Abstract
The biodegradability of a mixture of PAHs, namely fluorene (Fl), phenanthrene (Phe) and pyrene (Pyr), in mangrove sediment slurry was investigated. At the end of week 4, natural attenuation based on the presence of autochthonous microorganisms degraded more than 99% Fl and Phe but only around 30% of Pyr were degraded. Biostimulation with addition of mineral salt medium degraded over 97% of all three PAHs, showing that nutrient amendment could enhance Pyr degradation. Bioaugmentation with inoculation of a PAH-degrading bacterial consortium enriched from mangrove sediments did not show any promotion effect and the degradation percentages of three PAHs were similar to that by natural attenuation. Some inhibitory effect was observed in bioaugmentation treatment in week 1 with only 50% Fl and 70% Phe degraded. These results indicate that autochthonous microbes may interact and even compete with the enriched consortium during PAH biodegradation. Natural attenuation appeared to be the most appropriate way to remedy Fl- and Phe-contaminated mangrove sediments while biostimulation was more capable to degrade Pyr-contaminated sediments. The study also shows that although a large portion of the added PAHs (more than 95%) was adsorbed onto the sediments at the beginning of the experiment, most PAHs were degraded in 4 weeks, suggesting that the degraders could utilize the adsorbed PAHs efficiently.
Keywords: PAH-degraders, Fluorene, Phenanthrene, Pyrene, Bacterial consortium
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants. Excessive inputs from anthropogenic activities have caused serious contamination and adversely affect the health of aquatic life and human through bioaccumulation. PAHs are hydrophobic and readily adsorbed onto particulate matter, therefore, coastal and marine sediments become the ultimate sinks and elevated concentrations have been recorded (Hughes et al., 1997). Mangrove ecosystems, important inter-tidal estuarine wetlands along coastlines of tropical and subtropical regions, are closely tied to human activities and are subject to contamination. Tam et al. (2001) reported that concentrations of total PAHs (based on 16 USEPA priority PAHs) in some of the Hong Kong mangrove sediments exceeded the Lower Chemical Exceedance Level (LCEL) and even the Upper Chemical Exceedance Level (UCEL) developed from the sediment quality criteria by Hong Kong SAR government (website: http://www.etwb.gov.hk). Total PAHs concentrations in Hong Kong mangrove sediments were also comparable to that recorded in mangrove sediments receiving petroleum pollution in Puerto Rico (Klekowski et al., 1994). Proper remedial actions to remove PAHs from contaminated sediments are essential.
Microbial degradation is believed to be one of the major processes to clean up PAH-contaminated sediments (Hughes et al., 1997). Ramsay et al. (2000) reported that a large number and a wide diversity of PAH-degrading microorganisms were found in mangrove sediments. Ke et al. (2003) showed that over 90% pyrene was removed in contaminated mangrove microcosms in 6 months. In order to enhance the biodegradation efficiency, three remedial strategies, namely natural attenuation, bioaugmentation and biostimulation, have been proposed (Iwamoto and Nasu, 2001). Natural attenuation utilizes intrinsic degradation capability of the autochthonous microorganisms to degrade contaminants and is a natural degradation process (Mills et al., 2003). This strategy is advantageous as it avoids damaging the ecologically sensitive mangrove habitats (Dowty et al., 2001). However, intrinsic bioremediation often takes a long time to complete because of population size of the indigenous degrading microorganisms is low (Forsyth et al., 1995). Yu et al. (2005) reported that the most probable number of PAH-degraders in Ho Chung sediments was around 103 MPN per gram wet sediments. Bioaugmentation, inoculation of microorganisms with desired degradation capability, is a possible mean to enhance biodegradability of toxic contaminants (Vogel, 1996). Biostimulation, supplying additional nutrients or substrates to stimulate degradation of native microorganisms, is another strategy to promote biodegradation (Riser-Roberts, 1998), especially in environments such as mangrove sediments where nutrients are often limiting (Burns et al., 1999). Lee et al. (1993) reported that the microbial activity to degrade oil contamination was stimulated by addition of soluble inorganic fertilizer to mangrove sediments. However, the effectiveness of these strategies varies from sediments to sediments and from contaminants to contaminants (Balba et al., 1998). The present study therefore aims to compare the efficiency of degrading a PAH mixture by three bioremediation strategies, namely natural attenuation, bioaugmentation and biostimulation, in mangrove sediment slurry. The study will also evaluate the population sizes of PAH-degraders, the fate and mass balance of PAH compounds under these three strategies.
2. Materials and methods
2.1. Collection of sediments and analysis of PAHs in sediments
Three random replicated surface sediments (0–3 cm) were collected at the landward region (close to the discharge point of a public sewer) of the Ho Chung mangrove swamp in Hong Kong SAR, China during low tides. These samples were mixed thoroughly to form a composite sample and used to prepare the sediment slurry for biodegradation studies. Ho Chung swamp covers a mangrove area of 2.37 ha and has been affected by vehicle exhausting deposition, and discharge of industrial, livestock and household waste and waste-water. The ΣPAHs concentrations (sum of 16 USEPA priority PAHs) of surface sediments varied from 1162 to 3322 ng g−1 freeze-dry weight with mean and standard deviation values (based on three replicates) of 2202 ± 959 ng g−1 freeze-dry weight. The silt + clay percentage, organic matter content and pH in this mangrove sediment were 36 ± 12%, 4.59 ± 0.58% dry weight and 7.24 ± 0.24%, respectively (Yu et al., 2005).
2.2. Biodegradation studies
The sediment slurry was prepared by adding 4 g fresh sediments into a 100 ml conical flask containing 40 ml artificial seawater at a salinity of 10‰ (parts per thousands). Sediment slurries were then divided into sterile and non-sterile portions. For the sterile sediment slurry, the flask was autoclaved at 121 °C for 30 min, and used as the control for the determination of any abiotic loss of PAHs. The autoclaving step was omitted for the non-sterile sediment slurry. The non-sterile slurries were further divided into two sets: natural attenuation and bioaugmentation. The natural attenuation flasks, without any addition of nutrients and bacterial inoculum, indicate PAH degradation capability of microorganisms naturally present in sediments (i.e. the autochthonous microbes). For the bioaugmentation sediment slurry, 1 ml of the enriched bacterial consortium was inoculated to give an initial inoculum size of 5.77 × 104 cell ml−1 at the beginning of each degradation experiment, i.e. after the spiked PAHs were adsorbed onto the sediments. The bacterial consortium consisted of three bacterial isolates, namely Rhodococcus sp., Acinetobacter sp., and Pseudomonas sp., was enriched from Ho Chung mangrove sediments and had shown a good PAH degradation potential in liquid culture medium within 4 weeks (Yu et al., 2005). The biostimulation slurry was prepared in the same way as the natural attenuation flask except a mineral salt medium (MSM) instead of artificial seawater was used. The MSM, a complete nutrient solution, had the following composition (mg l−1): (NH4)2SO4, 1000; K2HPO4, 10,000; KH2PO4, 5000; MgSO4 · 7H2O, 200; CaCl2 · 2H2O, 100; and trace elements made up of FeSO4 · 7H2O, 5; MnSO4 · H2O, 3; ZnSO4 · 7H2O, 3; CoSO4 · 7H2O, 1; (NH4)6Mo7O24 · 7H2O, 1. The salinity of MSM was also adjusted to 10‰(parts per thousands), same as that in artificial seawater.
A stock PAH standard consisted of a mixture of fluorene (Fl), phenanthrene (Phe) and pyrene (Pyr), each at a concentration of 1000 mg l−1 dissolved in distilled acetone, was prepared. The stock standard (400 μg for each PAH compound) was added into the sediment slurry and shaken overnight to let the PAH adsorbed onto the sediments. The flasks, in triplicate for each treatment as well as for the abiotic control, were retrieved weekly during the 4-weeks experiment. The liquid and sediment phases were separated by centrifugation at 4000 rpm. The residual PAHs in each phase were extracted and analyzed following the standard protocol described by Tam et al. (2001, 2002). An internal standard, m-terphenyl, was added before extraction, and ethyl acetate was used for the liquid phase while dichloromethane and methanol were used to extract PAHs remaining in the sediment phase. The PAH compounds were identified and quantified by GC-FID (Hewlett–Packard 5890 gas chromatography with a flame ionization detector). Biodegradation percentage was calculated as: (amounts of residual PAH in the abiotic control—amounts of residual PAH in the degradation flask)/(amounts of residual PAH in the abiotic control) * 100%. The amounts of residual PAH in each flask were the sum of the amounts remained in the liquid phase and that adsorbed in the sediment phase.
2.3. Enumeration of total aerobic heterotrophs and PAH-degrading bacteria
The population sizes of total aerobic heterotrophs and PAH-degrading bacteria were determined by the most probable number (MPN) method described by Yu et al. (2005). A 10-fold serial dilution factor (from 10−1 to 10−8 dilution) was prepared for each retrieved sample. An aliquot of 10 μl diluted culture was inoculated as a single spot, and each dilution had five replicates for MPN calculation. The number of total aerobic heterotrophs was enumerated by positive growth on nutrient agar plates while the number of PAH-degraders was counted based on their growth on solidified MSM agar plates dropped with a mixed PAH standard, a technique modified from Kiyohara et al. (1982). Every PAH drop had 20 μl of a mixed Flu, Phe and Pyr standard, each at a concentration of 1000 mg l−1 in acetone, and the drop appeared as a white spot after acetone was evaporated. The plates were incubated at 28 °C for 3 weeks.
2.4. Statistical analysis
The mean and standard deviation of three replicates were calculated. The mean values were compared by parametric one way or two-way ANOVA tests at the level of P ≤ 0.05. The differences in PAH degradation percentages, population sizes of total aerobic heterotrophs, PAH-degraders and pH were tested by two-way ANONA with the three treatments and four sampling time as the factors. If sampling time is a significant factor, the treatment effect would be tested again by one-way ANOVA. If the ANOVA result is significant, Tukey multiple comparison would be used to determine where the difference were. All statistical analyses were performed using the software called Statistical Package for Social Sciences (SPSS 10.0 for Window, SPSS Inc. IL, USA).
3. Results
3.1. Biodegradation of mixed PAHs under different strategies
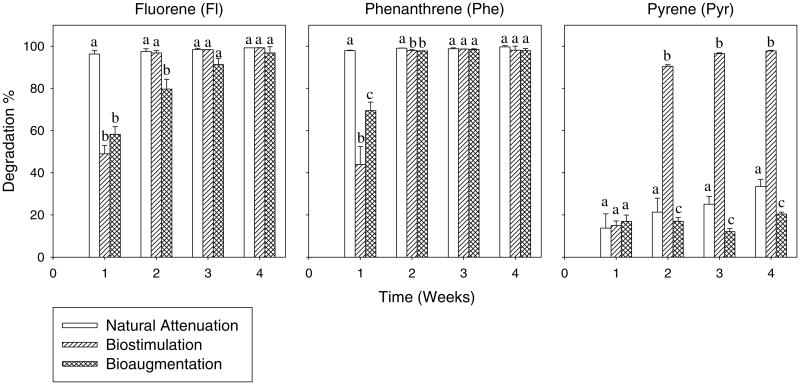
During the 4-weeks biodegradation experiment, losses of fluorene, phenanthrene and pyrene in the control flasks (sterile sediment slurries) were 10.64 ± 3.46%, 1.13 ± 0.77% and 0.88 ± 0.55%, respectively, indicating that abiotic losses of these three mixed PAHs in the sterile sediment slurry were minimal. On the contrary, most of the added PAHs were biodegraded in sediment slurries with microorganisms although the efficiency varied (Fig. 1). In week 1, Fl and Phe degradation percentages in natural attenuation sediment slurries were significantly higher than that in bioaugmentation and biostimulation (P < 0.001 according to one-way ANOVA test), suggesting that initial biodegradation of these two PAHs was inhibited by addition of an enriched PAH-degrading consortium or by supplements of nutrients. In week 2, both natural attenuation and biostimulation slurries achieved over 97% Fl degradation, significantly higher than that in bioaugmentation slurry. Natural attenuation degraded over 99% Phe in week 2, slightly higher than that in biostimulation and bioaugmentation. From week 3 onwards, Fl and Phe degradation percentages were not significantly different among the three slurries, and all had over 96% Fl and 97% Phe degradation at the end of the 4-weeks experiment. Pyrene degradation was small, only 15%, in week 1 and was not significantly different among the three slurries (Fig. 1). However, in week 2, biostimulation slurries degraded over 90% Pyr, which were significantly higher than that in natural attenuation (21%) and bioaugmentation (17%). In week 4, nearly 98% Pyr was degraded in biostimulation slurries, but degradation in natural attenuation (33%) and bioaugmentation (20%) was still low. These results indicate that pyrene was more difficult to degrade than fluorene and phenanthrene, but Pyr degradation was strongly enhanced by nutrient addition.
Fig. 1.
Biodegradation percentages of fluorene (Fl), phenanthrene (Phe) and pyrene (Pyr) in different sediment slurries during 4-weeks degradation. (Mean and standard deviation values of three replicates are shown; significant differences among treatments in each sampling time, according to one-way ANOVA test at P ≤ 0.05, are shown by different lowercase letters.)
3.2. Population sizes of total aerobic heterotrophs and PAH-degraders
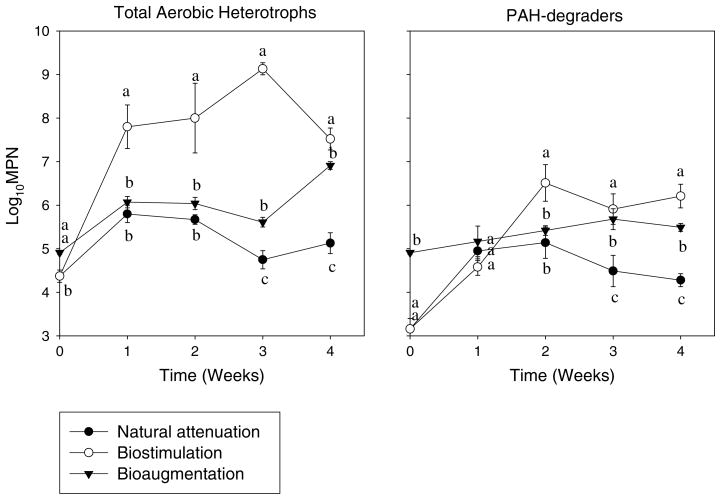
In week 1, biostimulation sediment slurries had the highest log10MPN values of the total aerobic heterotrophs, followed by bioaugmentation, and natural attenuation slurry had the lowest number, but there was no significant difference in log10MPN values of the PAH-degraders among three sediment slurries (Fig. 2). From week 2 onwards, the log10MPN values of total aerobic heterotrophs and PAH-degraders in biostimulation sediment slurries were significantly higher than that in bioaugmentation, and natural attenuation treatments had the smallest numbers (P < 0.001). These findings indicate that addition of nutrients or inoculation of the enriched consortium enhanced the sizes of total aerobic heterotrophic bacteria and PAH-degrading bacteria in the slurry.
Fig. 2.
Changes in population sizes (log10MPN) of total aerobic heterotrophs and PAH-degraders in different sediment slurries during 4-weeks degradation. (Mean and standard deviation values of three replicates are shown; significant differences among treatments in each sampling time, according to one-way ANOVA test at P ≤ 0.05, are shown by different lowercase letters.)
3.3. Mass balance of the spiked PAHs during 4-weeks degradation
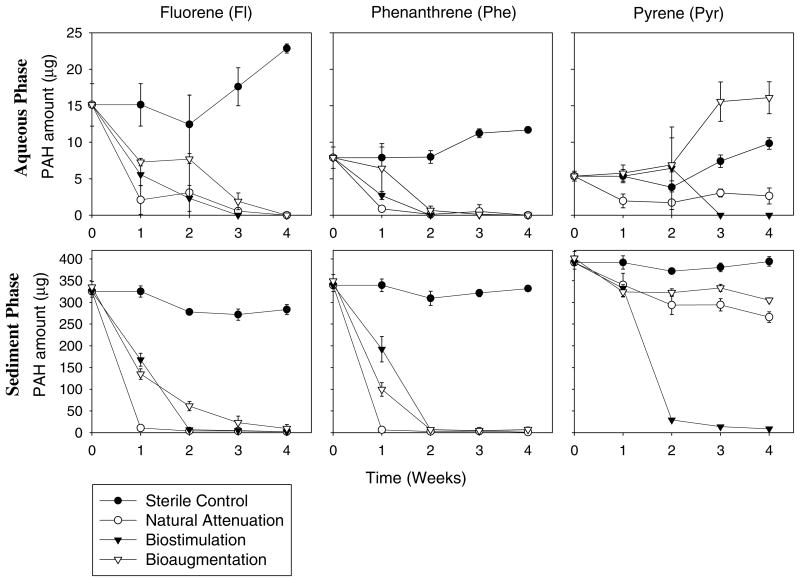
At the beginning of the experiment, 95.55 ± 3.79%, 97.73 ± 4.21% and 98.65 ± 3.84% of the added Fl, Phe and Pyr in the sterile sediment slurry were adsorbed onto the sediments, respectively. This indicates that evaporation of acetone during overnight shaking did not cause any significant loss of the spiked PAHs in sediment slurries. In sterile control (without any microorganisms), the amounts of PAHs in the sediment and the liquid phases maintained at relatively constant levels (Fig. 3). At the end of the 4-weeks experiment, most PAHs in the sterile sediment slurries were still remained in the sediment phase, and only a small fraction was dissolved in the liquid phase (Table 1). These results demonstrate that the spiked PAHs in the sterile sediment slurry were tightly adsorbed onto the sediments throughout the experiment, and percentages of Fl, Phe and Pyr in the sediment phase were 83%, 95% and 99%, respectively. Very little biodegradation occurred in the control as most bacteria had been inactivated or killed by autoclaving, and abiotic losses were also negligible (Table 1).
Fig. 3.
Amounts (μg) of fluorene (Fl), phenanthrene (Phe) and pyrene (Pyr) remained in liquid and sediment phases during 4-weeks degradation under different bioremediation strategies. (Mean and standard deviation values of three replicates are shown.)
Table 1.
Mass balance of fluorene (Fl), phenanthrene (Phe) and pyrene (Pyr), amounts remained (μg) and percentages in each fraction to total PAHs added (input), in different sediment slurries after 4 weeks of degradation
| PAH | Fate of PAHs | Sterile control |
Natural attenuation |
Biostimulation |
Bioaugmenation |
||||
|---|---|---|---|---|---|---|---|---|---|
| Amounts (μg) | % Input | Amounts (μg) | % Input | Amounts (μg) | % Input | Amounts (μg) | % Input | ||
| Fl | Input | 340.35 ± 15.82 | 340.35 ± 15.82 | 340.35 ± 15.82 | 340.35 ± 15.82 | ||||
| In liquid phase | 22.85 ± 0.51 | 6.7 | ND | 0 | ND | 0 | ND | 0 | |
| In sediment phase | 281.27 ± 9.33 | 82.7 | 2.52 ± 0.16 | 0.7 | 2.30 ± 0.25 | 0.7 | 6.70 ± 3.11 | 2.0 | |
| Losses | 36.23 ± 11.17 | 10.6 | 337.83 ± 0.19 | 99.3 | 338.05 ± 0.30 | 99.3 | 333.65 ± 3.81 | 98.0 | |
| Phe | Input | 347.33 ± 16.08 | 347.33 ± 16.08 | 347.33 ± 16.08 | 347.33 ± 16.08 | ||||
| In liquid phase | 11.69 ± 0.26 | 3.4 | ND | 0 | ND | 0 | ND | 0 | |
| In sediment phase | 331.72 ± 1.92 | 95.5 | 1.10 ± 0.56 | 0.3 | 6.59 ± 5.46 | 1.9 | 7.23 ± 2.95 | 2.1 | |
| Losses | 3.92 ± 2.67 | 1.1 | 346.23 ± 1.91 | 99.7 | 340.74 ± 6.69 | 98.1 | 340.10 ± 3.62 | 97.9 | |
| Pyr | Input | 397.05 ± 15.92 | 397.05 ± 15.92 | 397.05 ± 15.92 | 397.05 ± 15.92 | ||||
| In liquid phase | 9.84 ± 0.65 | 2.5 | 2.66 ± 0.90 | 0.7 | ND | 0 | 16.11 ± 1.79 | 4.1 | |
| In sediment phase | 383.73 ± 1.15 | 96.6 | 266.10 ± 10.03 | 67.0 | 8.89 ± 0.90 | 2.2 | 305.02 ± 4.42 | 76.8 | |
| Losses | 3.48 ± 2.00 | 0.9 | 128.29 ± 13.30 | 32.3 | 388.16 ± 1.10 | 97.8 | 75.92 ± 3.26 | 19.1 | |
Mean and standard deviation of three replicates are shown; ND: not detected.
In all non-sterile sediment slurries, irrespective to the bioremediation strategies, the amounts of fluorene and phenanthrene in both sediment and liquid phases dropped to very low levels during the experiment (Fig. 3). Less than 2% Fl and Phe were remained in the sediment phase at the end of the experiment, indicating that over 98% of the spiked Fl and Phe were lost by biodegradation. On the other hand, large fractions of the spiked pyrene were still remained in the sediment phase in natural attenuation and bioaugmentation sediment slurries (Fig. 3 and Table 1). In the biostimulation treatment, no pyrene was found in the liquid phase and only 2% of the spiked pyrene in the sediment phase. The mass balance results suggest that although the spiked PAHs were adsorbed tightly onto the sediments at the beginning of the experiment, PAH-degraders had the ability to utilize and degrade the sorbed PAHs efficiently.
3.4. Changes of pH during 4-weeks biodegradation
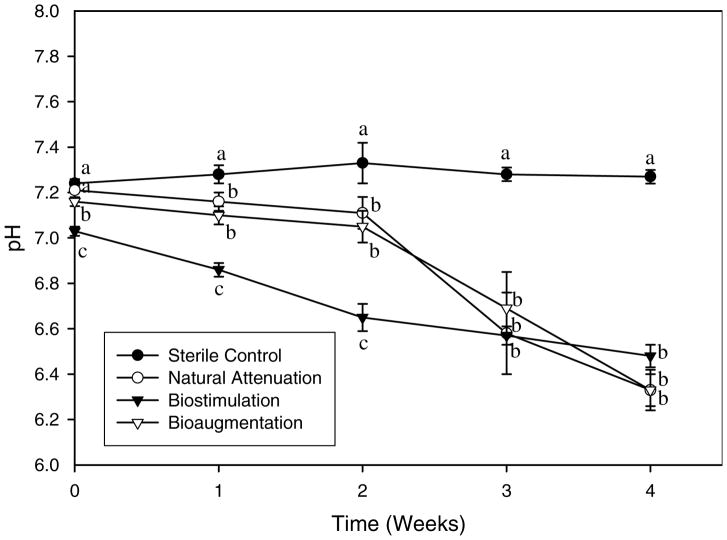
pH of the sterile sediment slurry was relatively constant and maintained at around 7.2 throughout the experiment while pH of the three non-sterile slurries dropped to around 6.30–6.50, significantly lower than that of the sterile slurry (Fig. 4), probably due to the release of proton ions formed during PAHs degradation. In spite of such drop, pH values were still at a range of 6.30–7.20, which should not cause any adverse effects on microbial growth.
Fig. 4.
Change of pH in sterile control, natural attenuation, biostimulation and bioaugmentation sediment slurries during 4-weeks degradation. (Mean and standard deviation values of three replicates are shown; significant differences among treatments in each sampling time, according to one-way ANOVA test at P ≤ 0.05, are shown by different lowercase letters.)
4. Discussion
Mangrove forests, adjacent to human activities, are susceptible to various contamination including PAHs from anthropogenic sources (Klekowski et al., 1994; Tam et al., 2001). Microorganisms in the contaminated mangrove sediments, because of acclimation and adaptation, may have developed some capability to degrade PAHs (Ke et al., 2003). Mills et al. (2003) found that over 95% of the aromatic hydrocarbons in a petroleum-contaminated wetland in southeast Texas were degraded by natural attenuation. In the present study, the natural attenuation sediment slurry demonstrated a high ability to degrade Fl and Phe, and surprisingly the degradation percentages were even higher than that in biostimulation and bioaugmentation slurries. However, pyrene degradation percentages in natural attenuation slurries were significantly lower than that in biostimulation.
Biostimulation has long been used as a strategy to enhance the biodegradation rate of contaminants in nutrient limited environments. Previous studies showed that biostimulation with addition of inorganic nutrients or fertilizers to oil-contaminated salt marsh sediments enhanced their biodegradation rates (Lee and Levy, 1991; Mills et al., 2004). The present study, however, shows that Fl and Phe degradation in biostimulation slurries were inhibited in week 1 although it significantly enhanced Pyr degradation. Similar negative effects of biostimulation on biodegradation had been reported by previous researchers. Braddock et al. (1997) found that the greatest stimulation of microbial activity in a fuel oil contaminated aquifer was at low nutrient addition levels, not at high nutrient addition levels. The mineralization rates of phenanthrene in two out of four tested soils were depressed when nitrogen and phosphorus were added (Johnson and Scow, 1999). Atagana et al. (2003) showed that nutrient supplementation at lower nitrogen concentration with C:N ratio of 25:1 was more effective in enhancing biodegradation of creosote and its components than at high N level with the desired C:N ratio of 10:1 for microbial growth.
The inconsistent effects of biostimulation, positive or negative, were explained by Smith et al. (1998) who proposed a resource-ratio theory to predict how competition for growth-limiting resources influenced biological diversity and function within a biological community. They suggested that biodegradation rate should be optimized by a specific nutrient ratio which may vary as different PAH-degrading microorganisms required different ratios, and some key PAH-degraders may not be selected if the nutrient ratio is inappropriate. The nutrient addition and the nutrient ratio in the present study might select Pyr-degraders but not Fl- and Phe-degraders. The effects of biostimulation, the role and ratio of different nutrients, and the selection of particular degraders required further research.
Bioaugmentation, with inoculation of the bacterial consortium enriched from the same Ho Chung mangrove sediments, was not effective in enhancing biodegradation of the mixed PAHs. The degradation percentages of Fl, Phe and Pyr were lower than that in natural attenuation slurries especially in the first 2 weeks. Although the consortium was enriched from the same piece of mangrove sediments and was able to degrade these three PAHs in liquid medium (Yu et al., 2005), the degradation ability of the inoculum was suppressed by other microorganisms in the non-sterile sediment slurry. Such negative interaction between inoculum and indigenous microbial community may be due to competition for resources, in particular nutrients, as the sediment slurry was prepared in artificial seawater and nutrients were limiting in mangrove sediments. The interactions between inoculum and indigenous microbes are complicated and further work on monitoring the survival and activity of the inoculum will be essential.
5. Conclusions
Mangrove sediments had a high potential to degrade PAHs by its autochthonous microorganisms. Natural attenuation sediment slurry degraded over 99% Fl and Phe while biostimulation using mineral salt medium as nutrient sources successfully degraded 98% Pyr within 4 weeks of degradation. Bioaugmentation with inoculation of an enriched bacterial consortium showed some inhibition effect on Fl, Phe and Pyr degradation in the first 2 weeks, suggesting some negative interaction occurred between inoculum and indigenous microbial community. The mass balance of PAHs indicates that the spiked PAHs were mainly adsorbed onto the sediments and PAH-degraders were still able to degrade them effectively.
Acknowledgments
The work described in this paper was financially supported by a grant from the Research Grant Council of the Hong Kong SAR (Project No. RGC Ref: CityU 1110/02M).
References
- Atagana HI, Haynes RJ, Wallis FM. Optimization of soil physical and chemical conditions for the bioremediation of creosote-contaminated soil. Biodegradation. 2003;14:297–307. doi: 10.1023/a:1024730722751. [DOI] [PubMed] [Google Scholar]
- Balba MT, Al-Awadhi N, Al-Daher R. Bioremediation of oil-contaminated soil: microbiological methods for feasibility assessment and field evaluation. Journal of Microbiological Methods. 1998;32:155–164. [Google Scholar]
- Braddock JF, Ruth ML, Catterall PH. Enhancement and inhibition of microbial activity in hydrocarbon-contaminated arctic soils: implications for nutrient-amended bioremediation. Environmental Science and Technology. 1997;31:2078–2084. [Google Scholar]
- Burns KA, Codi S, Swannell RJP, Duke NC. Assessing the petroleum hydrocarbon potential of endogenous tropical marine wetland microorganisms: flask experiments. Mangroves and Salt Marshes. 1999;3:67–83. [Google Scholar]
- Dowty RA, Shaffer GP, Hester MW, Childers GW, Campo FM, Greene MC. Phytoremediation of small-scale oil spills in fresh marsh environments: a mesocosm simulation. Marine Environmental Research. 2001;52:195–211. doi: 10.1016/s0141-1136(00)00268-3. [DOI] [PubMed] [Google Scholar]
- Forsyth JV, Tsao YM, Bleam RD. Bioremediation: when is augmentation needed. In: Hinchee RE, Fredrickson J, Alleman BC, editors. Bioaugmentation for Site Remediation; Columbus: Battelle Press; 1995. pp. 1–14. [Google Scholar]
- Hughes JB, Beckles DM, Chandra SD, Ward CH. Utilization of bioremediation processes for the treatment of PAH-contaminated sediments. Journal of Industrial Microbiology Biotechnology. 1997;18:152–160. doi: 10.1038/sj.jim.2900308. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Nasu M. Review: current bioremediation practice and perspective. Journal of Bioscience and Bioengineering. 2001;92:1–8. doi: 10.1263/jbb.92.1. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Scow KM. Effect of nitrogen and phosphorus addition on phenanthrene biodegradation in four soils. Biodegradation. 1999;10:43–50. doi: 10.1023/a:1008359606545. [DOI] [PubMed] [Google Scholar]
- Ke L, Wang WQ, Wong TWY, Wong YS, Tam NFY. Removal of pyrene from contaminated sediments by mangrove microcosms. Chemosphere. 2003;51:25–34. doi: 10.1016/S0045-6535(02)00811-1. [DOI] [PubMed] [Google Scholar]
- Kiyohara H, Nagao K, Yana K. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbon on agar plates. Applied and Environmental Microbiology. 1982;43:454–457. doi: 10.1128/aem.43.2.454-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klekowski, Corredor JE, Morell JM, DelCastello CA. Petroleum pollution and mutation in mangroves. Marine Pollution Bulletin. 1994;28:166–169. [Google Scholar]
- Lee K, Levy EM. Bioremeidation: wazy crude oils stranded on low-energy shorelines. Proceedings of the 1991 Oil Spill Conference (Prevention, Behaviour, Control, Cleanup); Washington, DC: American Petroleum Institute; 1991. pp. 541–547. [Google Scholar]
- Lee K, Tremblay GH, Levy EM. Bioremediation: application of slow-release fertilizers on low-energy shorelines. Proceedings of the 1993 Oil Spill Conference; Washington, DC: American Petroleum Institute; 1993. pp. 449–454. [Google Scholar]
- Mills MA, Bonner JS, McDonald TJ, Page CA, Autenrieth RL. Intrinsic bioremediation of a petroleum-impacted wetland. Marine Pollution Bulletin. 2003;46:887–899. doi: 10.1016/S0025-326X(02)00367-3. [DOI] [PubMed] [Google Scholar]
- Mills MA, Bonner JS, Page CA, Autenrieth RL. Evaluation of bioremediation strategies of a controlled oil release in a wetland. Marine Pollution Bulletin. 2004;49:425–435. doi: 10.1016/j.marpolbul.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Ramsay MA, Swannell RPJ, Shipton WA, Duke NC, Hill RT. Effect of bioremediation community in oiled mangrove sediments. Marine Pollution Bulletin. 2000;41:413–419. [Google Scholar]
- Riser-Roberts E. Remediation of Petroleum Contaminated Soils: Biological, Physical, and Chemical Processes. Boca Raton: Lewis Publishers; 1998. pp. 5–313. [Google Scholar]
- Smith VH, Graham DW, Cleland DD. Application of resource ratio theory to hydrocarbon biodegradation. Environmental Science and Technology. 1998;32:3386–3395. [Google Scholar]
- Tam NFY, Ke L, Wang XH, Wong YS. Contamination of polycyclic aromatic hydrocarbons in surface sediments of mangrove swamps. Environmental Pollution. 2001;114:255–263. doi: 10.1016/s0269-7491(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Tam NFY, Guo CL, Yau WY, Wong YS. Preliminary study on biodegradation of phenanthrene by bacteria isolated from mangrove sediments in Hong Kong. Marine Pollution Bulletin. 2002;45:316–324. doi: 10.1016/s0025-326x(02)00108-x. [DOI] [PubMed] [Google Scholar]
- Vogel TM. Bioaugmentation as a soil bioremediation approach. Current Opinion and Biotechnology. 1996;7:311–316. doi: 10.1016/s0958-1669(96)80036-x. [DOI] [PubMed] [Google Scholar]
- Yu SH, Ke L, Wong YS, Tam NFY. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by a consortium enriched from mangrove sediments. Environment International. 2005;31:149–154. doi: 10.1016/j.envint.2004.09.008. [DOI] [PubMed] [Google Scholar]