Abstract
In the kidney, defects in the regulation of urine salt excretion can result in extracellular fluid volume expansion, leading to salt-sensitive hypertension. Previous studies have demonstrated that when rats are maintained on a high sodium chloride (NaCl) diet, adenosine production increases in the renal medulla with parallel changes in adenosine receptor expression. These studies suggest that adenosine signaling in the kidney can respond to high NaCl loading; however, the functional consequences of these changes in adenosine signaling are not clear.
We used the immortalized cell line mIMCD-K2, a murine model system for the renal inner medullary collecting duct (IMCD), to study the direct effects of adenosine on NaCl transport across IMCD epithelium with an Ussing chamber system. When epithelial Na+ channels were inhibited, addition of adenosine to the apical side of mIMCD-K2 cell sheets stimulated short-circuit current (Isc) in a dose-dependent manner. This increase in Isc was inhibited by a CFTR Cl− channel inhibitor. Pharmacological studies with a panel of adenosine receptor agonists and antagonists demonstrated that adenosine activates apical A2b adenosine receptors to enhance Isc. Furthermore, adenosine application to mIMCD-K2 cell sheets increased intracellular cAMP, while inhibition of protein kinase A (PKA) completely blocked the adenosine response. Together, our findings indicate that adenosine stimulates Cl− secretion through CFTR in mIMCD-K2 cells by activating apical A2b receptors and signaling through cAMP/PKA. We propose that this adenosine receptor pathway may provide one mechanism for enhancing urine NaCl excretion in the setting of high dietary NaCl intake.
Keywords: adenosine, CFTR, A2b receptor, renal medulla, collecting duct
INTRODUCTION
The integrated control of natriuretic (sodium-excreting) and natriferic (sodium-retaining) systems by the kidney determines sodium chloride (NaCl) balance, extracellular fluid (ECF) volume, and ultimately blood pressure. The collecting duct of the kidney plays a critical role in the regulation and fine-tuning of NaCl transport and is subject to extensive hormonal regulation. For instance, in an immortalized murine cell line derived from the initial segment of the inner medullary collecting duct (IMCD), mIMCD-K2, hormones and signaling intermediates, such as vasopressin and cAMP, stimulate Cl− secretion through the Cl− channel, cystic fibrosis transmembrane conductance regulator (CFTR) 1, 2. In this same cell line, hormones that are up-regulated in response to low dietary salt intake, such as aldosterone, enhance Na+ absorption through the epithelial Na+ channel (ENaC) 3. These studies indicate that the mIMCD-K2 cultured cell line, a model system for the IMCD of the kidney, can respond to an array of hormonal inputs and control net NaCl excretion by integrating NaCl secretory and absorptive pathways.
One paracrine hormone that has been implicated in both NaCl secretory and absorptive pathways in the kidney is adenosine. Adenosine has several well-characterized functions in the kidney, including regulating renal blood flow, glomerular filtration, tubulo-glomerular feedback, and renin release 4. Adenosine can also exert direct effects on NaCl transport in the kidney tubule 4, 5. In particular, the collecting duct appears to be an important site for adenosine signaling because this segment has the capacity to produce high levels of extracellular adenosine 6 and expresses all four classes of adenosine receptors 4, 5. Adenosine receptor subtypes A1, A2a, A2b, and A3 are G protein-coupled receptors, with high (A1 and A2a) or low (A2b and A3) binding affinities for adenosine 7, 8. Adenosine receptors have been localized to the apical surface of numerous kidney tubule segments, including the collecting duct, suggesting a role for extracellular adenosine as a signaling molecule for direct regulation of tubular function 5, 9–11.
High dietary salt intake in rats stimulates production of adenosine in the urine and renal interstitium, with the highest levels of adenosine concentration in the renal medulla 12. In addition to an increase in adenosine production, the ratio of A2/A1 receptor expression in the kidney increases in response to high dietary NaCl intake 13. We hypothesized that the observed elevation in adenosine concentration in the renal medulla and the increase in the ratio of A2/A1 receptor expression could represent an adaptive response by the kidney to stimulate NaCl secretion across the IMCD as a means to enhance urine NaCl excretion. Indeed, under conditions of high dietary NaCl intake, NaCl and fluid secretion have been observed in the IMCD of rats, with the highest rate of fluid secretion in the initial segment of the IMCD 14.
The purpose of our study was to characterize mechanisms by which adenosine influences NaCl transport across the IMCD, under conditions where ENaC channel activity is diminished. We used the mIMCD-K2 cell line as our model system for the initial segment of the IMCD to study the direct effects of adenosine on NaCl transport across IMCD epithelium with an Ussing chamber system. We show that adenosine can act through apical A2b receptors to directly stimulate Cl− secretion through CFTR. Moreover, adenosine action in IMCD cells is associated with increases in intracellular cAMP and is blocked by the inhibition of protein kinase A (PKA), suggesting that adenosine signals through the cAMP/PKA pathway to activate CFTR. We propose that adenosine-stimulated Cl− secretion across the IMCD may have a role in modulating the final ionic composition and volume of urine under high dietary salt conditions and in protecting against the development of salt-sensitive hypertension.
METHODS
Please see the online Data Supplement at http://hyper.ahajournals.org for the Expanded Methods section.
Cell Culture
The mIMCD-K2 cell line was kindly provided by Dr. Bruce Stanton (Dartmouth Medical School, Hanover, NH). Cells (passage numbers 38 – 46) were expanded, plated on polycarbonate Snapwell inserts (Corning Costar, Corning, NY) coated with Vitrogen plating media, and maintained in modified Optimem medium (Invitrogen, Carlsbad, CA), as described previously 3.
Ussing Chamber Measurements
Transepithelial voltage (Vte) and resistance (Rte) were measured using an Evometer “chopstick voltmeter” (World Precision Instruments, Sarasota, FL). Cell sheets were studied in Ussing chambers (Physiological Instruments, San Diego, CA), as described previously 15.
Pharmacological Agents
Cell sheets were exposed to a series of pharmacological agents once short-circuit current (Isc) and Rte stabilized. The apical side of all cell sheets was first treated with 10−5 mol/L amiloride (Sigma, St. Louis, MO), an inhibitor of ENaC. Some cell sheets were exposed to increasing concentrations of adenosine (Sigma), from 10−9 to 10−4 mol/L, to generate a cumulative dose response curve. At 10−4 mol/L, adenosine induced a large transient increase in Isc, which was followed by a sustained increase in Isc. The vehicle control (containing 0.1N NH4OH), when administered at identical volumes, also induced a transient increase in Isc, but it did not cause a sustained change in Isc. Therefore, only sustained changes in Isc were considered when measuring adenosine-stimulated Isc (Iscadenosine).
A series of adenosine receptor agonists and antagonists were added to identify the receptors responsible for adenosine action. All of the following agonists and antagonists were commercially available from Tocris Bioscience (Ellisville, MO): CPA, CGS21680, NECA, DPCPX, ZM241385, SCH442416, and PSB603. Table S1 (available in the online Data Supplement) summarizes the relative binding affinities of the above agonists and antagonists for each class of adenosine receptors. Pharmacological blockers of anion secretion were added to identify the ion channels responsible for Iscadenosine: CFTR inhibitor-172 (Calbiochem, Gibbstown, NJ), 4,4-diisothiocyano-2, 2-stilbenedisulfonate (DIDS) (Sigma), and diphenylamine-2-carboxylate (DPAC) (Sigma). To determine whether Iscadenosine was dependent on PKA activity, cell sheets were pre-treated for 30 minutes with 10−5 mol/L H-89 (Sigma) to inhibit PKA.
Statistics
Statistical analyses for comparisons between different treatment groups of mIMCD-K2 cells were performed using paired or unpaired two-tailed Student’s t-tests. Differences were considered to be significant at P values < 0.05.
RESULTS
Baseline electrophysical properties of mIMCD-K2 cells
Evometer measurements of mIMCD-K2 cells grown on Snapwell inserts were recorded daily and reached resistance values between 800–1200 Ω.cm2 within 10–14 days before they were mounted in Ussing chambers for electrophysiological studies. For all filters (n = 105), the average baseline Rte in Ussing chambers was 971.6 ± 46.5 Ω.cm2, and the average baseline Isc was 1.04 ± 0.09 μA/cm2. The observed baseline electrophysiological properties closely resembled those originally described for mIMCD-K2 cells 3. Amiloride was added to the apical bath solution in all experiments to inhibit ENaC-mediated Na+ absorption. The addition of amiloride did not cause significant changes to Isc (0.01 ± 0.007 μA/cm2).
Apical adenosine stimulates basal electrogenic Cl− secretion through CFTR
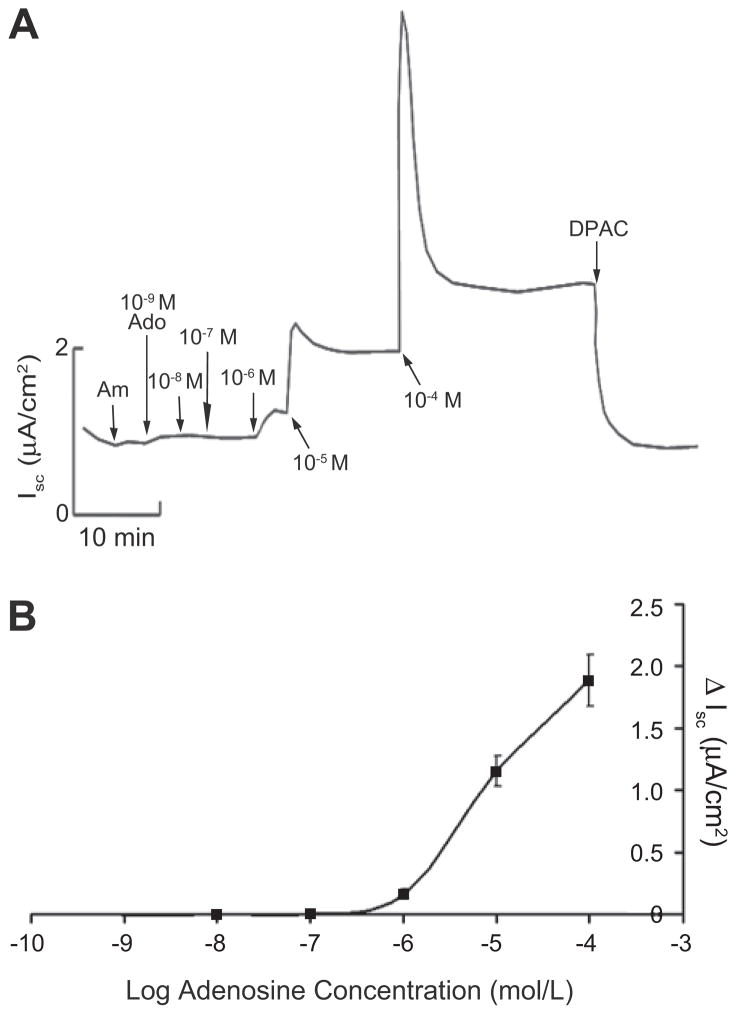
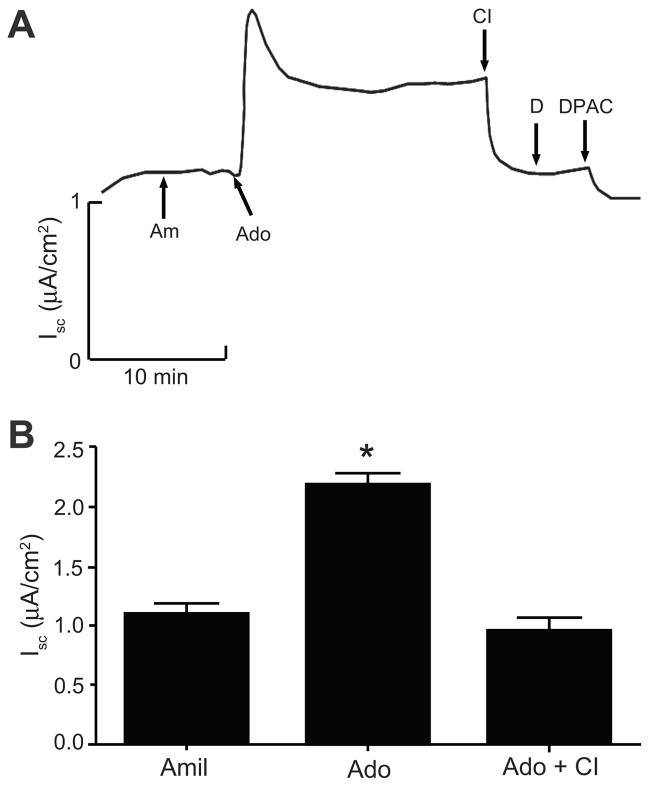
Addition of adenosine (10−5 mol/L) to both sides of cell sheets caused a sustained increase in Isc, which was blocked by the addition of the anion-transport inhibitor DPAC. Next, we generated an adenosine dose response curve, which showed that concentrations below 10−6 mol/L did not affect basal Isc. From 10−6 to 10−4 mol/L, adenosine stimulated Isc in a dose-dependent manner (Figure 1). To determine the sidedness of adenosine action, we administered adenosine to either the apical or basolateral bath of paired cell sheets. The addition of adenosine to the apical bath, and not to the basolateral bath, induced a sustained Isc increase (Figure S1). Finally, to identify the ion channels involved in adenosine-induced anion secretion, we treated mIMCD-K2 cells with CFTR-inhibitor-172 (a selective inhibitor of CFTR) and DIDS (an inhibitor of Ca2+- activated Cl− channels) following the addition of adenosine. Iscadenosine was completely blocked by CFTR inhibitor-172 but not by DIDS (Figure 2).
Figure 1.
A. Representative Isc trace of cumulative dose response to adenosine in mIMCD-K2 cells. Am, 10−5 mol/L amiloride (apical addition); Ado, adenosine concentrations in mol/L (M) as specified (addition to both sides); DPAC, 2 × 10−3 mol/L DPAC (apical addition). B. Cumulative dose response curve derived from average sustained Isc increases (ΔIsc) after adenosine addition. Values are mean ± SE (n = 24 cell sheets).
Figure 2.
A. Representative trace of Isc showing the effects of CFTR inhibitor-172 and DIDS on adenosine-induced Isc in mIMCD-K2 cells. Am, 10−5 mol/L amiloride (apical addition); Ado, 10−5 mol/L adenosine (apical addition); CI, 10−5 mol/L CFTR inhibitor-172 (apical addition); D, 10−5 mol/L DIDS (apical addition); DPAC, 2 × 10−3 mol/L DPAC (apical addition). B. Average Isc values after sequential addition of amiloride (Amil), adenosine (Ado), and CFTR inhibitor-172 (CI). Values are mean ± SE (n = 16 filters). Statistical significance between the different conditions was compared as indicated. *P<0.05.
Adenosine activates A2b receptors to stimulate Cl− secretion
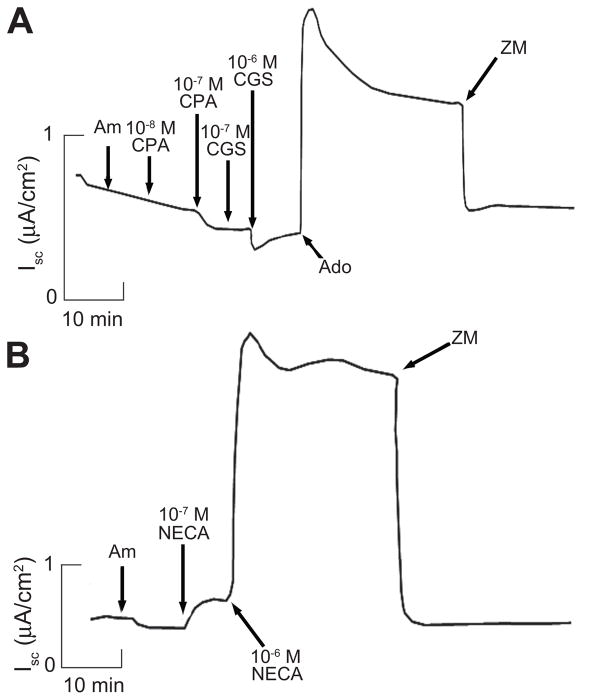
To identify which class of adenosine receptors was responsible for Iscadenosine, we used a series of adenosine receptor agonists and antagonists. Agonists specific to A1 and A2a receptors (CPA and CGS21680, respectively) failed to change Isc (Figure 3A). However, the non-selective adenosine receptor agonist, NECA, caused a sustained increase in Isc at concentrations of 10−7 mol/L and above (Figure 3B).
Figure 3.
A. Representative Isc trace showing actions of the A1 receptor-specific agonist CPA and the A2a receptor-specific agonist CGS21680 on Isc in mIMCD-K2 cells. Trace represents experiments performed on 16 filters. Am, 10−5 mol/L amiloride (apical addition); CPA, CPA concentrations mol/L (M) as specified; CGS, CGS21680 concentrations mol/L (M) as specified; 10−5 mol/L Ado, adenosine (apical addition); ZM, 10−5 mol/L ZM241385 (apical addition). B. Representative Isc trace showing action of the non-selective adenosine receptor agonist NECA. NECA concentrations mol/L (M) as specified. Trace represents experiments performed on 14 filters.
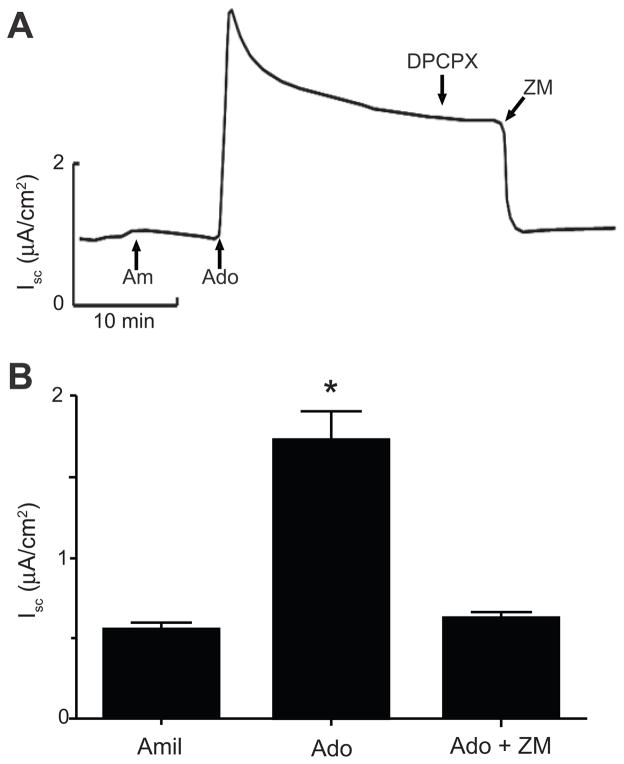
We next stimulated cell sheets with adenosine and then used adenosine receptor antagonists to block Iscadenosine as a complementary approach for identifying the adenosine receptors responsible for Iscadenosine. The specific A1 receptor antagonist DPCPX did not affect Iscadenosine (Figure 4A). In contrast, the non-selective A1/A2 receptor antagonist ZM241385, which has an extremely low affinity for the A3 receptor (Table S1), completely inhibited Iscadenosine (Figure 4).
Figure 4.
A. Representative Isc trace describing the action of the specific A1 receptor antagonist DPCPX and the non-selective A2 receptor antagonist ZM241385 on adenosine-induced Isc in mIMCD-K2 cells. Am, 10−5 mol/L amiloride (apical addition); Ado, 10−5 mol/L adenosine (apical addition); DPCPX, 2 × 10−8 mol/L DPCPX (apical addition); ZM, 10−5 mol/L ZM241385 (apical addition). B. Average Isc values after sequential addition of amiloride (Amil), adenosine (Ado), and ZM241385 (ZM). Values are mean ± SE (n = 23 cell sheets). Statistical significance between the different conditions was compared as indicated. *P<0.05.
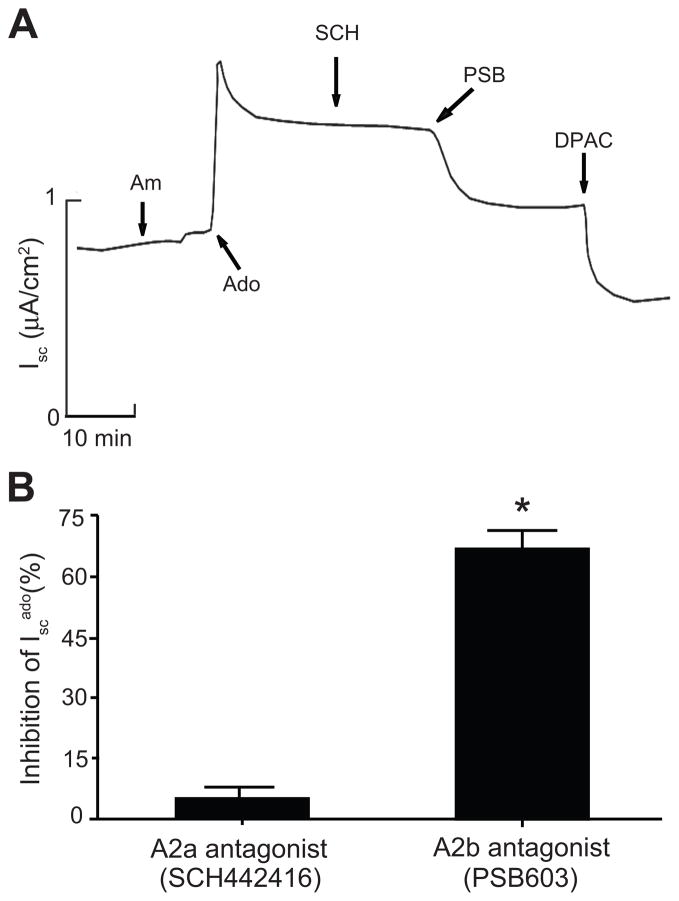
Given that the A1 (CPA) and A2a (CGS21680) receptor agonists both failed to stimulate basal Isc, we concluded that adenosine stimulates Cl− secretion by activating low affinity A2b receptors. To confirm this, we tested whether two highly specific A2a and A2b receptor antagonists could block Iscadenosine. Addition of PSB603 (A2b receptor antagonist) to the apical bath largely abolished Iscadenosine, whereas SCH442416 (A2a receptor antagonist) did not (Figure 5).
Figure 5.
A. Representative Isc trace showing actions of the specific A2a receptor antagonist SCH442416 and the specific A2b receptor antagonist PSB603 on adenosine-induced Isc in mIMCD-K2 cells. Am, 10−5 mol/L amiloride (apical addition); Ado, 10−5 mol/L adenosine (apical addition); SCH, 10−8 mol/L SCH442416 (apical addition); PSB, 10−8 mol/L PSB603 (apical addition); DPAC, 2 × 10−3 mol/L DPAC (apical addition). B. Percent inhibition of adenosine-induced Isc (Isc ado) upon the addition of SCH442416 or PSB603 to the apical bath. Values are mean ± SE (n = 19 filters). *P<0.05.
A2b receptors are expressed in mIMCD-K2 cells
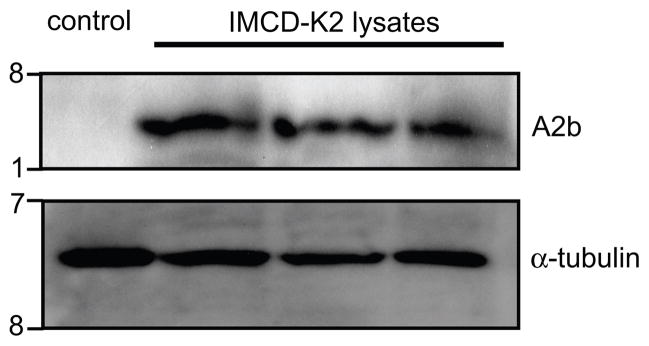
To verify that mIMCD-K2 cells express A2b receptors, we assessed A2b receptor expression by Western blotting using an anti-A2b receptor antibody. Western blotting showed an immunoreactive band of approximately 36 KDa, which matched the predicted size of mouse A2b receptor protein (Figure 6). Notably, mIMCD-K2 cells did not express A2a receptor by Western blotting (data not shown).
Figure 6.
A2b receptor protein expression in mIMCD-K2 cells by Western immunoblot. Representative immunoblot showing whole cell lysates from either negative control (Xenopus laevis oocyte) or 3 different passages of mIMCD-K2 cells probed with an anti-A2b receptor antibody. Blots were also probed with an anti-α-tubulin antibody to assess protein loading. kD, kiloDaltons.
A2b receptors signal through adenylate cyclase and PKA
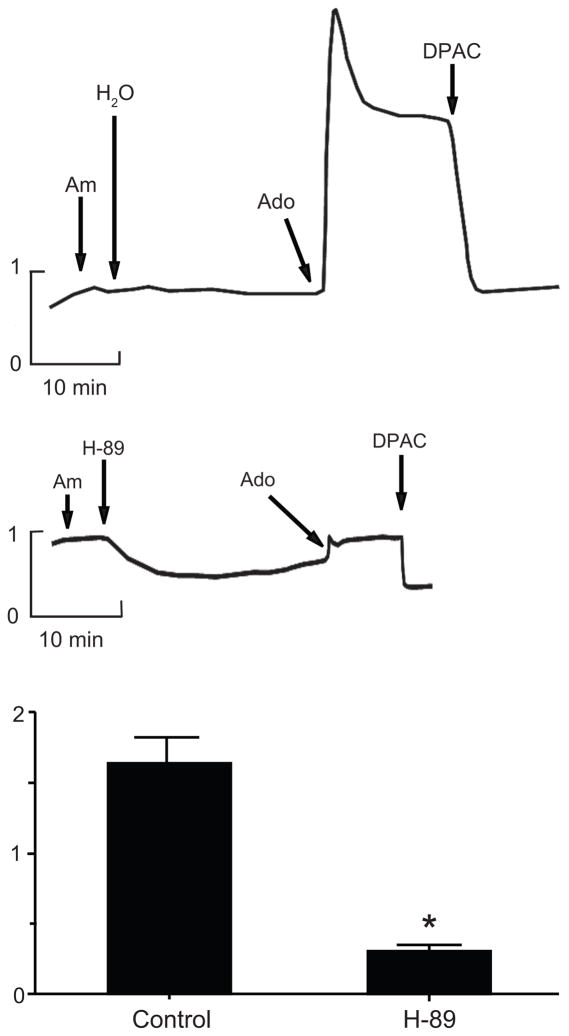
A2b receptors couple to the Gα protein subunit, Gαs, to stimulate adenylate cyclase and PKA catalytic activity 16. To show that A2b receptor activation signals through adenylate cyclase and PKA, we measured intracellular cAMP accumulation in response to apical application of adenosine to mIMCD-K2 cell sheets. Adenosine enhanced intracellular cAMP by over 40% compared to vehicle control (Figure S2). To determine if PKA activity is necessary for Iscadenosine, we incubated cell sheets with the PKA inhibitor H-89 prior to the addition of adenosine. H-89 completely blocked Iscadenosine (Figure 7).
Figure 7.
A, B. Representative traces of Isc showing the effect of pre-incubation with vehicle control (H2O) or PKA-inhibitor H-89 (10−5 mol/L) on adenosine-induced Isc in mIMCD-K2 cells. Am, 10−5 mol/L amiloride (apical addition); Ado, 10−5 mol/L adenosine (apical addition); DPAC, 2 × 10−3 mol/L DPAC (apical addition). C. Effect of H-89 on adenosine-induced Isc (Iscado). Values are mean ± SE (n = 17 cell sheets). *P < 0.05.
DISCUSSION
In this study, we used the mIMCD-K2 cell line to examine direct effects of adenosine on NaCl transport across IMCD epithelium. Our data show that adenosine stimulates Cl− secretion in mIMCD-K2 cells through CFTR by activating apical A2b receptors and signaling through the cAMP/PKA pathway. Our experiments further demonstrate that adenosine stimulates Cl− secretion in the presence of amiloride, suggesting that adenosine may play an important role in regulating ion transport under conditions of minimal ENaC activity. To our knowledge, this is the first demonstration of A2b receptor-mediated stimulation of Cl− secretion in a kidney model system.
Several lines of evidence support our conclusion that adenosine stimulates Cl− secretion by activating A2b adenosine receptors. First, adenosine and the potent adenosine analogue NECA stimulate Cl− secretion in the micromolar range, suggesting that adenosine action in mIMCD-K2 cells is mediated through low-affinity adenosine receptors. Second, the A1 receptor agonist CPA and the A2a receptor agonist CGS21680 both failed to reproduce the stimulatory effect of adenosine on Isc. Due to the lack of available A2b receptor agonists, A2b receptor activation must be inferred. The characteristic fingerprint of A2b receptor activation is stimulation with high concentrations of NECA but not with CGS21680 7, similar to what we observed in mIMCD-K2 cells. Third, both the non-selective A2 receptor antagonist ZM241385 and the specific A2b receptor antagonist PSB603 inhibited Iscadenosine, suggesting that A2b receptors are involved in Cl− secretion. Fourth, adenosine treatment of mIMCD-K2 cell sheets increased intracellular cAMP levels. Of the two types of low-affinity adenosine receptors, A2b and A3, only A2b receptors are coupled to Gαs and capable of increasing intracellular cAMP by stimulating adenylate cyclase activity 16. Fifth, mIMCD-K2 cells express A2b receptor immunoreactive protein as shown by Western blot analysis.
In an effort to provide additional support for our conclusion that A2b receptors stimulate Cl− secretion in mIMCD-K2 cells, we attempted to knock down A2b gene expression using lentiviral transduction of shRNAs. Although we could decrease A2b gene expression, we could not achieve knockdown in mIMCD-K2 cells without disturbing their intrinsic electrophysical properties. Cells treated with either scrambled control or A2b receptor-specific shRNAs failed to reach high Rte, likely reflecting significant cell toxicity. We were unable to make reliable Isc measurements (data not shown). Nevertheless, we believe our pharmacological and biochemical studies are sufficiently definitive to suggest an important role for A2b receptors in Cl− secretion in mIMCD-K2 cells.
A similar A2b receptor signaling pathway has been described in T84 intestinal epithelial cells 17–19. Strohmeier et al. have demonstrated that apical application of adenosine to T84 cell sheets activates A2b receptors to stimulate Cl− secretion 17. After several minutes of adenosine stimulation, A2b receptors are recruited to the apical plasma membrane, where it forms a receptor-signaling complex with the scaffold proteins E3KARP/ezrin, PKA, and CFTR 18. Thus, in both T84 and mIMCD-K2 cells, A2b receptors are linked to Cl− secretion at the apical side.
Our experiments demonstrate that adenosine stimulates a cellular response in mIMCD-K2 cells in the micromolar range, which is likely within the physiological range of adenosine concentration in the kidney medulla. Siragy et al. showed that the adenosine concentration in the interstitial fluid of the kidney medulla reaches 10−6 mol/L in rats maintained on a high salt diet 12. In human urine, adenosine concentrations have been reported to be between 0.9–9.9 × 10−6 mol/L20. Allowing for the possibility of cellular uptake, metabolism, and degradation of adenosine, we estimate that the local concentration of adenosine in the IMCD of human kidney may well exist in the range between 10−6 to 10−5 mol/L.
Moyer et al. have demonstrated that activation of basolateral A1 receptors inhibits arginine vasopressin (AVP)-stimulated Cl− secretionin mIMCD-K2 cells by reducing intracellular cAMP levels 11. In our study, we identify an additional adenosine receptor signaling pathway that stimulates Cl− secretion in mIMCD-K2 cells through the activation of apical A2b receptors. We believe our experimental findings expand the role of adenosine signaling in the IMCD, which could apply under conditions of high NaCl loading and serve as an adaptive response to stimulate Cl− transport and enhance urine NaCl excretion.
The IMCD is uniquely situated at the terminal end of the kidney tubular system, where it could have a significant role in the daily regulation of NaCl excretion. Although the classic view posits that net renal NaCl excretion is the difference between the quantity of filtered NaCl by the glomeruli and the quantity of reabsorbed NaCl by the kidney tubule, there is evidence for Cl− and fluid secretion in the rodent IMCD using retrograde microcatheterization 21 and isolated tubule microperfusion techniques 14. Sonnenberg and colleagues measured NaCl and fluid transport in rat IMCD in situ and observed that ECF volume expansion converted IMCD transport characteristics from net NaCl and fluid absorption to net secretion 21. According to Grantham et al., changes in net tubular excretion in the IMCD as low as 0.055% of the filtered NaCl load can be sufficient to cause significant changes in ECF volume over time 22. If our findings can be extrapolated to the in vivo setting, we estimate that adenosine-induced Cl− flux across mouse IMCD epithelium to be equivalent to 74 μEq Cl− secretion per day, which could constitute 0.261% of the filtered Cl− load (expanded calculations in Figure S3). Thus, the magnitude of the rates of adenosine-stimulated Cl− secretion in this study are in qualitative agreement with studies that support the view that the IMCD has an intrinsic capacity to secrete NaCl and fluid. However, the extent to which the observed rates of adenosine-stimulated secretion in mIMCD-K2 cells are quantitatively similar to the in vivo state remains to be determined.
PERSPECTIVES
In conclusion, we show that adenosine can activate apical A2b receptors to stimulate Cl− secretion through CFTR in mIMCD-K2 cells, a model system for murine IMCD. We propose that this adenosine receptor pathway may provide a mechanism for enhancing urine NaCl excretion in the face of high dietary NaCl intake in the whole animal. Adenosine-mediated stimulation of Cl− secretion in IMCD would induce chloriuresis (and subsequent natriuresis) in states of NaCl excess, which could help return the whole animal to salt balance. Moreover, defects in this pathway could contribute to the pathogenesis of salt-sensitive hypertension, since inappropriate control of renal salt handling may result in expansion of ECF volume and elevated blood pressure.
Supplementary Material
Acknowledgments
We are grateful to Dr. Bruce Stanton for providing mIMCD-K2 cells for our experiments. We thank Drs. Carol A. Charlton, Jonathan Widdicombe, Michael H. Humphreys, Glenn Chertow, and Vivek Bhalla for valuable discussions and helpful comments on the manuscript.
SOURCE OF FUNDING
This work was supported by grants from National Institutes of Health Grant K08-DK-073487 (A.C.P.), Amgen (2009 Amgen Nephrology Junior Faculty Research Support Program to A.C.P.), Satellite Healthcare (2008 Norman S. Coplon Extramural Grant to A.C.P.).
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
None.
References
- 1.Kizer NL, Vandorpe D, Lewis B, Bunting B, Russell J, Stanton BA. Vasopressin and cAMP stimulate electrogenic chloride secretion in an IMCD cell line. Am J Physiol. 1995;268:F854–861. doi: 10.1152/ajprenal.1995.268.5.F854. [DOI] [PubMed] [Google Scholar]
- 2.Vandorpe D, Kizer N, Ciampollilo F, Moyer B, Karlson K, Guggino WB, Stanton BA. CFTR mediates electrogenic chloride secretion in mouse inner medullary collecting duct (mIMCD-K2) cells. Am J Physiol. 1995;269:C683–689. doi: 10.1152/ajpcell.1995.269.3.C683. [DOI] [PubMed] [Google Scholar]
- 3.Kizer NL, Lewis B, Stanton BA. Electrogenic sodium absorption and chloride secretion by an inner medullary collecting duct cell line (mIMCD-K2) Am J Physiol. 1995;268:F347–355. doi: 10.1152/ajprenal.1995.268.2.F347. [DOI] [PubMed] [Google Scholar]
- 4.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 5.Di Sole F. Adenosine and renal tubular function. Curr Opin Nephrol Hypertens. 2008;17:399–407. doi: 10.1097/MNH.0b013e32830321e1. [DOI] [PubMed] [Google Scholar]
- 6.Jackson EK, Mi Z, Zhu C, Dubey RK. Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther. 2003;307:888–896. doi: 10.1124/jpet.103.057166. [DOI] [PubMed] [Google Scholar]
- 7.Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal. 2002;14:99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 8.Yaar R, Jones MR, Chen JF, Ravid K. Animal models for the study of adenosine receptor function. J Cell Physiol. 2005;202:9–20. doi: 10.1002/jcp.20138. [DOI] [PubMed] [Google Scholar]
- 9.Yagil Y. Interaction of adenosine with vasopressin in the inner medullary collecting duct. Am J Physiol. 1990;259:F679–687. doi: 10.1152/ajprenal.1990.259.4.F679. [DOI] [PubMed] [Google Scholar]
- 10.Yagil Y. Differential effect of basolateral and apical adenosine on AVP-stimulated cAMP formation in primary culture of IMCD. Am J Physiol. 1992;263:F268–276. doi: 10.1152/ajprenal.1992.263.2.F268. [DOI] [PubMed] [Google Scholar]
- 11.Moyer BD, McCoy DE, Lee B, Kizer N, Stanton BA. Adenosine inhibits arginine vasopressin-stimulated chloride secretion in a mouse IMCD cell line (mIMCD-K2) Am J Physiol. 1995;269:F884–891. doi: 10.1152/ajprenal.1995.269.6.F884. [DOI] [PubMed] [Google Scholar]
- 12.Siragy HM, Linden J. Sodium intake markedly alters renal interstitial fluid adenosine. Hypertension. 1996;27:404–407. doi: 10.1161/01.hyp.27.3.404. [DOI] [PubMed] [Google Scholar]
- 13.Zou AP, Wu F, Li PL, Cowley AW., Jr Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension. 1999;33:511–516. doi: 10.1161/01.hyp.33.1.511. [DOI] [PubMed] [Google Scholar]
- 14.Wallace DP, Rome LA, Sullivan LP, Grantham JJ. cAMP-dependent fluid secretion in rat inner medullary collecting ducts. Am J Physiol Renal Physiol. 2001;280:F1019–1029. doi: 10.1152/ajprenal.2001.280.6.F1019. [DOI] [PubMed] [Google Scholar]
- 15.Rajagopal M, Fischer H, Widdicombe JH. Hormonal and purinergic stimulation of bicarbonate secretion in oviducts of rhesus monkey. Am J Physiol Endocrinol Metab. 2008;295:E55–62. doi: 10.1152/ajpendo.00714.2007. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 18.Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem. 2002;277:33188–33195. doi: 10.1074/jbc.M202522200. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Kolachala V, Walia B, Balasubramanian S, Hall RA, Merlin D, Sitaraman SV. Agonist-induced polarized trafficking and surface expression of the adenosine 2b receptor in intestinal epithelial cells: role of SNARE proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1100–1107. doi: 10.1152/ajpgi.00164.2004. [DOI] [PubMed] [Google Scholar]
- 20.Barfuss DW, McCann WP, Katholi RE. Axial heterogeneity of adenosine transport and metabolism in the rabbit proximal tubule. Kidney Int. 1992;41:1143–1149. doi: 10.1038/ki.1992.174. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenberg H. Secretion of salt and water into the medullary collecting duct of Ringer-infused rats. Am J Physiol. 1975;228:565–568. doi: 10.1152/ajplegacy.1975.228.2.565. [DOI] [PubMed] [Google Scholar]
- 22.Grantham JJ, Wallace DP. Return of the secretory kidney. Am J Physiol Renal Physiol. 2002;282:F1–9. doi: 10.1152/ajprenal.2002.282.1.F1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.