Abstract
It has been accepted for a hundred years or more that rods and cones are the only photoreceptive cells in the retina. The light signals generated in rods and cones, after processing by downstream retinal neurons (bipolar, horizontal, amacrine and ganglion cells), are transmitted to the brain via the axons of the ganglion cells for further analysis. In the past few years, however, convincing evidence has rapidly emerged indicating that a small subset of retinal ganglion cells in mammals is also intrinsically photosensitive. Melanopsin is the signaling photopigment in these cells. The main function of the innerretina photoreceptors is to generate and transmit non-image-forming visual information, although some role in conventional vision (image detection) is also possible.
Introduction
Vertebrate eyes mediate both image-forming and non-image-forming visual functions. Image-forming vision, with its high spatial and temporal resolution, enables the animal to detect and track objects in the visual world. Non-image-forming vision, however, provides a measure of the ambient luminance for the purposes of synchronizing the animal’s biological clock with the surrounding light–dark cycle (circadian photoentrainment), controlling the pupil size, and other functions such as acute suppression of locomotor behavior (negative masking) in rodents (reviewed by Foster and Hankins [1]). Circadian photoentrainment, in turn, affects a whole host of functions such as melatonin release, body-temperature regulation and feeding behavior.
Retinal rods and cones, the classical ocular photoreceptors, are responsible for image-forming vision. In mammals, it now appears that non-image-forming vision is mediated not only by rods and cones but also by a small subset of retinal ganglion cells (RGCs) that is intrinsically photosensitive (ipRGCs). In lower vertebrates such as fish, a subset of inner retinal neurons (not necessarily RGCs) might also be photoreceptive [2]. Here, we summarize the current knowledge of the workings of these non-rod and non-cone ocular photoreceptors and their photopigments.
The quest for novel ocular photoreceptors
The quest for novel photoreceptors in the eye began in the field of circadian biology. Almost all organisms exhibit circadian rhythms in physiology and behavior, with roughly 24-hour cycles. Although many tissues are now known to have intrinsic rhythms [3,4], the overall bodily circadian rhythm in mammals is synchronized by a master pacemaker in the hypothalamus called the suprachiasmatic nucleus (SCN). To be in phase with the solar day, the biological time in the SCN in turn has to be synchronized with—or ‘entrained’ to—the environment.
Ambient light adjusts the phase of the circadian rhythm in the SCN via the retinohypothalamic tract, a monosynaptic pathway connecting a small population of RGCs to the SCN [5–7]. Circadian photoentrainment disappears after removal of the eyes, suggesting that ocular photoreceptors are exclusively responsible for photoentrainment [8–10]. Until recently, rods and cones were assumed to be the exclusive circadian photoreceptors, even though photoentrainment was known to exhibit certain properties different from those of rods and cones, such as long-term temporal integration [11]. Beginning in the 1980s, researchers started to challenge the ‘rod–cone dogma’ through a series of behavioral studies on genetic mouse lines with retinal degeneration [12,13]. The most convincing experiments were performed on transgenic mice with an almost complete elimination of rods and cones [14,15]. Surprisingly, these mice showed an apparently normal circadian photoresponse to light, strongly suggesting the existence of circadian photoreceptors other than rods and cones. These experiments set the stage for ‘hunting’ for the novel non-rod and non-cone photoreceptors.
Intrinsic photosensitivity of a subset of retinal ganglion cells
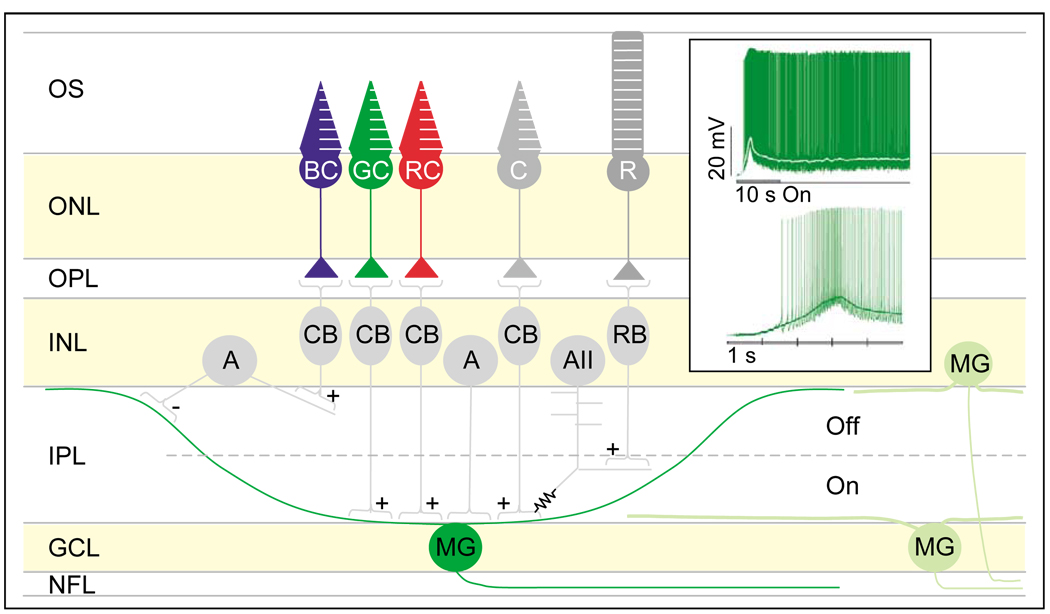
To look for the elusive circadian photoreceptors, Berson et al. [16] labeled the RGCs that project to the SCN by retrograde transport of fluorescent microspheres injected into the rat SCN. Remarkably, they found that this subset of RGCs remained light-sensitive even after synaptic inputs from rod and cone pathways had been pharmacologically blocked. Several key properties distinguish these intrinsically photosensitive RGCs from rods and cones: first, light depolarizes ipRGCs but hyperpolarizes rods and cones; second, the ipRGCs are much less sensitive to light than rods and cones; and third, the kinetics of the light responses of ip RGCs are ~100-fold slower than rod and cone responses, with an overall time course (triggered by a flash stimulus) lasting as long as 1 min. A subsequent study has identified giant primate ipRGCs with similar properties [17••]. These ipRGCs steadily fire action potentials throughout a light stimulus (Figure 1), thus faithfully encoding the irradiance over time and providing a neural representation of the steady light intensity that is useful for photoentraining the circadian system [16,17••].
Figure 1.
Schematic showing the synaptic circuitry of primate melanopsin-expressing RGCs in the retina. Melanopsin-expressing RGCs (MGs) are primarily (~95% in rodents, ~60–70% in primates) located in the ganglion cell layer, and the rest (~5% in rodents, ~30–40% in primates) are displaced to the inner nuclear layer (INL) (H-W Liao, unpublished; [17••,26]). MGs have sparse dendrites and extremely large dendritic fields. The dendrites arborize in the inner plexiform layer (IPL), forming a major (both in rodents and in primates) plexus in the outermost boundary of the IPL and a minor (even less prominent in rodents) plexus in the innermost boundary of the IPL. In primates, green and red cones provide excitatory inputs through bipolar cells to MG proximal dendrites [17••,60], and rods provide excitatory inputs through, presumably, rod bipolar cells, AII amacrine cells, and cone bipolar cells successively. Blue cones provide inhibitory inputs [17••], presumably through cone bipolar cells and inhibitory (probably GABAergic) amacrine cells [60,67]. Some amacrine cells of unknown identity also make synaptic contacts with MG somata [60]. Inset (adapted with permission from Nature [17••] copyright 2005 Macmillan Publishers Ltd; http://www.nature.com/): top panel shows the response of a primate MG cell to a 470 nm light pulse. The cell continued to fire action potentials for 30 s after the end of the light stimulus. White line shows membrane potential values averaged over 0.5 s sliding time windows. Bottom panel shows the first 5 s of the response shown on top panel. Abbreviations: +, excitatory input; −, inhibitory input; resistor symbol, electrical coupling; A, amacrine cell; AII, Type II amacrine cell; BC, blue cone; C, cone; CB, cone bipolar cell; GC, green cone; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; MG, melanopsin ganglion cell; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segment; R, rod; RB, rod bipolar cell; RC, red cone.
Identity of the circadian photopigment
Rod and cone visual pigments consist of an opsin, the protein moiety, covalently linked to a vitamin A-based chromophore, typically 11-cis-retinaldehyde. Upon photon absorption, 11-cis-retinaldehyde isomerizes to all-trans-retinaldehyde, after which the opsin undergoes conformational changes to become active as a signaling molecule, eventually splitting into opsin and all-trans-retinaldehyde. In the past decade, several novel, opsin-like proteins have been identified by molecular cloning from the eye and other tissues. One of these, melanopsin, was originally found in dermal melanophores of Xenopus laevis [18], but also localized by in situ hybridization to the iris and inner retina of Xenopus [18]. Subsequently, melanopsin was found in the inner retina of monkey and mouse [19]. These unusual findings no doubt prompted the pioneering experiments by Berson et al. [16] mentioned earlier. More recently, melanopsin has been found in the retina and brain of fish and chicken [2,20–22]. Melanopsin has also been reported to be present in the mouse retinal pigment epithelium [23], but this result has not been verified by others [24••].
In rodents, the expression of melanopsin in SCN-projecting RGCs was established by combined retrograde labeling from the SCN and in situ hybridization [25] or immunocytochemistry [26]. Evidence also came from co-immunolabeling of melanopsin and pituitary adeny-late cyclase-activating peptide (PACAP, which is present in SCN-projecting RGCs) [27], and from targeting of the axon-labeling marker tau-LacZ to the melanopsin gene locus [26]. As mentioned before, RGCs that project to the SCN show intrinsic photosensitivity [16,26]. In this review, melanopsin-expressing RGCs and ipRGCs will be used interchangeably. Genetic ablation of melanopsin eliminated the intrinsic photosensitivity of mouse ipRGCs without altering their genesis or axonal projections [28]. In addition, mice lacking melanopsin showed an incomplete pupil light response [28] and attenuated circadian photoentrainment [29,30]. Finally, removing melanopsin in mice with non-functional rods and cones [31] or with degenerated rods and cones [32] completely abolished the pupillary light reflex and circadian photoentrainment, indicating that ipRGCs, rods and cones together account for all major non-image-forming functions.
Although melanopsin is clearly essential for the intrinsic light response of ipRGCs, it was unclear for a while whether it is indeed the photopigment. One concern was that the first reported absorption spectrum of heterologously expressed melanopsin had a λmax at 424 nm, and an action spectrum with λmax in the same region [33]. These measures are both considerably shorter than the 480 nm value derived from the action spectra for the photosensitivity of ipRGCs [16,17••], circadian phase-shifting and pupillary light reflex in the absence of rods and cones [31,34]. This discrepancy, together with the fact that melanopsin shares only ~27% amino-acid identity with known vertebrate photopigments, has led to the suggestion that melanopsin is not the signaling pigment in question but, instead, a photoisomerase that binds all-trans-retinaldehyde and uses light energy to convert it back to 11-cis-retinaldehyde for regenerating the still-unidentified pigment in ipRGCs [35].
Contributing to the confusion about melanopsin is cryptochrome, a blue-light-sensitive flavoprotein that functions as a circadian photopigment in Arabidopsis and Drosophila (reviewed by Van Gelder [36]). Cryptochrome was first found in plants, with high sequence homology to DNA photolyases [37]. In contrast to opsin-based photopigments, which use retinaldehyde as chromophore, cryptochromes use flavin adenonucleotide and/or a pterin [38]. In mammals, there are two cryptochromes, both of which are widely expressed in the body, including in most cells in the inner retina [39]. This retinal location has prompted the proposal that cryptochromes function as circadian photopigments in mammals also, a notion apparently supported by the finding that the signaling of light to the SCN is preserved even when retinaldehyde is severely depleted by dietary vitamin-A deprivation [40]. The validity of this finding [40], however, has since been questioned by a recent study on a mouse model that lacks 11-cis-retinaldehyde production in the retina [24••]. Moreover, a flavin-based photopigment is unlikely to account for the opsin-like action spectrum of ipRGCs [41]. Furthermore, genetic deletion of cryptochromes results in complete circadian arrhythmicity in mice [42–44] but does not abolish light-induced expression of SCN clock genes [42]. Therefore, mammalian cryptochromes appear to have a much more important role in the generation of the circadian oscillation itself than in the presumptive circadian photoresponse. Finally, the mammalian cryptochromes in cultured cells function as light-independent components of the circadian clock [45]. It was reported that the deletion of cryptochromes reduces the sensitivity of the pupillary light reflex in mice with degenerated rods and cones [46], an observation again used for arguing for a role of cryptochromes in non-image-forming photoreception. Nonetheless, this interpretation is complicated by the fact that cryptochrome-knockout mice frequently show ocular inflammation [47], which might cause subtle changes in retinal function. Moreover, parallel experiments on mice lacking cryptochromes but having normal rods, cones and ipRGCs show no diminution in the sensitivity of the pupil reflex [46], a finding difficult to reconcile with the proposal of cryptochromes having a photoreceptive role. The hypothesis that cryptochromes are mammalian circadian photopigments is also inconsistent with the observation mentioned earlier that mice lacking melanopsin and functional rods and cones (but with intact cryptochromes) show no sign of any non-image-forming visual response [31,32]. Thus, taking all the evidence into account, cryptochromes are unlikely to be responsible for non-image-forming ocular photo-reception in mammals. Whether cryptochromes take on such a role in other vertebrate species or contexts, such as in chick iris constriction [48], remains to be examined further.
Any remaining doubt about melanopsin being the signaling photopigment was removed by several recent studies showing that heterologously expressed melanopsin confers photosensitivity to non-photosensitive cell lines [49••–51••]. Supporting evidence also came from experiments showing that exogenous chromophore was unable to restore the photosensitivity of ipRGCs in melanopsin-knockout mice (a result inconsistent with melanopsin acting solely as a photoisomerase), but was able to do so in mice having melanopsin but deficient in 11-cis-retinaldehyde [24••].
There is also some evidence to suggest that melanopsin exhibits bistability, that is, the ability to serve the dual function of photopigment and photoisomerase [24••, 50••, 51••]. To date, bistable pigments have only been found in invertebrates. They have two photoconvertible stable states, bound to 11-cis-retinaldehyde or all-trans-retinaldehyde. By contrast, vertebrate pigments have only one stable state (the state with 11-cis-retinaldehyde bound), and rely on the retinoid cycle in the retinal pigment epithelium for regeneration. A bistable melanopsin would mean that ipRGCs can regenerate their pigment autonomously, possibly explaining why melanopsin appears less susceptible to chromophore-deprivation than rod and cone pigments [24••, 40]. It is conceivable that a bistable melanopsin in the ipRGCs would be able to function continuously despite being physically far removed from the retinal pigment epithelium.
Heterologously expressed melanopsin is able to signal through Gαq or Gα11 and the TRPC3 ion channel ([49••, 50••] but see [51••]), analogous to an invertebrate photo-transduction cascade. Likewise, a phospholipase C signaling pathway triggered by light has been demonstrated in cultured Xenopus dermal melanophores, which express melanopsin [52]. However, whether these results implicate a G-protein-mediated phospholipase C pathway in ipRGCs remains unclear.
Morphology and axonal projections of melanopsin-expressing retinal ganglion cells
In rodents, roughly 1000–2000 RGCs (~ 1–3% of all RGCs) express melanopsin [26]. The number is about 3000 in primates (0.2% of total RGCs) [17••]. Most melanopsin-expressing RGCs reside in the ganglion cell layer but some are displaced to the inner nuclear layer [17••,26]. The melanopsin-expressing RGCs give rise to large and extensively overlapping dendritic fields, forming a photoreceptive net [17••,26,53]. Individual cells with respect to their dendritic aborizations are principally monostratified, creating two distinct subpopulations that send dendrites to the extreme inner or outer boundaries of the inner plexiform layer (Figure 1) [17••,26]. These RGCs also express PACAP (see earlier), a neuromodulator in retinohypothalamic transmission [27].
Melanopsin-expressing RGCs innervate a variety of brain regions (Table 1). Their projections were most readily visualized in a mouse line harboring the tau-lacZ marker gene (which codes for a β-galactosidase fused to a tauprotein sequence for axonal localization) targeted to the melanopsin gene locus [26]. The blue labeling of the β-galactosidase activity provides a vivid image of individual axons in this mouse. Using this and other techniques, investigators found that the majority of melanopsin-expressing RGCs innervate the SCN, and most of the RGCs innervating the SCN express melanopsin [25,54–57]. Melanopsin-expressing RGCs also send dense projections to the intergeniculate leaflet (IGL), which integrates photic and non-photic cues for the control of circadian rhythms, and the olivary pretectal nucleus (OPN), which controls the pupillary light reflex [17••,26,54,56]. Brain regions that receive melanopsin RGC fibers but are more heavily innervated by conventional RGCs include the ventrolateral preoptic nucleus (VLPO), which is implicated in sleep–wake regulation in mammals; the lateral hypothalamus (LH), which is involved in energy homeostasis; and the ventral subpar-aventricular zone (vSPZ), which is involved in circadian regulation and the acute suppression of locomotor activity by light [54,56–59]. The functional significance of the various minor melanopsin RGC projections remains to be elucidated.
Table 1.
Projections of melanopsin retinal ganglion cells.
| Melanopsin RGC target | Abbrev. | Function of target | Innervation | Species examined |
|---|---|---|---|---|
|
Highest density of melanopsin fibers |
||||
| Suprachiasmatic nucleus | SCN | Master regulation of circadian rhythms [68] | Dominant | Mouse [26,58], rat [25,54,56] and hamster [55,57] |
| Intergeniculate leaflet | IGL | Integration of photic and non-photic circadian cues [68,69] | Major | Mouse [26,58], rat [54,56] and hamster [57] |
| Olivary pretectal nucleus | OPN | Pupillary constriction [70] | Major | Mouse [26,58], rat [54,56], hamster [57] and macaque [17••] |
| Lateral habenula | LHb | Integration of limbic, motor and circadian systems [71] | Undetermined | Mouse [58] |
|
Lower density of melanopsin fibers |
||||
| Dorsal lateral geniculate nucleus |
dLGN | Image-forming vision [72] | Minor | Mouse [58] and macaque [17••] |
| Lateral hypothalamus | LH | Energy homeostasis [68] | Minor | Mouse [58] and rat [56] |
| Lateral posterior thalamic nucleus |
LP | Higher-order processing of thalamic, cortical and visual signals [73] | Minor | Mouse [58] and rat [56] |
| Posterior limitans thalamic nucleus |
PLi | Detection of rapid illumination changes for non-imaging vision [74] | Moderate | Rat [56] |
| Superior colliculus | SC | Integration of multiple modalities for gaze control [75] | Minor | Mouse [58], rat [56]and hamster [55,57] |
| Ventral lateral geniculate nucleus |
vLGN | Visuomotor function [69] | Minor | Mouse [26,58] and rat [56] |
| Ventral subparaventricular zone |
vSPZ | Circadian and direct regulation of locomotion and sleep [68] | Minor | Rat [54,56] |
| Ventrolateral preoptic nucleus | VLPO | Promotion of sleep [68] | Minor | Mouse [58] and rat [54,56] |
Reported central targets of melanopsin RGCs are listed with the general function of those brain regions. Areas receiving the highest absolute density of melanopsin fibers are grouped at the top of the table, and areas receiving a lower density are listed below in alphabetical order. ‘Innervation’ refers to the density of melanopsin fibers as compared with conventional retinohypothalamic fibers in each region. Melanopsin afferents from the contralateral eye predominate in all regions evaluated except for the SCN, which receives just slightly more fibers from the contralateral eye. This list of targets is not exhaustive, leaving out areas for which melanopsin innervation is suggested but less certain. Moreover, it does not describe the apparent innervation of multiple target regions by single melanopsin RGCs [54,57]. Results from mouse are from [26,58], rat from [54,56], hamster from [57], and primate from [17••].
Rod and cone inputs onto ipRGCs
Mice lacking melanopsin still show pupillary light response and can be photoentrained (although both functions become subnormal), indicating the involvement of rods and cones in these functions [28–30]. The most obvious source of rod and cone inputs for the ipRGCs would be through synapses from amacrine and bipolar cells [60] (Figure 1). The rod–cone influence on the ipRGCs was nicely demonstrated by a recent study in primates, showing that short-wavelength-sensitive cones inhibit, whereas rods and medium- and long- wavelength cones excite, ipRGCs ([17••] see also [61]; Figure 1). Rods and cones can provide the initial sensitivity and speed of the light signals for non-image-forming functions, whereas the intrinsic response of the ipRGCs can provide sustained signals throughout a light stimulus and long temporal integration [17••]. The fact that the ipRGCs in primates also project to the lateral geniculate nucleus, which is the thalamic relay to primary visual cortex, suggests that these cells might contribute to conscious visual perception [17••].
Inner-retina photoreceptors in fish
Compared with the situation with mammals, much less is known about ocular non-rod and non-cone photoreception in lower vertebrates, even though at least one photopigment (VA opsin, for vertebrate ancient opsin) had been found in their inner retinae before melanopsin was discovered [62]. VA opsin was first isolated from the eyes of Atlantic salmon and was localized to a subset of horizontal cells and to cells in the ganglion and amacrine cell layers [62]. When heterologously expressed, this pigment gave an absorption spectrum with λmax at ~460 nm [63]. Subsequent electrophysiological recordings from the intact roach retina suggested the presence of a subclass of intrinsically photosensitive horizontal cells, which gave a very slow, depolarizing light response with an action spectrum peaking at 477 nm [2]. However, blockade of synaptic transmission eliminated these responses [2], making the finding inconclusive. Even if real, the photopigment underlying this ‘intrinsic’ photo-response of horizontal cells remains unclear because both melanopsin and VA opsin were suggested to be present in these cells [2]. Several isoforms of VA opsin were subsequently found [64–66]. So far, VA opsin has only been found in fish.
Conclusions
The past decade has witnessed the fascinating discovery that a subset of inner retinal neurons in mammals is intrinsically photosensitive and is important for non-image-forming visual functions such as pupillary light reflex and circadian photoentrainment. Melanopsin appears to be the photopigment underlying this intrinsic light response. Moreover, melanopsin might be a bistable pigment, a feature found in many invertebrate pigments. If so, this property will benefit the ipRGCs by removing their need to compete with rods and cones for chromophore.
The next challenge will be to understand the phototransduction mechanism in these unusual RGCs, in addition to the detailed interactions between their intrinsic light responses and the rod-cone signals.
Update
After the completion of this review, two interesting studies relating to melanopsin function were published. In one report [76••], a homolog of melanopsin was found to be expressed in rhabdomeric photoreceptor cells of the amphioxus and shown to be a bistable pigment possibly coupled to a Gq-mediated transduction cascade. In the other [77••], mouse ipRGCs were found to be light-responsive from birth. Thus, this photosensitivity appears much earlier than that of rod and cone photoreceptors that starts at postnatal day 10.
Acknowledgements
We thank J Gooley for discussions on the axonal projections of the melanopsin-expressing RGCs.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Foster RG, Hankins MW. Non-rod, non-cone photoreception in the vertebrates. Prog Retin Eye Res. 2002;21:507–527. doi: 10.1016/s1350-9462(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins A, Munoz M, Tarttelin EE, Bellingham J, Foster RG, Hankins MW. VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr Biol. 2003;13:1269–1278. doi: 10.1016/s0960-9822(03)00509-8. [DOI] [PubMed] [Google Scholar]
- 3.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 4.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5699–5700. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 6.Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 7.Pickard GE. Morphological characteristics of retinal ganglion cells projecting to the suprachiasmatic nucleus: a horseradish peroxidase study. Brain Res. 1980;183:458–465. doi: 10.1016/0006-8993(80)90481-3. [DOI] [PubMed] [Google Scholar]
- 8.Nelson RJ, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp Biochem Physiol. 69A:145–148. [Google Scholar]
- 9.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., III Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- 11.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebihara S, Tsuji K. Entrainment of the circadian activity rhythm to the light cycle: effective light intensity for a Zeitgeber in the retinal degenerate C3H mouse and the normal C57BL mouse. Physiol Behav. 1980;24:523–527. doi: 10.1016/0031-9384(80)90246-2. [DOI] [PubMed] [Google Scholar]
- 13.Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol [A] 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 14.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 15.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 16.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 17. Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. The authors demonstrated that melanopsin-expressing retinal ganglion cells in primates exhibit intrinsic light responses in addition to rod- and cone-driven visual responses. The ipRGCs are color-selective, and their steady firing to light gives a faithful representation of the ambient luminance. The projection of these neurons to the lateral geniculate nucleus suggests that they might contribute to conscious visual perception.
- 18.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellingham J, Whitmore D, Philp AR, Wells DJ, Foster RG. Zebrafish melanopsin: isolation, tissue localisation and phylogenetic position. Brain Res Mol Brain Res. 2002;107:128–136. doi: 10.1016/s0169-328x(02)00454-0. [DOI] [PubMed] [Google Scholar]
- 21.Drivenes O, Soviknes AM, Ebbesson LO, Fjose A, Seo HC, Helvik JV. Isolation and characterization of two teleost melanopsin genes and their differential expression within the inner retina and brain. J Comp Neurol. 2003;456:84–93. doi: 10.1002/cne.10523. [DOI] [PubMed] [Google Scholar]
- 22.Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, et al. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- 23.Peirson SN, Bovee-Geurts PH, Lupi D, Jeffery G, DeGrip WJ, Foster RG. Expression of the candidate circadian photopigment melanopsin (Opn4) in the mouse retinal pigment epithelium. Brain Res Mol Brain Res. 2004;123:132–135. doi: 10.1016/j.molbrainres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24. Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment melanopsin. Proc Natl Acad Sci USA. 2005 doi: 10.1073/pnas.0501866102. in press. This study suggests that melanopsin is the photopigment in mouse ipRGCs, based on experiments using single-cell recordings and pupil light reflexes. IpRGC function could be rescued with exogenous chromophore in a mouse model deficient in 11-cis-retinaldehyde synthesis, but not in the melanopsin-null mouse. The chromophore-deficient melanopsin system also had the ability to utilize all-trans-retinal for light detection, suggesting that melanopsin could be a bistable pigment.
- 25.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 26.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 29.Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 30.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 31.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 33.Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 34.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 35.Foster RG, Bellingham J. Inner retinal photoreceptors (IRPs) in mammals and teleost fish. Photochem Photobiol Sci. 2004;3:617–627. doi: 10.1039/b400092g. [DOI] [PubMed] [Google Scholar]
- 36.Van Gelder RN. Tales from the crypt(ochromes) J Biol Rhythms. 2002;17:110–120. doi: 10.1177/074873002129002401. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 38.Thompson CL, Sancar A. Photolyase/cryptochrome blue-light photoreceptors use photon energy to repair DNA and reset the circadian clock. Oncogene. 2002;21:9043–9056. doi: 10.1038/sj.onc.1205958. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci USA. 1998;95:6097–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson CL, Blaner WS, Van Gelder RN, Lai K, Quadro L, Colantuoni V, Gottesman ME, Sancar A. Preservation of light signaling to the suprachiasmatic nucleus in vitamin A-deficient mice. Proc Natl Acad Sci USA. 2001;98:11708–11713. doi: 10.1073/pnas.201301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang RP, Todo T, Wei YF, Sancar A. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry. 1996;35:13871–13877. doi: 10.1021/bi962209o. [DOI] [PubMed] [Google Scholar]
- 42.Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, Muijtjens M, Hoeijmakers JH, van der Horst GT. Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science. 1999;286:2531–2534. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 43.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 44.Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griftin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 46.Van Gelder RN, Wee R, Lee JA, Tu DC. Reduced pupillary light responses in mice lacking cryptochromes. Science. 2003;299:222. doi: 10.1126/science.1079536. [DOI] [PubMed] [Google Scholar]
- 47.Van Gelder RN. Non-visual ocular photoreception. Ophthalmic Genet. 2001;22:195–205. doi: 10.1076/opge.22.4.195.2215. [DOI] [PubMed] [Google Scholar]
- 48.Tu DC, Batten ML, Palczewski K, Van Gelder RN. Nonvisual photoreception in the chick iris. Science. 2004;306:129–131. doi: 10.1126/science.1101484. [DOI] [PubMed] [Google Scholar]
- 49. Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. By transiently expressing melanopsin in human embryonic kidney 293 cells, the authors showed that light triggers a membrane depolarization in these cells with a spectral sensitivity matching that of ipRGCs. In addition, melanopsin can also couple to a Gq-mediated phospholipase C signaling cascade, activating co-expressed TRPC3 channels.
- 50. Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. By expressing melanopsin in Xenopus oocytes, the authors showed that melanopsin is able to couple to a Gαq or Gα11 pathway, resulting in light-dependent activation of membrane currents. When co-expressed with arrestins, melanopsin could use exogenous all-trans-retinaldehyde as a chromophore, suggesting that melanopsin might function as a bistable pigment.
- 51. Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. By expressing human melanopsin in a Neuro-2A mouse neuroblastoma cell line, the authors demonstrated that melanopsin renders these cells photosensitive, indicating that melanopsin is a bona fide photopigment. In addition, they showed that melanopsin has an intrinsic photoisomerase function.
- 52.Isoldi MC, Rollag MD, de Lauro Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci USA. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 54.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sollars P, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- 56.Hannibal J, Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 2004;316:99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- 57.Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- 58.Hattar S, Kumar M, Tung J, Park A, Tong P, Berson DM, Yau K-W. Diverse brain targets of melanopsin-expressing ganglion cells. Invest Ophthal Vis Sci. 2004;45:E-Abstract 660. [Google Scholar]
- 59.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 60.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 61.Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 62.Soni BG, Foster RG. A novel and ancient vertebrate opsin. FEBS Lett. 1997;406:279–283. doi: 10.1016/s0014-5793(97)00287-1. [DOI] [PubMed] [Google Scholar]
- 63.Soni BG, Philp AR, Foster RG, Knox BE. Novel retinal photoreceptors. Nature. 1998;394:27–28. doi: 10.1038/27794. [DOI] [PubMed] [Google Scholar]
- 64.Kojima D, Mano H, Fukada Y. Vertebrate ancient-long opsin: a green-sensitive photoreceptive molecule present in zebrafish deep brain and retinal horizontal cells. J Neurosci. 2000;20:2845–2851. doi: 10.1523/JNEUROSCI.20-08-02845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moutsaki P, Bellingham J, Soni BG, David-Gray ZK, Foster RG. Sequence, genomic structure and tissue expression of carp (Cyprinus carpio L.) vertebrate ancient (VA) opsin. FEBS Lett. 2000;473:316–322. doi: 10.1016/s0014-5793(00)01550-7. [DOI] [PubMed] [Google Scholar]
- 66.Minamoto T, Shimizu I. A novel isoform of vertebrate ancient opsin in a smelt fish, Plecoglossus altivelis. Biochem Biophys Res Commun. 2002;290:280–286. doi: 10.1006/bbrc.2001.6186. [DOI] [PubMed] [Google Scholar]
- 67.Wu SM, Gao F, Pang J-J. Synaptic circuitry mediating light-evoked signals in dark-adapted mouse retina. Vision Res. 2004;44:3277–3288. doi: 10.1016/j.visres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 68.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- 70.Clark RJ, Zhang H, Gamlin PD. Primate pupillary light reflex: receptive field characteristics of pretectal luminance neurons. J Neurophysiol. 2003;89:3168–3178. doi: 10.1152/jn.01130.2002. [DOI] [PubMed] [Google Scholar]
- 71.Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–RA273. [PubMed] [Google Scholar]
- 72.Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci. 1998;21:47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- 73.Guillery RW. Anatomical evidence concerning the role of thalamus in corticocortical communication: a brief review. J Anat. 1995;187:583–592. [PMC free article] [PubMed] [Google Scholar]
- 74.Prichard JR, Stoffel RT, Quimby DL, Obermeyer WH, Benca RM, Behan M. Fos Immunoreactivity in rat subcortical visual shell in response to illuminance changes. Neuroscience. 2002;114:781–793. doi: 10.1016/s0306-4522(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 75.King AJ. The superior colliculus. Curr Biol. 2004;14:R335–R338. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 76. Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. The authors showed that a homolog of melanopsin was localized to the rhabdomeric photoreceptor cells the amphioxus by in situ hybridization. Heterologously expressed amphioxus melanopsin showed bistability by absorption spectrum measurements. Furthermore, immunocytochemistry data suggested that melanopsin was colocalized with Gq, but not with other G proteins, such as Gs, Gi or Gt, and Go. This indicates that melanopsin possibly couples to an invertebrate-like phototransduction cascade.
- 77. Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. Using the technique of calcium imaging, the authors demonstrated that mouse ipRGCs were photosensitive from birth (P0), 10 days earlier than the rod and cone photoreceptors become light responsive. Interestingly, the number of ipRGCs at P0 is more than five times that in the adult retina, reflecting an initial overproduction of melanopsin-expressing cells during development. The significance of the early emergence of photosensitivity of the ipRGCs is unclear.