Abstract
Pancreatic beta cells are specialised endocrine cells that continuously sense the levels of blood sugar and other fuels and, in response, secrete insulin to maintain normal fuel homeostasis. During postprandial periods an elevated level of plasma glucose rapidly stimulates insulin secretion to decrease hepatic glucose output and promote glucose uptake into other tissues, principally muscle and adipose tissues. Beta cell mitochondria play a key role in this process, not only by providing energy in the form of ATP to support insulin secretion, but also by synthesising metabolites (anaplerosis) that can act, both intra- and extramitochondrially, as factors that couple glucose sensing to insulin granule exocytosis. ATP on its own, and possibly modulated by these coupling factors, triggers closure of the ATP-sensitive potassium channel, resulting in membrane depolarisation that increases intracellular calcium to cause insulin secretion. The metabolic imbalance caused by chronic hyperglycaemia and hyperlipidaemia severely affects mitochondrial metabolism, leading to the development of impaired glucose-induced insulin secretion in type 2 diabetes. It appears that the anaplerotic enzyme pyruvate carboxylase participates directly or indirectly in several metabolic pathways which are important for glucose-induced insulin secretion, including: the pyruvate/malate cycle, the pyruvate/citrate cycle, the pyruvate/isocitrate cycle and glutamate-dehydrogenase-catalysed α-ketoglutarate production. These four pathways enable ‘shuttling’ or ‘recycling’ of these intermediate(s) into and out of mitochondrion, allowing continuous production of intracellular messenger(s). The purpose of this review is to present an account of recent progress in this area of central importance in the realm of diabetes and obesity research.
Keywords: Coupling factors, Glucose-stimulated insulin secretion, Insulin secretion, Mitochondrial metabolism, Pyruvate cycling, Review
Glucose-stimulated insulin secretion
Several nutrients can act as insulin secretagogues, including glucose and some amino acids such as leucine alone, glutamine in combination with leucine, and NEFA [1]. However, glucose is the most potent secretagogue for insulin secretion as it induces robust insulin secretion within a few minutes of the onset of the stimulus and the stimulatory effect lasts as long as the plasma glucose is elevated. Secretion of insulin in response to glucose occurs in two phases, i.e. it is a biphasic response, in which the first release begins within a few minutes of stimulation, after which it declines. The second phase of insulin secretion begins a few minutes after the first phase and secretion gradually increases to a peak within 30–40 min [2]. The beta cell senses an elevated level of glucose in the plasma by glucokinase. Rapid entry of glucose through solute carrier family 2 (facilitated glucose transporter), member 2 (GLUT2) for rodents and solute carrier family 2 (facilitated glucose transporter), member 1 (GLUT1) for humans is followed by phosphorylation of glucose which increases glycolytic flux, producing pyruvate as the terminal product of the pathway [3]. Pyruvate then enters the mitochondrion and is decarboxylated to acetyl-CoA, which enters the tricarboxylic acid cycle (TCA), resulting in the production of NADH and FADH2 [4]. These reducing equivalents are subsequently oxidised in the respiratory chain to enable ATP production. The rise of the ATP:ADP ratio in the cytoplasm closes the plasmalemmal ATP-sensitive potassium channel (KATP), resulting in depolarisation of the plasma membrane, which triggers the opening of the voltage-gated Ca2+ channel [5]. This results in an influx of Ca2+ into the cell that triggers the exocytosis of insulin granules. This glucose-stimulated insulin secretion (GSIS) is known as the KATP-dependent GSIS (Fig. 1).
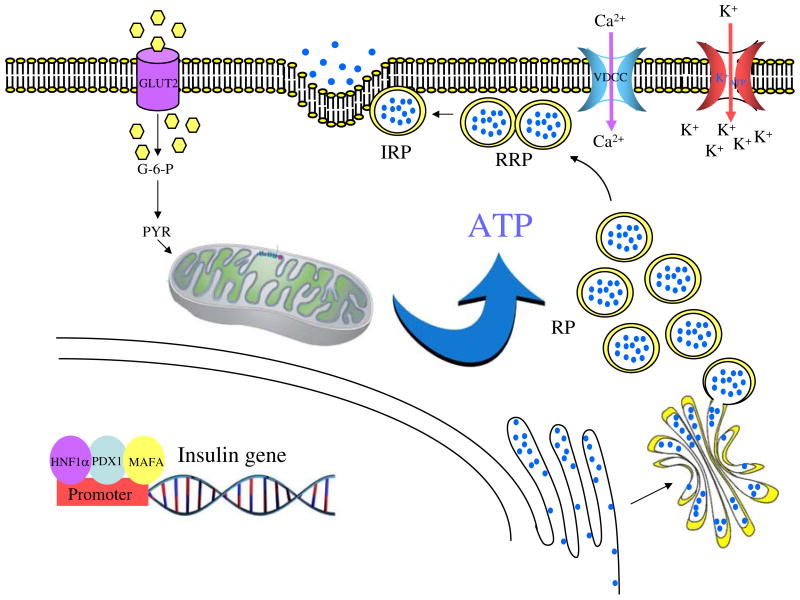
Fig. 1.
KATP-dependent GSIS. Insulin gene transcription is regulated by three transcription factors specific to the beta cell: HNF1α, PDX1 and MAFA [100]. An elevated level of extracellular glucose is taken up by GLUT2 before being rapidly phosphorylated to glucose-6 phosphate (G-6-P) by glucokinase. Pyruvate (PYR), the terminal product of glycolysis, is oxidised in the mitochondrion, yielding a large amount of ATP [101]. The increased cellular ATP:ADP ratio closes KATP-sensitive channels, resulting in membrane depolarisation, followed by Ca2+ influx through voltage-gate-dependent Ca2+ channels [5]. This drives the exocytosis of insulin granules. IRP, immediately releasable pool; RP, reserve pool; RRP, readily releasable pool; VDCC, voltage-gate-dependent calcium channel
However, it is clear that GSIS can also occur independently of KATP channel activity and this is known as the KATP-independent GSIS. The evidence in favour of a KATP-independent GSIS comes from two independent studies. The first study used diazoxide, an agent that maintains the KATP channel in the open state thus eliminating the KATP-dependent GSIS mechanism. GSIS was still detected in the diazoxide-treated islets that had been depolarised with K+, suggesting that a KATP-independent GSIS mechanism must be operating [6]. The second piece of evidence was obtained with mice in which the KATP channels were disrupted. A KATP channel is composed of two subunits, the sulfonylurea receptor 1 (SUR1) and the inward rectifying K+ channel. Mice with targeted disruptions of SUR1 [7, 8] or the inward rectifying K+ channel [9, 10] were still capable of secreting insulin in response to glucose stimulation, suggesting that GSIS does not solely operate through the KATP channel. It was later recognised that KATP-dependent and KATP-independent GSIS are coordinated to ensure the maximum secretion of insulin in response to glucose [2]. The KATP-dependent mechanism may act as the ‘triggering signal’ responsible for the first phase of insulin secretion. In contrast, the KATP-independent mechanism may provide an ‘amplifying signal’, which supports the longer-lasting second phase of insulin secretion. The common feature of the KATP-dependent and KATP-independent pathways is that both require an influx of Ca2+ to the cytosol through the Ca2+ channel [2]. As the KATP-dependent GSIS is mostly understood, many attempts have been made to identify the cellular or biochemical pathways that are associated with KATP-independent GSIS. In this review, we will discuss many of the key enzymes or biochemical pathways underlying the control of GSIS.
KATP-independent GSIS and the role of mitochondria
The role of mitochondria in GSIS was first demonstrated in the INS-1 (rat insulinoma) and MIN6 β cell lines depleted of mitochondrial DNA. These cell lines have defects affecting the enzymes involved in the respiratory chain and are, therefore, unable to generate an electrochemical gradient across the mitochondrial membrane, concomitant with the loss of the glucose-induced increase of intracellular Ca2+ [11–13]. In humans, a point mutation and deletion of the mitochondrial-encoding transfer RNA genes have also been known to associate with a specific form of diabetes known as ‘mitochondrial diabetes’ [14]. These mutations severely affect mitochondrial protein synthesis, which may result in a diminished number of functional beta cells and insulin secretion [14].
Loss of mitochondrial structure and function leading to impaired GSIS may be also caused by mutations of the nuclear-encoded genes. For example, these gene products may be the transcriptional regulators of mitochondrial gene expression [15, 16].
NADH shuttles
The NADH redox shuttles are a means to regenerate NAD+ for glycolysis and to transport electrons to the mitochondrial inner membrane for oxidative phosphorylation. Unlike many types of cells, pancreatic beta cells express very low levels of lactate dehydrogenase (LDH) [17] and do not regenerate NAD+ via lactate formation. Because an increased NADH:NAD ratio will inhibit glycolysis, beta cells possess high activity of two redox shuttles, the glycerol-3-phosphate shuttle [18] and the malate/aspartate shuttle to regenerate NAD+ via mitochondrial oxidation [19]. The glycerol-3-phosphate shuttle consists of the cytosolic glycerol-3-phosphate dehydrogenase (cGPDH) and the mitochondrial glycerol-3-phosphate dehydrogenase (mGPDH), which work in concert [20]. The cGPDH catalyses the conversion of dihydroxyacetone phosphate to glycerol-3-phosphate coupled with the oxidation of NADH to NAD+. Glycerol-3-phosphate is then converted back to the dihydroxyacetone phosphate coupled with the formation of FADH2 by mGPDH. In the malate/aspartate shuttle, NADH is transported into the mitochondrion by the interconversion of malate and aspartate via oxaloacetate in the cytoplasm and the mitochondrion [19].
The activity and the level of mGPDH is exceptionally high in pancreatic islets of rodents [17, 18] and humans [21], suggesting an association of this enzyme with GSIS in pancreatic beta cells. Indeed, decreased activity of mGPDH or the glycerol-3-phosphate shuttle is associated with type 2 diabetes in both rodents [22, 23] and humans [24]. Even though low mGPDH is associated with type 2 diabetes, disruption of the gene encoding it in mice does not impair GSIS, suggesting the malate/aspartate shuttle may compensate for the lack of glycerol-3-phosphate shuttle [25, 26]. However, inhibition of the malate/aspartate shuttle in mGpdh−/− (also known as Gpd2−/−) mice lacking mGPDH does severely impair GSIS [19]. Inhibition of these two NADH shuttle systems not only prevents the closure of the KATP channels required for membrane depolarisation, but also inhibits steps distal to Ca2+ influx that are required for the exocytosis of insulin granules in response to glucose [25]. In INS-1E cells, silencing of the malate/aspartate transporter Aralar1 in the mitochondrial inner membrane that is necessary for the malate/aspartate shuttle partly impairs GSIS [27], further suggesting a role for the malate/aspartate shuttle in GSIS.
Pyruvate carboxylation and pyruvate dehydrogenation
Because pancreatic beta cells produce relatively low levels of LDH, pyruvate is able to enter the mitochondrion for the subsequent oxidation [28]. As the levels and the activities of pyruvate carboxylase (PC) and pyruvate dehydrogenase (PDH) are high in beta cells, these enzymes provide two routes for metabolism of glycolysis-derived pyruvate: either oxidation by PDH to acetyl-CoA or carboxylation by PC to oxaloacetate [29, 30] (Fig. 2). It is notable that PC is as highly abundant in beta cells as it is in the gluconeogenic tissues, i.e. liver and kidney cortex. However, beta cells lack cytosolic phosphoenolpyruvate carboxykinase and fructose-1,6-bisphosphatase, indicating that PC does not serve a gluconeogenic role in these cells. In addition, the rate of pyruvate carboxylation, but not pyruvate decarboxylation, is correlated with the degree of insulin release in rat pancreatic islets [29, 31]. 13C nuclear magnetic resonance (NMR) isotopomer analysis studies show that GSIS is well correlated with pyruvate carboxylation, but not pyruvate oxidation [32]. Furthermore, PC production is induced by glucose above the normal physiological concentration [33]. The key piece of evidence to support the role of PC in GSIS comes from the studies of two independent groups. Suppression of PC production by small interfering RNA (siRNA) impairs GSIS in both insulinoma cells [34, 35] and isolated rat islets [35], and also reduced cell proliferation [35], while overproduction of PC in INS-1 cells increases GSIS and cell proliferation [35]. These data strongly suggest the supportive role of PC in GSIS and beta cell proliferation. In addition, non-carbohydrate metabolisable secretagogues also failed to stimulate GSIS in cells with Pc (also known as Pcx) knockdown, indicating that PC is required for both carbohydrate and non-carbohydrate secretagogues [34]. It is noteworthy that impaired GSIS was only observed when PC activity was lower than 60% of normal [34]. Insufficient lowering of PC may trigger compensatory mechanisms to prevent impaired GSIS in INS-1 cells and islets. A compensatory response resulting from an increased level of acetyl-carnitine—which reflects cellular acetyl-CoA, a potent allosteric activator of PC—was reported in both INS-1 cells and isolated islets with moderately lowered PC [36].
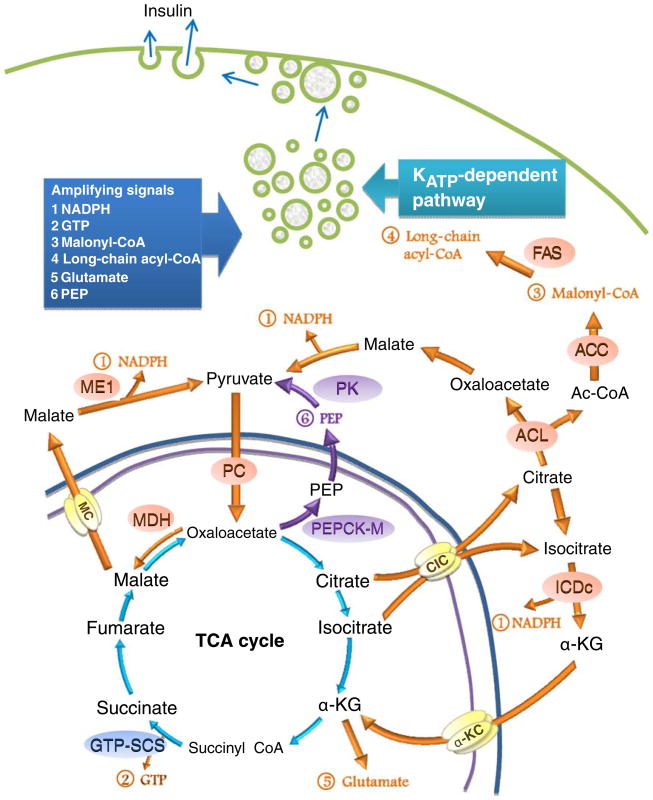
Fig. 2.
Mitochondrial biochemical pathways that are involved in KATP-independent GSIS. Pyruvate enters the mitochondrion through the pyruvate transporter to undergo a PDH-catalysed reaction to form acetyl-CoA or an anaplerotic reaction catalysed by PC [29, 30]. There are two routes for oxaloacetate. The first route involves its conversion to malate, which exits the mitochondrion via the malate carrier (MC) before being converted back to pyruvate. Pyruvate is then cycled back to the mitochondrion to form the ‘pyruvate/malate cycle’ [41]. The second route involves its conversion to citrate, which exits the mitochondrion and is converted to oxaloacetate and acetyl-CoA (Ac-CoA). Acetyl-CoA is converted to malonyl-CoA and long-chain acyl-CoA, while ME oxaloacetate is converted to malate and pyruvate by malate dehydrogenase and cytosolic ME, respectively, as part of the ‘pyruvate/citrate cycle’ [40]. Citrate in the mitochondrion can be converted to isocitrate, which exits the mitochondrion through the citrate/isocitrate carrier (CIC) to the cytoplasm, where it is further converted to α-ketoglutarate (α-KG) by NADP-dependent isocitrate dehydrogenase (ICDc). α-Ketoglutarate then re-enters the mitochondrion as part of the ‘pyruvate/isocitrate’ cycle [62]. The above three cycles share the common terminal step, i.e. the conversion of malate to pyruvate concomitant with the production of cytosolic NADPH by cytosolic ME. Among other amplifying signals, NADPH is thought to be the consensus factor for KATP-independent GSIS. Oxaloacetate may be removed from the mitochondrion as PEP by PEPCK-M. Cytosolic PEP is converted to pyruvate by pyruvate kinase before being recycled back to the mitochondrion [65]. Other amplifying signals include mitochondrial GTP produced by GTP-SCS, and glutamate produced by GDH. NADPH, GTP, malonyl-CoA, long-chain acyl-CoA, glutamate and PEP are thought to be the amplifying signals that control the KATP-dependent pathway. These amplifying signals are suggested to drive exocytosis of insulin granules
Besides supplying acetyl-CoA for the citric acid cycle, a specific role of PDH in GSIS remains unclear. Although inhibition of PDH activity by overproducing pyruvate dehydrogenase kinase 4 in INS-1 cells did not affect GSIS [35], it is possible that PDH may play a supportive role for GSIS by providing acetyl-CoA to condense with the oxaloacetate produced by PC, to form citrate for downstream shuttles. PDH may also supply the acetyl-CoA required to allosterically activate PC during GSIS. The importance of PC in supporting GSIS is well documented in various rodent models. In the islets of obese rats, Pc expression remains normal because of a compensatory mechanism [37], while downregulation of Pc is observed once the impaired GSIS has progressed [23, 38]. In humans, downregulation of PC and its mRNA was observed in type 2 diabetic patients [39]. These data suggest that anaplerosis via PC is important to support insulin secretion. Subsequent studies have clearly shown that cataplerosis, primarily through the export of mitochondrial metabolites to the cytosol, occurs to balance the increased mitochondrial oxaloacetate. Several studies using islets and insulinoma cell lines have clearly demonstrated that GSIS elevates the level of several intermediates of the TCA cycle and promotes the export of some of them from mitochondria to the cytoplasm. Those intermediates include malate, citrate, α-ketoglutarate and succinate [40, 41]. The specific cataplerosis of various intermediates of the TCA cycle is discussed below.
Pyruvate/malate shuttle
In pancreatic beta cells, GSIS stimulates the export of malate from mitochondria to the cytoplasm [41]. The abundance of PC in beta cells and the high rate of malate export from mitochondria to cytoplasm following GSIS suggests the presence of the pyruvate/malate shuttle [41]. In this shuttle, the oxaloacetate produced by PC is converted to malate by mitochondrial malate dehydrogenase (Fig. 2). Malate exits the mitochondria to the cytoplasm where it is subsequently oxidised to pyruvate concomitant with the production of NADPH by cytosolic malic enzyme (ME1). Pyruvate then re-enters mitochondria for the next round of carboxylation by PC. As this occurs in a cyclic fashion, significant amounts of NADPH can be generated in proportion to the expenditure of mitochondrial ATP and NADH. The necessity of ME1 with respect to GSIS is still debated. Using the rat insulinoma cell line INS-1 as the model, Pongratz et al. [42] employed siRNA to suppress production of ME1 or mitochondrial malic enzyme (ME2), and found that only the ME1, and not the ME2, regulates GSIS in these cells. A similar result was obtained when the ME1 was knocked down in the INS-1 832/13 cell line [43]. Contrary to these results another group observed that suppression of ME1 in isolated rat islets did not have an impact on GSIS, although suppression of this enzyme in the INS-1 832/13 cell line yielded results similar to the two previous studies [44]. However, the latter observation was associated with a slow rate of growth of the targeted cells [44]. Although there are many reports that ME1 is very low or absent in mouse islets, there is a report that suppression of ME1 activity by 50% resulted in the reduction of GSIS by half [45], while overproduction of ME1 did not affect GSIS [46]. In the Mod-1 mouse and the mmgg mouse, two strains of mice that lack ME1 in all tissues, GSIS is normal [44, 47]. In line with the in vivo mouse data, a recent study using the INS-1 832/13 cell line in which either Me1 or Me2 was chronically knocked down by 80–90%, which is much greater than in the previous studies, insulin release was not decreased [48]. It is possible that redundancy of the cytosolic pathways for NADPH formation may permit normal GSIS in the Me1-null mice and ME1-deficient cell lines.
Pyruvate/citrate shuttle and acyl-CoAs
The pyruvate/citrate shuttle is another route that uses oxaloacetate formed by PC. This concept was supported by the observation that INS-1 cells stimulated with a high glucose concentration exhibited an increased efflux of citrate from mitochondria to the cytoplasm [40]. In this shuttle (Fig. 2), oxaloacetate condenses with acetyl-CoA to form citrate, mediated by citrate synthase. Citrate then exits the mitochondrion to the cytoplasm where it is converted back to oxaloacetate and acetyl-CoA by ATP-citrate lyase (ACL). Oxaloacetate is converted by cytosolic malate dehydrogenase to malate before being converted to pyruvate by ME1. The latter enzyme produces NADPH as described for the pyruvate/malate cycle [40]. Acetyl-CoA formed by ACL is subsequently carboxylated by acetyl-CoA carboxylase (ACC) to form malonyl-CoA for conversion to long-chain acyl-CoA by fatty acid synthase (FAS). The operation of this cycle was supported by the parallel increases of ACC, FAS and malic enzyme (ME) [49].
Pharmacological inhibition of the tricarboxylate transporter in the mitochondrial outer membrane inhibits GSIS in INS-1 832/13 cells, indicating that the export of citrate from mitochondria to cytosol is crucial for downstream metabolic pathways [43]. MacDonald et al. [50] observed oscillations of citrate in the mitochondria of rat islets and INS-1 cells in the presence of pyruvate and in intact INS-1 cells exposed to glucose. These oscillations were synchronous with the oscillations of other mitochondrial factors, including ATP and NAD(P) [50]. These synchronous oscillations are believed to orchestrate the oscillations of glycolytic intermediates, especially fructose bisphosphate. This, in turn, could affect Ca2+oscillations, causing secretion of insulin in a pulsatile manner [51].
Suppression of Acl (also known as Acly) mRNA by 67% in INS-1 832/13 had a modest effect on the reduction of GSIS, accompanied by an increase in fatty acid oxidation [43]. However, it has also been reported that siRNA-mediated suppression of Acl mRNA by 80% did not affect GSIS in INS-1 cells [52]. Similarly, suppression of Acl mRNA by 90% and ACL activity by 95% had no impact on GSIS in the INS-1 832/13 cell line and in isolated rat islets [53].
It is particularly interesting that ACC production in islets is as highly abundant as in lipogenic tissues [54] and there is a great deal of evidence to indicate that malonyl-CoA can act as a coupling factor that regulates insulin secretion [55, 56]. Malonyl-CoA inhibits carnitine palmitoyl-transferase (CPT I), which transports fatty acyl-CoA into mitochondria where it is oxidised. Inhibition of CPT I should cause an increase in long-chain acyl-CoAs in the cytosol. Glucose rapidly induces ACC production, [54] with a concomitant rapid rise in malonyl-CoA concentration preceding insulin secretion. Furthermore, acute exposure of the permeabilised clonal beta cell line, HIT, to long-chain acyl-CoA also stimulates Ca2+-evoked insulin release [57]. Exogenous NEFAs also enhance GSIS in this cell line. This fatty-acid-potentiated GSIS was suggested to be mediated through the formation of long-chain acyl-CoAs [56]. Although malonyl-CoA and long-chain acyl-CoA are believed to regulate GSIS, abrogation of malonyl-CoA formation by overproducing malonyl-CoA decarboxylase in beta cells did not interfere with GSIS, [58] whereas chronic, but not acute, suppression of ACC production by 60–80% in INS-1 832/13 cells and isolated islets significantly reduced GSIS [59]. However, it is unclear whether the lower GSIS was primarily due to the suppression of ACC activity per se because glucose oxidation, glycolytic flux, glycogen synthesis, TCA cycle intermediates and ATP production were also decreased [59]. The role of long-chain acyl-CoA in GSIS is not well understood, although it is proposed to have a signalling role in regulating KATP channel and insulin exocytosis [56, 57]. A recent study has shown that suppression of FAS production has no impact on GSIS in INS-1 832/13 cells and in isolated islets [53]. This evidence may argue against the role of long-chain acyl-CoAs in controlling GSIS.
Although acute suppression of ACC1 and of FAS by siRNA does not affect GSIS, pharmacological inhibition of ACC1 and FAS markedly reduced GSIS in the INS-1 832/13 cells [52]. de novo fatty acid synthesis occurs rapidly in INS-1 832/13 cells upon stimulation with a high glucose concentration [52]. Glucose stimulation also altered membrane lipid composition including cholesteryl esters, phospholipids, NEFAs and triacylglycerols. Modification of the lipid species could alter the structure of the plasma membrane and membrane-bound vesicles which, in turn, could affect insulin exocytosis [52]. Apart from rapid lipogenesis and alteration of lipid content in INS-1 832/13 cells, the export of rapidly produced short-chain acyl-CoAs as acetoacetate from mitochondria to cytoplasm was also observed in beta cells upon stimulation by glucose [60]. The acetyl group derived from acetoacetate might be more important for GSIS than that derived from ACL, because suppression of acetoacetyl-CoA synthetase, a cytosolic enzyme that converts acetoacetate to acetoacetyl-CoA, markedly reduced GSIS [60]. In contrast, suppression of ACL, which potentially reduces citrate-derived acetyl-CoA, does not inhibit GSIS [60]. It appears that the supply of acetyl-CoA via the transfer of acyl groups from mitochondria in the form of acetoacetate is important for de novo fatty acid synthesis, and alterations of lipid content and lipid species may influence insulin secretion in beta cells [52].
Pyruvate/isocitrate (pyruvate/α-ketoglutarate) shuttle
In this shuttle, oxaloacetate is condensed with acetyl-CoA to form citrate by citrate synthase before being converted to isocitrate. Isocitrate then exits the mitochondrion to the cytoplasm via the citrate/isocitrate transporter and is converted to α-ketoglutarate by the cytosolic NADP-dependent isocitrate dehydrogenase (cICD). α-Ketoglutarate is further converted to oxaloacetate via the malate/aspartate shuttle as mentioned earlier in the NADH shuttle system. Similar to the two previous cycles, the cICD reaction of this pathway produces NADPH (Fig. 2). Two lines of evidence demonstrate the necessity of this pathway. First, siRNA-mediated suppression of the citrate/isocitrate transporter results in a reduction of citrate accumulation in the cytoplasm, concomitant with a modest reduction of GSIS in INS-1 832/13 cells and in isolated rat islets [61]. This is also accompanied by only a 25% reduction in the NADPH:NADP+ ratio [61]. Similarly, suppression of the cICD results in a 50% reduction in GSIS in INS-1 832/13 cells and in isolated rat islets [62]. This reduction in GSIS is accompanied by only a slightly decreased NADPH:NADP+ ratio [62].
TCA cycle and GTP production
SCS catalyses the conversion of succinyl-CoA to succinate coupled with the formation of GTP or ATP, depending on the enzyme isoforms [63]. The importance of mitochondrial GTP in insulin secretion was demonstrated by the fact that patients who carry a mutation in the inhibitory GTP-binding domain of glutamate dehydrogenase have hyperinsulinaemia and hypoglycaemia [64]. Silencing of the GTP-SCS in the INS-1 832/13 cells or in isolated rat islets impaired GSIS by 50% accompanied by an impaired glucose-stimulated increase in the level of cytosolic Ca2+ which, in turn, inhibits insulin secretion [63]. This supports the idea that mitochondrial GTP may be one of the coupling factors that regulates GSIS [63].
It appears that the TCA cycle is not the only source of GTP for GSIS. This is supported by the recent finding that the phosphoenolpyruvate (PEP) cycle is associated with the GTP-SCS-catalysed reaction [65]. This cycle involves the mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) which, in islets and insulinoma cell lines, is about 50% of the level in liver. Unlike the cytosolic PEPCK (PEPCK-C), which is absent from pancreatic islets [65, 66], PEPCK-M catalyses the GTP-driven conversion of oxaloacetate to PEP. Using 13C NMR analysis, it was shown that 30% of cytosolic PEP is associated with PEPCK-M activity [65]. Because mitochondrial GTP is only available from GTP-SCS activity, this provides the link between GTP-SCS and PEPCK-M.
Glutamate dehydrogenase and glutamate production
The evidence that glutamate may serve as a second messenger to amplify insulin secretion comes from identifying a gain-of-function mutation in the gene encoding glutamate dehydrogenase (GDH), an enzyme that catalyses conversion of α-ketoglutarate to glutamate. Mutations in the inhibitory GTP-binding domain cause a hyperinsulinaemia/hypoglycaemia syndrome, suggesting the involvement of GDH in insulin secretion [64]. Maechler et al. [67] have suggested that intracellular glutamate produced from α-ketoglutarate by GDH is a second messenger that triggers insulin secretion. When permeabilised INS-1 cells, perfused with an elevated fixed concentration of Ca2+ and ATP, were exposed to a high concentration of glucose, insulin secretion was stimulated, accompanied by an increased intracellular glutamate concentration [67]. Glutamate is thought to enter insulin secretory vesicles and to promote exocytosis of the vesicles [67]. Evidence favouring this hypothesis includes: (1) overproduction in INS-1E cells or in rat pancreatic islets of glutamate decarboxylase, an enzyme that decarboxylates glutamate in the cytoplasm, led to a reduced cellular glutamate content that correlated with the impaired GSIS [68]; (2) overproduction of GDH in INS-1E cells and in rat and mouse pancreatic islets increased GSIS at high glucose concentrations, suggesting GDH is a rate-limiting step in GSIS [69].
Although the above data support glutamate being an important coupling factor to enhance insulin secretion, there are several issues regarding its importance [70–72]. Experiments performed elsewhere did not find an increase in cellular glutamate content upon GSIS in either INS-1 cells or isolated rat islets [70]. Although treating these cells with high concentrations of glutamine alone does increase the cellular content of glutamate (through glutaminase action), this does not stimulate insulin secretion [70]. When Bertrand et al. [72] used high glucose concentrations to stimulate insulin secretion, cellular glutamate levels increased, but did not correlate with the level of insulin secretion [72]. Glutamine markedly increased cellular glutamate content, but it did not augment insulin secretion. The combination of high glucose and glutamine enhanced insulin secretion without influencing cellular glutamate levels [72]. Contrary to the findings of Maechler et al. [67], overproduction of the gain-of-function GDH mutant in insulinoma MIN6 cells increased GSIS at low glucose concentrations (2 mmol/l and 5 mmol/l) without affecting insulin release at high glucose concentrations (8–25 mmol/l) [73]. Re-examination of the experiment by the same laboratory indicated that overproduction in MIN6 and rat islets of GDH indeed promoted conversion of glutamate to α-ketoglutarate, thereby increasing TCA cycle activity without increasing the level of glutamate [74]. A recent study has also suggested that GDH functions solely in glutamate oxidation rather than glutamate synthesis [75]. In addition, flux analysis by NMR of INS-1 cells stimulated by glucose did not support the idea that anaplerosis through GDH is associated with GSIS [76]. These experiments therefore argue against the idea that glutamate is a second messenger required for GSIS. A recent study involving mice with Gdh (also known as Glud1) null beta cells has clearly shown that these mice exhibited only a 37% loss of GSIS [77]. Similarly, silencing of the glutamate carrier GC1 of INS-1E cells caused only a 23% reduction in insulin secretion upon stimulation by a high glucose concentration (15 mmol/l) and had no effect at an intermediate glucose concentration (8 mmol/l) [78].
Downregulation of metabolic enzymes in diabetes
From the above evidence, various biochemical pathways have been shown to link with mitochondrial metabolism and to be required for normal GSIS in beta cells. In the Zucker fatty rat, which is hyperinsulinaemic but has normal blood glucose, the activities of PC, MDH and ME are increased to support the pyruvate cycling activity required to increase GSIS during this metabolic overload [37]. In humans with type 2 diabetes, islet mitochondria show large structural and biochemical changes. The biochemical changes include impaired GSIS and impaired hyperpolarisation of the mitochondrial membranes [79]. The mitochondrial enzymes that are significantly reduced in a mouse model that develops type 2 diabetes progressively include PC, cICD, NADH dehydrogenase, citrate synthase and ATP synthase [80]. Of particular interest, PC and cICD have previously been shown to be necessary for GSIS. More specifically, MacDonald et al. [39] compared the expression and activity of mitochondrial enzymes from the islets of normal and type 2 diabetic patients. Again, production or activities of PC and cICD were lower in individuals with type 2 diabetes. Other mitochondrial enzymes, also produced in low quantities, included mGPDH, NADH dehydrogenase and ACL [39]. Malmgren et al. [81] have shown a negative correlation of the expression of genes involved in mitochondrial metabolism and HbA1c, while the expression of genes for enzymes involved in glycolysis was positively correlated with HbA1c. This indicates that, in the absence of diabetes, a poorer glycaemic control is associated with downregulation of mitochondrial metabolic genes while those involved in glycolysis are upregulated.
Table 1 summarises the effect of suppression or overproduction of various mitochondrial enzymes on GSIS.
Table 1.
The effect of suppression or overproduction of mitochondrial enzymes on GSIS in beta cells
| Enzyme | Method | Beta cell type |
|||||
|---|---|---|---|---|---|---|---|
| INS-1 | INS-1E | INS-1 832/13 | r-islets | m-islets | Authors [reference no.] | ||
| PC | Silencing | – | – | Jensen et al. [36] | |||
| Silencing | ↓ | Hasan et al. [34] | |||||
| Silencing | ↓ | ↓ | Xu et al. [35] | ||||
| Overproduction | ↑ | Xu et al. [35] | |||||
| Enzyme inhibition | ↓ | Liu et al. [37] | |||||
| Enzyme inhibition | ↓ | Farfari et al. [40] | |||||
| PDH | Overproduction PDK4 | – | Xu et al. [35] | ||||
| mGPDH | KO | – | Eto et al. [19] | ||||
| KO | – | Brown et al. [26] | |||||
| cME | Silencing | ↓ | Pongratz et al. [42] | ||||
| Silencing | ↓ | Guay et al. [43] | |||||
| Silencing | – | Brown et al. [48] | |||||
| Silencing | ↓ | – | Ronnebaum et al. [44] | ||||
| Silencing | ↓ | Xu et al. [45] | |||||
| KO | – | Ronnebaum et al. [44] | |||||
| mME | Silencing | – | Pongratz et al. [42] | ||||
| Silencing | ↓ | – | Ronnebaum et al. [44] | ||||
| Silencing | – | Brown et al. [48] | |||||
| ACL | Silencing | ↓ | Guay et al. [43] | ||||
| Silencing | – | MacDonald et al. [60] | |||||
| Silencing | – | – | Joseph et al. [53] | ||||
| ACC1 | Silencing | ↓ | Ronnebaum et al. [59] | ||||
| Enzyme inhibition | ↓ | ↓ | MacDonald et al. [52] | ||||
| FAS | Silencing | – | – | Joseph et al. [53] | |||
| Enzyme inhibition | ↓ | ↓ | MacDonald et al. [52] | ||||
| ICD | Silencing | ↓ | ↓ | Ronnebaum et al. [62] | |||
| CIC | Silencing | ↓ | ↓ | Joseph et al. [61] | |||
| ATP-SCS | Silencing | – | – | Kibbey et al. [63] | |||
| GTP-SCS | Silencing | ↓ | ↓ | Kibbey et al. [63] | |||
| PEPCK-M | Silencing | ↓ | Stark et al. [65] | ||||
| GDH | Overproduction | ↑ | ↑ | ↑ | Carobbio et al. [69] | ||
| Gain of function mutant | – | Tanizawa et al. [73] | |||||
| Beta cell KO | Partial reduction | Carobbio et al. [77] | |||||
| GC1 | Silencing | Partial reduction | Casimir et al. [78] | ||||
↑ increased; ↓ decreased; – not changed; ATP-SCS, ATP-dependent succinyl-CoA synthetase; CIC, citrate/isocitrate carrier; GC1, glutamate carrier 1; ICD, NADP-dependent isocitrate dehydrogenase; KO, knockout
Reactive oxygen species and uncoupling protein
Exposure of pancreatic islets to a high concentration of glucose not only stimulates respiratory-chain activity but also stimulates production of reactive oxygen species (ROS). Beta cells are prone to the oxidative damage caused by production of ROS because they express limited levels of antioxidant enzymes [82]. Although ROS appear to have an adverse effect on beta cell function, there is evidence that ROS may be obligatory signals for GSIS [83, 84]. The production of ROS upon stimulation of islets with a high concentration of glucose appears transiently because ROS are rapidly removed by antioxidant enzymes [84]. The production of ROS during chronic exposure of beta cells to an elevated level of glucose also stimulates expression of the uncoupling protein 2 (UCP2) [85]. The role of UCP2 in controlling GSIS was thought to dissipate proton conductance and hence inhibit a rise in cytosolic ATP, blunting insulin secretion [85]. Although Ucp2 knockout mice showed a higher level of cellular ATP production and enhanced GSIS, [86] which tends to support the above idea, two independent studies reported that Ucp2-ablated fully back-crossed mice failed to show improved GSIS [87, 88]. It appears that the role of UCP2 in regulation of GSIS is still unclear.
Novel coupling factors
Although the discussion above suggests many mitochondrial biochemical pathways produce molecules that can act as the coupling factors to amplify insulin secretion, a prominent candidate is the NADPH produced by pyruvate cycling. Previous work [89] has shown that glucose acutely stimulates a sharp increase in the NADPH:NADP+ ratio concomitant with insulin secretion. This is mediated by glutaredoxin 1 (GRX1). GRX1, together with thioredoxin 1 (TRX1) and glutathione (GS), form extramitochondrial redox pairs to maintain an appropriate cellular redox within the cell (Fig. 3). These redox pairs require NADPH to cycle their oxidised/reduced states. Injection of additional GRX1 into beta cells stimulates GSIS in a manner similar to that by NADPH [90]. Silencing of GRX1 but not TRX1 in INS-1 832/13 cells or rat islets dramatically reduces GSIS without affecting the global cellular redox state, suggesting GRX1 acts to support insulin secretion locally [90]. Overproduction of GRX1 in INS-1 832/13 cells and isolated rat islets also stimulates insulin exocytosis [90].
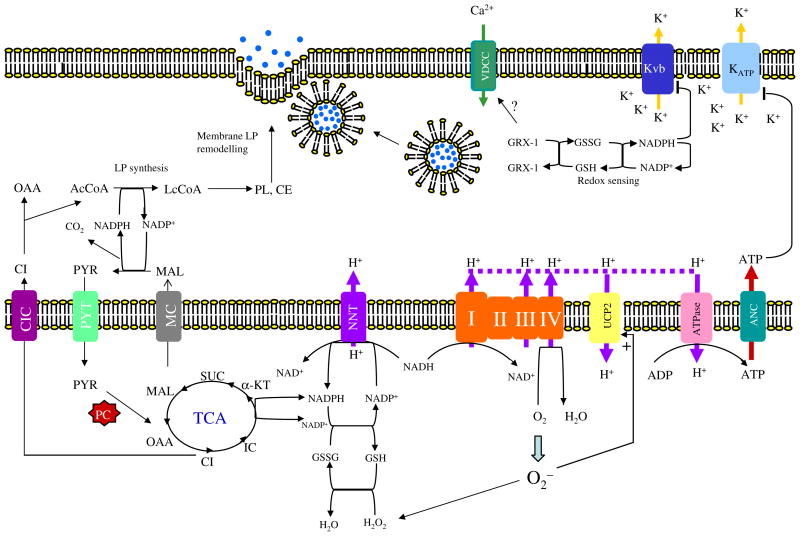
Fig. 3.
The role of lipogenesis and NADPH in insulin exocytosis. The NADPH produced from the pyruvate cycle is used for de novo fatty acid synthesis in pancreatic β-cells. This rapid lipogenesis alters cellular lipid composition, especially cholesteryl ester (CE) and phospholipids (PL) [52]. Modification of cholesterol content of the plasma membrane at the SNARE protein complex can potentiate insulin secretion [97]. The NADPH is also required for maintaining the cytoplasmic redox state through glutaredoxin GRX1, which acts as the molecular redox sensor [90]. The NADPH also regulates intracellular K+ by binding to its β-subunit (Kvβ), inhibiting K+ efflux through this channel and hence causing prolonged depolarisation of the intracellular membrane potential. The NADPH produced by NNT transhydrogenase is also required to detoxify ROS produced by respiratory chain activity [94]. The accumulation of ROS stimulates UCP2 activity, dissipating ATP production required to close the KATP channel. NADPH may also be transported to cytoplasm through the conversion of α-ketoglutarate to isocitrate catalysed by the mitochondrial NADP-dependent isocitrate dehydrogenase. NADPH can be reformed in the cytoplasm by cytosolic ME that converts the isocitrate-derived malate to pyruvate in the cytoplasm. ANC, adenosine nucleotide carrier; CE, cholesteryl ester; CI, citrate; IC, isocitrate; CIC, citrate/isocitrate carrier; GSSH, oxidised glutathione; GSH, reduced glutathione; α-KT, α-ketoglutarate; LP, lipid; MAL, malate; MC, malate carrier; OAA, oxaloacetate; PL, phospholipid; PYR, pyruvate; PYT, pyruvate transporter; SUC, succinate; VDCC, voltage-gate-dependent calcium channel
NADPH is not only required for insulin granule exocytosis via the GRX1 redox pair in the cytoplasm but is also required to maintain the redox state in mitochondria. In mitochondria, NADPH is formed by reduction of NADP+ by NADH, catalysed by nicotinamide nucleotide transhydrogenase (NNT) [91] (Fig. 3). This is a reversible reaction and also a redox-driven proton pump. In beta cell mitochondria, ROS produced during oxidative phosphorylation are detoxified by the glutathione redox system, which requires NADPH to maintain its redox state [92]. Silencing of Nnt in MIN6 cells results in impaired GSIS [92]. Increased NNT production in mice also predisposes to insulin hypersecretion [93]. However, it is not known whether NADPH produced by NNT is present just to detoxify ROS because NADPH may be exported to the cytoplasm as isocitrate via the reaction catalysed by mitochondrial NADP-dependent isocitrate dehydrogenase (Fig. 3). NADPH can then be regenerated via the cICD-catalysed reaction, and participate in the NADPH-dependent insulin exocytosis.
NADPH has also been reported to be associated with the redox state of the voltage-dependent potassium channel (Kv). This channel works in the opposite way to the KATP-sensitive channel. It serves as the outward rectifying channel allowing K+ efflux and thus causing membrane repolarisation for the next cycle of GSIS [94]. Kv is composed of two subunits, the α-subunit (Kvα) forms a pore structure, while the β-subunit functions as the regulatory subunit [94]. The β-subunit (Kvβ1.1) exhibits the oxidoreductase activity and contains the NADPH-binding site, which is thought to be the sensor of intracellular redox potential that in turn regulates the channel [95]. Binding of NADPH to the β-subunit reduces the efficacy of this channel in repolarisation of the membrane potential. This inhibits K+ influx, and therefore favours K+ efflux through the KATP-sensitive channel during GSIS [96]. This mode of action is believed to be modulated by the cellular NADPH:NADP+ ratio [96].
The alteration of lipid content as the result of anaplerosis [52] may also serve as a long-term signal to promote insulin exocytosis. Vikman et al. [97] have reported that the cholesterol content of the SNARE protein complex is crucial for insulin exocytosis. The SNARE protein complex is composed of synaptobrevin, syntaxin 1 and SNAP25 [98]. The synaptobrevin is located on the insulin-containing vesicles while syntaxin 1 and SNAP25 are located on the plasma membrane. Formation of the SNARE complex is associated with cholesterol in the lipid bilayer. Depletion of the cholesterol content of the SNAP25 complex markedly affects insulin granule exocytosis accompanied by the altered dynamic of insulin release and reduced GSIS [97]. In addition, there is a great deal of evidence showing that reorganisation of phospholipids, especially the phosphatidyl-inositol phosphate, in the plasma membrane can affect insulin granule exocytosis [99]. The alteration of cholesteryl esters and phospholipid, especially the phosphatidyl-inositol, in INS-1 832/13 cells during GSIS [52] provides the link between lipogenesis and insulin exocytosis.
Conclusion
In conclusion, a unique pattern of enzymes is produced in the pancreatic beta cell that permits the synthesis by mitochondria of metabolites that are exported to the cytosol to support insulin secretion. A major player in this process is PC, an anaplerotic enzyme that enables the beta cells to convert half of glucose-derived pyruvate into metabolites that participate in shuttles or cycles that provide intracellular messengers including NADPH, GTP, precursors of short-chain acyl-CoAs, lipids and amino acids which support KATP-independent insulin secretion that may be equal in importance to ATP production by mitochondria for insulin secretion. Lowering of the activity of PC and other enzymes of these pathways inhibits secretagogue-stimulated insulin secretion and the levels of many of these enzymes are decreased in the islets of rodents and humans with type 2 diabetes, reflecting their critical involvement in insulin secretion. Research to further understand the roles of these pathways may provide strategies for future therapies of type 2 diabetes.
Acknowledgments
Research in S. Jitrapakdee’s laboratory is supported by the Thailand Research Fund and the Faculty of Science, Mahidol University. Research in the laboratory of M. J. MacDonald is supported by NIH Grant DK28348. The authors apologise in advance for any inadvertent oversight in the selection of relevant work due to a limitation of space.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- ACL
ATP-citrate lyase
- cGPDH
Cytosolic glycerol-3-phosphate dehydrogenase
- cICD
Cytosolic NADP-dependent isocitrate dehydrogenase
- CPT I
Carnitine palmitoyl-transferase I
- FAS
Fatty acid synthase
- GDC
Glutamate decarboxylase
- GDH
Glutamate dehydrogenase
- GLUT1
Solute carrier family 2 (facilitated glucose transporter), member 1
- GLUT2
Solute carrier family 2 (facilitated glucose transporter), member 2
- GRX1
Glutaredoxin 1
- GSIS
Glucose-stimulated insulin secretion
- GTP-SCS
GTP-isoformed succinyl-CoA synthetase
- KATP
ATP-sensitive potassium channel
- LDH
Lactate dehydrogenase
- MDH
Malate dehydrogenase
- ME
Malic enzyme
- ME1
Cytosolic malic enzyme
- ME2
Mitochondrial malic enzyme
- mGPDH
Mitochondrial glycerol-3-phosphate dehydrogenase
- NMR
Nuclear magnetic resonance
- NNT
Nicotinamide nucleotide transhydrogenase
- PC
Pyruvate carboxylase
- PDH
Pyruvate dehydrogenase
- PEP
Phosphoenolpyruvate
- PEPCK
Phosphoenolpyruvate carboxykinase
- PEPCK-M
Mitochondrial PEPCK
- PEPCK-C
Cytosolic PEPCK
- ROS
Reactive oxygen species
- siRNA
Small interfering RNA
- SUR1
Sulfonylurea receptor 1
- TCA
Tricarboxylic acid
- TRX1
Thioredoxin 1
- UCP2
Uncoupling protein 2
Glossary
- Anaplerosis
a biochemical reaction which replenishes TCA cycle intermediates when they are removed for biosynthetic purposes. This permits the TCA cycle to operate without disruption
- Cataplerosis
a biochemical reaction in which the TCA cycle intermediates are removed for biosynthetic purposes
- Glucose-stimulated insulin secretion (GSIS)
secretion of insulin from beta cells in response to elevated glucose concentration
- Pyruvate cycling
a pathway by which the intracellular pyruvate is converted via pyruvate carboxylation into TCA cycle intermediates which are re-converted to pyruvate in the cytosol to participate in metabolite shuttles and cycles
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contributor Information
S. Jitrapakdee, Email: scsji@mahidol.ac.th, Molecular Metabolism Research Group, Department of Biochemistry, Faculty of Science, Mahidol University, Rama 6 Road, Phya-Thai, Bangkok 10400, Thailand
A. Wutthisathapornchai, Molecular Metabolism Research Group, Department of Biochemistry, Faculty of Science, Mahidol University, Rama 6 Road, Phya-Thai, Bangkok 10400, Thailand
J. C. Wallace, School of Molecular and Biomedical Science, University of Adelaide, Adelaide, SA, Australia
M. J. MacDonald, Department of Pediatrics, Children’s Diabetes Center, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA
References
- 1.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 2.Straub SG, Sharp GW. Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. Am J Physiol Cell Physiol. 2004;287:C565–571. doi: 10.1152/ajpcell.00079.2004. [DOI] [PubMed] [Google Scholar]
- 3.Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990;39:647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald MJ, Kaysen JH, Moran SM, Pomije CE. Pyruvate dehydrogenase and pyruvate carboxylase. Sites of pretranslational regulation by glucose of glucose-induced insulin release in pancreatic islets. J Biol Chem. 1991;266:22392–22397. [PubMed] [Google Scholar]
- 5.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 6.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275:9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 8.Shiota C, Larsson O, Shelton KD, et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277:37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- 9.Miki T, Nagashima K, Tashiro F, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remedi MS, Rocheleau JV, Tong A, et al. Hyperinsulinism in mice with heterozygous loss of K(ATP) channels. Diabetologia. 2006;49:2368–2378. doi: 10.1007/s00125-006-0367-4. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy ED, Maechler P, Wollheim CB. Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes. 1998;47:374–380. doi: 10.2337/diabetes.47.3.374. [DOI] [PubMed] [Google Scholar]
- 12.Tsuruzoe K, Araki E, Furukawa N, et al. Creation and characterization of a mitochondrial DNA-depleted pancreatic beta-cell line: impaired insulin secretion induced by glucose, leucine, and sulfonylureas. Diabetes. 1998;47:621–631. doi: 10.2337/diabetes.47.4.621. [DOI] [PubMed] [Google Scholar]
- 13.Noda M, Yamashita S, Takahashi N, et al. Switch to anaerobic glucose metabolism with NADH accumulation in the beta-cell model of mitochondrial diabetes. Characteristics of betaHC9 cells deficient in mitochondrial DNA transcription. J Biol Chem. 2002;277:41817–41826. doi: 10.1074/jbc.M207690200. [DOI] [PubMed] [Google Scholar]
- 14.Maassen JA, t Hart LM, Janssen GM, Reiling E, Romijn JA, Lemkes HH. Mitochondrial diabetes and its lessons for common type 2 diabetes. Biochem Soc Trans. 2006;34:819–823. doi: 10.1042/BST0340819. [DOI] [PubMed] [Google Scholar]
- 15.Silva JP, Kohler M, Graff C, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000;26:336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier BR, Wiederkehr A, Baquie M, et al. PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab. 2009;10:110–118. doi: 10.1016/j.cmet.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine N, Cirulli V, Regazzi R, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 18.MacDonald MJ. High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J Biol Chem. 1981;256:8287–8290. [PubMed] [Google Scholar]
- 19.Eto K, Tsubamoto Y, Terauchi Y, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald MJ. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990;39:1461–1466. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald MJ, Warner TF, Pellett JR. Increased mitochondrial glycerol phosphate dehydrogenase activity in insulinomas of two hypoglycemic infants. J Clin Endocrinol Metab. 1983;57:662–664. doi: 10.1210/jcem-57-3-662. [DOI] [PubMed] [Google Scholar]
- 22.Rasschaert J, Malaisse-Lagae F, Sener A, Leclercq-Meyer V, Herberg L, Malaisse WJ. Impaired FAD-glycerophosphate dehydrogenase activity in islet and liver homogenates of fa/fa rats. Mol Cell Biochem. 1994;135:137–141. doi: 10.1007/BF00926516. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald MJ, Tang J, Polonsky KS. Low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of Zucker diabetic fatty rats. Diabetes. 1996;45:1626–1630. doi: 10.2337/diab.45.11.1626. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Alvarez J, Conget I, Rasschaert J, Sener A, Gomis R, Malaisse WJ. Enzymatic, metabolic and secretory patterns in human islets of type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1994;37:177–181. doi: 10.1007/s001250050090. [DOI] [PubMed] [Google Scholar]
- 25.Eto K, Suga S, Wakui M, et al. NADH shuttle system regulates K(ATP) channel-dependent pathway and steps distal to cytosolic Ca(2+) concentration elevation in glucose-induced insulin secretion. J Biol Chem. 1999;274:25386–25392. doi: 10.1074/jbc.274.36.25386. [DOI] [PubMed] [Google Scholar]
- 26.Brown LJ, Koza RA, Everett C, et al. Normal thyroid thermogenesis but reduced viability and adiposity in mice lacking the mitochondrial glycerol phosphate dehydrogenase. J Biol Chem. 2002;277:32892–32898. doi: 10.1074/jbc.M202408200. [DOI] [PubMed] [Google Scholar]
- 27.Rubi B, del Arco A, Bartley C, Satrustegui J, Maechler P. The malate-aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells. J Biol Chem. 2004;279:55659–55666. doi: 10.1074/jbc.M409303200. [DOI] [PubMed] [Google Scholar]
- 28.Zhao C, Wilson MC, Schuit F, Halestrap AP, Rutter GA. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes. 2001;50:361–366. doi: 10.2337/diabetes.50.2.361. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald MJ. Metabolism of the insulin secretagogue methyl succinate by pancreatic islets. Arch Biochem Biophys. 1993;300:201–205. doi: 10.1006/abbi.1993.1028. [DOI] [PubMed] [Google Scholar]
- 30.Schuit F, de Vos A, Farfari S, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald MJ. Estimates of glycolysis, pyruvate (de) carboxylation, pentose phosphate pathway, and methyl succinate metabolism in incapacitated pancreatic islets. Arch Biochem Biophys. 1993;305:205–214. doi: 10.1006/abbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 32.Lu D, Mulder H, Zhao P, et al. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc Natl Acad Sci U S A. 2002;99:2708–2713. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald MJ. Influence of glucose on pyruvate carboxylase expression in pancreatic islets. Arch Biochem Biophys. 1995;319:128–132. doi: 10.1006/abbi.1995.1274. [DOI] [PubMed] [Google Scholar]
- 34.Hasan NM, Longacre MJ, Stoker SW, et al. Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J Biol Chem. 2008;283:28048–28059. doi: 10.1074/jbc.M804170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Han J, Long YS, Epstein PN, Liu YQ. The role of pyruvate carboxylase in insulin secretion and proliferation in rat pancreatic beta cells. Diabetologia. 2008;51:2022–2030. doi: 10.1007/s00125-008-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen MV, Joseph JW, Ilkayeva O, et al. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem. 2006;281:22342–22351. doi: 10.1074/jbc.M604350200. [DOI] [PubMed] [Google Scholar]
- 37.Liu YQ, Jetton TL, Leahy JL. beta-Cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002;277:39163–39168. doi: 10.1074/jbc.M207157200. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald MJ, Efendic S, Ostenson CG. Normalization by insulin treatment of low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of the GK rat. Diabetes. 1996;45:886–890. doi: 10.2337/diab.45.7.886. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald MJ, Longacre MJ, Langberg EC, et al. Decreased levels of metabolic enzymes in pancreatic islets of patients with type 2 diabetes. Diabetologia. 2009;52:1087–1091. doi: 10.1007/s00125-009-1319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270:20051–20058. [PubMed] [Google Scholar]
- 42.Pongratz RL, Kibbey RG, Shulman GI, Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem. 2007;282:200–207. doi: 10.1074/jbc.M602954200. [DOI] [PubMed] [Google Scholar]
- 43.Guay C, Madiraju SR, Aumais A, Joly E, Prentki M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem. 2007;282:35657–35665. doi: 10.1074/jbc.M707294200. [DOI] [PubMed] [Google Scholar]
- 44.Ronnebaum SM, Jensen MV, Hohmeier HE, et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem. 2008;283:28909–28917. doi: 10.1074/jbc.M804665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Han J, Long YS, et al. Malic enzyme is present in mouse islets and modulates insulin secretion. Diabetologia. 2008;51:2281–2289. doi: 10.1007/s00125-008-1155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heart E, Cline GW, Collis LP, Pongratz RL, Gray JP, Smith PJ. Role for malic enzyme, pyruvate carboxylation, and mitochondrial malate import in glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2009;296:E1354–1362. doi: 10.1152/ajpendo.90836.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacDonald MJ, Marshall LK. Survey of normal appearing mouse strain which lacks malic enzyme and Nad+-linked glycerol phosphate dehydrogenase: normal pancreatic beta cell function, but abnormal metabolite pattern in skeletal muscle. Mol Cell Biochem. 2001;220:117–125. doi: 10.1023/a:1010821821921. [DOI] [PubMed] [Google Scholar]
- 48.Brown LJLM, Hasan NM, Kendrick MA, Stoker SW, MacDonald MJ. Chronic reduction of the cytosolic or NAD(P)-mitochondrial malic enzymes does not affect insulin secretion in a rat insulinoma cell line. J Biol Chem. 2009;284:35359–35367. doi: 10.1074/jbc.M109.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roche E, Farfari S, Witters LA, et al. Long-term exposure of beta-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes. 1998;47:1086–1094. doi: 10.2337/diabetes.47.7.1086. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald MJ, Fahien LA, Buss JD, Hasan NM, Fallon MJ, Kendrick MA. Citrate oscillates in liver and pancreatic beta cell mitochondria and in INS-1 insulinoma cells. J Biol Chem. 2003;278:51894–51900. doi: 10.1074/jbc.M309038200. [DOI] [PubMed] [Google Scholar]
- 51.Deeney JT, Kohler M, Kubik K, et al. Glucose-induced metabolic oscillations parallel those of Ca(2+) and insulin release in clonal insulin-secreting cells. A multiwell approach to oscillatory cell behavior. J Biol Chem. 2001;276:36946–36950. doi: 10.1074/jbc.M105056200. [DOI] [PubMed] [Google Scholar]
- 52.MacDonald MJ, Dobrzyn A, Ntambi J, Stoker SW. The role of rapid lipogenesis in insulin secretion: Insulin secretagogues acutely alter lipid composition of INS-1 832/13 cells. Arch Biochem Biophys. 2008;470:153–162. doi: 10.1016/j.abb.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph JW, Odegaard ML, Ronnebaum SM, et al. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem. 2007;282:31592–31600. doi: 10.1074/jbc.M706080200. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, Kim KH. Glucose activation of acetyl-CoA carboxylase in association with insulin secretion in a pancreatic beta-cell line. J Endocrinol. 1995;147:33–41. doi: 10.1677/joe.0.1470033. [DOI] [PubMed] [Google Scholar]
- 55.Corkey BE, Glennon MC, Chen KS, Deeney JT, Matschinsky FM, Prentki M. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J Biol Chem. 1989;264:21608–21612. [PubMed] [Google Scholar]
- 56.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem. 1992;267:5802–5810. [PubMed] [Google Scholar]
- 57.Deeney JT, Gromada J, Hoy M, et al. Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting cells (HIT T-15 and NMRI beta-cells) J Biol Chem. 2000;275:9363–9368. doi: 10.1074/jbc.275.13.9363. [DOI] [PubMed] [Google Scholar]
- 58.Mulder H, Lu D, Finley J, 4th, et al. Overexpression of a modified human malonyl-CoA decarboxylase blocks the glucose-induced increase in malonyl-CoA level but has no impact on insulin secretion in INS-1-derived (832/13) beta-cells. J Biol Chem. 2001;276:6479–6484. doi: 10.1074/jbc.M010364200. [DOI] [PubMed] [Google Scholar]
- 59.Ronnebaum SM, Joseph JW, Ilkayeva O, et al. Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J Biol Chem. 2008;283:14248–14256. doi: 10.1074/jbc.M800119200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacDonald MJ, Smith AD, 3rd, Hasan NM, Sabat G, Fahien LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem. 2007;282:30596–30606. doi: 10.1074/jbc.M702732200. [DOI] [PubMed] [Google Scholar]
- 61.Joseph JW, Jensen MV, Ilkayeva O, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006;281:35624–35632. doi: 10.1074/jbc.M602606200. [DOI] [PubMed] [Google Scholar]
- 62.Ronnebaum SM, Ilkayeva O, Burgess SC, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281:30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 63.Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5:253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacMullen C, Fang J, Hsu BY, et al. Hyperinsulinism/hyperammonemia syndrome in children with regulatory mutations in the inhibitory guanosine triphosphate-binding domain of glutamate dehydrogenase. J Clin Endocrinol Metab. 2001;86:1782–1787. doi: 10.1210/jcem.86.4.7414. [DOI] [PubMed] [Google Scholar]
- 65.Stark R, Pasquel F, Turcu A, et al. Phosphoenolpyruvate cycling via mitochondrial pepck links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem. 2009;284:26578–26590. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacDonald MJ, Chang CM. Do pancreatic islets contain significant amounts of phosphoenolpyruvate carboxykinase or ferroactivator activity? Diabetes. 1985;34:246–250. doi: 10.2337/diab.34.3.246. [DOI] [PubMed] [Google Scholar]
- 67.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 68.Rubi B, Ishihara H, Hegardt FG, Wollheim CB, Maechler P. GAD65-mediated glutamate decarboxylation reduces glucose-stimulated insulin secretion in pancreatic beta cells. J Biol Chem. 2001;276:36391–36396. doi: 10.1074/jbc.M104999200. [DOI] [PubMed] [Google Scholar]
- 69.Carobbio S, Ishihara H, Fernandez-Pascual S, Bartley C, Martin-Del-Rio R, Maechler P. Insulin secretion profiles are modified by overexpression of glutamate dehydrogenase in pancreatic islets. Diabetologia. 2004;47:266–276. doi: 10.1007/s00125-003-1306-2. [DOI] [PubMed] [Google Scholar]
- 70.MacDonald MJ, Fahien LA. Glutamate is not a messenger in insulin secretion. J Biol Chem. 2000;275:34025–34027. doi: 10.1074/jbc.C000411200. [DOI] [PubMed] [Google Scholar]
- 71.Yamada S, Komatsu M, Sato Y, Yamauchi K, Aizawa T, Hashizume K. Glutamate is not a major conveyer of ATP-sensitive K+ channel-independent glucose action in pancreatic islet beta cell. Endocr J. 2001;48:391–395. doi: 10.1507/endocrj.48.391. [DOI] [PubMed] [Google Scholar]
- 72.Bertrand G, Ishiyama N, Nenquin M, Ravier MA, Henquin JC. The elevation of glutamate content and the amplification of insulin secretion in glucose-stimulated pancreatic islets are not causally related. J Biol Chem. 2002;277:32883–32891. doi: 10.1074/jbc.M205326200. [DOI] [PubMed] [Google Scholar]
- 73.Tanizawa Y, Nakai K, Sasaki T, et al. Unregulated elevation of glutamate dehydrogenase activity induces glutamine-stimulated insulin secretion: identification and characterization of a GLUD1 gene mutation and insulin secretion studies with MIN6 cells overexpressing the mutant glutamate dehydrogenase. Diabetes. 2002;51:712–717. doi: 10.2337/diabetes.51.3.712. [DOI] [PubMed] [Google Scholar]
- 74.Anno T, Uehara S, Katagiri H, et al. Overexpression of constitutively activated glutamate dehydrogenase induces insulin secretion through enhanced glutamate oxidation. Am J Physiol Endocrinol Metab. 2004;286:E280–285. doi: 10.1152/ajpendo.00380.2003. [DOI] [PubMed] [Google Scholar]
- 75.Li C, Matter A, Kelly A, et al. Effects of a GTP-insensitive mutation of glutamate dehydrogenase on insulin secretion in transgenic mice. J Biol Chem. 2006;281:15064–15072. doi: 10.1074/jbc.M600994200. [DOI] [PubMed] [Google Scholar]
- 76.Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem. 2004;279:44370–44375. doi: 10.1074/jbc.M311842200. [DOI] [PubMed] [Google Scholar]
- 77.Carobbio S, Frigerio F, Rubi B, et al. Deletion of glutamate dehydrogenase in beta-cells abolishes part of the insulin secretory response not required for glucose homeostasis. J Biol Chem. 2009;284:921–929. doi: 10.1074/jbc.M806295200. [DOI] [PubMed] [Google Scholar]
- 78.Casimir M, Lasorsa FM, Rubi B, et al. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J Biol Chem. 2009;284:25004–25014. doi: 10.1074/jbc.M109.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anello M, Lupi R, Spampinato D, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48:282–289. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 80.Lu H, Yang Y, Allister EM, Wijesekara N, Wheeler MB. The identification of potential factors associated with the development of type 2 diabetes: a quantitative proteomics approach. Mol Cell Proteomics. 2008;7:1434–1451. doi: 10.1074/mcp.M700478-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malmgren S, Nicholls DG, Taneera J, et al. Tight coupling between glucose and mitochondrial metabolism in clonal beta-cells is required for robust insulin secretion. J Biol Chem. 2009;284:32395–32404. doi: 10.1074/jbc.M109.026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 83.Leloup C, Tourrel-Cuzin C, Magnan C, et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pi J, Bai Y, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 85.Krauss S, Zhang CY, Scorrano L, et al. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 87.Pi J, Bai Y, Daniel KW, et al. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology. 2009;150:3040–3048. doi: 10.1210/en.2008-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parker N, Vidal-Puig AJ, Azzu V, Brand MD. Dysregulation of glucose homeostasis in nicotinamide nucleotide transhydrogenase knockout mice is independent of uncoupling protein 2. Biochim Biophys Acta. 2009;1787:1451–1457. doi: 10.1016/j.bbabio.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ivarsson R, Quintens R, Dejonghe S, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 90.Reinbothe TM, Ivarsson R, Li DQ, et al. Glutaredoxin-1 mediates NADPH-dependent stimulation of calcium-dependent insulin secretion. Mol Endocrinol. 2009;23:893–900. doi: 10.1210/me.2008-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jackson JB. Proton translocation by transhydrogenase. FEBS Lett. 2003;555:176–177. doi: 10.1016/s0014-5793(03)01123-2. [DOI] [PubMed] [Google Scholar]
- 92.Freeman H, Shimomura K, Horner E, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a key role in insulin secretion. Cell Metab. 2006;3:35–45. doi: 10.1016/j.cmet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Aston-Mourney K, Wong N, Kebede M, et al. Increased nicotinamide nucleotide transhydrogenase levels predispose to insulin hypersecretion in a mouse strain susceptible to diabetes. Diabetologia. 2007;50:2476–2485. doi: 10.1007/s00125-007-0814-x. [DOI] [PubMed] [Google Scholar]
- 94.MacDonald PE, Wheeler MB. Voltage-dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia. 2003;46:1046–1062. doi: 10.1007/s00125-003-1159-8. [DOI] [PubMed] [Google Scholar]
- 95.McCormack T, McCormack K. Shaker K+ channel beta subunits belong to an NAD(P)H-dependent oxidoreductase superfamily. Cell. 1994;79:1133–1135. doi: 10.1016/0092-8674(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 96.Tipparaju SM, Liu SQ, Barski OA, Bhatnagar A. NADPH binding to beta-subunit regulates inactivation of voltage-gated K (+) channels. Biochem Biophys Res Commun. 2007;359:269–276. doi: 10.1016/j.bbrc.2007.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vikman J, Jimenez-Feltstrom J, Nyman P, Thelin J, Eliasson L. Insulin secretion is highly sensitive to desorption of plasma membrane cholesterol. FASEB J. 2009;23:58–67. doi: 10.1096/fj.08-105734. [DOI] [PubMed] [Google Scholar]
- 98.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis—roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berggren PO, Barker CJ. A key role for phosphorylated inositol compounds in pancreatic beta-cell stimulus-secretion coupling. Adv Enzyme Regul. 2008;48:276–294. doi: 10.1016/j.advenzreg.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J. 2008;415:1–10. doi: 10.1042/BJ20081029. [DOI] [PubMed] [Google Scholar]
- 101.Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]