doi: 10.1097/00004703-198306000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Halbower AC, Degaonkar M, Barker PB, Earley CJ, Marcus CL, Smith PL. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3(8):e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 pt 1):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 9.Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169(8):950–956. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 10.Enright PL, Goodwin JL, Sherrill DL, Quan JR, Quan SF. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and Hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Arch Pediatr Adolesc Med. 2003;157(9):901–904. doi: 10.1001/archpedi.157.9.901. [DOI] [PubMed] [Google Scholar]
- 11.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;57(4 pt 1):1098–1103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 12.Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109(8):951–957. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 13.Blunden S, Lushington K, Lorenzen B, Martin J, Kennedy D. Neuropsychological and psychosocial function in children with a history of snoring or behavioral sleep problems. J Pediatrics. 2004;146(6):780–786. doi: 10.1016/j.jpeds.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44–49. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122(1) doi: 10.1542/peds.2007-3398. Available at: www.pediatrics.org/cgi/content/full/122/1/e149. [DOI] [PubMed]
- 16.Guilleminault C, Li K, Khramtsov A, Pelayo R, Martinez S. Sleep disordered breathing: surgical outcomes in prepubertal children. Laryngoscope. 2004;114(1):132–137. doi: 10.1097/00005537-200401000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Rosen G, Ward S, Halbower AC, Halbower AC, Sterni L, Lutz J. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117(3) doi: 10.1542/peds.2005-1634. Available at: www.pediatrics.org/cgi/content/full/117/3/e442. [DOI] [PubMed]
- 19.McGinley BM, Patil SP, Kirkness JP, Smith PL, Schwartz AR, Schneider H. A nasal cannula can be used to treat obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(2):194–200. doi: 10.1164/rccm.200609-1336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162(pt 2):682–686. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 21.Marcus CL. Obstructive sleep apnea syndrome: differences between children and adults. Sleep. 2000;23(suppl 4):S140–S141. [PubMed] [Google Scholar]
- 22.Marcus CL, Moreira GA, Bamford O, Lutz J. Response to inspiratory resistive loading during sleep in normal children and children with obstructive apnea. J Appl Physiol. 1999;87(4):1448–1454. doi: 10.1152/jappl.1999.87.4.1448. [DOI] [PubMed] [Google Scholar]
- 23.Badr MS, Skatrud JB, Dempsey JA, Begle RL. Effect of mechanical loading on expiratory and inspiratory muscle activity during NREM sleep. J Appl Physiol. 1990;68(3):1195–1202. doi: 10.1152/jappl.1990.68.3.1195. [DOI] [PubMed] [Google Scholar]
- 24.Schneider H, Patil SP, Canisius S, Gladmon EA, Schwartz AR, O’Donnell CP. Hypercapnic duty cycle is an intermediate physiological phenotype linked to mouse chromosome 5. J Appl Physiol. 2003;95(1):11–19. doi: 10.1152/japplphysiol.01144.2002. [DOI] [PubMed] [Google Scholar]
- 25.Iber C, Ancoli-Israel S, Chesson A Jr, Quan S, editors. The AASM Manual for the Scoring of Sleep and Associated Events Rules, Terminology and Technical Specifications. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 26.Ayappa I, Norman RG, Krieger A, Rosen A, O’malley RL, Rapoport DM. Non-invasive detection of respiratory effort-related arousals (RERAS) by a nasal cannula/pressure transducer system. Sleep. 2000;23(6):763–771. doi: 10.1093/sleep/23.6.763. [DOI] [PubMed] [Google Scholar]
- 27.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med. 1998;157(5 pt 1):1461–1467. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 28.Serebrisky D, Cordero R, Mandeli J, Kattan M, Lamm C. Assessment of inspiratory flow limitation in children with sleep-disordered breathing by a nasal cannula pressure transducer system. Pediatr Pulmonlol. 2002;33(5):380–387. doi: 10.1002/ppul.10096. [DOI] [PubMed] [Google Scholar]
- 29.Pichard LE, Patil SP, Gladmon E, Smith PL, Schwartz AR, Schneider H. Women have a greater ventilatory responses to upper airway obstruction than men. Conf Proc IEEE Eng Med Biol Soc. 2004;5:3878–3880. doi: 10.1109/IEMBS.2004.1404085. [DOI] [PubMed] [Google Scholar]
- 30.Boudewyns A, Punjabi N, Van de Heyning PH, DeBacker WA, O’Donnell CP, Scheider H. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118(4):1031–1041. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 31.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984;57(2):520–527. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 32.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57(1):99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 33.Marcus CL, Fernandes Do, Prado LB, Lutz J, Katz ES, Black CA. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97(1):98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 34.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 35.Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol. 1995;78(1):179–184. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- 36.Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol. 1981;51(3):557–564. doi: 10.1152/jappl.1981.51.3.557. [DOI] [PubMed] [Google Scholar]
- 37.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130(2):175–178. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 39.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;98(5):2159–2164. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 40.Series F, Cormier Y, Lampron N, La Forge J. Increasing the functional residual capacity may reverse obstructive sleep apnea. Sleep. 1988;11(4):349–353. [PubMed] [Google Scholar]
- 41.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105(1):197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stradling JR, Thomas G, Warley AR, Williams P, Freeland A. Effect of adenotonsillectomy on nocturnal hypoxaemia, sleep disturbance, and symptoms in snoring children. Lancet. 1990;335(8684):249–253. doi: 10.1016/0140-6736(90)90068-g. [DOI] [PubMed] [Google Scholar]
- 43.Section on Pediatric Pulmonology and Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):704–712. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 44.Sans Capdevila O, Kheirandish-Gozal L, Gozal D. Pediatric obstructive sleep apnea. complications, management, and long-term outcomes. Proc Am Thoracic Soc. 2008;5(2):274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]