Abstract
Purpose: The purpose of this study was to evaluate the age- and race-dependence of the breast fibroglandular tissue density based on three-dimensional breast MRI.
Methods: The normal breasts of 321 consecutive patients including Caucasians, Asians, and Hispanics were studied. The subjects were separated into three age groups: Younger than 45, between 45 and 55, and older than 55. Computer algorithms based on body landmarks were used to segment the breast, and fuzzy c-means algorithm was used to segment the fibroglandular tissue. Linear regression analysis was applied to compare mean differences among different age groups and race∕ethnicity groups. The obtained parameters were not normally distributed, and the transformed data, natural log (ln) for the fibroglandular tissue volume, and the square root for the percent density were used for statistical analysis.
Results: On the average, the transformed fibroglandular tissue volume and percent density decreased significantly with age. Racial differences in mean transformed percent density were found among women older than 45, but not among women younger than 45. Mean percent density was higher in Asians compared to Caucasians and Hispanics; the difference remained significant after adjustment for age, but not significant after adjusted for both age and breast volume. There was no significant difference in the density between the Caucasians and the Hispanics.
Conclusions: The results analyzed using the MRI-based method show age- and race-dependence, which is consistent with literature using mammography-based methods.
Keywords: three-dimensional MRI, breast segmentation, fibroglandular tissue segmentation, fuzzy C-means, quantitative breast density
INTRODUCTION
The measurement of breast density based on mammography suffers from several problems, and results may vary with different degrees of compression, positioning, and x-ray calibration. A recent review article by Kopans1 raised concerns about the accuracy of breast density determined by mammography. Breast MRI takes a three-dimensional (3D) view of the breast without compression and provides superior soft tissue contrast differentiating between fibroglandular and fatty tissues. Several early studies have investigated breast density measurements using MRI, but mainly reporting methodological development using a small number of selected subjects.2, 3, 4, 5, 6, 7, 8 Breast MRI became a clinical modality for screening of high-risk women in 2007, and in recent 2 years, larger series comparing between the density measured by mammography and MRI have been reported.9, 10
We have developed a comprehensive method using computer algorithms to quantitatively measure the total breast volume based on body landmarks of each individual woman, and further segment the fibroglandular tissue in the whole breast to measure the total fibroglandular tissue volume and the percent density.8 The purpose of this study is to apply the technique to analyze breast densities in the normal breasts of a cohort of 321 consecutive patients. Since it is well known that the density is associated with age, we evaluate whether the density analyzed based on MRI in our cohort also reveals the age dependence effect, showing lower density with older age. Lastly, the densities from women of different racial∕ethnic backgrounds including Caucasian (non-Hispanic White), Hispanic, and Asian women are analyzed and compared.
MATERIALS AND METHODS
Patient population
During 2004–2006, breast MRI studies from 509 consecutive patients were recorded in our database. Of these, 321 patients who had unilateral breast disease and for whom age and race∕ethnicity information was available were included in this study. The mean age of patients at the time of the MRI scans was 54 yr old ±12 [standard deviation] (ranging from 25 to 84 yr old). The radiology and pathology reports for each patient were reviewed to confirm that no bilateral disease was present. Only the normal breast was used for the density analysis in this study. This study was approved by the Institutional Review Board and was in compliance with the Health Insurance Portability and Accountability Act (HIPAA). All patients gave written informed consent to participate in an MRI research study.
In order to investigate the dependence of breast density on age and race∕ethnicity, the cohort of 321 patients was separated into three age groups: <45 yr old, between 45 and 55 yr old, and >55 yr old and three major race∕ethnicity groups: Non-Hispanic White (Caucasian), Asian, and Hispanic. The mean age was 55 yr old in the Caucasian group, 52 yr old in the Asian group, and 54 yr old in the Hispanic group. The number of subjects in each age and race∕ethnicity category is summarized in Table 1. We only had a small number of African American women in our database and could not include them in the analysis.
Table 1.
Number of patients in each age and race∕ethnicity category (N=321).
| Race∕ethnicity | Age group | |||
|---|---|---|---|---|
| <45 yr | 45–55 yr | >55 yr | Total | |
| Caucasian | 33 | 59 | 88 | 180 |
| Asian | 22 | 20 | 29 | 71 |
| Hispanic | 22 | 18 | 30 | 70 |
| Total | 77 | 97 | 147 | 321 |
MRI acquisition
The study was performed on a clinical 1.5 T system (Eclipse; Philips Medical System, Cleveland, OH). A body coil was used for transmission, and a dedicated four-channel phased-array breast coil (USA Instruments, Aurora, OH) was used for receiving. The detailed imaging protocol was described in our other studies.11 The breast density was analyzed on the precontrast images acquired in the dynamic contrast enhanced study using a 3D gradient echo radio frequency spoiled fast acquisition in the steady state pulse sequence without fat saturation. Thirty-two axial slices with thickness of 4 mm were used to cover both breasts. The imaging parameters were as follows: TR∕TE=8.1∕4.0 ms, flip angle=20°, acquisition matrix size=256×128, and FOV=32–38 cm. The scan time was 42 s per acquisition. The sequence was repeated 16 times to acquire four sets of precontrast images and 12 sets of postcontrast images. The contrast agent (Omniscan; 1 ml∕10 lb body weight) was manually injected at the beginning of the fifth acquisition. The precontrast images acquired in the third frame of the dynamic sequence (which usually had the least motion artifact) were used for measurement of breast volume and density.
Quantitative breast MRI density assessment
The detailed quantitative density analysis method has been described by Nie et al.8 Briefly, the first step was to segment the breast from the body based on the body landmarks of each individual woman. On the image where the aortic arch was seen, the operator defined two lines connecting the spinous process of the thoracic spine and the lateral margin of the bilateral pectoralis muscles to form the V-shaped cut. This cut was applied to all 32 slices. The next procedure was to define the chest wall muscle within the remaining region. This was done using a fuzzy c-means (FCM) based segmentation algorithm with the B-spline curve fitting, and all tissues posterior to the boundary between the breast and the chest wall muscle were excluded. Next, the layer of the skin on the surface of the breast was identified using the dynamic searching algorithm and excluded. After the breast segmentation procedure was completed, the total breast volume (BV) was calculated by summing over all breast volumes from 32 slices. For fibroglandular tissue segmentation, the adaptive FCM was applied for simultaneous bias field correction (to remove image intensity nonuniformities) and segmentation of fibroglandular and fatty tissues. After completing the segmentation from all 32 imaging slices, the total fibroglandular tissue volume (FV) was calculated, and the percent breast density (% BD) was obtained by normalizing FV to the BV.
Although the procedures were mostly done using computer algorithms, some operator inputs were required. In performing the FCM segmentation, the operator needed to decide the number of clusters and how many should be combined as the dense tissue. In a previous work, we have studied intra- and interoperator variability using 11 cases and showed that the average standard deviation for the percent density was 2.8% for intraobserver comparison and 3.9% for interobserver comparison.8
Statistical analyses
The mean and standard deviation of age, breast volume, fibroglandular volume, and the percent density were calculated for all patients and in each subgroup based on age and race∕ethnicity. Due to lack of normality, as determined by the Kolmogorov–Smirnov test, the transformed data were used for group comparisons. Before application of statistical tests, the natural logarithm (ln) transformation was applied to breast volume and fibroglandular tissue volume, and the square-root transformation was applied to the percent density. Two-way analysis of variance (ANOVA) was used to examine mean differences among the three age groups and three race∕ethnicity groups using the transformed data.
It has been reported that age is a strong predictor of density and that lower body-mass index, or smaller breast (or cup) size, might explain the higher breast density found in some individuals within certain race∕ethnicity groups. Multiple linear regression analysis was performed to examine group differences in fibroglandular tissue volume and in percent density. Race∕ethnicity group, age, and the transformed breast volume were used as predictors, and the transformed fibroglandular tissue volume and transformed percent density as outcome variables. Regression models were examined: (1) Without adjustment for age or transformed breast volume, (2) with adjustment for age, and (3) with adjustment for both age and transformed breast volume. Pairwise differences in means between any two race∕ethnicity groups (Caucasian vs Asian, Caucasian vs Hispanic, and Asian vs Hispanic) were also compared using the multiple comparisons procedure of Tukey’s least significant differences. For fibroglandular tissue volume and percent density in each group, the antilog of least squares means and end points of the 95% confidence intervals were reported.
RESULTS
Overall population distribution
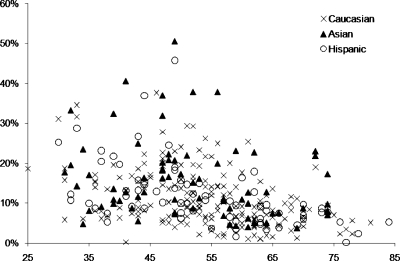
The mean untransformed breast volume of all analyzed patients was 779 cm3 and the mean untransformed fibroglandular tissue volume was 86 cm3, with the mean untransformed percent breast density of 12.1%. Table 2 lists the group means and standard deviations for untransformed data from all patients and for each of the age and race∕ethnicity groups. Figure 1 shows the distribution of the untransformed percent fibroglandular tissue density with age of all 321 patients.
Table 2.
Mean and standard deviation of untransformed breast volume, fibroglandular tissue volume, and percent density for the study population in different age and race groups. (C: Caucasian, A: Asian, and H: Hispanic.)
| Variable | All | C | A | H |
|---|---|---|---|---|
| Age (yr old) | 54±12 | 55±11 | 52±12 | 54±13 |
| Breast volume (cm3) | ||||
| All | 779±313 | 815±348 | 617±223 | 840±240 |
| <45 yr | 728±284 | 804±338 | 602±226 | 725±203 |
| 45–55 yr | 743±306 | 744±347 | 617±160 | 881±207 |
| >55 yr | 829±326 | 872±346 | 627±262 | 893±258 |
| Fibroglandular volume (cm3) | ||||
| All | 86±59 | 82±61 | 94±61 | 94±60 |
| <45 yr | 119±70 | 116±67 | 122±77 | 121±72 |
| 45–55 yr | 98±60 | 93±64 | 103±47 | 110±56 |
| >55 yr | 61±38 | 58±38 | 66±45 | 62±31 |
| Percent density (%) | ||||
| All | 12.1±8.1 | 11.4±8.2 | 15.1±8.2 | 11.5±7.7 |
| <45 yr | 16.7±8.6 | 15.0±7.4 | 20.0±10.4 | 16.3±8.1 |
| 45–55 yr | 14.2±8.3 | 13.9±9.4 | 16.9±6.8 | 12.5±5.0 |
| >55 yr | 8.3±5.7 | 7.7±5.1 | 11.7±7.9 | 7.0±3.6 |
Figure 1.
The scatterplot between the fibroglandular tissue percentage (%) and age of all 321 patients, including 180 Caucasians, 71 Asians, and 70 Hispanics. There is a wide distribution within each race∕ethnicity group. It is noted that in the upper part of the figure with the highest densities, the Asian women are the dominating group. Also noted is the clear trend of decreasing density over the age of 55.
Analysis of age groups
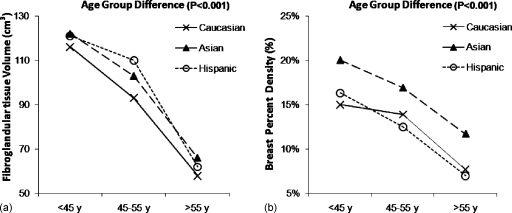
The age dependence was analyzed among three groups: <45, 45–55, and >55 yr old. There were no significant differences in the transformed breast volume with regard to age group, but the transformed fibroglandular tissue volume and transformed percent density showed statistically significant age dependence (p<0.001 for both). We also evaluated the age dependence in each race∕ethnicity group separately (Fig. 2, untransformed data are shown). As in the whole population, both fibroglandular tissue volume and percentage density decreased significantly with age within each race∕ethnicity group. Due to lack of normality of the parameters, the statistical analysis was performed based on an ANOVA of the transformed data. Both fibroglandular tissue volume and percent density decreased significantly with age in the Asian, Caucasian, and Hispanic groups, with p<0.001 for all analyses. These analyses were done, considering all three age groups together, not pairwise.
Figure 2.
Distribution of the (a) mean fibroglandular tissue volume and (b) the mean percent density in the three age groups (<45, 45–55, and >55 yr old) within the Caucasian (non-Hispanic White), Asian, and Hispanic race∕ethnicity groups. The original, untransformed data are plotted. Due to lack of normality of the parameters, the statistical analysis was performed based on an ANOVA of the transformed data. The fibroglandular tissue volume and the percent density in Caucasian, Asian, and Hispanic women all decrease across the three age groups (p<0.001).
Analysis of race∕ethnicity groups
Comparing the three race∕ethnicity groups, the Asian women had relatively smaller breast volume (617±223 cm3) than the Caucasian (815±348 cm3) women or the Hispanic women (840±240 cm3). Regarding the untransformed fibroglandular volume, the Asian had 94±61 cm3, Caucasian had 82±61 cm3, and Hispanic had 94±60 cm3. Regarding untransformed percent density, the Asian women had the highest mean density of 15.1±8.2% [full range, 1.1%–34.6%] among the three groups. The Caucasian women had the mean density of 11.4±8.2% [4.4%–40.6%], and the Hispanic women had a similar mean density of 11.5±7.7% [3.7%–39.5%]. The Caucasian and Hispanic groups did not show any significant difference in all comparisons done in this study. Considering all three race groups together, there were significant differences in the mean transformed densities (square root of the percent density) among races (p<0.01). Within each age group, the difference among mean transformed percent density in three races was significant for women >55 yr old (p=0.05), and women 45–55 yr old (p<0.05), but not in younger women <45 yr old (p=0.29).
Age- and breast volume-adjusted comparison between three race∕ethnicity groups
To further understand whether the higher density in the Asian women was related to their younger age (52 compared to 54 for Hispanic and 55 for Caucasian), or smaller breast volume, multiple linear regression analyses were performed with adjustment for covariates. The results of the transformed fibroglandular tissue volume in three analyses—unadjusted, age-adjusted, and age- and breast volume-adjusted—are summarized in Table 3. The transformed fibroglandular tissue volumes in three race groups did not show significant differences. The adjustment by age or by additional breast volume had little effect on the comparison and remained nonsignificant.
Table 3.
Comparison of fibroglandular tissue volume in three race∕ethnicity groups. (CI: confidence interval.)
| Least-squares means for fibroglandular volume in cm3 (95% CI)a | ||||
|---|---|---|---|---|
| Caucasian | Asian | Hispanic | p -valuea | |
| Unadjusted | 82.4 (73.5–91.3) | 94.0 (79.9–108.1) | 94.1 (80.1–108.1) | 0.20 |
| Age-adjusted | 83.9 (75.7–103.6) | 90.7 (77.8–103.6) | 93.7 (80.9–106.5) | 0.28 |
| Age-, breast size-adjusted | 82.1 (74.3–90.0) | 95.1 (82.0–108.3) | 89.9 (77.6–102.2) | 0.13 |
Because of the non-normal distribution of the fibroglandular tissue volume, the ANOVA was performed after applying the natural logarithm transformation. The antilog of the least-squares means and end points of the 95% confidence intervals are presented.
The results based on the analysis of the transformed percent density are shown in Table 4. The antilog of least-squares means and end points of the 95% confidence intervals are presented. There was a significant difference in unadjusted transformed percent density among three groups (p<0.01). After adjusting for age, the estimated percent density decreased from 15.1% to 14.6% in the Asian women, which was still significantly higher than that in the Caucasian women (11.6%) and the Hispanic women (11.4%) withp=0.01. However, after further adjustment for differences in breast volume, there was no significant difference in the mean transformed percent density among racial groups (p=0.25).
Table 4.
Comparison of the percent fibroglandular density in three race∕ethnicity groups.
| Least-squares means for percent density in % (95% CI)a | |||||||
|---|---|---|---|---|---|---|---|
| Caucasian | Asian | Hispanic | p -valuea | C-Ab | C-Hb | A-Hb | |
| Unadjusted | 11.4 (10.2–12.6) | 15.1 (13.3–17.0) | 11.5 (9.6–13.3) | 0.003 | 0.001 | 0.98 | 0.002 |
| Age-adjusted | 11.6 (10.6–12.7) | 14.6 (12.8–16.3) | 11.4 (9.7–13.1) | 0.01 | 0.005 | 0.97 | 0.005 |
| Age-, breast size-adjusted | 12.1 (11.0–13.1) | 13.7 (12.6–16.0) | 11.8 (10.2–13.4) | 0.25 | 0.10 | 0.80 | 0.80 |
Because of the non-normal distribution of the percent density, ANOVA was performed after applying the square-root transformation. The antilog of least-squares means and end points of the 95% confidence intervals are presented.
Pairwise comparison between two ethnic groups. C: Caucasian, A: Asian, and H: Hispanic, using the Tukey’s test.
Age- and breast volume-adjusted comparison between pairwise race∕ethnicity groups
In addition to ANOVA analysis of mean differences across the three age groups, the pairwise differences were analyzed between two race∕ethnicity groups using the least significant differences method. No significant differences were observed in the mean transformed fibroglandular tissue volume between any two racial groups in unadjusted or adjusted models.
For the percent density, as shown in Table 4, the Asian women had significantly higher mean transformed percent density (p=0.001) compared to the Caucasian women. After adjustment for differences in age, the estimated means between them remained significantly different (p=0.005); however, after further adjusting for differences in breast volume, there was no difference in the mean transformed percent density between them (p=0.10). The comparison between the Asian and the Hispanic groups showed similar results that Asians had higher density but the difference became insignificant after adjusting for breast volume. Collectively, these results suggest that the Asian women had a higher percent density compared to the Caucasian or Hispanic women, which was due to a similar fibroglandular tissue volume in a smaller breast. For comparison between the Caucasian and Hispanic groups, no significant differences were found in the mean transformed percent density in the unadjusted or adjusted models.
DISCUSSION
Many studies have reported the dependence of mammographic density on age using qualitative methods such as BI-RADS or quantitative percent density.12, 13, 14, 15, 16, 17, 18, 19, 20, 21 It has been found consistently that women of younger age or premenopausal women have denser breasts compared to older or postmenopausal women. The mammographic density decreased gradually when age increased from 45 to 65 yr old.13, 17, 18, 19 In our study using three age groups (<45, 45–55, and >55 yr old), we found a significant difference in the fibroglandular tissue volume and the percent density across the three age groups (Fig. 2), which was consistent with previous reports.
For race dependence, there were some studies investigating the differences between Hispanic and Caucasian women,20, 21, 22, 23 and reporting no significant difference. In our study, the mean percent density was comparable in all age groups between the Caucasian and the Hispanic women. Conflicting results have been reported between the Asian women and the Caucasian women,21, 23, 24, 25, 26 some showing significant differences while others not. A large study using BI-RADS score to classify the subjects into fatty (BI-RADS 1-2) and dense (BI-RADS 3-4) breasts found that the Asian women had significantly denser breasts than the Caucasian women, and that the differences were greatest in the older age groups.23
Only a few studies have applied quantitative analysis methods to investigate the differences in the density of different race∕ethnicity groups based on mammography.27, 28, 29, 30 Chen et al.30 used a thresholding method to compare among Asian, African American, and Caucasian women. They did not find significant differences in the dense tissue area between the Asian and the Caucasian women, but the percent density showed significant differences. The mean differences remained significant after adjustment for age, but became insignificant when further adjusting for the breast size. The results indicated that the mean percent breast density was higher in the Asian women, but this came from a similar dense tissue area in a smaller breast area on mammography.
Our study also showed similar results. As shown in Table 3, the mean transformed fibroglandular tissue volume did not differ significantly between the three racial groups. In Table 4 the mean transformed percent density in the Asian women was significantly higher compared to the Caucasian women in the unadjusted or age-adjusted analysis; but the estimated mean differences were no longer statistically significant after further adjustment for breast volume. The results also suggest that Asian women had comparable fibroglandular tissue volumes as Caucasian or Hispanic women, but they had small breasts, thus yielding a higher percent density.
Many studies have investigated the association between mammographic density and the risk of developing cancer, and with the overwhelming evidence, the Breast Cancer Prevention and Collaborative Group has recommended that quantitative breast density should be incorporated into the risk prediction model.31 Since these studies analyzed breast density on mammography, the percent mammographic density was used to correlate with cancer risk. In addition to percent density, 3D MRI can be used to measure the dense tissue volume and the morphology distribution pattern,32 and whether they can be used to improve the accuracy of predicting cancer risk needs to be investigated. In recent 2 years, with the established MRI density analysis methods and the large database of breast MRI patient cohorts, more studies reporting MRI-based density were published.9, 10, 32, 33, 34 One major research focus was to compare the density measured based on mammography and MRI for a better understanding of their correlations as well as differences.9, 10, 33 Although, in general, there was a significant association between the percent density analyzed on mammography and MRI, there were cases that showed a high discrepancy (up to 50% difference between mammography and MRI densities).10 The relationship in women with high and low breast densities may be different.10, 33 One main source of the discrepancy comes from the analysis based on a projection mammography vs the 3D MRI, which is an active research area undertaken by many research groups. In addition to predicting cancer risk, another clinical application of breast density is to evaluate the changes induced by chemoprevention drugs, and hopefully to use this change as a surrogate marker for predicting the efficacy of chemoprevention.5, 35
One limitation is associated with the binary segmentation. Each pixel within the breast is either classified as dense tissue or fatty tissue. The blood vessels and intramammary nodes have similar signal intensities as that of fibroglandular tissues, and they will be classified with the fibroglandular tissue as dense tissues and be a part of the analyzed breast density. We have recently published one paper36 describing the segmentation of blood vessels on breast MRI; however, contrast injection is needed and only large vessels can be segmented. Therefore, it is not reliable to quantify and exclude all vascular pixels. Similarly, intramammary lymph nodes cannot be reliably detected and excluded from the dense tissue.
In conclusion, in this study we reported the density analyzed from 321 women based on three-dimensional MRI. We found that the fibroglandular tissue volume and the percent density were strongly dependent on age. On the average, the Asian women had higher transformed percent density compared to the Caucasian and Hispanic women, but the differences reflected the smaller breast volume in the Asian women containing comparable fibroglandular tissue volume. These results were consistent with studies analyzed based on mammography, and that further established the MRI-based density analysis method. With the role of breast density in cancer risk prediction and chemoprevention gradually being established, the results of density in different age and race∕ethnic groups reported in this work may be used to understand the confounding effects coming from these factors.
ACKNOWLEDGMENT
This work was conducted at Tu and Yuen Center for Functional Onco-Imaging at University of California, Irvine.
References
- Kopans D. B., “Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk,” Radiology 246, 348–353 (2008). 10.1148/radiol.2461070309 [DOI] [PubMed] [Google Scholar]
- Lee N. A., Rusinek H., Weinreb J., Chandra R., Toth H., Singer C., and Newstead G., “Fatty and fibroglandular tissue volumes in the breasts of women 20–83 years old: Comparison of x-ray mammography and computer assisted MR imaging,” AJR, Am. J. Roentgenol. 168, 501–506 (1997). [DOI] [PubMed] [Google Scholar]
- van Engeland S., Snoeren P. R., Huisman H., Boetes C., and Karssemeijer N., “Volumetric breast density estimation from full-field digital mammograms,” IEEE Trans. Med. Imaging 25, 273–282 (2006). 10.1109/TMI.2005.862741 [DOI] [PubMed] [Google Scholar]
- Yao J., Zujewski J. A., Orzano J., Prindiville S., and Chow C., “Classification and calculation of breast fibroglandular tissue volume on SPGR fat suppressed MRI.” Proc. SPIE 5747, 1942–1949 (2005). 10.1117/12.594671 [DOI] [Google Scholar]
- Eng-Wong J., Orzano-Birgani J., Chow C. K., Venzon D., Yao J., Galbo C. E., Zujewski J. A., and Prindiville S., “Effect of raloxifene on mammographic density and breast magnetic resonance imaging in premenopausal women at increased risk of breast cancer,” Cancer Epidemiol. Biomarkers Prev. 17, 1696–1701 (2008). 10.1158/1055-9965.EPI-07-2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klifa C., Carballido-Gamio J., Wilmes L., Laprie A., Lobo C., Demicco E., Watkins M., Shepherd J., Gibbs J., and Hylton N., “Quantification of breast tissue index from MR data using fuzzy cluster,” Proceedings of IEEE Engineering in Medicine and Biology Society, 2004, Vol. 3, pp. 1667–1670 (unpublished). [DOI] [PubMed]
- Wei J., Chan H. P., Helvie M. A., Roubidoux M. A., Sahiner B., Hadjiiski L. M., Zhou C., Paquerault S., Chenevert T., and Goodsitt M. M., “Correlation between mammographic density and volumetric fibroglandular tissue estimated on breast MR images,” Med. Phys. 31, 933–942 (2004). 10.1118/1.1668512 [DOI] [PubMed] [Google Scholar]
- Nie K., Chen J. H., Chan S., Chau M. K., Yu H. J., Bahri S., Tseng T., Nalcioglu O., and Su M. Y., “Development of a quantitative method for analysis of breast density based on three-dimensional breast MRI,” Med. Phys. 35, 5253–5262 (2008). 10.1118/1.3002306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazen M., Warren R. M., Boggis C. R., Bryant E. C., Reed S., Warsi I., Pointon L. J., Kwan-Lim G. E., Thompson D., Eeles R., Easton D., Evans D. G., Leach M. O., and Collaborators in the United Kingdom Medical Research Council Magnetic Resonance Imaging in Breast Screening (MARIBS) Study, “A pilot study of compositional analysis of the breast and estimation of breast mammographic density using three-dimensional T1-weighted magnetic resonance imaging,” Cancer Epidemiol. Biomarkers Prev. 17, 2268–2274 (2008). 10.1158/1055-9965.EPI-07-2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. J., Leach M. O., Kwan-Lim G., Gayther S. A., Ramus S. J., Warsi I., Lennard F., Khazen M., Bryant E., Reed S., Boggis C. R., Evans D. G., Eeles R. A., Easton D. F., Warren R. M., and The UK Study of MRI Screening for Breast Cancer in Women at High Risk (MARIBS), “Assessing the usefulness of a novel MRI-based breast density estimation algorithm in a cohort of women at high genetic risk of breast cancer: The UK MARIBS study,” Breast Cancer Research 11, R80 (2009). 10.1186/bcr2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Feig B., Agrawal G., Yu H., Carpenter P. M., Mehta P. S., Nalcioglu O., and Su M. Y., “MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy,” Cancer 112, 17–26 (2008). 10.1002/cncr.23130 [DOI] [PubMed] [Google Scholar]
- El-Bastawissi A. Y., White E., Mandelson M. T., and Taplin S. H., “Reproductive and hormonal factors associated with mammographic breast density by age (United States),” Cancer Causes Control 11, 955–963 (2000). 10.1023/A:1026514032085 [DOI] [PubMed] [Google Scholar]
- Vachon C. M., Pankratz V. S., Scott C. G., Maloney S. D., Ghosh K., Brandt K. R., Milanese T., Carston M. J., and Sellers T. A., “Longitudinal trends in mammographic percent density and breast cancer risk,” Cancer Epidemiol. Biomarkers Prev. 16, 921–928 (2007). 10.1158/1055-9965.EPI-06-1047 [DOI] [PubMed] [Google Scholar]
- Perry N. M., Allgood P. C., Milner S. E., Mokbel K., and Duffy S. W., “Mammographic breast density by area of residence: Possible evidence of higher density in urban areas,” Curr. Med. Res. Opin. 24, 365–368 (2008). 10.1185/030079908X260907 [DOI] [PubMed] [Google Scholar]
- Caire-Juvera G., Arendell L. A., Maskarinec G., Thomson C. A., and Chen Z., “Associations between mammographic density and body composition in Hispanic and non-Hispanic white women by menopause status,” Menopause 15, 319–325 (2008). 10.1097/gme.0b013e3181405b8a [DOI] [PubMed] [Google Scholar]
- Kerlikowske K., Ichikawa L., Miglioretti D. L., Buist D. S., Vacek P. M., Smith-Bindman R., Yankaskas B., Carney P. A., Ballard-Barbash R., and National Institutes of Health Breast Cancer Surveillance Consortium, “Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk,” J. Natl. Cancer Inst. 99, 386–395 (2007). 10.1093/jnci/djk066 [DOI] [PubMed] [Google Scholar]
- Maskarinec G., Pagano I., Lurie G., and Kolonel L. N., “A longitudinal investigation of mammographic density: The multiethnic cohort,” Cancer Epidemiol. Biomarkers Prev. 15, 732–739 (2006). 10.1158/1055-9965.EPI-05-0798 [DOI] [PubMed] [Google Scholar]
- Gapstur S. M., López P., Colangelo L. A., Wolfman J., Van Horn L., and Hendrick R. E., “Associations of breast cancer risk factors with breast density in Hispanic women,” Cancer Epidemiol. Biomarkers Prev. 12, 1074–1080 (2003). [PubMed] [Google Scholar]
- Ursin G., Ma H. Y., Wu A. H., Bernstein L., Salane M., Parisky Y. R., Astrahan M., Siozon C. C., and Pike M. C., “Mammographic density and breast cancer in three ethnic groups,” Cancer Epidemiol. Biomarkers Prev. 12, 332–338 (2003). [PubMed] [Google Scholar]
- Bartow S. A., Pathak D. R., Mettler F. A., Key C. R., and Pike M. C., “Breast mammographic pattern: A concatenation of confounding and breast cancer risk factors,” Am. J. Epidemiol. 71, 647–650 (1975). [DOI] [PubMed] [Google Scholar]
- Hart B. L., Steinbock R. T., Mettler F. A., Pathak D. R., and Bartow S. A., “Age and race related changes in mammographic parenchymal patterns,” Cancer 63, 2537–2539 (1989). [DOI] [PubMed] [Google Scholar]
- White E., Velentgas P., Mandelson M. T., Lehman C. D., Elmore J. G., Porter P., Yasui Y., and Taplin S. H., “Variation in mammographic breast density by time in menstrual cycle among women aged 40–49 years,” J. Natl. Cancer Inst. 90, 906–910 (1998). 10.1093/jnci/90.12.906 [DOI] [PubMed] [Google Scholar]
- Bartow S. A., Pathak D. R., and Mettler F. A., “Radiographic microcalcification and parenchymal patterns as indicators of histologic high-risk benign breast disease,” Cancer 66, 1721–1725 (1990). [DOI] [PubMed] [Google Scholar]
- El-Bastawissi A. Y., White E., Mandelson M. T., and Taplin S., “Variation in mammographic breast density by race,” Ann. Epidemiol. 11, 257–263 (2001). 10.1016/S1047-2797(00)00225-8 [DOI] [PubMed] [Google Scholar]
- Turnbull A. E., Kapera L., and Cohen M. E., “Mammographic parenchymal patterns in Asian and Caucasian women attending for screening,” Clin. Radiol. 48, 38–40 (1993). 10.1016/S0009-9260(05)80105-9 [DOI] [PubMed] [Google Scholar]
- Gravelle I. H., Bullbrook R. D., Wang D. Y., Allen D., Hayward J. L., Bulstrode J. C., and Takatani O., “A comparison of mammographic parenchymal patterns in premenopausal Japanese and British women,” Breast Cancer Res. Treat. 18, S93–S95 (1991). 10.1007/BF02633537 [DOI] [PubMed] [Google Scholar]
- Maskarinec G., Lyu L. C., Meng L., Theriault A., and Ursin G., “Determinations of mammographic density among women of Asian, Native Hawaiian, and Caucasian ancestry,” Ethn Dis. 11, 44–50 (2001). [PubMed] [Google Scholar]
- Maskarinec G., Meng L., and Ursin G., “Ethnic differences in mammographic densities,” Int. J. Epidemiol. 30, 959–965 (2001). 10.1093/ije/30.5.959 [DOI] [PubMed] [Google Scholar]
- Maskarinec G., Nagata C., Shimmizu H., and Kashiki Y., “Comparison of mammographic densities and their determinates in women from Japan and Hawaii,” Int. J. Cancer 102, 29–33 (2002). 10.1002/ijc.10673 [DOI] [PubMed] [Google Scholar]
- Chen Z., Wu A. H., Gauderman W. J., Bernstein L., Ma H., Pike M. C., and Ursin G., “Does mammographic density reflect ethnic differences in breast cancer incidence rates?,” Am. J. Epidemiol. 159, 140–147 (2004). 10.1093/aje/kwh028 [DOI] [PubMed] [Google Scholar]
- Santen R. J., Boyd N. F., Chelbowski R. T., Cummings S., Cuzick J., Dowsett M., Easton D., Forbes J. F., Key T., Hankinson S. E., Howell A., and Ingle J., “Critical assessment of new risk factors for breast cancer: Considerations for development of an improved risk prediction model,” Endocrine Related Cancer 14, 169–187 (2007). 10.1677/ERC-06-0045 [DOI] [PubMed] [Google Scholar]
- Nie K., Chen J. H., Chang D., Hsu C. C., Nalcioglu O., and Su M. Y., “Quantitative analysis of breast parenchymal patterns using 3D fibroglandular tissue segmentation based on MRI,” Med. Phys. 37, 217–226 (2010). 10.1118/1.3271346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klifa C., Carballido-Gamio J., Wilmes L., Laprie A., Shepherd J., Gibbs J., Fan B., Noworolski S., and Hylton N., “Magnetic resonance imaging for secondary assessment of breast density in a high-risk cohort,” Magn. Reson. Imaging 28, 8–15 (2010). 10.1016/j.mri.2009.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie K., Chang D., Chen J. H., Hsu C. C., Shih T. C., Nalcioglu O., and Su M. Y., “Segmentation of skin for quantitative measurement of breast density on MRI,” Med. Phys. 37, 227–233 (2010). 10.1118/1.3271353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J., Warwick J., Pinney E., Warren L. R. M., and Duffy S. W., “Change in breast density as a biomarker of breast cancer risk reduction: Results from IBIS-1,” Proceedings of the San Antonio Breast Cancer Symposium (SABCS), Abstract, 2008, p. 61 (unpublished).
- Lin M., Chen J. H., Ni K., Chang D., Nalcioglu O., and Su M. Y., “An algorithm-based method for the detection of blood vessels in breast MRI for the development of computer-aided diagnosis,” J. Magn. Reson Imaging 30, 817–824 (2009). 10.1002/jmri.21915 [DOI] [PMC free article] [PubMed] [Google Scholar]