Abstract
Background
In utero alcohol exposure can lead to fetal alcohol spectrum disorders characterized by cognitive and behavioral deficits. In vivo and in vitro studies have shown that ethanol alters neuronal development. One mechanism through which ethanol has been shown to exert its effects is the perturbation of activated signaling cascades. The cholinergic agonist carbachol has been shown to induce axonal outgrowth through intracellular calcium mobilization, PKC activation, and ERK1/2 phosphorylation. This study investigated the effect of ethanol on the differentiation of rat hippocampal pyramidal neurons induced by carbachol as a possible mechanism involved in the developmental neurotoxicity of ethanol.
Methods
Prenatal rat hippocampal pyramidal neurons were treated with ethanol (50–75 mM) in the presence or absence of carbachol for 24 h. Neurite outgrowth was assessed spectrophotometrically; axonal length was measured in neurons fixed and immunolabeled with the neuron-specific βIII tubulin antibody; cytotoxicity was analyzed using the MTT assay. The effect of ethanol on carbachol-stimulated intracellular calcium mobilization was assessed utilizing the fluorescent calcium probe, Fluo-3AM. The PepTag® Assay for Non-Radioactive Detection of Protein Kinase C from Promega was used to measure PKC activity, and ERK1/2 activation was determined by densitometric analysis of Western blots probed for phospo-ERK1/2.
Results
Ethanol treatment (50–75 mM) caused an inhibition of carbachol-induced axonal growth, without affecting neuronal viability. Neuron treatment for 15 min with ethanol did not inhibit the carbachol-stimulated rise in intracellular calcium, while inhibiting PKC activity at the highest tested concentration and ERK1/2 phosphorylation at both the concentrations used in this study. On the other hand, neuron treatment for 24 h with ethanol significantly inhibited carbachol-induced increase in intracellular calcium.
Conclusions
Ethanol inhibited carbachol-induced neurite outgrowth by inhibiting PKC and ERK1/2 activation. These effects may be, in part, responsible for some of the cognitive deficits associated with in utero alcohol exposure.
Keywords: hippocampus, pyramidal cells, muscarinic receptors, neurite outgrowth, ethanol
Introduction
Maternal alcohol consumption during pregnancy can produce a variety of central nervous system abnormalities resulting in a broad spectrum of cognitive and behavioral impairments that constitute the most severe and long-lasting effects observed in fetal alcohol spectrum disorder (FASD) (Bertrand et al., 2005; Jones and Smith, 1975; Wattendorf and Muenke, 2005). Prenatal alcohol exposure affects several brain regions including the hippocampus, the cerebellum and the neocortex (Berman and Hannigan, 2000; Fabregues et al., 1985; Hammer and Scheibel, 1981; Smith et al., 1986). The hippocampus is the portion of the brain necessary for the formation, storage, and processing of memories, and animal models of FAS provide in vivo evidence that prenatal alcohol exposure may cause abnormal hippocampal architecture, including decreased numbers of pyramidal neurons, abnormal axon projections and dendritic arbors, and aberrant hippocampal electrophysiology (Berman and Hannigan, 2000).
Several in vivo studies have investigated the effect of in utero alcohol exposure on neuronal development. Prenatal ethanol exposure has an inhibitory effect on dendritic arbor size in the hippocampus, neocortex, and cerebellum of rodents (Davies and Smith, 1981; Hammer and Scheibel, 1981; Smith and Davies, 1990; Smith et al., 1986), and causes a decrease in dendrite number and branching in guinea-pig cortical layer V pyramidal neurons (Fabregues et al., 1985), chick spinal cord serotonergic neurons (Mendelson and Driskill, 1996), and rat dopaminergic neurons of the substantia nigra (Shetty et al., 1993). In vivo prenatal ethanol exposure has been shown to reduce the size of the hippocampal commissure (Livy and Elberger, 2001), but to increase the number of neurons projecting from rat somatosensory cortex to the spinal cord (Miller, 1987), to increase the total axoplasmic volume in layer V of the somatosensory cortex (al-Rabiai and Miller, 1989), and to increase axonal growth in the rat pyramidal tract (Miller and al-Rabiai, 1994). Additionally, hypertrophic axonal projections have been observed in axons extending from the granule cells of the dentate gyrus to apical dendrites of hippocampal pyramidal neurons (West et al., 1981). The different effects exerted by ethanol in vivo may reflect species or strain differences in sensitivity to ethanol or differences in the dose, timing, or route of ethanol administration (Lindsley, 2006).
In vitro studies further support the hypothesis that prenatal ethanol exposure may interfere with the development of neuronal processes. Indeed, ethanol has been shown to increase neurite outgrowth in cerebellar neurons (Zou et al., 1993), and in PC12 cells (Messing et al., 1991a; Roivainen et al., 1993). Only very high concentrations of ethanol inhibit neurite extension and viability of primary culture hippocampal neurons (Heaton et al., 1994). On the other hand, ethanol has been shown to inhibit neurite outgrowth in cerebellar granule neurons (Liesi, 1997), and LA-N-5 human neuroblastoma cells (Saunders et al., 1995), and to decrease neurite outgrowth and dendritic branching in primary cultures of fetal cortical neurons grown in close proximity of glial cells monolayer (Bingham et al., 2004). Ethanol has also been reported to increase the number of minor processes and the number of cells with more than one axon, and to accelerate the development of hippocampal pyramidal neurons in the early phase of development (first 24 h in culture), while inhibiting the development of dendrites and synapses of later stages of development (Clamp and Lindsley, 1998; Yanni and Lindsley, 2000). Furthermore, ethanol strongly inhibits neurite outgrowth mediated by the cell adhesion molecule L1CAM in cerebellar granule cells (Bearer et al., 1999), but not in cortical neurons (Hoffman et al., 2008).
Recently, it has also been reported that ethanol inhibits neurite outgrowth mediated by astrocytes; indeed, neuritogenesis is inhibited in cortical neurons grown in the presence of astrocytes prepared from rats prenatally exposed to ethanol in comparison to neurons incubated with astrocytes from unexposed animals (Pascual and Guerri, 2007). Ethanol also inhibits axonal growth of cerebellar neurons induced by the active fragment of the astrocyte-released activity-dependent neuroprotective protein (Chen and Charness, 2008). In addition, we have recently reported that the stimulation of muscarinic receptors in astrocytes induces neurotogenesis in hippocampal neurons (Guizzetti et al., 2008) and that this effect is inhibited by ethanol (Guizzetti et al., manuscript in preparation).
Taken together, these studies suggest that ethanol alters neuronal morphogenesis both in vivo and in vitro with effects that range from inhibition to overstimulation of neuronal differentiation. The variety of effects exerted by ethanol depends on the brain region from which the investigated neurons derive, the developmental stage of neurons, and the presence or absence of glial cells.
In several instances, the effect of ethanol on neuronal development has been ascribed to an interference exerted by ethanol on specific signal transduction pathways. Indeed, it was recently reported that alcohol inhibition of L1CAM-induced neurite outgrowth was due to inhibition of the activation of extracellular signal-regulated kinase (ERK) 1/2 (Tang et al., 2006). Alcohol-induced inhibition of axonal growth stimulated by the active fragment of the activity-dependent neuroprotective protein is due to the inhibition of the tyrosine phosphorylation of Fyn kinase and Crk-associated substrate (Chen and Charness, 2008). Furthermore, inhibition by ethanol of retinoic acid-induced differentiation of human SH-SY5Y neuroblastoma cells is due to inhibition of protein kinase C (PKC) and ERK1/2 signaling (Hellmann et al., 2008).
We have recently reported that the cholinergic agonist carbachol induces axonal growth in prenatal rat hippocampal pyramidal neurons through the stimulation of M1 muscarinic receptors that lead to an increase in intracellular calcium and activation of PKC and ERK1/2 (VanDeMark et al., 2009). In previous studies we have found that ethanol inhibits muscarinic receptor signaling both in vitro and in vivo and that this effect is associated with microencephaly and inhibition of astrocytes proliferation (Balduini and Costa, 1989; Balduini et al., 1994; Costa and Guizzetti, 1999; Guizzetti and Costa, 2000; Guizzetti and Costa, 2002; Guizzetti et al., 2003). In this study, we investigated the hypothesis that ethanol may inhibit the neuritogenic effect of carbachol by inhibiting the signaling pathway activated by cholinergic stimulation in hippocampal neurons.
Materials and Methods
Materials
Time-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). Neurobasal-A medium, Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and trypsin were from Invitrogen (Carlsbad, CA). Cell culture inserts and nylon mesh filters were from BD Falcon™ (Franklin Lakes, NJ); glass coverslips were from Fisher Scientific (Federal Way, WA), and plastic coverslips from Nunc™ (Rochester, NY). The antibodies against tubulin, βIII isoform, microtubule-associated protein 2 (MAP2), and Tau1 were purchased from Chemicon® (Temecula, CA). The Alexa Fluor® 488 and 555 secondary antibodies, Hoechst 33342, and Fluo-3AM were purchased from Molecular Probes™ Invitrogen (Carlsbad, CA). The antibodies against phospho-ERK1/2 and ERK1/2 were purchased from Cell Signaling (Danvers, MA). The PepTag® Assay for Non-Radioactive Detection of Protein Kinase C was from Promega (Madison, WI). Ethanol determination kits were purchased from r-Biopharm (Marshall, MI). Carbachol and all other reagents were purchased from Sigma Chemical Company (St. Louis, MO)
Culture of hippocampal neurons
Primary cultures of hippocampal neurons were prepared from 21 day-old rat fetuses as previously described (Brewer et al., 1993; Guizzetti et al., 2008; VanDeMark et al., 2009), with minor modifications. Briefly, a pregnant dam was euthanized with carbon dioxide, and the uterine horns were removed by using a protocol approved by the University of Washington Institutional Animal Care and Use Committee. Fetuses were removed and sacrificed by decapitation. The hippocampi were removed from the cerebral hemispheres, stripped of meninges, placed in Hank’s balanced salt solution, cut into small pieces, and treated with papain (2 mg/ml HBSS) in the presence of DNase (40 μg/ml) and MgCl2 (5 mM) for 30 min at 37°C. The tissue was spun down, and resuspended in Neurobasal-A complete media (Neurobasal-A medium supplemented with 10% FBS, 30 mM glucose, 3 mM GlutaMAX, 1% gentamicin, and 0.5% fungizone) and DNase (40 μg/ml). Tissue was further dissociated by repeated passages through a Pasteur pipette, and cells were filtered through a nylon mesh of 40 μm pore size. Cells were then spun down and resuspended in Neurobasal-A complete media. For the neurite extension assays, cells were seeded on cell culture porous inserts at the density 2 × 105 per insert. For morphometric analysis, cells were seeded on round glass coverslips placed in 24 well plates at 1 × 104 cells per coverslip. Inserts and coverslips were coated overnight with 100 μg/ml poly-D-lysine at 37°C. Neurons were allowed to attach in Neurobasal-A complete media for at least 30 min, after which they were switched to astrocyte-conditioned medium (ACM) containing the various treatments for 24 hours. No differences in cell survival were noticed between neurons maintained for 24 hours in ACM compared to cells maintained in Neurobasal-A medium.
For Western blot analysis and PKC activity, cells were plated in 35 mm dishes (2 × 106 cells/dish for Western blot experiments and 5 × 106 cells/dish for PKC activity assays). Cells were allowed to attach overnight in Neurobasal-A complete and then switched to ACM containing the different treatments.
Preparation of Astrocyte-Conditioned Medium
Primary astrocyte cultures were prepared from cerebral cortex of 21-day-old rat fetuses as previously described (Guizzetti et al., 1996; 2008), and maintained for 10–14 days in DMEM/10% FBS. Cells were passed in 100 mm dishes and cultured for 4 days in DMEM/10%FBS. Two days before astrocyte-conditioned medium was needed, astrocytes were switched to DMEM supplemented with 0.1% bovine serum albumin (BSA). After 48 hours, the medium was collected and spun for 10 min at 200 g to remove any debris and floating cells. This medium was utilized for treatment of neurons.
Carbachol and Ethanol Treatments
The general experimental design adopted in this study is the following: hippocampal neurons were incubated in ACM alone (control) or in ACM containing 100 μM carbachol without or with 50 or 75 mM ethanol, or ethanol alone (50 or 75 mM). In neuritogenesis and cytotoxicity experiments, incubations with carbachol and/or ethanol were carried out for 24 h (unless otherwise indicated). The release in intracellular Ca++, an event occurring rapidly after muscarinic receptor stimulation, was measured every 10 sec during the first 200 sec after carbachol addition; PKC activation was measured 15 min after carbachol treatments; ERK1/2 activation was measured after 30 min treatments with carbachol. Ethanol was added to the treatment medium; each plate was treated with only one concentration of ethanol, as ethanol redistributes in all the wells within the same plate uniformly over the course of the incubation. To minimize ethanol evaporation from 24-well plates, water containing ethanol at the same concentration present in the treatments was added in the interwell spaces and to all the wells that did not have cells, and Parafilm was wrapped around the two long sides of the plates closing part of the space between the lid and the dish without totally stopping the air exchange. In order to reduce ethanol evaporation from dishes during short term exposure to ethanol (less than 1 hour), Parafilm was wrapped around two third of the perimeter of the Petri dishes, closing part of the space between the lid and the dish without totally stopping the air exchange. We found that in these conditions ethanol loss does not exceed 15%, cells do not suffer from lack of oxygen, and no pH changes occur. In our hands, this method of ethanol exposure is comparable to the use of sealed chambers (also in use in our laboratory) for treatments up to 24 h (Guizzetti et al., 2007; Guizzetti and Costa, 1996; Guizzetti and Costa, 2002). Ethanol concentrations at the beginning and the end of the incubation were determined in some experiments using a commercially available kit from r-Biopharm (Marshall, MI).
Neurite Extension Assay
Neurite extension was assessed spectrophotometrically following a previously described method (Guizzetti et al., 2008; Smit et al., 2003; VanDeMark et al., 2009). Briefly, hippocampal neurons were plated on inserts with a 3 μm pore membrane at their base coated with poly-D-lysine; the inserts were then placed into 24-well plates. During the 24 h incubation with carbachol and/or ethanol, neurons extended their neurites through the microporous membrane to the lower chamber. At the end of the incubation, membrane inserts were then removed, neurons were fixed in methanol and neurites were stained with a cresyl violet solution (0.09%) (Jin et al., 2006). Cresyl violet is a synthetic dye that is widely used to stain neuronal tissue. It binds to the acidic components of the neuronal cytoplasm, such as RNA-rich ribosomes, and to the nuclei and nucleoli. Cell bodies are removed from the top of the membrane using a cotton swab and the dye is extracted from the neurites in the underside of the porous inserts and quantified at 562 nm on a spectrophotometer using a SPECTRAmax® PLUS microplate spectrophotometer. Because cell bodies are separated from neurites by the porous membrane, they are easily removed from the top of the membrane while leaving undisturbed the neurites on the lower side of the membrane. By the time cell bodies are removed, cells are not anymore in medium and are fixed in methanol. Because of all these reasons, it is very unlikely that the dye from the cell body will “leak” into the neurites. With this method, neurite outgrowth is not measured in individual cells, but in the whole well and therefore the absorbance value represents an average of all the cells plated..
Immunocytochemistry
In order to measure cell morphology, neurons were plated on coverslips placed in 24-well tissue culture plates. After treatment, the cells were fixed in 4% paraformaldehyde, then permeablized in 0.1% Triton X-100 for 10 minutes at room temperature, and blocked in 3% BSA for 30 minutes. The coverslips were then incubated for at least 18 hours with the neuron-specific mouse anti-tubulin, beta III isoform monoclonal antibody (Chemicon®, Temecula, CA). After primary antibody incubations, coverslips were incubated for 1 hour with Alexa Fluor® 488. Nuclei were then stained with 5 μg/ml Hoescht for 5 minutes and coverslips were mounted onto glass slides with Vectashield® mounting gel (Vector Laboratories, Inc, Burlingame, CA), covered with cover glass (Corning, Acton, MA), and sealed with nail polish.
Morphometric Analysis
The slides were viewed with a fluorescence microscope (Nikon™, Melville, NY) and pictures were obtained using a SPOT-RT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI). The images were analyzed with the MetaMorph 6.1 software (Molecular Devices, Sunnyvale, CA). Hippocampal neuron analysis was limited to cells that were identifiable as pyramidal, and were not in contact with any other cells. The criteria used for distinguishing pyramidal neurons from non-pyramidal cells and for determining the developmental stage of the pyramidal cell were based on nomenclature described previously (Dotti et al., 1988), used by others (Clamp and Lindsley, 1998), and by us (VanDeMark et al., 2009). Briefly, hippocampal pyramidal neurons were those with 3 or more extensions after 24 hours in culture. Further characterization into stage 3 pyramidal neurons required a cell body diameter of 10–15 μm, 2–5 undifferentiated neurites, and a single axon with length ≥ 40 μm. In addition, the extensions had to be visible for unambiguous measurement for the duration of the analysis, and neurites that overlapped with other cells were excluded (Lindsley et al., 2003).
MTT assay
Cell viability was measured using Thiazolyl Blue Tetrazolium Bromide (MTT). At the end of ethanol treatments, hippocampal neurons plated in 24-well plates (2 × 105 cells/well) were washed and incubated with 250 μg/ml MTT in PBS for 1 hour at 37°C followed by a 5 min-wash in DMSO to solubilize the MTT-formazan. The absorbance of the MTT-fomazan complex was measured by a spectrophotometer set to detect 562 nm wavelengths.
Intracellular Calcium Mobilization
Changes in the levels of intracellular Ca++ were measured as previously described (Giordano et al., 2007; VanDeMark et al., 2009). Briefly, neurons were plated in 35-mm glass bottom dishes and were loaded with the intracellular Ca++-sensitive fluorescent dye Fluo-3/AM (3 μM) for 30 min. Fluo-3 is a fluorescein-based calcium indicator that exhibits large intensity increases in fluorescence upon Ca++ binding. In this study the cell-permeable acetoxymethyl (AM) ester form of Fluo-3 was utilized. Once Fluo-3/AM enters the cell, the ester bond is cleaved by endogenous esterases in the cytosol and the dye is trapped within the cytosol as it is no longer cell permeable. At the end of the incubation with Fluo-3/AM, cells were washed and incubated for an additional 30 min in a Fluo-3/AM–free Locke’s buffer to remove extracellular traces of the dye and to complete intracellular de-esterification. The cells were then placed on the stage of a fluorescence microscope and the dye was excited with a continuous fluorescence light source (excitation wavelength: 488nm); images of the emitted fluorescence at wavelength 520 nm were captured at 10-s intervals by a charge-coupled device camera (Princeton Instruments, Trenton NJ) using MetaMorph software (Molecular Devices, Sunnyvale, CA). 50 cells in each treatment group were analyzed. Fluorescence measurements were normalized as ΔF/F (Manning and Sontheimer, 1999). Cells were classified as responders or non-responders depending on the size of the carbachol-induced rise in intracellular calcium. Only cells responding with a 10% or greater rise in intracellular calcium were analyzed for their average peak response. Data are expressed as the mean of three separate experiments.
PKC Activity
PKC activity was determined using a non-radioactive method as previously described (VanDeMark et al., 2009). Briefly, after treatment, neurons were lysed in a modified PKC extraction buffer (25 mM Tris, 0.05% Triton X-100, 10 mM β-mercaptoethanol, protease and phosphatase inhibitors). After protein extraction and quantification, equal amounts of protein were used in each PKC reaction following the PepTag® Assay for Non-Radioactive Detection of Protein Kinase C protocol (Promega, Madison, WI). Samples were then incubated with a positively-charged, fluorescent, PKC-specific peptide for 30 min and separated on agarose gels. The phosphorylated, negatively-charged peptide was separated from the non-phosphorylated, positively-charged peptide and visualized under UV light. Resulting bands were quantified by densitometry and normalized to controls.
Protein Isolation and Western Blot
Neurons were plated and treated as described for PKC activity assays. After treatment, cells were lysed with cell lysis buffer in the presence of protease and phosphatase inhibitors, and the protein content was quantified. Equal amounts of proteins were loaded in 10% Bi-Tris gels and separated by electrophoresis. Proteins were then transferred to PVDF membranes. Membranes were blocked in 5% milk and immunolabeled with an anti- phospho-ERK1/2 antibody, followed by a HRP-conjugated secondary antibody. Membranes were stripped and reprobed with a β-actin antibody to control for loading and an ERK1/2 antibody to control for total ERK1/2 levels Resulting bands were quantified by densitometry and normalized to controls.
Statistical Analysis
Each experiment was carried out at least three times. In the morphometric analyses at least 60 cells per treatment were measured (n ≥ 60). All statistical tests were carried out using KaleidaGraph 4.0. Unless otherwise stated, for comparing multiple treatments to controls, one–way ANOVA followed by Dunnett’s test was used to determine significant differences. Values are expressed as mean ± S.E.M.
Results
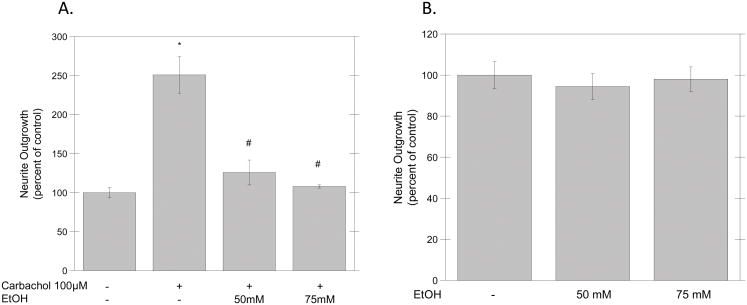
In order to determine the effect of ethanol on carbachol-induced neurite outgrowth, primary hippocampal neurons plated in transwell cell culture inserts were treated in ACM for 24 hours with or without carbachol (100 μM) in the presence of ethanol (50–75 mM) and neurite outgrowth was assessed using a spectrophotometric method (see Materials and Methods). Carbachol alone increased neurite outgrowth by 2.5-fold, and this effect was significantly inhibited by both ethanol concentrations (Fig. 1A), whereas ethanol alone did not affect neurite length (Fig. 1B). The effect of ethanol was not due to increased cytotoxicity, measured by the MTT assay; indeed, neurons incubated for 24 h in the presence of 75 mM ethanol exhibited 94 % ± 8 of the incorporation of MTT-formazan observed in control cells (n=3; p=0.542).
Figure 1. Effect of Ethanol on Carbachol-Stimulated Neurite Outgrowth.
Hippocampal neurons plated on porous inserts were incubated for 24 hrs with (A) or without (B) carbachol (100 μM) in the presence of different concentrations of ethanol (50–75 mM), and neurite outgrowth was assessed spectrophotometrically, as described in Materials and Methods. Results represent the average of three experiments and are expressed as mean ± S.E.M. (*p<0.05 compared to control; #p<0.05, compared to carbachol alone).
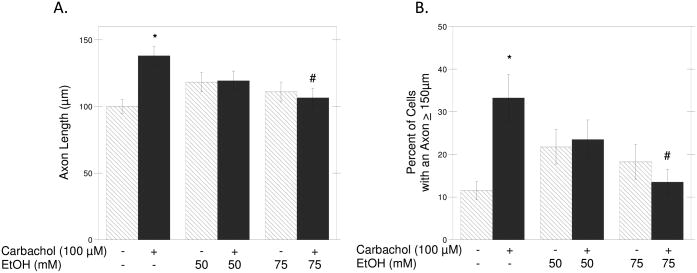
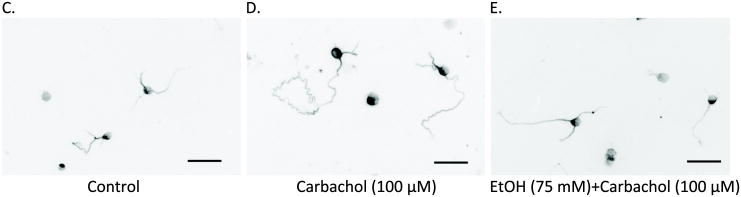
As the described method used for the determination of neurite outgrowth does not provide information on neuronal morphology, we also confirmed these results using a morphometric approach. The axonal length in neurons incubated for 24 h in the presence of carbachol and/or ethanol (50 and 75 mM) and then fixed and immunolabeled with a β-tubulin antibody was measured using the MetaMorph software. We limited this analysis to axonal length because we have previously shown that carbachol increases the length of axons without affecting the length of minor neurites (VanDeMark et al., 2009). We confirmed that carbachol induced a significant increase in the length of axons when compared to control cells, and found that this effect was inhibited by ethanol in a concentration-dependent manner (Fig. 2A; solid bars). We also calculated the percentage of neurons whose axons were longer than 150 μm as an additional index of carbachol-induced hippocampal neuron differentiation, and found that carbachol increased by more than two fold the number of cells with longer axons when compared to control cells (first 2 bars in Fig. 2B), thus confirming our previous results (VanDeMark et al., 2009); this effect of carbachol was also inhibited by ethanol (Fig. 2B; solid bars). Ethanol alone had no significant effects on either axon length or the percentage of cells with axons longer than 150 μm (Figs. 2A, B; striped bars). Representative fields from untreated (C), carbachol-treated (D) and carbachol-treated in the presence of 75 mM ethanol (E) neurons are shown.
Figure 2. Effect of Ethanol on Carbachol-Stimulated Axonal Growth.
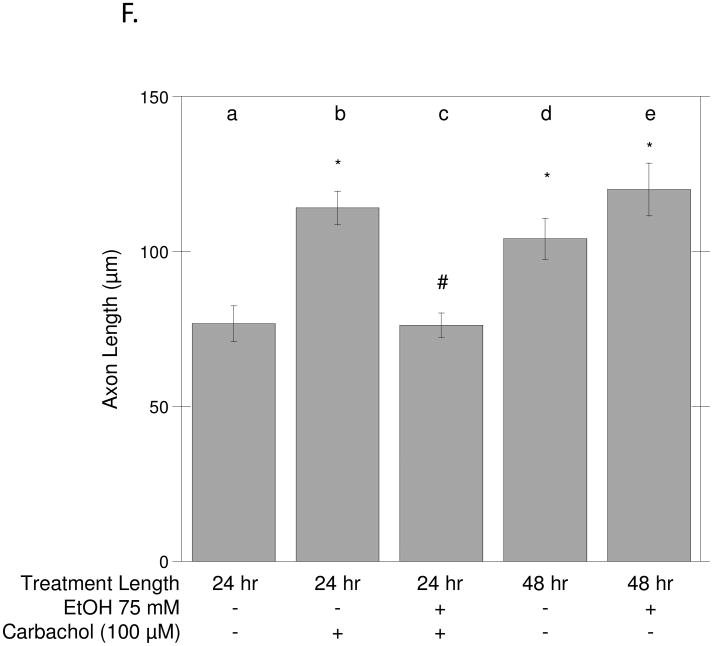
(A): Hippocampal neurons were incubated for 24 h in the presence of the indicated concentrations of ethanol in the absence (striped bars) or presence (solid bars) of 100 μM carbachol. Neurons were then fixed and stained with a β-tubulin antibody followed by a fluorescent secondary, as described in Materials and Methods. Axonal length was quantified using the MetaMorph software. (B): Percent of hippocampal neurons with an axon ≥ 150 μm upon incubation with 50 or 75 mM ethanol alone (striped bars) or with 100 μM carbachol and ethanol (solid bars). Results are from 60 cells/treatment and are expressed as mean ± S.E.M. (*p<0.05 compared to control; #p<0.05, compared to carbachol alone). (C-E): Representative fields of control hippocampal neurons (C), neurons treated for 24 h with carbachol (100 μM) (D), and neurons treated for 24 h with carbachol + EtOH 75 mM (E). (Magnification 20x, scale bar = 25 μm). (F) Hippocampal neurons were incubated for 24 h in ACM (a), ACM supplemented with 100 μM carbachol (b), ACM with carbachol and 75 mM ethanol (c), or for 48 h in ACM (d) or ACM containing 75 mM ethanol (e). Neurons were then fixed and stained as described above. Results are from 60 cells/treatment, and are expressed as mean ± S.E.M. (*p<0.05 compared to control; #p<0.05, compared to carbachol alone).
In our in vitro system, neurons continue to develop over several days. To test whether the effect of ethanol was related to the developmental stage of neurons or whether its effect was exerted on muscarinic signaling, we investigated the effect of ethanol on unstimulated neurons cultured for 48 h in the presence or absence of ethanol. The length of the axons in neurons incubated for 48 h in astrocyte-conditioned medium was similar to the length of the axons of neurons incubated for 24 h in the presence of carbachol (Fig. 2F). Ethanol, while inhibiting axonal elongation stimulated by carbachol (Fig. 2F and 2 A), did not affect the maturation of neurons occurring after 48 h in the absence of stimuli (Fig. 2F). These results suggest that the effect of ethanol on carbachol-induced axonal growth is likely due to the inhibition of signaling pathways activated by carbachol leading to neuronal maturation.
We have previously shown that in pyramidal hippocampal neurons the mobilization of Ca++ from intracellular stores, the activation of PKC, and the phosphorylation of ERK1/2 are involved in the neuritogenic effect of carbachol (VanDeMark et al., 2009).
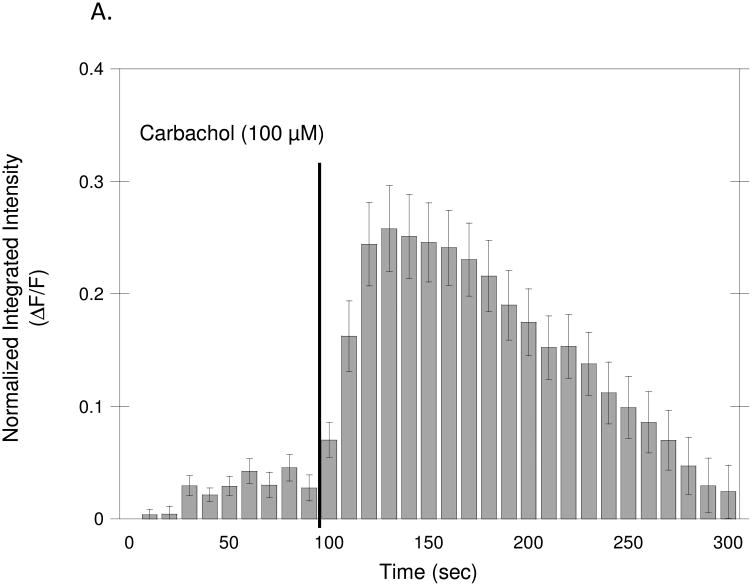
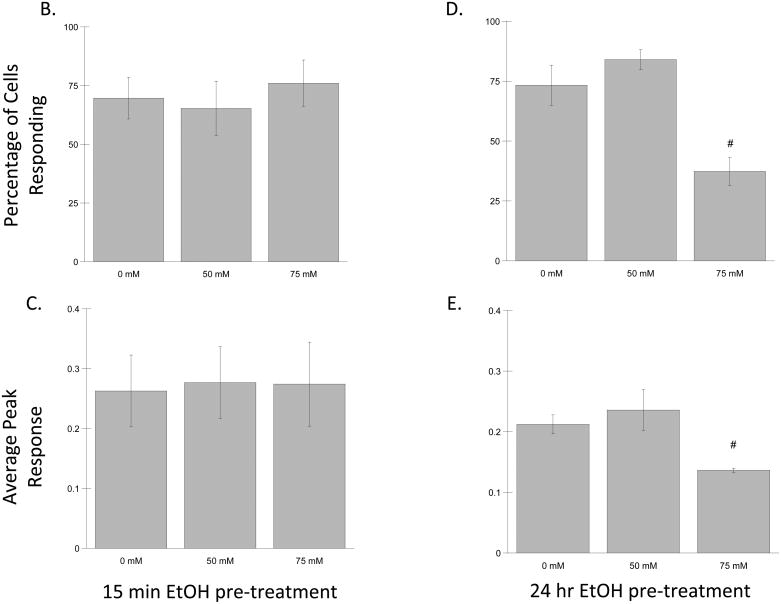
To measure calcium mobilization, neurons were pre-incubated with the intracellular calcium indicator Fluo3-AM. Images of the cells were captured every 10 sec by an inverted fluorescent microscope attached to a digital camera. Ethanol (50 and 75 mM) was added to the cultures 15 min before beginning recording; carbachol (100 μM) was added to the cultures after 100 sec of recording and image capture continued for an additional 200 sec; the fluorescence intensity of the Ca++-bound dye Fluo3 was quantified in at least 50 cells/experiment. Figure 3A shows the average normalized fluorescence intensity before and after carbachol treatment. Seventy percent of the cells (+/− 8.84) (Fig. 3B) responded to carbachol with a rapid increase in intracellular Ca++ that returned to control levels within 200 sec. Ethanol did not affect the percentage of responding neurons, nor the average peak response after 15 min pre-incubation at either of the tested concentrations (Fig. 3B, C), suggesting that ethanol does not directly interfere with carbachol-stimulated intracellular calcium mobilization. On the other hand, pre-incubation with 75 mM ethanol for 24 hr significantly inhibited the percentage of neurons responding to carbachol and the average peak response (Fig. 7D, E), similarly to what has been reported in astrocyte purified cultures and in astrocyte/neuron mixed cultures (Catlin et al., 2000; Kovacs et al., 1995).
Figure 3. Effect of Ethanol on Carbachol-Stimulated Intracellular Calcium Mobilization.
Hippocampal neurons plated on glass bottom dishes and incubated with the intracellular calcium indicator Fluo3-AM, as described in Materials and Methods. (A) Average normalized fluorescence intensity before and after carbachol (100 μM) treatment. Each bar represents the mean (± SEM) of 50 cells. (B–E) Effect of ethanol on carbachol-induced increases in intracellular calcium after a 15 min (B, C) or 24 h (D, E) incubation with ethanol. (B, D) Percentage of cells responding to carbachol after treatment with ethanol. (C, E) Average peak response induced by carbachol after treatment with ethanol. Results are shown as the means (± SEM) of three separate experiments. (#p<0.05 compared to carbachol alone).
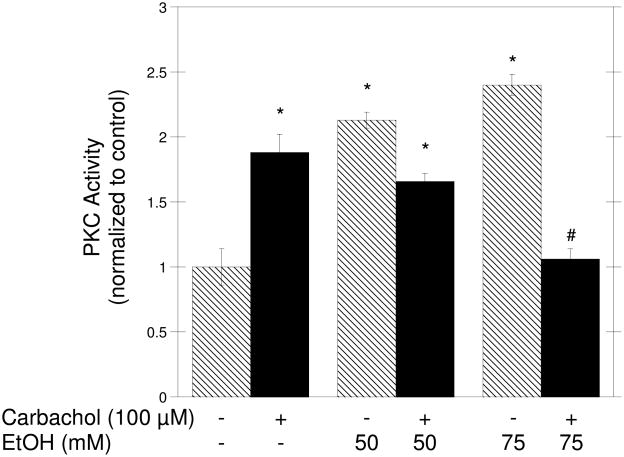
We also tested the effect of ethanol on PKC activation, as we have previously shown that it is involved in carbachol-induced axonal growth (VanDeMark et al., 2009). To measure PKC activation, a non-radioactive kit that quantifies the ability of PKC to phosphorylate a fluorescent substrate was used. Pre-incubation of neurons with 75 mM ethanol for 15 min inhibited PKC activation induced by 100 μM carbachol, while 50 mM ethanol slightly reduced PKC activation in comparison to carbachol alone, but not in a statistically significant manner (Fig. 4, solid bars). Ethanol (50 and 75 mM) alone, in the absence of carbachol, caused an increase in PKC activity in comparison to control cells (Fig. 4, striped bars).
Figure 4. Effect of Ethanol on Carbachol-Stimulated PKC Activity.
Hippocampal neurons were pretreated with 50 or 75 mM ethanol for 15 min; 100 μM carbachol was then added to some of the samples (solid bars), and cells were incubated for an additional 30 min. Cell lysates were collected and equal amounts of proteins were incubated with a fluorescent PKC substrate as described in Materials and Methods. At the end of the reaction the mixture was separated on an agarose gel and the bands corresponding to the phosphorylated peptide were quantified and normalized to controls. Results are the mean (± SEM) of three independent experiments. (*p<0.05 compared to control; #p<0.05, compared to carbachol alone).
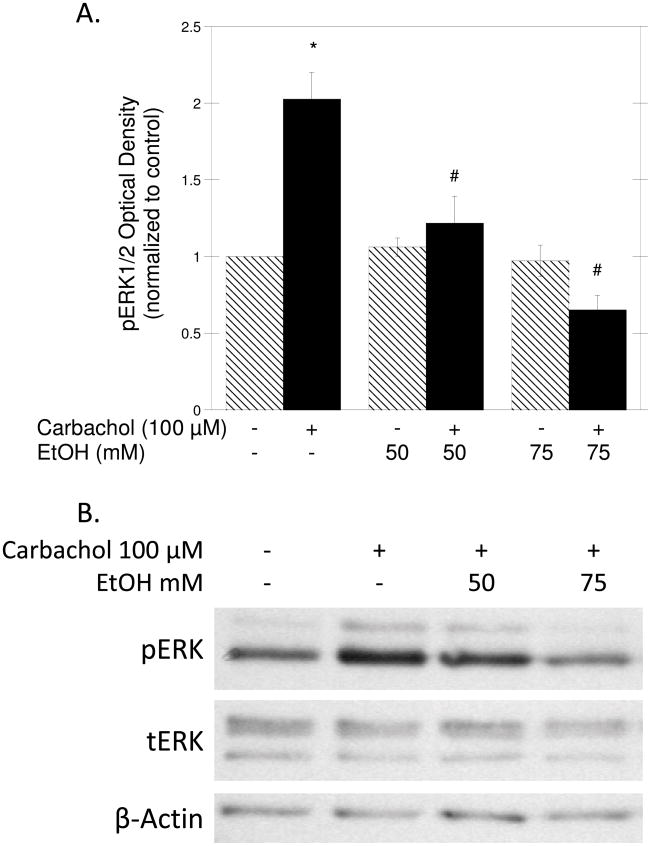
Finally, we previously identified ERK1/2 activation as the key event in carbachol-induced axon growth in hippocampal neurons (VanDeMark et al., 2009). We thus investigated the effect of ethanol on carbachol-stimulated ERK1/2 phosphorylation in hippocampal pyramidal neurons by Western blot, using ERK1/2 phospho-specific antibodies. Densitometric analysis revealed that carbachol (100 μM) caused a 2-fold increase in the levels of phosphorylated (active) ERK1/2 in hippocampal neurons after 30 min incubation. Ethanol inhibited carbachol-induced phosphorylation of ERK1/2 at both tested concentrations (Fig. 5A, solid bars), but did not have any effect on ERK1/2 activity in the absence of carbachol stimulation (Fig. 5A, striped bars). Figure 5B shows a representative immunoblot of the effect of ethanol on carbachol-stimulated ERK1/2 phosphorylation.
Figure 5. Effect of Ethanol on Carbachol-Induced ERK1/2 Phosphorylation.
Hippocampal neurons were pretreated with 50 or 75 mM ethanol for 15 min; 100 μM carbachol was then added to some of the samples (solid bars) and cells were incubated for an additional 30 min. Cell lysates were collected, proteins were quantified, separated, transferred to PVDF membranes and incubated with phospho-ERK1/2, ERK1/2, or β-actin antibodies as described in Materials and Methods. (A) Average optical density of phosphorylated ERK1/2 normalized to controls (mean ± SEM; n=3; *p<0.05 compared to control; #p<0.05, compared to carbachol alone). (B) Representative immunoblot derived from a membrane labeled with phosphoERK1/2 (pERK), total ERK1/2 (tERK), and β-actin (loading control) antibodies.
Discussion
While it is generally accepted that alcohol exposure affects neuronal development both in vivo and in vitro, a review of the literature reveals that contrasting effects of ethanol have been reported, with studies indicating that ethanol increases the elongation of neurites and studies showing an inhibitory effect of alcohol. Increases in axonal growth in the rat pyramidal tract (Miller and al-Rabiai, 1994) and in the corpus callosum of nonhuman primates (Miller et al., 1999) have been observed, along with increases in the density of callosal neurons (Miller, 1997). In contrast, other in vivo animal models have shown decreased callosal size with prenatal ethanol exposure (Moreland et al., 2002; Zimmerberg and Mickus, 1990), similarly to what has been observed clinically in FAS children. Indeed, magnetic resonance images and autopsies of children with known prenatal alcohol exposure have shown significantly smaller callosal areas, especially of the posterior region, and agenesis of the corpus callosum (Coulter et al., 1993; Pratt and Doshi, 1984; Riley et al., 1995; Sowell et al., 2001; Swayze et al., 1997).
In addition, prenatal alcohol exposure has been shown to cause optic nerve hypoplasia in children (Stromland, 1987). This effect has been reproduced in animal models in which alcohol exposure during myelination reduces the number of myelinated fibers (Phillips, 1989). In utero ethanol exposure leads to a loss of myelinated axons in the optic nerve of mice (Parson and Sojitra, 1995), and to smaller optic nerves, degenerating and atrophic optic axons, reduced axonal densities, and decreased numbers of small axonal-diameter myelinated fibers in ethanol-exposed rats (Pinazo-Duran et al., 1997; Sawada et al., 2002). The results of these studies suggest that axonal growth is affected by ethanol exposure in both human FAS cases and in vivo models of FAS.
The effect of ethanol on neurite outgrowth has been investigated in several in vitro systems, that also reported inhibitory and stimulatory effects (Bingham et al., 2004; Liesi, 1997; Messing et al., 1991a; Roivainen et al., 1993; Saunders et al., 1995; Zou et al., 1993). It is likely that the contrasting results reported in different studies reflect the heterogeneity of brain regions, as suggested by the observation that ethanol inhibits neurite outgrowth stimulated by L1CAM in cerebellar granule neurons but not in cortical neurons (Bearer et al., 1999; Hoffman et al., 2008). Furthermore, the developing brain undergoes continuous and rapid changes; therefore, the timing of ethanol exposure plays a pivotal role in the type of response ethanol may trigger. For instance, some of the inhibitory effects of ethanol on neuronal development have been shown to be mediated by astrocytes (Chen and Charness, 2008; Guizzetti et al., manuscript in preparation; Pascual and Guerri, 2007), which, however, proliferate and increase in number relatively late in development (during the third trimester of gestation in human and postnatally in rats); therefore, the effects of earlier ethanol exposure are unlikely to be mediated by astrocytes. Finally, ethanol has been shown to activate signaling molecules involved in neuronal development, such as PKC and ERK1/2 (Chaturvedi and Sarkar, 2005; Messing et al., 1991b), but to inhibit the activation of these enzymes mediated by membrane receptors, such is the case of L1CAM and Brain-Derived Neurotrophic Factor (Hellmann et al., 2008; Tang et al., 2006).
Because of the diversity of effects triggered by ethanol in developing neurons, it is important to examine the consequences of ethanol exposures under different conditions that mimic processes occurring during specific phases of brain development, in order to fully understand the relationship between in utero alcohol and neuronal development.
We and others have been pursuing the hypothesis that acetylcholine, through the activation of muscarinic receptors, may play an important role in brain development, and particularly in neuronal maturation (Costa, 1993; Lauder and Schambra, 1999; Mount et al., 1994; Owen and Bird, 1995). We have shown that the activation of M3 muscarinic receptors in astrocytes induces neuritogenesis in primary hippocampal neurons, through the release of neuritogenic factors (Guizzetti et al., 2008), and that the direct stimulation of M1 muscarinic receptors in hippocampal neurons increases axonal length (VanDeMark et al., 2009). In our cell culture system, hippocampal neurons maintained for 24 h in vitro have not developed synapses yet (Dotti et al., 1988), therefore, the reported effect of carbachol is extrasynaptic, and is likely localized at the level of the growing axon. The concentration of carbachol that induces neurite outgrowth (100 μM) is lower than the concentration of acetylcholine found in synaptic vesicles, which are in the millimolar range (Dunant and Israel, 2000). This would be in agreement with the hypothesis that acetylcholine released by growing axons before synaptogenesis occurs (Yao et al., 2000) may be responsible for neuritogenesis of neighboring neurons in the developing brain.
As we have previously shown that ethanol inhibits muscarinic receptor signaling (Balduini and Costa, 1989; Balduini et al., 1994; Costa and Guizzetti, 1999; Guizzetti and Costa, 2000; Guizzetti and Costa, 2002; Guizzetti et al., 2003), we hypothesized that ethanol may reduce axonal growth induced by cholinergic stimulation by interfering with muscarinic signaling. In this study we report that ethanol inhibited carbachol-stimulated axonal growth. The alcohol concentrations used in this study, 50 and 75 mM, corresponding 0.23 g/dl and 0.35 g/dl respectively, are found in the blood of individuals after moderate to high ethanol intake and are in the range of concentrations recommended for in vitro studies (Deitrich and Harris, 1996), underlying the relevance of this in vitro study to the pathological effects caused by prenatal alcohol exposure. Both ethanol concentrations inhibited carbachol-induced neurite outgrowth measured by a spectrophotometric method, while only the higher ethanol concentration significantly inhibited the effect of ethanol on axonal growth, measured by morphometric analysis. These differences may reflect a difference in sensitivity of the two methods, while supporting each other in the conclusion that ethanol inhibits neuronal differentiation stimulated by carbachol.
The inhibition of carbachol-stimulated neurite outgrowth by ethanol was not a result of cytotoxicity, in agreement with previous results (Clamp and Lindsley, 1998; Heaton et al., 1994; Saunders et al., 1997) in which hippocampal neurons survived concentrations of 2.4 g/dl, 1.0 g/dl, and 0.8 g/dl of ethanol, respectively.
Ethanol alone did not inhibit neuronal development occurring in the absence of cholinergic stimulation after 48 h in vitro, when neurons were more developed and similar in morphology to the neurons cultured for 24 h in the presence of carbachol, suggesting that the effect of ethanol was not dependent on the differentiation stage of neurons, but rather that it may interfere with the muscarinic M1 receptor-stimulated signaling cascade. As we have previously shown that carbachol induces increase in intracellular Ca++, and PKC and ERK1/2 activation in hippocampal neurons, and that these events are important for carbachol-induced axonal growth (VanDeMark et al., 2009), we investigated the effect of ethanol on each of these signaling molecules. The increase in intracellular Ca++ was not affected by a 15 min incubation with ethanol, similarly to what previously reported in astrocytes and mixed cultures (Catlin et al., 2000; Kovacs et al., 1995). In contrast, ethanol pre-incubation for 24 h inhibited the percentage of cells responding to carbachol with an increase in intracellular Ca++, and the amplitude of the response, also in agreement with previous findings (Catlin et al., 2000; Kovacs et al., 1995). This may suggest that the mechanisms involved in Ca++ release are not directly affected by ethanol, but that alcohol, instead, may alter the levels or activity of proteins involved in Ca++ release through changes in their gene expression or post-translational modification, as these effects are not immediate and require several hours to occur. This delayed effect of ethanol on Ca++ release may also contribute to the inhibition of carbachol-stimulated axonal growth by alcohol.
Both PKC and ERK1/2 activation were inhibited by ethanol, although PKC activation was inhibited only at 75 mM ethanol, while ERK1/2 activation was inhibited at both the ethanol concentrations. We have shown that PKC activation is up-stream to ERK1/2 activation in pyramidal hippocampal neurons stimulated by carbachol and that the activation of both these kinases is involved in carbachol-induced neuritogenesis (VanDeMark et al., 2009), therefore suggesting that ethanol may inhibit neurite outgrowth by inhibiting PKC or up-stream activators of PKC leading then to inhibition of ERK1/2. However, we cannot exclude that additional effects of ethanol may be involved in the inhibition by ethanol of carbachol-induced neuritogenesis in hippocampal pyramidal neurons. The fact that ERK1/2 appears to be more sensitive to ethanol inhibition than PKC may be due to a difference in the sensitivity of the methods used for measuring activation. Another possibility is that more than one mechanism may be involved in the effect of ethanol on ERK1/2, one acting on PKC or upstream to PKC, another acting downstream. Our findings are in agreement with previous results showing that ethanol inhibits neuronal differentiation by inhibiting PKC (Hellmann et al., 2008) and ERK1/2 (Tang et al., 2006).
It should be also noted that, while ethanol inhibited the activation of PKC stimulated by carbachol, it activated PKC when present alone. However, ethanol-induced PKC activation did not lead to MAPK activation. Ethanol-induced activation of PKC has previously been reported, and it has been implicated in ethanol-induced neuritogenesis in PC12 cells (Messing et al., 1991b; Roivainen et al., 1993). Similarly, we also observed a trend toward an increase of axonal length in neurons exposed to ethanol (in the absence of carbachol), although this effect was not statistically significant in our experiments. These observations underscore the complexity of the responses triggered by ethanol in developing neurons, and the fact that these responses depend largely on the factors and stimuli to which neurons are exposed, which, in vivo, change widely during the different phases of brain development.
Our results suggest that the inhibition of neurite outgrowth induced by M1 muscarinic receptor activated signaling in hippocampal neurons may be responsible, at least in part, for the cognitive deficits associated with in utero alcohol exposure. In support to this hypothesis, M1 knockout mice have been found to present impairments in certain memory functions, such as spatial working memory and social discrimination, which are processes dependent on the interaction between the neocortex and the hippocampus, although no global deficits in hippocampal-dependent tasks were reported (Anagnostaras et al., 2003). M1 knockout mice also present behavioral abnormalities associated with memory dysfunction, which are also observed in FAS. It has been proposed that the lack of dramatic effects in cognitive functions observed in these mice may be due to compensating mechanisms that may intervene; if this is the case, the full effect of M1 receptor elimination on hippocampal functions could be evident only after disruption of these compensatory processes (Bymaster et al., 2003). The effect of ethanol on brain development appears to be more severe than the effect of knocking out M1 muscarinic receptors, likely because ethanol may interfere with multiple signaling pathways at the same time, thereby reducing the aforementioned compensatory processes that take place when a single receptor is deleted. Finally, it should also be pointed out that the effects of ethanol on muscarinic receptor-mediated neuritogenesis in hippocampal neurons may provide a mechanistic explanation to the observations that choline supplementation appears to ameliorate learning deficits caused by ethanol exposure during brain development (Ryan et al., 2008).
Acknowledgments
This research was supported in part by grants from the National Institutes of Health AA08154, ES07033 and ES07032.
References
- al-Rabiai S, Miller MW. Effect of prenatal exposure to ethanol on the ultrastructure of layer V of mature rat somatosensory cortex. J Neurocytol. 1989;18:711–29. doi: 10.1007/BF01187226. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–8. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Balduini W, Costa LG. Effects of ethanol on muscarinic receptor-stimulated phosphoinositide metabolism during brain development. J Pharmacol Exp Ther. 1989;250:541–7. [PubMed] [Google Scholar]
- Balduini W, Reno F, Costa LG, Cattabeni F. Developmental neurotoxicity of ethanol: further evidence for an involvement of muscarinic receptor-stimulated phosphoinositide hydrolysis. Eur J Pharmacol. 1994;266:283–9. doi: 10.1016/0922-4106(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O’Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep. 2005;54:1–14. [PubMed] [Google Scholar]
- Bingham SM, Mudd LM, Lopez TF, Montague JR. Effects of ethanol on cultured embryonic neurons from the cerebral cortex of the rat. Alcohol. 2004;32:129–135. doi: 10.1016/j.alcohol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, McKinzie DL, Felder CC, Wess J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res. 2003;28:437–42. doi: 10.1023/a:1022844517200. [DOI] [PubMed] [Google Scholar]
- Catlin MC, Guizzetti M, Costa LG. Effect of ethanol on muscarinic receptor-induced calcium responses in astroglia. J Neurosci Res. 2000;60:345–355. doi: 10.1002/(SICI)1097-4547(20000501)60:3<345::AID-JNR9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chaturvedi K, Sarkar DK. Role of protein kinase C-Ras-MAPK p44/42 in ethanol and transforming growth factor-{beta}3-induced basic fibroblast growth factor release from folliculostellate cells. J Pharmacol Exp Ther. 2005;314:1346–1352. doi: 10.1124/jpet.105.088302. [DOI] [PubMed] [Google Scholar]
- Chen S, Charness ME. Ethanol inhibits neuronal differentiation by disrupting activity-dependent neuroprotective protein signaling. Proc Natl Acad Sci U S A. 2008;105:19962–19967. doi: 10.1073/pnas.0807758105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp PA, Lindsley TA. Early events in the development of neuronal polarity in vitro are altered by ethanol. Alcohol Clin Exp Res. 1998;22:1277–84. [PubMed] [Google Scholar]
- Costa LG. Muscarinic receptors and the developing nervous system. In: Zagon IS, McLaughlin PJ, editors. Receptors in the developing nervous system. Vol. 2. Chapman & Hall; London: 1993. pp. 21–62. [Google Scholar]
- Costa LG, Guizzetti M. Muscarinic cholinergic receptor signal transduction as a potential target for the developmental neurotoxicity of ethanol. Biochem Pharmacol. 1999;57:721–726. doi: 10.1016/s0006-2952(98)00278-0. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA. Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Arch Neurol. 1993;50:771–5. doi: 10.1001/archneur.1993.00540070083022. [DOI] [PubMed] [Google Scholar]
- Davies DL, Smith DE. A Golgi study of mouse hippocampal CA1 pyramidal neurons following perinatal ethanol exposure. Neurosci Lett. 1981;26:49–54. doi: 10.1016/0304-3940(81)90424-9. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y, Israel M. Neurotransmitter release at rapid synapses. Biochimie. 2000;82:289–302. doi: 10.1016/s0300-9084(00)00194-2. [DOI] [PubMed] [Google Scholar]
- Fabregues I, Ferrer I, Gairi JM, Cahuana A, Giner P. Effects of prenatal exposure to ethanol on the maturation of the pyramidal neurons in the cerebral cortex of the guinea-pig: a quantitative Golgi study. Neuropathol Appl Neurobiol. 1985;11:291–8. doi: 10.1111/j.1365-2990.1985.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Giordano G, White CC, Mohar I, Kavanagh TJ, Costa LG. Glutathione levels modulate domoic acid induced apoptosis in mouse cerebellar granule cells. Toxicol Sci. 2007;100:433–44. doi: 10.1093/toxsci/kfm236. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Chen J, Oram JF, Tsuji R, Dao K, Moller T, Costa LG. Ethanol Induces Cholesterol Efflux and Up-regulates ATP-binding Cassette Cholesterol Transporters in Fetal Astrocytes. J Biol Chem. 2007;282:18740–18749. doi: 10.1074/jbc.M702398200. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Inhibition of muscarinic receptor-stimulated glial cell proliferation by ethanol. J Neurochem. 1996;67:2236–2245. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Muscarinic receptors, protein kinase C isozymes and proliferation of astroglial cells: effects of ethanol. Neurotoxicology. 2000;21:1117–21. [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Effect of ethanol on protein kinase C zeta and p70S6 kinase activation by carbachol: a possible mechanism for ethanol-induced inhibition of glial cell proliferation. J Neurochem. 2002;82:38–46. doi: 10.1046/j.1471-4159.2002.00942.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moller T, Costa LG. Ethanol inhibits muscarinic receptor-mediated DNA synthesis and signal transduction in human fetal astrocytes. Neurosci Lett. 2003;344:68–70. doi: 10.1016/s0304-3940(03)00431-2. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. J Biol Chem. 2008;283:31884–31897. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer RP, Scheibel AB. Morphologic evidence for a delay of neuronal maturation in fetal alcohol exposure. Exper Neurol. 1981;74:587–596. doi: 10.1016/0014-4886(81)90193-x. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Swanson DJ, Walker DW. Responsiveness of cultured septal and hippocampal neurons to ethanol and neurotrophic substances. J Neurosci Res. 1994;39:305–18. doi: 10.1002/jnr.490390308. [DOI] [PubMed] [Google Scholar]
- Hellmann J, Rommelspacher H, Wernicke C. Long-term ethanol exposure impairs neuronal differentiation of human neuroblastoma cells involving neurotrophin-mediated intracellular signaling and in particular protein kinase C. Alcohol Clin Exp Res. 2008;33:1–13. doi: 10.1111/j.1530-0277.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- Hoffman EJ, Mintz CD, Wang S, McNickle DG, Salton SRJ, Benson DL. Effects of ethanol on axon outgrowth and branching in developing rat cortical neurons. Neuroscience. 2008;157:556–565. doi: 10.1016/j.neuroscience.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. The fetal alcohol syndrome. Teratology. 1975;12:1–10. doi: 10.1002/tera.1420120102. [DOI] [PubMed] [Google Scholar]
- Kovacs KA, Kavanagh TJ, Costa LG. Ethanol inhibits muscarinic receptor-stimulated phosphoinositide metabolism and calcium mobilization in rat primary cortical cultures. Neurochem Res. 1995;20:939–49. doi: 10.1007/BF00970740. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ Health Perspect. 1999;107:65–9. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P. Ethanol-exposed central neurons fail to migrate and undergo apoptosis. J of Neurosci Res. 1997;48:439–448. doi: 10.1002/(sici)1097-4547(19970601)48:5<439::aid-jnr5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lindsley TA. Effects of ethanol on mechanisms regulating neuronal process outgrowth. In: Miller MW, editor. Brain development: normal processes and the effects of alcohol and nicotine. Oxford University Press; New York: 2006. pp. 230–244. [Google Scholar]
- Lindsley TA, Kerlin AM, Rising LJ. Time-lapse analysis of ethanol’s effects on axon growth in vitro. Dev Brain Res. 2003;147:191–199. doi: 10.1016/j.devbrainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Elberger AJ. Effect of prenatal alcohol exposure on midsagittal commissure size in rats. Teratology. 2001;63:15–22. doi: 10.1002/1096-9926(200101)63:1<15::AID-TERA1003>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Manning TJ, Jr, Sontheimer H. Recording of intracellular Ca2+, Cl−, pH and membrane potential in cultured astrocytes using a fluorescence plate reader. J Neurosci Methods. 1999;91:73–81. doi: 10.1016/s0165-0270(99)00083-7. [DOI] [PubMed] [Google Scholar]
- Mendelson B, Driskill A. Ethanol exposure alters the development of serotonergic neurons in chick spinal cord. Alcohol. 1996;13:431–441. doi: 10.1016/0741-8329(96)00028-6. [DOI] [PubMed] [Google Scholar]
- Messing RO, Henteleff M, Park JJ. Ethanol enhances growth factor-induced neurite formation in PC12 cells. Brain Res. 1991a;565:301–311. doi: 10.1016/0006-8993(91)91662-k. [DOI] [PubMed] [Google Scholar]
- Messing RO, Petersen PJ, Henrich CJ. Chronic ethanol exposure increases levels of protein kinase C delta and epsilon and protein kinase C-mediated phosphorylation in cultured neural cells. J Biol Chem. 1991b;266:23428–23432. [PubMed] [Google Scholar]
- Miller MW. Effect of prenatal exposure to alcohol on the distribution and time of origin of corticospinal neurons in the rat. J Comp Neurol. 1987;257:372–82. doi: 10.1002/cne.902570306. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of prenatal exposure to ethanol on callosal projection neurons in rat somatosensory cortex. Brain Res. 1997;766:121–8. doi: 10.1016/s0006-8993(97)00533-7. [DOI] [PubMed] [Google Scholar]
- Miller MW, al-Rabiai S. Effects of prenatal exposure to ethanol on the number of axons in the pyramidal tract of the rat. Alcohol Clin Exp Res. 1994;18:346–54. doi: 10.1111/j.1530-0277.1994.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Astley SJ, Clarren SK. Number of axons in the corpus callosum of the mature macaca nemestrina: increases caused by prenatal exposure to ethanol. J Comp Neurol. 1999;412:123–31. [PubMed] [Google Scholar]
- Moreland N, La Grange L, Montoya R. Impact of in utero exposure to EtOH on corpus callosum development and paw preference in rats: protective effects of silymarin. BMC Complement Altern Med. 2002;2:10. doi: 10.1186/1472-6882-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount HTJ, Dreyfus CF, Black IB. Muscarinic stimulation promotes cultured Purkinje cell survival: a role for acetylcholine in cerebellar development? J Neurochem. 1994;63:2065–2073. doi: 10.1046/j.1471-4159.1994.63062065.x. [DOI] [PubMed] [Google Scholar]
- Owen A, Bird M. Acetylcholine as a regulator of neurite outgrowth and motility in cultured embryonic mouse spinal cord. Neuroreport. 1995;6:2269–72. doi: 10.1097/00001756-199511270-00001. [DOI] [PubMed] [Google Scholar]
- Parson SH, Sojitra NM. Loss of myelinated axons is specific to the central nervous system in a mouse model of the fetal alcohol syndrome. J Anat. 1995;187 ( Pt 3):739–48. [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Guerri C. The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem. 2007;103:557–568. doi: 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- Phillips DE. Effects of limited postnatal ethanol exposure on the development of myelin and nerve fibers in rat optic nerve. Exp Neurol. 1989;103:90–100. doi: 10.1016/0014-4886(89)90190-8. [DOI] [PubMed] [Google Scholar]
- Pinazo-Duran MD, Renau-Piqueras J, Guerri C, Stromland K. Optic nerve hypoplasia in fetal alcohol syndrome: an update. Eur J Ophthalmol. 1997;7:262–70. doi: 10.1177/112067219700700311. [DOI] [PubMed] [Google Scholar]
- Pratt OE, Doshi R. Range of alcohol-induced damage in the developing central nervous system. Ciba Found Symp. 1984;105:142–56. doi: 10.1002/9780470720868.ch9. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Roivainen R, McMahon T, Messing RO. Protein kinase C isozymes that mediate enhancement of neurite outgrowth by ethanol and phorbol esters in PC12 cells. Brain Res. 1993;624:85–93. doi: 10.1016/0006-8993(93)90063-s. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DE, DiCerbo JA, Williams JR, Hannigan JH. Alcohol reduces neurofilament protein levels in primary cultured hippocampal neurons. Alcohol. 1997;14:519–526. doi: 10.1016/s0741-8329(97)00043-8. [DOI] [PubMed] [Google Scholar]
- Saunders DE, Zajac CS, Wappler NL. Alcohol inhibits neurite extension and increases N- Myc and c-Myc proteins. Alcohol. 1995;12:475–483. doi: 10.1016/0741-8329(95)00034-o. [DOI] [PubMed] [Google Scholar]
- Sawada K, Sakata-Haga H, Komatsu S, Ohta K, Jeong YG, Fukui Y. A selective loss of small-diameter myelinated optic nerve axons in rats prenatally exposed to ethanol. Congenit Anom (Kyoto) 2002;42:125–9. doi: 10.1111/j.1741-4520.2002.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Burrows RC, Phillips DE. Alterations in neuronal development in the substantia nigra pars compacta followingin utero ethanol exposure: immunohistochemical and Golgi studies. Neuroscience. 1993;52:311–322. doi: 10.1016/0306-4522(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Smit M, Leng J, Klemke RL. Assay for neurite outgrowth quantification. BioTechniques. 2003;35:254–256. doi: 10.2144/03352bm01. [DOI] [PubMed] [Google Scholar]
- Smith DE, Davies DL. Effect of perinatal administration of ethanol on the CA1 pyramidal cell of the hippocampus and Purkinje cell of the cerebellum: an ultrastructural survey. J Neurocytol. 1990;19:708–717. doi: 10.1007/BF01188039. [DOI] [PubMed] [Google Scholar]
- Smith DE, Foundas A, Canale J. Effect of perinatally administered ethanol on the development of the cerebellar granule cell. Exper Neurol. 1986;92:491–501. doi: 10.1016/0014-4886(86)90291-8. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–44. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Stromland K. Ocular involvement in the fetal alcohol syndrome. Surv Ophthalmol. 1987;31:277–84. doi: 10.1016/0039-6257(87)90028-2. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–40. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Tang N, He M, O’Riordan MA, Farkas C, Buck K, Lemmon V, Bearer CF. Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases. J Neurochem. 2006;96:1480–90. doi: 10.1111/j.1471-4159.2006.03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDeMark KL, Guizzetti M, Giordano G, Costa LG. The activation of M1 muscarinic receptor signaling induces neuronal differentiation in pyramidal hippocampal neurons. J Pharmacol Exp Ther. 2009;329:532–542. doi: 10.1124/jpet.108.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattendorf DJ, Muenke M. Fetal alcohol spectrum disorders. Am Fam Physician. 2005;72:279–82. [PubMed] [Google Scholar]
- West JR, Hodges CA, Black AC., Jr Prenatal exposure to ethanol alters the organization of hippocampal mossy fibers in rats. Science. 1981;211:957–9. doi: 10.1126/science.7466371. [DOI] [PubMed] [Google Scholar]
- Yanni PA, Lindsley TA. Ethanol inhibits development of dendrites and synapses in rat hippocampal pyramidal neuron cultures. Brain Res Dev Brain Res. 2000;120:233–243. doi: 10.1016/s0165-3806(00)00015-8. [DOI] [PubMed] [Google Scholar]
- Yao W-D, Rusch J, Poo M-M, Wu C-F. Spontaneous acetylcholine secretion from developing growth cones of drosophila central neurons in culture: effects of cAMP-pathway mutations. J Neurosci. 2000;20:2626–2637. doi: 10.1523/JNEUROSCI.20-07-02626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Mickus LA. Sex differences in corpus callosum: influence of prenatal alcohol exposure and maternal undernutrition. Brain Res. 1990;537:115–22. doi: 10.1016/0006-8993(90)90347-e. [DOI] [PubMed] [Google Scholar]
- Zou J-Y, Rabin RA, Pentney RJ. Ethanol enhances neurite outgrowth in primary cultures of rat cerebellar macroneurons. Brain Res Dev Brain Res. 1993;72:75–84. doi: 10.1016/0165-3806(93)90161-3. [DOI] [PubMed] [Google Scholar]